Abstract
Purpose
Our objective is to describe the most prevalent electroencephalographic findings in COVID-19 hospitalized patients, and to determine possible predictors of mortality including EEG and clinical variables.
Methods
A multicentric prospective observational study in patients with COVID-19 requiring EEG during hospitalization.
Results
We found 94 EEG from 62 patients (55 % men, mean age 59.7 ± 17.8 years) were analyzed. Most frequent comorbidity was cardiac (52 %), followed by metabolic (45 %) and CNS disease (39 %). Patients required ICU management by 60 %, with a mortality of 27 % in the whole cohort. The most frequent EEG finding was generalized continuous slow-wave activity (66 %). Epileptic activity was observed in 19 % including non-convulsive status epilepticus, seizures and interictal epileptiform discharges. Periodic patterns were observed in 3 patients (3.2 %). Multivariate analysis found that cancer comorbidity and requiring an EEG during the third week of evolution portended a higher risk of mortality
Conclusion
We observed that the most prevalent EEG finding in this cohort was generalized continuous slow-wave activity, while epileptic activity was observed in less than 20 % of the cases. Mortality risk factors were comorbidity with cancer and requiring an EEG during the third week of evolution, possibly related to the hyperinflammatory state.
Keywords: COVID-19, Electroencephalogram, Mortality
1. Introduction
Coronavirus (COVID-19) is a novel severe acute respiratory syndrome caused by coronavirus 2 (SARS-CoV-2). Mortality is associated with risk factors such as older age, male gender, cancer, pulmonary and cardiovascular comorbidities [1].
Neurological involvement in COVID-19 has been described before and includes dizziness, headache, anosmia and ageusia [2]. Severe involvement of the central nervous system presents itself as encephalopathy, stroke, seizures [3], encephalitis and meningitis [4]. The last two affecting also young patients [5].
The electroencephalogram (EEG) is crucial when assessing patients with seizures and encephalopathy in COVID-19 disease. To date, small series show that epileptiform discharges or severe/critical EEG abnormalities occur in 30–40 % of patients with COVID-19 [6,7]. However, it is still unknown whether specific EEG findings could predict patients' outcomes.
The aim of our study is to describe the most prevalent electroencephalographic findings during COVID-19 and to analyze whether these findings, together with other clinical variables, are predictors of mortality in this inpatient cohort.
2. Material and methods
2.1. Patients
Demographic, clinical and paraclinical data from patients were prospectively registered in a database from May 1 st to June 15th, 2020 at the Pontificia Universidad Católica de Chile Clinical Hospital (UC, private health system) and the Hospital Dr. Sótero del Río (HSR, public health system). Adults (>18 years old) with COVID-19 were confirmed with positive detection by nucleic acid-based polymerase chain reaction (PCR), amplifying a specific genetic sequence of SARS-CoV-2, from nasopharyngeal swab (CDC qPCR Integrated DNA Technologies). All patients were studied with at least one portable EEG or EEG monitoring within four weeks from the onset of symptoms. Database collection for research was approved by the local Ethics Committee.
2.2. EEG
For portable EEG (duration of recording from 30 min to 1 h) and EEG monitoring (recording over 12 h) a Cadwell’s Easy II EEG system at UC and a Natus NeuroWorks at HSR with 21 electrodes using 10–20 montage at 200 Hz sampling rate plus electrocardiogram were used. EEGs were indicated in patients with unexplained loss of consciousness without major abnormalities on blood tests and/or neuroimaging. Seizures or suspicious events were also indication. We used the standardized terminology of the American Clinical Neurophysiology Society. Recordings were examined by two trained clinical neurophysiologists (I.S. and R.U-S-M) to define the main EEG findings using bipolar and monopolar montage.
2.3. Statistical analysis
Results were reported using mean ± standard deviation and percentages. Two groups, UC and HSR Centers were compared. Differences in qualitative variables between the groups were established using Fisher exact test. For quantitative variables, Mann–Whitney U test was used. Potential risk factors for mortality among recognized prognostic clinical variables and EEG findings were assessed. Binomial logistic regression was performed to analyze the probability of death using Relative Risk (RR) and 95 % confidence interval (95 % CI). First, we performed a univariate analysis and then selected for the multivariable analysis, all variables that had p < 0.1. Differences were considered significant at p < 0.05. For statistical analysis, IBM SPSS Statistics 21 was used.
3. Results
3.1. Clinical characteristics
Ninety-four EEGs from 62 patients were included in the study. Mean age was 59.7 ± 17.8 years and males represented 55 %. Cardiac comorbidity was the most frequent (51.6 %) including coronary heart disease, hypertension, and arrhythmias. These were followed by metabolic comorbidities (45.2 %, diabetes mellitus 2, hypothyroidism and obesity), and central nervous system (CNS) disease (38.7 %, brain tumours, stroke, traumatic brain injury, encephalitis and epilepsy) (Table 1 ).
Table 1.
Demographic, clinical and paraclinical variables.
| Total (EEG: 94) | UC (EEG: 72) | HSR (EEG: 22) | p-Value | |
|---|---|---|---|---|
| Patients | 62 | 44 | 18 | |
| Gender* (F/M) | 28 / 34 (45 % / 55 %) | 17 / 27 (39 % / 61 %) | 11 / 7 (61 % / 39 %) | 0.160 |
| Age* (years, X + SD) | 59.7 + 17.8 | 60.5 + 19.2 | 57.8 + 14.2 | 0.438 |
| Comorbidities* | ||||
| - Respiratory | 10 (16.1 %) | 5 (11.4 %) | 5 (27.8 %) | 0.137 |
| - Cardiac | 32 (51.6 %) | 22 (50 %) | 10 (55.6 %) | 0.786 |
| - Hepatic | 4 (6.5 %) | 2 (4.5 %) | 2 (11.1 %) | 0.573 |
| - Renal | 14 (22.6 %) | 8 (18.2 %) | 6 (33.3 %) | 0.315 |
| - Metabolic | 28 (45.2) | 18 (40.9 %) | 10 (55.6 %) | 0.400 |
| - Autoimmune systemic | 2 (3.2 %) | 1 (2.3 %) | 1 (5.6 %) | 0.500 |
| - CNS | 24 (38.7 %) | 11 (25 %) | 13 (72.2 %) | 0.001 |
| - Cancer | 4 (6.5 %) | 3 (6.8 %) | 1 (5.6 %) | 1.000 |
| - Others | 21 (33.9 %) | 11 (25 %) | 10 (55.6 %) | 0.038 |
| Type EEG# | ||||
| - Portable EEG | 87 (92.6 %) | 70 (97.2 %) | 17 (77.3 %) | 0.007 |
| - EEG Monitoring | 7 (7.4 %) | 2 (2.8 %) | 5 (22.7 %) | |
| EEG duration# (min, X + SD) | 89.3 + 189.7 | 48.9 + 113.5 | 221.4 + 303.1 | <0.001 |
| N° EEG per patients# (X + SD) | 1.5 + 1.1 | 1.6 + 1.3 | 1.2 + 0.4 | 0.422 |
| COVID-19 disease duration at EEG# | ||||
| - Time to EEG (days, X + SD) | 11.9 + 9.2 | 13.5 + 8.6 | 6.7 + 9.3 | <0.001 |
| - 1st week | 35 (37.2 %) | 21 (29.2 %) | 14 (63.6 %) | 0.005 |
| - 2nd week | 27 (28.7 %) | 24 (33.3 %) | 3 (13.6 %) | 0.106 |
| - 3rd week | 16 (17.0 %) | 13 (18.1 %) | 3 (13.6 %) | 0.755 |
| - 4th week | 18 (19.1 %) | 16 (22.2 %) | 2 (9.1 %) | 0.225 |
| EEG Findings# | ||||
| - Non-convulsive status epilepticus | 2 (2.1 %) | 1 (1.4 %) | 1 (4.5 %) | 0.415 |
| - Seizures | 2 (2.1 %) | 1 (1.4 %) | 1 (4.5 %) | 0.415 |
| - Interictal epileptiform discharges | 14 (14.9 %) | 9 (12.5 %) | 5 (22.7 %) | 0.304 |
| - Focal intermittent slow waves | 13 (13.8 %) | 10 (13.9 %) | 3 (13.6 %) | 1.000 |
| - Generalized intermittent slow waves | 34 (36.2) | 29 (40.3 %) | 5 (22.7 %) | 0.204 |
| - Continuous focal slowness | 3 (3.2 %) | 3 (4.2 %) | 0 (0%) | 1.000 |
| - Slow theta background with reactivity | 17 (18.1 %) | 14 (19.4 %) | 3 (13.6 %) | 0.754 |
| - Generalized continuous slow-wave (delta) | 62 (66.0 %) | 44 (61.1 %) | 18 (81.8 %) | 0.121 |
| - Generalized low voltage | 10 (10.6 %) | 7 (9.7 %) | 3 (9.7 %) | 0.555 |
| - Periodic pattern | 3 (3.2 %) | 2 (2.8 %) | 1 (4.5 %) | 0.694 |
| - Normal | 9 (9.6 %) | 8 (11.1 %) | 1 (4.5 %) | 0.680 |
| Neuroimaging* | ||||
| - No | 8 (12.9 %) | 7 (15.9 %) | 1 (5.6 %) | 0.418 |
| - CT | 42 (67.7 %) | 27 (61.4 %) | 15 (83.3 %) | 0.136 |
| - MRI | 16 (25.8 %) | 13 (29.5 %) | 3 (16.7 %) | 0.355 |
| - Neuroimaging injury | 26 (41.9 %) | 15 (34.1 %) | 11 (61.1 %) | 0.087 |
| Concordant lateralization of abnormalities between EEG and MRI# | 28 (29.8 %) | 17 (23.6 %) | 11 (50 %) | <0.001 |
| Sedation during EEG# | 38 (40.4 %) | 22 (30.6 %) | 16 (72.7 %) | 0.001 |
| Requirements* | ||||
| - ICU | 37 (59.7 %) | 22 (50 %) | 15 (83.3 %) | 0.022 |
| - IMV | 27 (43.5 %) | 16 (36.4 %) | 11 (61.1 %) | 0.095 |
| Hospital stay* (days, X + SD) | 23.8 + 18.3 | 23.4 + 20.9 | 23.8 + 18.4 | 0.520 |
| - Discharge | 32 (51.6 %) | 21 (47.7 %) | 11 (61.1 %) | 0.408 |
| - Transfer | 2 (3.2 %) | 1 (2.3 %) | 1 (5.6 %) | 0.500 |
| Mortality* | 17 (27.4 %) | 8 (18.2 %) | 9 (50 %) | 0.025 |
*analysis performed with all patients (n: 62). #analysis performed with all EEG (n: 94). UC: Clinical Hospital of the Pontificia Universidad Católica de Chile. HSR: Hospital Dr. Sótero del Río. F: Female. M: Male. X: mean. SD: standard deviation. Respiratory (asthma, COPD, smoking, pulmonary fibrosis). Cardiac (coronary heart disease, hypertension, arrhythmias). Hepatic (chronic liver disease, acute hepatic failure). Renal (chronic renal disease, acute renal disease). Metabolic (type 2 diabetes, hypothyroidism, Obesity). Autoimmune systemic (systemic lupus erythematosus, rheumatoid arthritis). Central nervous system, CNS (tumours, stroke, brain traumatic brain injury, encephalitis, epilepsy). Cancer (solid and haematological). CT: computed tomography. MRI: magnetic resonance imaging. ICU: intensive care unit. IMV: invasive mechanical ventilation.
Most of the patients (93.5 %) were studied with neuroimaging and 41.9 % (26 patients) had pathological findings. Acute findings were found in 12 patients (UC: 5 and HSR:7) including stroke n = 3, parenchymal hematomas n = 2, acute/chronic subdural hematomas n = 2, subarachnoid haemorrhage n = 2, cerebral venous thrombosis n = 2, brain oedema n = 2 and reversible posterior encephalopathy syndrome n = 1. Chronic findings were found in 16 patients (UC: 12 and HSR: 4) with non-specific white matter hyperintensities n = 10, stroke n = 7, parenchymal hematomas n = 1 and other n = 2.
In patients who required antiepileptic drugs (n = 12), the most frequent treatment was levetiracetam (58 %), followed by phenytoin (40 %), clobazam (25 %), valproic acid (8%) and lacosamide (8%). Sixty percent of the patients required management in critical care units (ICU) and 43.5 % required invasive ventilation. The mortality in this cohort reached 27.4 % (Table 1).
Some differences were found between the two centers (UC and HSR). CNS comorbidities were more frequent in HSR, 72.2 % versus 25 % in UC, p: 0.001. In HSR, a higher percentage of patients were in ICU, 83.3 % versus 50 %, p: 0.022 and mortality was also higher, 50 % versus 18.2 %, p: 0.025 (Table 1).
3.2. EEG findings
Most recordings were portable EEGs (92.6 %) with a mean duration of 89.3 + 189.7 min corresponding to 1.5 + 1.1 EEG per patient. Most EEG (65.9 %) were performed during the first 2 weeks of COVID-19 with a mean of 11.9 + 9.2 days from symptoms onset (Table 1).
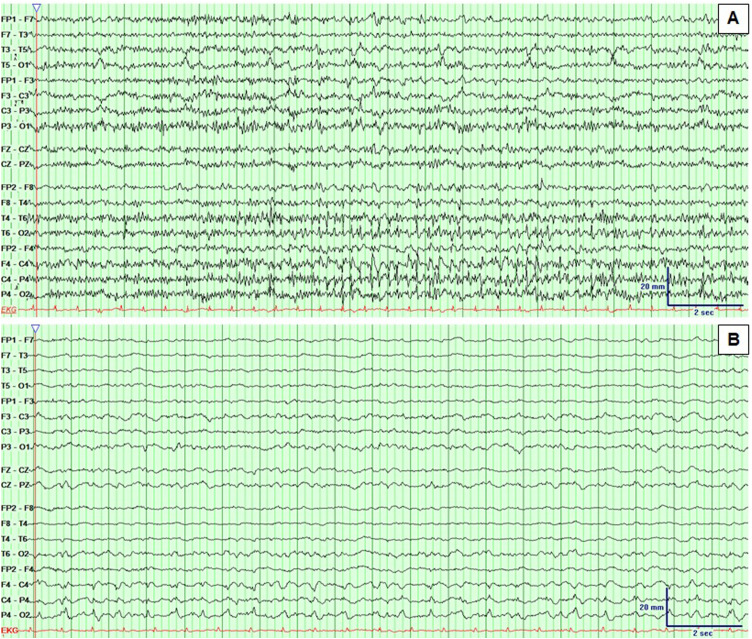
The most frequent EEG finding was the generalized continuous slow-wave delta activity (66 %) followed by generalized intermittent slow waves (36.2 %). Epileptic activity corresponded to 19.1 % and included non-convulsive status epilepticus, seizures and interictal epileptiform discharges. Periodic patterns were observed in 3 patients (3.2 %) (Table 1). The localization of the interictal activity (from 14 EEG) was generalized in 21 %. The rest was focal and corresponded to the frontal lobe 86 %, temporal lobe 36 %, parietal lobe 21 % and occipital lobe 21 %. The ictal onset zone of the two patients who had seizures was focal. Bifrontal and right temporoparietal lobes respectively. Non-convulsive status epilepticus was bifrontal in one patient, and right frontal in other patient (Supplementary figure 1). When correlating the EEG findings, 28 EEGs (30 %) were spatially concordant with the abnormalities visible in the neuroimaging.
There were differences between both centers. A higher percentage of EEG monitoring was performed at HSR, 22.7 % versus 2.8 % at UC, p: 0.007, presenting a longer duration of recordings 221.4 + 303.1 min versus 48.9 + 113.5 min at UC, p < 0.001. EEGs were performed earlier at HSR compared to UC, 6.7 + 9.3 days versus 13.5 + 8.6 days, p < 0.001, the majority at HSR during the first week (63.3 %). The concordant lateralization of the abnormalities between EEG and neuroimaging was greater at HSR, 50 % vs. 23.6 % in UC, p < 0.001. EEGs were more frequently performed under sedation at HSR compared to UC, 72.7 % versus 30.6 %, p: 0.001 (Table 1).
3.3. Risk factors for mortality
In the univariate analysis, we found 4 risk factors for mortality: The HSR center, respiratory and cancer comorbidities and requiring an EEG during the third week of COVID-19 evolution. One variable presented to elicit a more favorable outcome: the presence of generalized intermittent slow waves in EEG (Table 2 ). Multivariate analysis found that cancer and requiring an EEG during the third week of evolution had a higher risk of mortality (Table 2).
Table 2.
Univariate and multivariate analysis of factors associated with mortality.
| Univariate analysis (p < 0.1) |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variables | RR | 95 %CI | p-value | RR | 95 %CI | p-value |
| HSR Center | 5.0 | 1.8−14 | 0.002 | 4.8 | 0.46−49 | 0.190 |
| Respiratory comorbidity | 7.3 | 1.9−28 | 0.004 | 2.9 | 0.37−23 | 0.313 |
| Cancer comorbidity | 12.2 | 2.3−65 | 0.004 | 56 | 3.5−903 | 0.004 |
| EEG at 3rd week | 4.2 | 1.4−13 | 0.013 | 11 | 1.3−93 | 0.027 |
| Generalized intermittent slow waves | 0.3 | 0.09−0.9 | 0.038 | 0.12 | 0.01−1.5 | 0.102 |
| EEG monitoring | 4.8 | 0.9−23 | 0.053 | 3.1 | 0.13−74 | 0.488 |
| Generalized low voltage | 3.7 | 0.96−14 | 0.058 | 3.8 | 0.53−27 | 0.185 |
| Sedation during EEG | 2.4 | 0.92−6.2 | 0.074 | 2.3 | 0.37−14 | 0.376 |
| Concordant lateralization of abnormalities between EEG and MRI | 2.4 | 0.88−6.7 | 0.087 | 5.3 | 0.58−49 | 0.138 |
4. Discussion
The main findings in this study showed that the most frequent EEG abnormality was generalized continuous slow-wave activity and that epileptic activity occurred in almost 20 % of cases. We found on multivariate analysis that cancer as a comorbidity and an EEG required during the third week of COVID-19 infection were independent risk factors for mortality in this specific cohort including mainly critical ill patients.
Our prevalence of non-convulsive status epilepticus and seizures on EEG was 4.2 %, below that observed in other infectious pathologies that directly affect the CNS with prevalence near to 30 % [8] and also below the 10–15 % found in patients hospitalized for medical conditions without CNS involvement [9]. The same occurred in terms of epileptic activity, we found a prevalence of 19 % compared to 30–40 % in recent reports of COVID-19 [6,7]. Although a small case series (10 patients with COVID-19) found interictal discharges in only one patient [10]. In our case, one patient with non-convulsive status epilepticus and one patient with seizures were managed in ICU, the other 2 patients were managed in intermediate care units.
The finding of requiring an EEG at the third week of COVID-19 as an independent risk factor is interesting. It is known that this period can be related to a hyperinflammatory phase with a cytokine storm, which accompanies the acute respiratory distress syndrome, alterations of consciousness and multiple organ failure [11]. Requiring an EEG study during that time might be a marker of altered consciousness, which might be multifactorial, especially in the more severe patients, leading to worse prognosis associated to mortality.
In the univariate analysis, we found that HSR center had a higher risk of mortality, which could be explained by a greater number of seriously ill patients, who had more comorbidities affecting the CNS and required ICU management and sedation, although this variable was not statistically significant in the multivariate analysis. Comorbidities were similar to those published in previous reports (respiratory and cancer) [1] which supports the external validity of our findings.
Other variables have been described as prognostic in patients with other brain or systemic conditions that have required EEG studies [12], such as requiring EEG monitoring, sedation with a lower voltage EEG and concordant lateralization of abnormalities between EEG and neuroimaging, although we did not observe statistical significance results in the multivariate analysis. Also, we did not find that age or gender were prognostic factors as in other reports [1], probably due to our smaller number of patients.
There are several limitations to our study. The most important is the small sample size and the lack of inclusion of some recognized prognostic variables in critically ill patients such as the high fraction of inspired oxygen (FiO2), high positive end-expiratory pressure or low PaO2:FiO2 ratio and patients admitted to hospitals with fewer ICU beds [1]. Prospective studies including some of these variables or others from ICU would provide more information for the recognition of portended mortality outcomes and to validate the importance and reliability of our results for its use in clinical practice.
5. Conclusion
Our results show a lower prevalence of epileptic activity than previously reported in patients with COVID-19, and we found that the presence of cancer and the need of an electroencephalographic study during the third week of COVID-19 evolution were independent risk factors for mortality.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure
All authors have nothing to disclose.
Acknowledgements
We thank the staff of electroencephalography readers and the electroencephalography laboratory team for their permanent dedication to patients in these difficult times. We also thank Ethel Ciampi and Christian Cantillano for helping with the manuscript editing.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.seizure.2020.10.007.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Gupta S., Hayek S.S., Wang W., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US [published online ahead of print, 2020 jul 15] JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China [published online ahead of print, 2020 apr 10] JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review [published online ahead of print, 2020 May 29] JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benameur K., Agarwal A., Auld S.C., et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, georgia, USA, 2020 [published online ahead of print, 2020 jun 2] Emerg Infect Dis. 2020;26(9) doi: 10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanopoulou A.S., Ferastraoaru V., Correa D.J., et al. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open. 2020;5(2):314–324. doi: 10.1002/epi4.12399. Published 2020 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrescu A.M., Taussig D., Bouilleret V. Electroencephalogram (EEG) in COVID-19: A systematic retrospective study [published online ahead of print, 2020 Jun 25] Neurophysiol Clin. 2020;S0987-7053(20) doi: 10.1016/j.neucli.2020.06.001. 30057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taraschenko O., Gaspard N. In: Handbook of ICU EEG monitoring. 2nd ed. LaRoche S., Haider H., editors. Springer Publishing Company; New York: 2018. Infectious and inflammatory conditions; pp. 76–91. [Google Scholar]
- 9.Dhakar M., Hantus S., Gilmore E. In: Handbook of ICU EEG monitoring. 2nd ed. LaRoche S., Haider H., editors. Springer Publishing Company; New York: 2018. EEG monitoring in the medical ICU; pp. 123–131. [Google Scholar]
- 10.Canham L.J.W., Staniaszek L.E., Mortimer A.M., et al. Electroencephalographic (EEG) features of encephalopathy in the setting of Covid-19: a Case Series. Clin Neurophysiol Pract. 2020 doi: 10.1016/j.cnp.2020.06.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudzinski L. In: Handbook of ICU EEG monitoring. 2nd ed. LaRoche S., Haider H., editors. Springer Publishing Company; New York: 2018. Prognosis in patients without cardiac arrest; pp. 116–122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.