Abstract
Background
Reversible splenial lesion syndrome (RESLES) is characterized by a temporary lesion in the splenium of the corpus callosum, emerging related to encephalitis, seizures, antiepileptic drug withdrawal, or metabolic disturbances. Among RESLES, mild encephalitis/encephalopathy with reversible splenial lesion (MERS) has been defined as a distinct clinicoradiologic syndrome associated with viral infections.
Case presentation
We report two children with multisystem inflammatory syndrome-children related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) who developed RESLES during the disease course. Encephalopathy was the main central nervous system symptom. Both of the children showed a rapid recovery, and brain magnetic resonance imaging revealed complete resolution of the splenial lesion within 1 week.
Conclusion
The complete resolution of the splenial lesion and rapid recovery from encephalopathy in RESLES associated with SARS CoV-2 were similar to observed in MERS.
Keywords: Coronavirus, Corpus callosum, Child, Encephalitis
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been defined as a pandemic since March 11, 2020, by the World Health Organization [1]. On the same days, the first case with COVID-19 was confirmed in Turkey, and as of Sep 7, 2020, 279,806 confirmed cases and 6673 deaths related to the COVID-19 pandemic were reported [2]. Besides respiratory symptoms, neurological disturbances consisted of acute cerebrovascular events, Guillain-Barre syndrome, hemophagocytic lymphohistiocytosis, encephalitis, acute necrotizing hemorrhagic encephalopathy, and mild encephalitis/encephalopathy with reversible splenial lesion (MERS) were defined related to COVID-19 in adults [3], [4], [5]. In children with COVID-19, an immune-mediated syndrome called pediatric multisystem inflammatory syndrome was described, and some of these children developed neurological symptoms and splenial changes on brain imaging [6].
Reversible splenial lesion syndrome (RESLES) is characterized by a temporary lesion in the splenium of the corpus callosum, emerging related to encephalitis, seizures, antiepileptic drug withdrawal, or metabolic disturbances [7]. Among RESLES, MERS has been defined as a distinct clinicoradiologic syndrome associated with viral infections, including mumps, influenza A, adenovirus, varicella-zoster virus, dengue virus, and echovirus 6 [8], [9], [10]. MERS is the most common form of RESLES in childhood [11]. The pathophysiology of the disease is unclear. However, the clinical spectrum of the disease seems highly identical irrespective of causative microorganism [8]. Herein, we aim to define the clinical and radiologic features of two children with RESLES related to SARS-CoV-2 infection.
2. Case report
The written informed consent to publication has been obtained from the parents on behalf of the patients.
2.1. Case 1
A 10-year-old previously healthy boy presented with visual hallucinations and diarrhea after 2 days of fever, diffuse rash, swelling in his hands and feet. His immunization status was appropriate to his age. There was no sick contact history.
On admission, he was agitated and disoriented in time and place. Physical examination showed a diffuse erythematous rash that localized on his trunk, bilateral axillary and inguinal regions spreading up to his thighs and arms. The nasopharyngeal swab for SARS CoV-2 with real-time polymerase chain reaction (RT-PCR) with one week apart remained negative. Clinical, laboratory, and imaging findings are shown in Table 1 .
Table 1.
Clinical, laboratory and imaging findings of children with SARS CoV-2.
| Patient 1 | Patient 2 | ||
|---|---|---|---|
| Age (years) | 10 | 11 | |
| Sex | Male | Female | |
| Initial central nervous system manifestations | Personality changes Hallucinations |
Personality changes | |
| Respiratory symptoms | Dyspnea, tachypnea | Dyspnea, tachypnea, subcostal retractions | |
| Blood pressure (mm HG) | 99/62 | 91/45 | |
| Pulse rate (per min) | 136 | 140 | |
| SaO2 | 98 | 98 | |
| Laboratory findings | White blood cell (per μL) | 8990 | 6920 |
| Platelet (per μL) | 124,000 | 113,000 | |
| CRP (mg/L) | 392 | 456 | |
| Procalcitonin (ng/mL) (N: 0–0.5) |
114 | 32 | |
| IL-6 (pg/mL) (N: 0–7) |
66 | 898 | |
| Ferritin (µg/L) (N: 24–336) |
341 | 533 | |
| Troponin I (ng/ml) (N: 0–17.5) |
321 | 182 | |
| Pro-BNP (ng/L) (N: 0–133) |
13,800 | 35,000 | |
| Fibrinogen (mg/dL) (N: 200–400) |
615 | 794 | |
| D-dimer (µg FEU/mL) (N: 0–0.5) |
1.19 | 1.7 | |
| Albumin (g/L) | 33 | 21 | |
| Natrium (mmol/L) | 131 | 132 | |
| AST (U/L) | 34 | 37 | |
| ALT (U/L) | 24 | 37 | |
| CSF | Glucose (mg/dL) | 92 | N/A |
| Protein (mg/dL) | 20 | N/A | |
| Cell count (/mm3) | none | N/A | |
| Oligoclonal band | negative | N/A | |
| Chest X-ray | Prominent bronchovascular markings | Prominent bronchovascular markings | |
| EEG | Diffuse slowing | Diffuse slowing | |
| Brain MRI | on admission | Hyperintensity in the splenium of the corpus callosum | Hyperintensity in the splenium of the corpus callosum |
| on day 7 | normal | normal | |
| Immune treatment | IVIG (2 g/kg, 1 dose) MP (1 mg/kg/day) |
IVIG (2 g/kg, 1 dose) MP (20 mg/kg/day, 2 days)MP (1 mg/kg/day) |
Abbreviations: AST = Aspartate aminotransferase, ALT = Alanine aminotransferase, CSF = Cerebrospinal fluid, CRP = C-reactive protein, EEG = Electroencephalography, IL-6 = Interleukin 6, IVIG = Intravenous immunoglobulin, MP = Methylprednisolone, MRI = Magnetic resonance imaging, N/A = not available, pro-BNP = Pro–B-type natriuretic peptide.
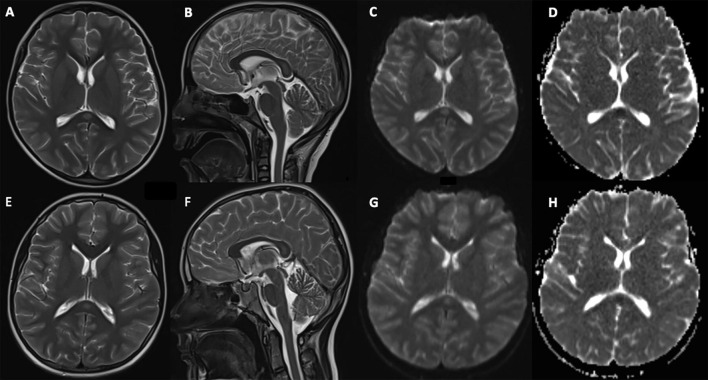
An echocardiogram revealed a reduction of ventricular systolic functions and mitral insufficiency. Brain magnetic resonance imaging (MRI) showed hyperintensity on T2-weighted images in the splenium of the corpus callosum with restricted diffusion (Fig. 1 ). Electroencephalography revealed diffuse slowed background activity. He was admitted to the pediatric intensive care unit (PICU) with a diagnosis of multisystem inflammatory syndrome in children (MIS-C). Intravenous immunoglobulin (IVIG) (2 g/kg) was administered, which was followed by subfebrile fever. He was started on a 7-day course of oral prednisone (1 mg/kg/day for 1 week) on day 3, then prednisone was tapered over 1 week. His hallucinations, agitation, and personality changes regressed on day 3. Rashes and fever regressed after methylprednisolone treatment. Repeated brain MRI on day 7 was normal, indicating the resolution of the lesion in the splenium of the corpus callosum. He discharged from the hospital on day 11, recovering completely. One week after discharge, anti-SARS-CoV-2 IgM and IgG were detected in his serum.
Fig. 1.
Brain magnetic resonance imaging of children. T2-weighted axial (A), sagital (B), diffusion-weighted image (C), and apparent diffusion coefficient (D) images of patient 1 show hyperintensity and restricted diffusion in the splenium of the corpus callosum. T2-weighted axial (E), sagital (F), and diffusion-weighted image (G), and apparent diffusion coefficient (H) images of patient 2 reveal hyperintensity and restricted diffusion in the splenium of the corpus callosum.
2.2. Case 2
A previously healthy 11-year-old girl presented with personality changes following 6 days of fever, 3 days of diarrhea, and rash. Her immunization status was appropriate for her age. There was no sick contact history.
On admission, she was conscious but agitated. She had conjunctivitis, diffuse rash on her trunk and extremities, and decreased breath sounds at the lung bases. On admission, she was negative for SARS CoV-2 on nasopharyngeal swab with RT-PCR but positive for anti-SARS CoV-2 IgM and IgG.
Reduction in systolic functions and mitral insufficiency were detected with echocardiography. Brain MRI revealed hyperintensity on T2-weighted images in the splenium of the corpus callosum with restricted diffusion (Figure I). She was hospitalized in PICU with a diagnosis of MIS-C. Milrinone (0.5 μg/kg/min) and noradrenaline (0.05 µg/kg/min) infusion was started due to diastolic hypotension. She was treated with IVIG (2 g/kg), then fever persisted. Methylprednisolone (20 mg/kg/day, 2 days) therapy was administered due to the persistence of fever and myocardial dysfunction despite increased infusion rate of milrinone treatment (0.75 µg/kg/min) on day 3. Agitation and personality changes resolved on day 4. She treated with low-dose methylprednisolone (2 mg/kg/day) for 1 week, then the dose was reduced gradually. She improved gradually. Brain MRI showed no abnormalities on day 7. She discharged home on day 10, recovering completely.
3. Discussion
We, herein, describe two children with RESLES while suffering from MIS-C associated with SARS CoV-2. Both cases were diagnosed with SARS CoV-2 infection based on the detection of anti-SARS-CoV-2 IgG and IgM. In a single-center case-series study focusing on neuroimaging findings of children with SARS CoV-2 infection, the presence of splenial lesions was notified in 4 children with MIS-C. In this report, all 4 children had neurologic findings, and the main central nervous system manifestation was encephalopathy [6]. The encephalopathy presenting with agitation, visual hallucinations, and personality changes were the common finding in our cases, in keeping with the previous report.
We did not observe any difference between RESLES associated with SARS CoV-2 infection and MERS in terms of clinical and radiological features. The typical findings were the complete resolution of the splenial lesion, relatively rapid and complete recovery from encephalopathy [8]. However, our patients received immune therapy due to MIS-C which makes it is difficult to interpret whether the spontan resolution of the splenial lesion will occur in children with SARS CoV-2. Hayashi et al. reported an adult patient with preceding neurologic comorbidities developing MERS associated with COVID-19. They notified the resolution of neurologic symptoms in 1 week [4]. EL Aoud S et al. described the improvement in neurologic disturbances in 1 week in an adult patient with MERS related to SARS CoV-2 infection and show the resolution of the splenial lesion at 1-month follow-up MRI without any immune-modulatory therapy [5].
In our study, the children with RESLES already had a diagnosis of MIS-C related to SARS CoV-2. Besides various microbial agents, children developing MERS as a neurologic complication of Kawasaki disease (KD) were reported [13]. MIS-C, characterized by multisystem involvement, is associated with prolonged fever and rash, resembling KD [14]. One could be hypothesized that the underlying pathophysiologic mechanism of the occurrence of the splenial lesion in the corpus callosum for both KD and MIS-C may be similar. However, it is unknown whether the splenial lesion in the corpus callosum emerges without any neurologic symptoms in children with MIS-C, as described in patients with KD [13].
Our case series has a limitation. Both cases were diagnosed with SARS CoV-2 infection based on the detection of anti-SARS-CoV-2 IgG and IgM. Single IgG and IgM tests have been defined as efficient to diagnose SARS CoV-2 infection in the clinical practice [12]. However, it should be stated that the SARS CoV-2 infection was confirmed neither by RT-PCR nor paired serology.
In conclusion, we described the clinical course of children with RESLES associated with SARS CoV-2 infection in the spectrum of MIS-C. The complete resolution of the splenial lesion and rapid recovery from encephalopathy in RESLES associated with SARS CoV-2 were similar to observed in MERS.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.World Health Organization (WHO) Coronavirus disease (COVID-19) Situation Report 2020-104 [Internet]. [cited 2020 May 7]. Available at https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200503-covid-19-sitrep-104.pdf?sfvrsn=53328f46_2.
- 2.World Health Organization (WHO) Coronavirus Disease (COVID-19) Dashboard-Turkey [Internet]. [cited 2020 Sep 7]. Avaliable at https://covid19.who.int/region/euro/country/tr.
- 3.Bridwell R., Long B., Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38:1549.e3–1549.e7. doi: 10.1016/j.ajem.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi M., Sahashi Y., Baba Y., Okura H., Shimohata T. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J Neurol Sci. 2020;415:116941. doi: 10.1016/j.jns.2020.116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Aoud S., Sorial D., Selmaoui A., Menif I., Lazard M., Si Hocine M. A first case of Mild Encephalitis with Reversible Splenial Lesion (MERS) as a presenting feature of SARS-CoV-2. Rev Neurol. 2020 doi: 10.1016/j.neurol.2020.06.001. S0035-3787(20)30606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Mannan O., Eyre M., Löbel U., Bamford A., Eltze C., Hameed B. Neurologic and radiographic findings associated With COVID-19 infection in children. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2687. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Monco J.C., Cortina I.E., Ferreira E., Martinez A., Ruiz L., Cabrera A. Reversible splenial lesion syndrome (RESLES): what’s in a name? J Neuroimaging. 2011;21:e1–e14. doi: 10.1111/j.1552-6569.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 8.Tada H., Takanashi J., Barkovich A.J., Oba H., Maeda M., Tsukahara H. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63:1854–1858. doi: 10.1212/01.wnl.0000144274.12174.cb. [DOI] [PubMed] [Google Scholar]
- 9.Fong C.Y., Khine M.M., Peter A.B., Lim W.K., Rozalli F.I., Rahmat K. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) due to dengue virus. J Clin Neurosci. 2017;36:73–75. doi: 10.1016/j.jocn.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 10.Masiello E., Gatto A., Lazzaresc I., Rigante D., Mariotti P., Valentini P. Mild encephalopathy with reversible splenial lesion associated with echovirus 6 infection: a case report and review of the literature. Turk J Pediatr. 2020;62:293–309. doi: 10.24953/turkjped.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Chen W.X., Liu H.S., Yang S.D., Zeng S.H., Gao Y.Y., Du Z.H. Reversible splenial lesion syndrome in children: Retrospective study and summary of case series. Brain Dev. 2016;38:915–927. doi: 10.1016/j.braindev.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z.L., Hou Y.L., Li D.T., Li F.Z. Diagnostic efficacy of anti-SARS-CoV-2 IgG/IgM test for COVID-19: a meta-analysis. J Med Virol. 2020;22 doi: 10.1002/jmv.26211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato T., Ushiroda Y., Oyama T., Nakatomi A., Motomura H., Moriuchi H. Kawasaki disease-associated MERS: Pathological insights from SPECT findings. Brain Dev. 2012;34:605–608. doi: 10.1016/j.braindev.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021756. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]