Abstract
Purpose of review
Exploring the molecular details of the coevolution of HIV-1 Envelope with broadly neutralizing antibodies (bNAbs) in infected individuals over time provides insights for vaccine design. Since mid-2017, the number of individuals described in such publications has nearly tripled. New publications have extended such studies to new epitopes on Env and provided more detail on previously known sites.
Recent findings
Studies of two donors – one of them an infant, the other with three lineages targeting the same site – has deepened our understanding of V3-glycan-directed lineages. A V2-apex-directed lineage showed remarkable similarity to a lineage from a previously described donor, revealing general principles for this class of bNAbs. Understanding development of CD4 binding site antibodies has been enriched by the study of a VRC01-class lineage. Finally, the membrane-proximal external region is a new addition to the set of epitopes studied in this manner, with early development events explored in a study of three lineages from a single donor.
Summary
These studies provide templates for immunogen design to elicit bNAbs against a widened set of epitopes, generating new directions in the quest for an HIV vaccine.
Keywords: evolution, HIV, neutralizing antibody, next-generation sequencing
INTRODUCTION
The last decade was marked by the groundbreaking discovery of HIV-1 broadly neutralizing antibodies (bNAbs) with exceptional potency and breadth, neutralizing most circulating HIV-1 strains [1]. Their effectiveness at preventing infection and reducing viral load in passive transfer animal models renewed hope that an HIV-1 vaccine might be achievable [2]. However, unusual features exhibited by many bNAbs including long heavy chain complementarity determining region 3s (CDRH3s), high levels of somatic hypermutation (SHM), insertion–deletion events (indels), and auto- or poly-reactivity, suggested long and complex maturation pathways not easily reproducible by vaccination. These observations, along with the failure of traditional vaccine strategies, led the field to consider vaccine regimens based on detailed studies of bNAb ontogeny during infection [3]. This approach relies on the identification and characterization of antibody precursors (unmutated common ancestors or UCAs) and key intermediates, as well as the relevant viral Envelope (Env) variants eliciting and driving the maturation of bNAb lineages. Enabled by technologies include rapid mAb isolation from single B-cells, next-generation sequencing (NGS) of the B-cell repertoire and viral Env populations, and functional and structural analyses of antibody/Env interactions, such studies reveal a roadmap for vaccine design.
Given the limited number of donors for whom samples exist allowing full Env/bNAb coevolution studies – the small fraction of individuals that develop bNAbs, the small number of longitudinal cohorts of untreated individuals, and the lack of new cohorts with the advent of early antiretroviral treatment – many studies lack one or more of these elements. Some use NGS samples from chronic-infection timepoints, or cloned antibodies only, to reconstruct precursors known as germline revertants [4-10]. Such constructs generally use mature CDRH3s and are therefore less relevant than true UCAs, yet some have provided important insights later validated by coevolution studies. This review focuses on studies of donors with longitudinal samples from the time of infection, in which both Envs and bNAb lineages were evaluated at a richly detailed molecular level.
INSIGHTS INTO SPECIFIC EPITOPES
The Env/bNAb coevolution studies reported to date and reviewed here describe the development of bNAb lineages targeting four epitope regions [11-14] (Fig. 1 and Table 1): the CD4-binding site (CD4bs) (CH103 and CH235 from donor CH505; PCIN63 from donor PC63), the V2-apex (CAP256-VRC26 from donor CAP256; PCT64 from donor PC64), the V3-glycan high-mannose patch (PCDN from donor PC76; DH270 from donor CH848; BF520.1 from infant donor BF520; PCIN39 from donor PC39), and the membrane-proximal external region (MPER) (VRC42, VRC43, and VRC46 from donor RV217-40512).
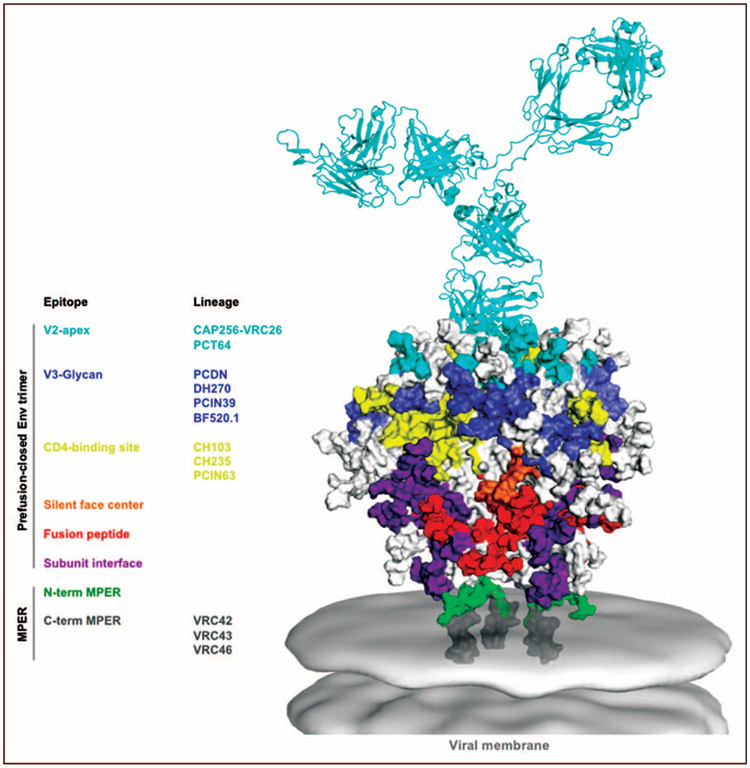
FIGURE 1.
Sites of vulnerability on the HIV-1 Env trimer. Model shows Env trimer as determined by cryo-electron microscopy and X-ray crystallography. Epitopes of known bNAbs are shown with their footprints color-coded. Viral membrane is shown in light gray. bNAbs from lineages described in the text are color-coded by epitope. N-terminal and C-terminal portions of MPER are distinguished by color. The CAP256-VRC26.09 bNAb (cyan) is shown for scale. bNAbs, broadly neutralizing antibodies; MPER, membrane-proximal external region. Source: Image adapted from Refs. [11-14].
Table 1.
Characteristics of broadly neutralizing antibody lineages that have been the subject of Env/antibody coevolution studies
| Epitope | Characteristics of bNAbs |
Donor | Sampling | Ab lineage |
Breadth median IC50 |
VH-gene SHM (nt) |
VL-gene | CDR3 length |
indels | Poly- auto- reactivity |
Lineage emergence |
Breadth in lineage |
Serum breadth |
Viral subtype |
Viral sequencing |
UCA binds/ neutralizes T/F |
Escape | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4bs | Multiple classes:-VH-generestricted: conserved angle of approach * VRC01-class: VH1-2, 5aa CRDL3, flexible/short CDRL1 loop *VH1-46 restricted - CDRH3 binder: Various angles of approach, gene usage and structural characteristics Highly somatically mutated (mean 28.4%] Need to accomodate surrounding glycans (N276 in loopD, N462 in V5-loop] | CH505 | 4–160 wpi | CH103 | 55% 4.54 μg/ml | VH4-59 14% | VL3-1 | H: 15 aa L: 10 aa | Yes Del CDRL1 | ++ | 14 wpi | 144 wpi | 84 wpi | C | Yes | Neutralization | LoopD V5 loop CD4bs loop | [24-25] |
| CH505 | 4–323 wpi | CH235 | 90% 0.6 μg/ml | VH1-46 28% | VK3-15 | H: 15 aa L: 8 aa | No | ++ | 14 wpi | 152 wpi | 84 wpi | C | Yes | Weak binding | LoopD V5 loop CD4bs loop | [23,24] | ||
| PC063 | 7–322 wpi | PCIN63 | 80% 0.24 μg/ml | VH1-2 15% | VK1-5 | H: 15 aa L: 5 aa | No | Variable | 168 wpi | 289 wpi | 266 wpi | C | Yes | No binding | LoopD V5 loop CD4bs loop | [26■■] | ||
| V2-glycan | Long (25–39aa) anionic CDHR3 Majority VH3 + DH3 genes Limited angles of approach Glycan binding (N160, N156) V2 C-strand K/R residues (166, 169, 171] | CAP256 | 24–206 wpi | VRC26-CAP256 | 63% 0.003 μg/ml | VH3-30 12% | VL1-51 | H: 36 aa L: 13 aa | No | - | 38 wpi | 119 wpi | 84 wpi | C | Yes | Neutralization | 166 and 169 | [11,28,30] |
| PC064 | 14–200 wpi | PCT64 | 37% 0.42 μg/ml | VH3-15 13% | VK3-20 | H: 25aa L: 8 aa | No | +/− | 43 wpi | 80 wpi | 80 wpi | A | Yes | Binding to 293S-expressed Env | 166 and 169 | [34■■,35■■] | ||
| V3-glycan | Diverse gene families Frequent indels Diverse angles of approach N332-glycan + GDIR dependant V3-glycans promiscuity | PC076 | 23–192 wpi | PCDN | 47% 0.53 μg/ml | VH4-34 16% | VK3-20 | H: 22 aa L: 8aa | No | +/− | 72 wpi | 120 wpi | 120 wpi | C | Yes | No binding | N328, N332 | [41] |
| PC039 | 15–265 wpi | PCN39 | 45% 0.03 μg/ml | VH4-34 16% | VK3-20 | H: 22 aa L: 10aa | Yes Ins CDRH1 | ++ | 37 wpi | 72 wpi | 84 wpi | C | Yes | No binding | 2 pathways–GDIR, 301, 332–V3 deletion | Landais, Poignard, Murrell, Wilson et al., unpublished | ||
| BF520 | 14–26 wpi | BF520.1 | 58% 1.95 μg/ml | VH1-2 7% | VK3-15 | H: 20 aa L: 7aa | No | 26 wpi | 62 wpi | 62 wpi | A | Yes | No autologous Env binding but binds BG505-SOSIP | [39,43■■] | ||||
| CH848 | 11–246 wpi | DH270 | 55% 0.08 μg/ml | VH1-2 13% | VL2-23 | H: 20aa L: 9 aa | No | +/− | 186 wpi | 205 wpi | n.a. | C | Yes | Weak binding to V3-peptide mimic | V1-loop GDIR N332 > N334 | [42■■] | ||
| MPER | Auto/poly-reactivity Narrow angle of approach Bind viral membrane | RV217-40512 | 4–163 wpi | RV217-VRC42 | 96% 4.7 μg/ml | VH1-69 11% | VK3-20 | H: 15 aa L: 9 aa | No | + | 22 wpi | 69 wpi | AE | Yes | Binding to scaffolded MPER peptide on 60-mer | [44■■] | ||
| RV217-40512 | 4–163 wpi | RV217-VRC43 | 65% 1.7 μg/ml | VH4-4 11% | VL7-23 | H: 19 aa L: 9 aa | No | +/− | 22 wpi | 69 wpi | AE | Yes | [44■■] | |||||
| RV217-40512 | 4–163 wpi | RV217-VRC46 | 30% 11 μg/ml | VH1-69 9% | VK1-39 | H: 14 aa L: 9 aa | No | ++ | 22 wpi | 69 wpi | AE | Yes | [44■■] |
CD4bS
BNAbs to the CD4bs typically develop after more than 5 years of infection and are typically highly somatically hypermutated – often 30% or more [15] – suggesting a long and complex maturation process. Two types of CD4bs bNAbs have been identified: CD4bs bNAbs classically using CDRH3 for epitope recognition; and CD4bs bNAbs with heavy chain variable gene (VH)-restricted recognition, including the VRC01 multidonor class, mostly relying on V-gene encoded structural features mimicking CD4 [15,16]. Immunogens eOD-GT8 and 426c, designed to bind VRC01-class germline revertants [5,6] have already succeeded in eliciting VRC01-like antibody responses in transgenic mouse models [17-21]. However, defining boosting immunogens capable of driving lineage maturation toward neutralization breadth remains a challenge. A major roadblock is the acquisition of mutations in the light-chain complementarity determining region 1 (CDRL1) loop to avoid structural clashes with Env V5-loops and Loop D glycans [22].
The detailed development of CD4bs bNAbs was first described in a series of articles demonstrating how two CD4bs bNAb lineages, CH103 (CDRH3 binder) and CH235 (VH1-46 gene restriction) cooperated in donor CH505 by restricting the possibilities of escape through distinct binding modes [23-25]. Each lineage induced an intense epitope diversification supporting the maturation of the other lineage. These groundbreaking studies were the first to trace Env/bNAb coevolution, and several features observed in the most recent studies were first noted for CH505.
The genetic and structural similarities shared by VRC01-class bNAbs, along with their breadth and potency, make them a particularly attractive target [15]. The first coevolution study of a VRC01-class bNAb, PCIN63 [26■■], showed that unlike most VRC01-class bNAbs, the PCIN63 antibodies achieved equivalent neutralization with only 13% SHM, mostly focused on residues previously shown to be minimally required for VRC01 neutralization. Although the heavy chain UCA sequence was determined with near certainty, several unmutated light chain sequences could correspond to a putative UCA; only two of the reconstructed UCA candidates bound with low affinity to eOD-GT8. One Env variant lacking both the N276-glycan and V5-glycans isolated ~6 months before the first detection of the lineage appeared to be the eliciting variant. However, in contrast to most VRC01-class bNAbs, PCIN63 antibodies appear to be partially dependent on the presence of the N276-glycan. These data suggest a shorter pathway more compatible with vaccination.
V2-apex
The V2-apex bNAbs are characterized by long CDRH3s (24–39 amino acids) which are typically very rare in the human repertoire. Nevertheless, such bNAbs are detected in ~10% of broad neutralizers [27] and include some of the most potent bNAbs isolated to date [28,29]. These bNAbs target V2 loop-strand C peptide positively charged residues and typically require binding to the N160 and/or N156-glycans as well as accommodation to V1-loop glycans. Located at the trimer apex, their epitopes often span more than one protomer such that these antibodies prefer (PG9, VRC38, and CH01) or require (PGT145 and CAP256) intact trimer for binding.
The initial study of V2-apex bNAb development described one of the most potently neutralizing bNAb lineages, CAP256-VRC26 [11,28,30]. The CAP256 donor was infected and superinfected by clade C viruses but the Env variants implicated in the elicitation and development of breadth derived entirely from the superinfecting virus [30,31■]. Viral diversity – overall and at the epitope – coincided with diversification in the antibody lineage and the appearance of breadth. Early escape mutations at key epitope residues 166 and 169 were tolerated by the broader lineage members, suggesting a mechanism for acquisition of breadth [30,31■]. Additional studies suggested a role for sialic-acid binding [32] and Env escape fitness cost [33] in supporting CAP256-VRC26 lineage maturation, although the association with breadth was unclear.
The study of PCT64 bNAb lineage development [34■■,35■■] confirmed the critical role of localized diversity at key V2 strand C residues in driving bNAb maturation and highlighted the role of glycans. The Env evolution pattern at key V2 C-strand 166/169 residues supporting PCT64 bNAbs maturation was remarkably similar to the one described in CAP256, suggesting a roadmap for immunogen design. Evaluation of PCT64-UCA binding to early autologous Env trimers suggested that Env glycoform heterogeneity played a critical role in the lineage initiation. With its 25-aa CDRH3 and lower SHM, PCT64 may also represent a more achievable target for vaccine design.
V3-glycan high-mannose patch
The high-mannose patch at the base of the V3-loop, also called the V3-glycan supersite, is the most commonly targeted epitope in broadly neutralizing sera [27]. This maybe associated with reduced structural constraints for this epitope, as bNAbs targeting this region show multiple angles of approach, gene usage, and subtle differences in epitope and glycan requirements. These properties suggest that V3-glycan bNAbs might represent a more achievable target. Many [36] but not all [37-39] of these bNAbs have indels in CDR loops which actively engage the glycan shield to access the V3-loop peptide containing the co-receptor binding site.
The development of N332-glycan dependent bNAbs in donors PC076 (PCDN lineage) and PC039 (PCIN39 lineage) was characterized by parallel maturation to breadth in multiple branches, supporting the hypothesis that several structural solutions to neutralization breadth exist even within a single lineage. While PCIN39 bNAbs all independently acquired insertions in the CDRL1 [40], the PCDN lineage achieved breadth without indels in any branches [41]. This not only confirms that indels may not be strictly required but also suggests that indels may not be as rare as anticipated and could readily be selected by the appropriate immunogens. Interestingly, both PCDN76 and PCIN39 used the VH4-34 gene, previously associated with autoimmune disorders but both lineages matured away from autoreactive features.
Three lineages that all targeted the high-mannose patch were isolated from donor CH848 [42■■]. The combined selective pressure of the non-bNAb DH475 and DH272 antibody lineages on the autologous Env led to a significant shortening of the V1-loop, which supported the DH270 bNAb lineage maturation. Interestingly, DH270 early intermediates acquired an improbable mutation in the CDRH2 introducing a coldspot for SHM and thereby fixing a mutation ultimately critical for development of heterologous breadth.
All three studies isolated bNAbs with relatively low levels of SHM (13–16%), driven by variation of V3-loop (mostly anchored at the V3 base) and in the V1-loop (glycans). Most recently, the maturation of BF520.1, an infant bNAb [39,43■■] was found to require as little as 2% SHM without any indels to achieve neutralization breadth within 6 months of infection.
Membrane-proximal external region
Similar to the CD4bs, the MPER is a major target of antibody responses in infected individuals although but broad MPER responses are rare. bnAbs to the C-terminus of MPER can be extremely broad [1] and possess lipid-binding properties allowing access to their epitope adjacent to the viral membrane, but also associated with auto and polyreactivity, raising questions about their compatibility with vaccination.
In the first coevolution study for MPER bNAbs, three MPER-targeting bNAb lineages were identified from a single superinfected individual (RV217-40512) in the prospective RV217 cohort [44■■]. Antibodies RV217-VRC42.01, VRC43.01, and VRC46.01 all had modest levels of somatic hypermutation (~10%) and normal antibody-loop lengths but used distinct recognition modes. The broadest lineage, VRC42, was similar to the known bNAb 4E10 in gene usage and mode of binding to the MPER peptide. A multimeric immunogen containing the founder virus MPER sequence could activate B cells bearing the VRC42-UCA. Furthermore, a VRC42 intermediate with only 3% SHM showed 50% breadth, demonstrating that a few changes can have an outsized impact. Although autoreactivity was detected for all three lineages, VRC42 and VRC43 were less autoreactive than 4E10 and were not associated with autoimmune disorder in this individual, raising cautious optimism for vaccination.
PRINCIPLES IN COMMON FROM DIFFERENT STUDIES
While each lineage has unique features, several common principles have emerged from studies of lineages targeting the same site and even across epitopes.
The naive B cell engages the early virus
Although this may seem an obvious conclusion, several studies have suggested an alternative origin for HIV antibodies via activation of memory B cells directed against gut microflora that cross-reacted with Env [45,46]. Indeed, for several lineages, UCA autologous binding and neutralization were not observed (PCT64, PCDN, DH270, and PCIN63), although this may be a limitation of the assayed binding partners. In contrast, some studies make the case for a more classical origin of these antibodies via engagement of naive B cells by early viruses. This has been observed for CH103, CAP256-VRC26, and VRC42 [11,25,44■■]. The eliciting Env is not necessarily the transmitted-founder virus, but may be a variant arising weeks or months after infection [26■■,30,41].
Furthermore, the availability of longitudinal samples does not completely alleviate the uncertainty of UCA determination as reported for the DH270, VRC42, and PCIN63 lineages [26■■,42■■,44■■]. These studies also highlighted how single nucleotide variations can have substantial functional impact, warranting caution for using functional evidence to determine which candidate UCA represents the true precursor.
Relatively few mutations are needed to gain breadth
The high levels of SHM detected in the first extremely broad bNAbs [47,48] raised concerns that only highly somatically mutated antibodies could exhibit breadth. However, subsequent discoveries have shown that antibodies with 7–15% mutation [35■■,36] can be broad and potent. In addition, some early intermediates from the BF520.1 and VRC42 lineages [43■■,44■■] are surprisingly broad despite having just 2–3% SHM. Virtually all of these studies have shown that shorter pathways compatible with vaccination are achievable. In fact, not only are not all mutations necessary for breadth [26■■,49] but the level of SHM does not translate to breadth: mature antibodies with varying degrees of breadth were interspersed along the phylogenetic trees of CAP25-VRC26, PCDN, PCT76, and PC64 [26■■,28,34■■,41]. This observation inspired the concept of ‘off-track’ antibodies [30] that are selected during infection for binding to contemporaneous viral variants but not for heterologous neuralization breadth. Thus, coevolution studies not only provide insights on which immunogens to choose, but also which to avoid, for eliciting bNAbs.
Improbable mutations are selected
Somatic hypermutations are called ‘improbable’ [50■] when, prior to selection, they: occur at unfavorable sequences (coldspots) for activation-induced cytosine deaminase (AID), the enzyme responsible for SHM; require multiple base substitutions for a specific amino-acid change; or require multiple mutations simultaneously to be structurally viable (e.g. a CDHR3 disulfide bond in CAP256-VRC26 [11]). In some lineages, amino-acid changes that are critical for the development of breadth are encoded by improbable mutations (DH270, CH235, and BF520.1) [42■■,50■]. The low frequency of such mutations may contribute to the rarity of bNAb lineage development in natural infection. As for rare precursors, the significance of this low frequency for vaccination is uncertain as relevant immunogens will be designed to increase the odds of positive selection.
Antibody lineages mature in the context of virus diversification by overcoming early viral escape
Several longitudinal studies noted that breadth is concomitant with viral diversification [25,30,34■■,42■■] suggesting the ability to tolerate amino-acid variation at critical epitope sites as a mechanism for imparting breadth [24,30,34■■] in successive waves of Env escape and antibody adaptation. In several cases (CH505-CH235, CH848, and PCDN), the epitope diversity was driven by escape from different cooperating autologous neutralizing antibody responses [23-25,41,42■■]. A previous observation that development of bNAbs is also associated with overall higher anti-Env IgG responses suggests a more global mechanism in which a diverse autologous antibody response may limit the Env overall escape landscape [25,51]. Immune pressure from maternal antibodies may similarly favor the rapid development of bNAbs in infants [39]. Superinfection, which may be associated with increased neutralization breadth [52,53], may also contribute to epitope diversity via recombination between strains. Of note, this may only aid the development of breadth when the targeted epitope is present in both the primary and superinfecting viruses allowing selection of cross-reactive B cells [31■].
CONCLUSION
Although features of the first bNAbs – high levels of SHM, indels, long CDR loops, and autoimmunity – were initially thought to be unattainable by vaccination, these apparent roadblocks are beginning to fall with the studies reviewed herein and the discovery of bNAbs with more normal features. Comparisons of the developmental pathways in multiple individuals with shared or different specificities are beginning to reveal similarities in bNAb maturation and in the nature of the Env triggering the broad lineages, indicating potential paths for immunogen design. Given the level of variability observed for some epitopes, it still remains highly important to study the development of bNAbs targeting various epitopes as additional lineages may refine immunogen strategies. Importantly, no such studies have yet addressed the gp120–gp41 interface, fusion peptide, and silent face regions. Finally, analysis of the developmental pathway of non-bNAbs would shed light on the molecular events limiting the acquisition of neutralization breadth. Immunization studies in animals have begun evaluating immunogens based on sequences and structures identified in coevolution studies. Overall, these studies have not only been valuable to the HIV vaccine effort but provided unprecedented details of immune mechanisms that could be applied to other pathogens.
KEY POINTS.
The coevolution of HIV-1 virus and broadly neutralizing antibodies has been studied in nine HIV-infected individuals.
While each lineage has unique features, several common principles have emerged from studies of lineages targeting the same site and even across epitopes.
These studies reveal pathways that are less complex than originally thought, providing optimism for eliciting them via vaccination.
Acknowledgements
We would like to thank Jonathan Stuckey and Peter Kwong for assistance with Fig. 1, and Kenneth Zhou for helpful comments on the manuscript.
Financial support and sponsorship
N. D. R.: Funding provided by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
E. L.: Funding provided by the International AIDS Vaccine Initiative (IAVI) and by the IAVI Neutralizing Antibody Consortium through the Collaboration for AIDS Vaccine Discovery grants OPP1084519 and OPP1115782. This work was also supported by National Institute of Health (NIH) Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery Grant UM1AI100663. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Sok D, Burton DR. Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol 2018; 19:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev 2017; 275:296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton DR, Ahmed R, Barouch DH, et al. A blueprint for HIV vaccine discovery. Cell Host Microbe 2012; 12:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrabi R, Voss JE, Liang CH, et al. Identification of common features in prototype broadly neutralizing antibodies to HIV envelope V2 apex to facilitate vaccine design. Immunity 2015; 43:959–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jardine J, Julien JP, Menis S, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science 2013; 340:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire AT, Hoot S, Dreyer AM, et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med 2013; 210:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonsignori M, Scott E, Wiehe K, et al. Inference of the HIV-1 VRC01 Antibody lineage unmutated common ancestor reveals alternative pathways to overcome a key glycan barrier. Immunity 2018; 49:1162–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soto C, Ofek G, Joyce MG, et al. Developmental Pathway of the MPER-Directed HIV-1-Neutralizing Antibody 10E8. PLoS One 2016; 11:e0157409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steichen JM, Kulp DW, Tokatlian T, et al. HIV vaccine design to target germline precursors of glycan-dependent broadly neutralizing antibodies. Immunity 2016; 45:483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams LD, Ofek G, Schatzle S, et al. Potent and broad HIV-neutralizing antibodies in memory B cells and plasma. Sci Immunol 2017; 2:eaal2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doria-Rose NA, Schramm CA, Gorman J, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 2014; 509:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon YD, Chuang GY, Zhang B, et al. Surface-matrix screening identifies semi-specific interactions that improve potency of a near pan-reactive HIV-1-neutralizing antibody. Cell Rep 2018; 22:1798–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong PD, Mascola JR. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity 2018; 48:855–871. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Bartesaghi A, Borgnia MJ, et al. Molecular architecture of native HIV-1 gp120 trimers. Nature 2008; 455:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou T, Lynch RM, Chen L, et al. Structural repertoire of HIV-1-neutralizing antibodies targeting the CD4 supersite in 14 donors. Cell 2015; 161:1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 2010; 329:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briney B, Sok D, Jardine JG, et al. Tailored immunogens direct affinity maturation toward HIV neutralizing antibodies. Cell 2016; 166:1459.e11–1470.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dosenovic P, von Boehmer L, Escolano A, et al. Immunization for HIV-1 broadly neutralizing antibodies in human Ig knockin mice. Cell 2015; 161:1505–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jardine JG, Ota T, Sok D, et al. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 2015; 349:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sok D, Briney B, Jardine JG, et al. Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice. Science 2016; 353:1557–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian M, Cheng C, Chen X, et al. Induction of HIV neutralizing antibody lineages in mice with diverse precursor repertoires. Cell 2016; 166:1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong L, Ju B, Chen Y, et al. Key gp120 glycans pose roadblocks to the rapid development of VRC01-class antibodies in an HIV-1-infected Chinese donor. Immunity 2016; 44:939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonsignori M, Zhou T, Sheng Z, et al. Maturation pathway from germline to broad HIV-1 neutralizer of a CD4-mimic antibody. Cell 2016; 165:449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao F, Bonsignori M, Liao HX, et al. Cooperation of B cell lineages in induction of HIV-1 -broadly neutralizing antibodies. Cell 2014; 158:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao HX, Lynch R, Zhou T, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 2013; 496:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.■■.Umotoy J, Bagaya B, Joyce C, et al. Rapid and focused maturation of a VRC01-class broadly neutralizing antibody lineage involves both binding and accommodation of the N276-glycan. Manuscript submitted and under revision, 2019PCIN63 monoclonal antibodies have the hallmark VRC01-class features and demonstrate neutralization breadth similar to the prototype VRC01 antibody but are three to four-fold less mutated. Maturation occurred rapidly within ~24 months of the lineage emergence with somatic hypermutations specifically accumulating at residues critical for breadth. In contrast to most VRC01-class bNAbs, PCIN63 Abs appear to be partially dependent on the presence of the N276-glycan.
- 27.Landais E, Huang X, Havenar-Daughton C, et al. Broadly neutralizing antibody responses in a large longitudinal Sub-Saharan HIV primary infection cohort. PLoS Pathog 2016; 12:e1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doria-Rose NA, Bhiman JN, Roark RS, et al. New member of the V1V2-Directed CAP256-VRC26 lineage that shows increased breadth and exceptional potency. J Virol 2016; 90:76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sok D, van Gils MJ, Pauthner M, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci USA 2014; 111:17624–17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhiman JN, Anthony C, Doria-Rose NA, et al. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat Med 2015; 21:1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.■.Sheward DJ, Marais J, Bekker V, et al. HIV superinfection drives de novo antibody responses and not neutralization breadth. Cell Host Microbe 2018; 24:593.e3–599.e3.This study found that superinfection failed to boost memory B cells primed by the first infection and did not promote the development on bNAbs, in two individuals that had bNAbs and two that did not.
- 32.Andrabi R, Su CY, Liang CH, et al. Glycans function as anchors for antibodies and help drive HIV broadly neutralizing antibody development. Immunity 2017; 47:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reh L, Magnus C, Kadelka C, et al. Phenotypic deficits in the HIV-1 envelope are associated with the maturation of a V2-directed broadly neutralizing antibody lineage. PLoS Pathog 2018; 14:e1006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.■■.Landais E, Murrell B, Briney B, et al. HIV Envelope glycoform heterogeneity and localized diversity govern the initiation and maturation of a V2 apex broadly neutralizing antibody lineage. Immunity 2017; 47:990.e9–1003.e9.The authors find that glycan heterogeneity played a role in the activation of the V2-apex-directed PCT64 bnAbs precursor. Viral evolution and escape in this donor were remarkably similar to those of CAP256, another donor with V2 apex bnAbs.
- 35.■■.Rantalainen K, Berndsen ZT, Murrell S, et al. Co-evolution of HIV envelope and apex-targeting neutralizing antibody lineage provides benchmarks for vaccine design. Cell Rep 2018; 23:3249–3261.This article describes the structural coevolution of HIV envelope glycoprotein and a V2-apex-directed antibody response in donor PC63. The coevolutionary mechanisms include antibody binding angle maturation, gradual loop rigidification, surface charge modulation, and changes in glycan contacts.
- 36.Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011; 477:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freund NT, Wang H, Scharf L, et al. Coexistence of potent HIV-1 broadly neutralizing antibodies and antibody-sensitive viruses in a viremic controller. Sci Transl Med 2017; 9:eaal2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Panda H, Makhdoomi MA, et al. An HIV-1 broadly neutralizing antibody from a clade C-infected pediatric elite neutralizer potently neutralizes the contemporaneous and autologous evolving viruses. J Virol 2019; 93:e01495–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonich CA, Williams KL, Verkerke HP, et al. HIV-1 neutralizing antibodies with limited hypermutation from an infant. Cell 2016; 166:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landais E, Moore PL. Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers. Retrovirology 2018; 15:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacLeod DT, Choi NM, Briney B, et al. Early antibody lineage diversification and independent limb maturation lead to broad HIV-1 neutralization targeting the Env high mannose patch. Immunity 2016; 44:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.■■.Bonsignori M, Kreider EF, Fera D, et al. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci Transl Med 2017; 9:.The CH848 donor developed three lineages targeting the V3-glycan site: two nonbNabs that selected an Env variant which in turn allowed a bNAb to develop. Early in development of the DH270 bNAb, a rare mutation became fixed in the lineage which was critical for the subsequent development of breadth.
- 43.■■.Simonich C, Doepker L, Ralph D, et al. Kappa chain maturation helps drive rapid development of an infant HIV-1 broadly neutralizing antibody lineage. Nature Communications 2019: in pressThis article describes the first example of bNAb evolution in an infant. Heterologous cross-clade neutralizing activity evolved in the BF520.1 V3-glycan directed lineage within 6 months of infection and that only 2% SHM is needed to achieve the full breadth of the mature antibody.
- 44.■■.Krebs SJ, Kwon YD, Schramm CA, et al. Longitudinal analysis reveals early development of three MPER-directed neutralizing antibody lineages from an HIV-1-infected individual. Immunity 2019; 50:677–691.This article describes the initiation and early development of three MPER-directed HIV broadly neutralizing antibody lineages from a single donor. One of the antibody lineages, VRC42, achieved high neutralizing breadth with low mutation from germline, and is a member of a multidonor class.
- 45.Liao HX, Chen X, Munshaw S, et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med 2011; 208:2237–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trama AM, Moody MA, Alam SM, et al. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell Host Microbe 2014; 16:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker LM, Phogat SK, Chan-Hui PY, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 2009; 326:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jardine JG, Kulp DW, Havenar-Daughton C, et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 2016; 351:1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.■.Wiehe K, Bradley T, Meyerhoff RR, et al. Functional relevance of improbable antibody mutations for HIV broadly neutralizing antibody development. Cell Host Microbe 2018; 23:759.e6–765.e6.This analysis showed that bNAbs are enriched with low-probability mutations and that these improbable mutations are often critical for HIV-1 bnAb neutralization breadth.
- 51.Lynch RM, Wong P, Tran L, et al. HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies. J Virol 2015; 89:4201–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cortez V, Odem-Davis K, McClelland RS, et al. HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response. PLoS Pathog 2012; 8:e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams KL, Wang B, Arenz D, et al. Superinfection drives HIV neutralizing antibody responses from several B cell lineages that contribute to a polyclonal repertoire. Cell Rep 2018; 23:682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]