Abstract
The management of patients infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be difficult due to the need for dedicated in-hospital pathways, protective measures for healthcare professionals and isolated beds of intensive care, particularly in areas overwhelmed by wide viral spread. Although pneumonia is the most common clinical manifestation in coronavirus disease 2019 (COVID-19), a variety of cardiovascular complications have been reported. An integrated diagnostic algorithm in SARS-CoV-2-infected patients with suspected cardiac involvement (laboratory findings of myocardial injury and electrocardiographic changes) may help to avoid unnecessary examinations and minimize the risk of operator infection. Due to its mobility and bedside feasibility, echocardiography is the first-line imaging technique in this clinical setting. It quickly provides information on ventricular functions, pulmonary hypertension, valve disease and pericardial effusion. In case of ST-segment elevation (STE), urgent coronary angiography should be performed. Cardiac ultrasound helps distinguish between ischemic and non-ischemic myocardial disease and may detect pericardial disease. Transmural ischemic electrocardiographic changes, with or without early elevated troponin levels or echocardiographic wall motion abnormalities, will determine the need for early invasive coronary angiography.
Computed tomography (CT) through its multiple applications (chest CT; CT pulmonary angiography and coronary CT angiography; late iodine enhancement CT) and cardiac magnetic resonance might be helpful in reinforcing or redirecting diagnostic hypothesis emerged by other clinical, electrocardiographic and echocardiographic findings. The current pandemic makes it challenging to perform serial invasive and non-invasive diagnostic testing in COVID-19 patients and high serum troponin level. Nevertheless, thoughtful and systematic use of an appropriate multimodality imaging strategy is clinically relevant to detect cardiac injury and distinguish myocardial infarction from, myocarditis, takotsubo syndrome and pulmonary embolism.
Keywords: COVID-19, SARS-CoV-2, Multimodality imaging, Myocardial injury, Myocarditis, Myocardial infarction
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory infectious disease first recognized in Wuhan, Hubei, China, which has spread to other provinces in China and within a few months propagated to every country in the world, causing a pandemic and a public health crisis of global proportions in 2020. To date, millions of patients around the world have been infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and hundreds of thousands have died. Initial epidemiologic data indicate that about 80% of COVID-19 patients are asymptomatic or with mild symptoms. Conversely, in the remaining cases, patients can develop severe respiratory disease complicated by systemic inflammatory response and multiorgan failure, requiring intensive care treatment. Given the lack of specific anti-viral treatment, critically ill patients are at high risk of in-hospital death despite the adoption of aggressive therapies. Although pneumonia is the most common clinical manifestation in COVID-19, a variety of cardiovascular complications have been reported [1].
Pathophysiology of cardiac damage in COVID-19
Despite rapid early advancements, the pathogenetic mechanisms responsible for cardiovascular complications in SARS-CoV-2 are still not fully understood. Indirect and direct pathways of myocardial damage have been hypothesized. Macrophage activation induced by viral invasion may precipitate an abnormal immuno-mediated host response leading to a cytokine storm. Huang et al. reported high plasma levels of various cytokines, including interleukin (IL)-2, IL-6, IL-7, IL-10 and tumor necrosis factor-α in patients admitted to the intensive care unit [2]. These cytokines may precipitate a systemic inflammatory response associated with apoptosis or necrosis of myocardial cells as well as with hypercoagulability [3,4].
Viral particles were observed in interstitial cytopathic macrophages but not inside the myocytes, with histological damage generally characterized by focal myofibrillar lysis [5]. While a clear cardiotropism of the SARS-Cov-2 has not yet been demonstrated, a direct cellular damage has been advocated. SARS-CoV-2 binds the angiotensin-converting enzyme 2 (ACE2), a surface molecule t that mediates virus entry into the target cells and is expressed in a variety of cells including pneumocytes, macrophages, enterocytes, cardiac myocyte, pericytes and endothelial cells [6]. Direct viral infection and diffuse inflammation involving endothelial cells of the heart and several organs have been detected by autopsy [7]. In myocardial tissue, diffuse endothelial inflammation and pericytes injury may be responsible for coronary circulatory dysfunction, increased vascular tone and vasoconstriction. These mechanisms may favor hypoperfusion and hypercoagulability, both inducing myocardial ischemia. Moreover, hypoxemia secondary to lung involvement may further deteriorate myocardial function. Depending on the type (inflammatory, ischemic) and extension of myocardial damage, a wide range of cardiovascular clinical manifestations can be observed.
Cardiac injury, defined as increased troponin level above 99th percentile of the upper reference limit, associated with electrocardiographic (ECG) and/or echocardiographic abnormalities is highly prevalent in patients with COVID-19 and is associated with more severe disease and worse prognosis [8]. As described above, cardiac injury is believed to be related to non-ischemic inflammatory myocardial processes and/or myocardial ischemia mostly resulting from microvascular and endothelial dysfunction or thrombotic events [9].
Among 416 patients with confirmed diagnosis of COVID-19, Shi et al. have reported a prevalence of around 20% of cardiac injury [8]. In this study, patients with cardiac injury had higher levels of C-reactive protein and N-terminal pro-B-type natriuretic peptide, when compared to those without cardiac injury. In addition, they had a higher prevalence of multiple mottling and ground-glass opacity in their chest X-ray, they were more likely to require non-invasive or invasive ventilation, and had a higher risk of death during hospitalization. These findings were consistent with those of Guo et al., which showed a significant association between myocardial injury and fatal outcome, particularly in patients with underlying cardiovascular disease [10].
Others have reported infiltration of the myocardium by interstitial mononuclear inflammatory cells in histological findings consistent with myocarditis [5,11]. Myocardial inflammation results in focal or global myocarditis and/or necrosis, possibly leading to ventricular dysfunction [12]. Patterns of myocardial diffuse interstitial edema and fibrosis have been documented in T2 and T1-weighted images, respectively, by cardiac magnetic resonance (CMR) in COVID-19 patients with severely reduced left ventricular ejection fraction [11,13]. Myocarditis may trigger life-threatening arrhythmias and, especially if associated with pericardial effusion, further deteriorate hemodynamics eventually leading to acute heart failure (HF) and cardiogenic shock [14,15]. In a cohort of 191 patients from Wuhan, HF occurred in 44 cases (23%) [16]. HF and acute cardiac injury were significantly more prevalent among patients who eventually died compared with those who survived (52% vs 12% and 59% vs 1%, respectively).
Acute coronary syndrome presenting with ST-segment elevation (STE) due to plaque instability, hypercoagulability and augmented sympathetic stimulation (type 1 myocardial infarction) has been reported in a minority of cases [17]. In instances, COVID-19 patients presenting with STE have non-significant coronary artery disease (CAD). In a series of 18 patients with STE, nine underwent coronary angiography, only six had obstructive CAD and five required percutaneous coronary intervention. In the remaining cases with non-obstructive CAD or without significant wall motion abnormalities by echocardiography, albeit in the absence of angiography, myocardial injury related to microvascular dysfunction was presumed [18]. Type 2 myocardial infarction, in contrast, may occur due to an imbalance in oxygen supply/demand favored by hypoxemia and tachycardia in the context of systemic inflammation [19].
A hypercoagulable state and thrombotic events are believed to be secondary to systemic inflammatory response as they are associated with markedly elevated D-dimer and fibrin degradation products [20]. In a study by Chen et al. including 1008 COVID-19 patients, pulmonary embolism (PE) was confirmed in 10 of 25 suspected cases by computed tomography pulmonary angiography (CTPA) [21]. Anecdotal cases of stroke, deep venous thrombosis and other thromboembolic events have been increasingly reported [22].
As is the case for other hyper-adrenergic conditions, Takotsubo syndrome (TTS) can develop in the setting of severe sepsis, hypoxemia or metabolic acidosis, all complications often detected in SARS-CoV-2 patients [23]. To date, only a few cases of typical TTS triggered by SARS-CoV-2 have been reported [1,24]. Since some authors detected myocardial inflammatory infiltrates in patients admitted for TTS, one might hypothesize that neurohumoral and inflammatory mechanisms coexist and play a role also in subjects with SARS-CoV-2 experiencing myocardial injury [25]. The pathogenetic mechanisms and clinical presentations of cardiovascular involvement in COVID-19 patients are summarized in Table 1 .
Table 1.
Pathogenetic mechanisms and clinical presentations of cardiovascular involvement seen in COVID-19 patients.
| Cardiovascular disease | Pathogenetic mechanism | Clinical presentation |
|---|---|---|
| ACS STE | Cytokine storm, hypercoagulability, plaque instability | Typical chest pain or atypical pain and/or dyspnea, elevated troponin, ECG changes and LV WMAs related to specific coronary artery territory distribution |
| ACS non-STE | Typical chest pain or atypical pain and/or dyspnea, elevated troponin, ECG changes (possible), LV WMAs (possible) related to specific coronary artery territory distribution | |
| Myocarditis | Cytokine storm, direct cellular damage (possible) | Chest pain (possible), dyspnea (possible), elevated troponin, ECG changes (possible), diffuse LV WMAs not related to specific coronary artery territory distribution |
| Pericarditis | Cytokine storm, direct cellular damage (possible) | Chest pain, dyspnea (possible), elevated troponin, ECG changes, impaired LV diastolic function and/or pericardial effusion |
| TTS | Emotional stress, microvascular and endothelial dysfunction, sepsis, acidosis | Chest pain and/or dyspnea, elevated troponin, ECG changes, LV WMAs not related to specific coronary artery territory distribution (circumferential pattern, apical ballooning most frequently) |
| PE | Hypercoagulability | Chest pain and/or dyspnea, elevated troponin (possible), ECG changes (possible), RV enlargement and dysfunction (McConnell sign, 60/60 sign) |
| Decompensated chronic HF | Hypoxia, elevated metabolic demand | Dyspnea, elevated troponin (possible), LV WMAs without de novo abnormalities |
| Acute myocardial injury | Cytokine storm, direct cellular damage (possible), microvascular and endothelial dysfunction, hypoxia | Chest pain and/or dyspnea (possible), elevated troponin, ECG changes (possible), LV WMAs (possible) not related to specific coronary artery territory distribution (if absence of coexistent CAD) |
ACS, acute coronary syndrome; CAD, coronary artery disease; CMR, cardiac magnetic resonance; CT, computed tomography; ECG, electrocardiogram; HF, heart failure; ICA, invasive coronary angiography; LV, left ventricular; PE, pulmonary embolism; RV, right ventricular; STE, ST-segment elevation; TTE, transthoracic echocardiography; TTS, takotsubo syndrome, WMAs, wall motion abnormalities.
Multimodality imaging in diagnostic work-up of cardiovascular involvement
The management of patients infected by SARS-CoV-2 is challenging due to the complexity and severity of their multi-organ disease. Moreover, the pandemic context makes the employment of cardiac imaging modalities difficult due to the risk of infection for imagers, nurses, and technicians related to the close patient contact. The reasons for conducting an exam need to be balanced with the risks for personnel and should be thoroughly evaluated on a case-by-case basis.
Specific recommendations by the international imaging societies and local protocols include the use of personal protective equipment as well as the adherence to disinfection procedures for imaging rooms and equipment (probe, scanner, etc.) [26,27]. Other important factors may complicate patient care, such as the need for dedicated in-hospital pathways and isolated rooms or beds in intensive care units, particularly in areas overwhelmed by wide viral spread.
These difficulties must be overcome to properly implement the best clinical practices, including early detection of cardiovascular complications and the adoption of adequate medical or interventional therapeutic strategies.
An integrated diagnostic algorithm in patients with suspected cardiovascular involvement will help optimize available resources and patient outcomes, while avoiding unnecessary examinations and procedures and minimizing the risk of health care workers infection [26,28].
Echocardiography or point of care ultrasound are the first-line cardiac imaging techniques in this clinical setting, due to its portability, bedside feasibility in emergency settings and low cost, which allow having a dedicated machine in COVID-19 units or the emergency department [[29], [30], [31], [32]]. The advantage of portability and bed-side feasibility, however, brings a higher risk of transmission to the sonographer or physician performing the study. Therefore it is important to minimize time of exposure (perform focused exams rather than full echocardiograms in COVID or patients under investigation, whenever possible) and practice extreme care on use of protective equipment, as recently proposed by the American Society of Echocardiography [26]. Transesophageal echocardiograms, on the other hand, should be practiced with extreme caution and reserved only as a last resource, as the action of intubation is of the highest transmission risk. Atrial fibrillation is the most frequent incident sustained arrhythmia, being reported in 17% of COVID-19 patients during hospitalization [33]. For stable patients needing cardioversion, cardiac CT can be used instead of transesophageal echocardiography to rule out atrial thrombi and avoid aerosol-generating procedure. Common echocardiographic findings in patients with acute COVID-19 are listed in Table 2 . The European Association of Cardiovascular Imaging recommends performing echocardiography in patients with abnormal cardiac biomarkers and/or ECG signs of myocardial damage, while acknowledging that other imaging diagnostic tests are not routinely used in the emergency context of the COVID-19 pandemic [27,34]. In patients with non-STE and/or equivocal ECG presentation, a rapid bedside echocardiogram showing regional wall motion abnormalities play an important role in detecting acute coronary syndromes early and triaging patients for invasive or conservative strategies. In cases of STE (Fig. 1 ), however, patients should be referred for primary percutaneous coronary intervention without delay. Although obsolete, thrombolysis might also be considered as bail-out option in specific patients, provided that an invasive strategy should be preferred whenever possible [35].
Table 2.
Common echocardiographic findings in patients with acute COVID-19.
| Normal heart (or unchanged from prior exams) |
| Global left ventricular dysfunction (EF and/or strain) |
| Regional left ventricular dysfunction |
| Right ventricular dilatation and/or dysfunction |
| Pulmonary hypertension |
| Pericardial effusion |
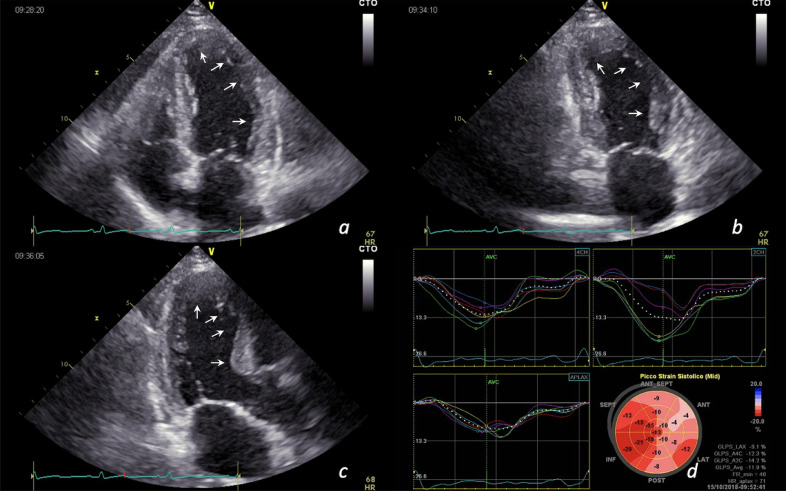
Fig. 1.
ST-elevation myocardial infarction in a 68-year-old man with positive SARS-CoV-2 nasopharyngeal swab. Apical, anterior and lateral wall akinesia in apical 4-chambers view (a), 2-chambers view (b), long axis view (c); longitudinal strain curve and bull's eye showing reduced myocardial deformation of apex and anterior wall segments (average global longitudinal strain = -11.9%) (d).
In case of diffuse WMAs, pericardial effusion (Fig. 2 ) and/or low pre-test probability of acute coronary syndrome, CT coronary angiography (CTA) could be combined with myocardial late iodine enhancement (LIE) to detect areas of myocardial fibrosis, inflammation or diffuse ischemia, which are important findings when considering myocarditis or type 2 myocardial infarction. In this scenario, multimodality imaging represents a useful alternative approach to recognize COVID-19 patients more prone to develop cardiogenic shock, avoiding invasive endomyocardial biopsy and hemodynamic measurements that expose additional health care personnel and are difficult to perform. In such circumstances, close monitoring is crucial to promptly identify patients requiring pharmacological or mechanical inotropic support, anticoagulation or other therapies.
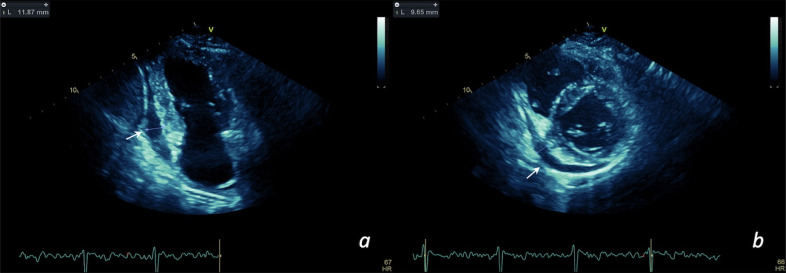
Fig. 2.
COVID-19 patient with preserved ejection fraction and slight increment of troponin (myocardial injury). Pericardial effusion (see arrows) in apical 2-chambers view (a) and parasternal short-axis view (b) can be appreciated.
Moreover, in patients with extensive apical akinesis resembling apical ballooning and without evidence of obstructive CAD or myocardial LIE, TTS should be suspected (Fig. 3 ) [[36], [37], [38]].
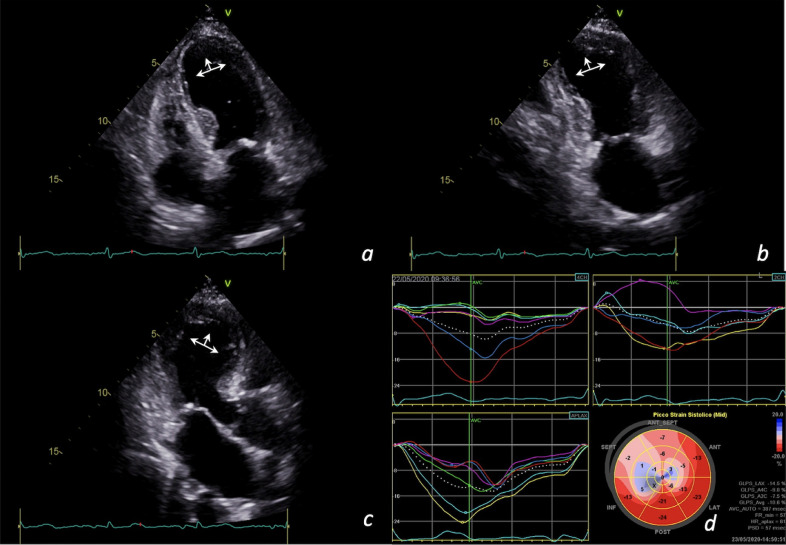
Fig. 3.
Takotsubo syndrome in a 75-year-old woman infected by SARS-CoV-2. Apical akinesia and circumferential pattern in apical 4-chambers view (a), 2-chambers view (b), long axis view (c); longitudinal strain curve and bull's eye showing reduced myocardial deformation of apex and interventricular septum (average global longitudinal strain = -10.6%) (d).
Patients with COVID-19 and dyspnea or hypoxia are most commonly affected by pneumonia. Therefore, their initial evaluation should include a lung ultrasound to identify pleural effusions and lung consolidation (abnormal B-lines), or chest computed tomography (CT). While lung ultrasound is conveniently fast and portable, advanced CT modalities allow for a comprehensive evaluation of lung disease and cardiac morphology and function. Indeed, the diagnostic accuracy of CT in detection of pneumonia and pulmonary embolism and its capability to rule out CAD make it a convenient one-stop-shop in COVID-19 patients. Although sometimes co-existing, the differential diagnosis between decompensated chronic HF and COVID-19 interstitial pneumonia is challenging but crucial to establish an appropriate treatment. Bedside cardiac and lung ultrasound or CT together with plasma B-type natriuretic peptide are generally helpful in clarifying this differential diagnosis [39,40]. Zhu et al. retrospectively analyzed 7 patients with HF and 12 patients with COVID-19 and compared baseline clinical and CT features [41]. Although ground-glass opacity and interlobular septal thickening were similar in both groups, the ratio of central and gradient distribution and the ratio of the expansion of small pulmonary veins were higher among HF patients, while CT evidence of lesions with rounded morphology was most common in the COVID-19 group.
Acute deterioration of right ventricular (RV) function is common in the setting of COVID-19, therefore accurate evaluation of RV morphology and function by echocardiography is warranted [42,43]. Detection of RV dysfunction and/or dilatation by echocardiography (Fig. 4 ), combined with Doppler evidence of pulmonary hypertension and elevated levels of serum D-dimer, are highly suggestive of PE. Additional findings such as McConnell's or the 60/60 signs could also support the suspicion of PE [44]. In this setting, CTPA is the standard of care to confirm diagnosis. However, other causes of RV dysfunction must be considered, such as underlying lung disease, myocarditis, or the mode of mechanical ventilation.
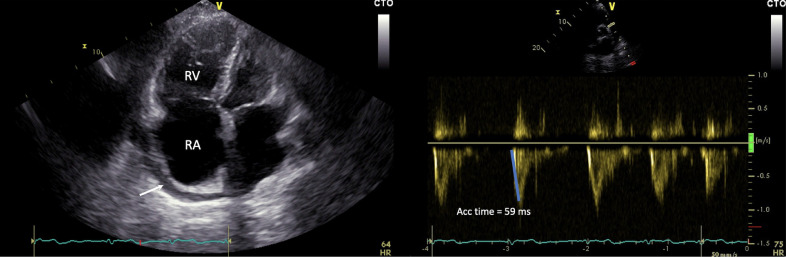
Fig. 4.
Recurrence of pulmonary embolism in a 64-years-old man with positive SARS-CoV-2 nasopharyngeal swab and chronic thromboembolic pulmonary hypertension. Right ventricle enlargement with retro-atrial pericardial effusion (see arrow) (a); reduced Doppler acceleration time on pulmonary valve, echocardiographic sign of pulmonary hypertension (b). RA, right atrium; RV, right ventricle.
Radiologic imaging in inflammatory myocardial damage
Owing to the wide spectrum of myocardial involvement in COVID-19, a deep comprehension of the underlying tissue damage is required. Unfortunately, echocardiography suffers from limited ability in tissue characterization. Conversely, CMR represents the non-invasive gold standard technique for myocardial tissue characterization, due to its high accuracy in detecting edema and fibrosis, with the latter further differentiated in ischemic and non-ischemic patterns [45]. Non-contrast T2-weighted spin-echo, T1, T2 and LGE sequences with cine loops allow for detection of focal subepicardial or middle-wall edema (suggesting myocarditis), intense edema usually involving the apex (classic TTS), or subendocardial to transmural edema affecting myocardial segments according to coronary distribution (myocardial infarction).
However, in addition to traditional disadvantages of CMR (scarce availability, exam length, claustrophobia, contraindication with specific devices), new obstacles limit the use of CMR during COVID-19 pandemic, such as assessment of critically ill patients and proper disinfection of scanner and room, a critical safety measure that applies to MR, CT and nuclear scanners alike. Therefore, CMR is frequently not a possibility in the critically ill patient and has to be held until the patient is clinically stable.
This potential diagnostic gap can be filled by CT, as it can be used for describing pulmonary parenchymal involvement, coronary anatomy and pulmonary vasculature, as well as for evaluation of wall motion abnormalities (if acquisition of an entire cardiac cycle is obtained during first-pass enhancement) and myocardial tissue characterization [46]. By using dedicated LIE datasets, generally acquired 6 to 8 minutes after iodinated contrast agent injection, it is possible to assess the presence of myocardial damage and myocardial infarction characterized by increased myocardial signal density. Since patterns of LIE consistent with acute myocarditis have been described in COVID-19 and non–COVID-19 cases [[47], [48], [49]], this test could be used to assess myocardial damage in patients with COVID-19 and serum evidence of cardiac injury as well as in those undergoing computed tomography for non-cardiac reasons [50].
In the clinical context of COVID-19 patients and cardiovascular involvement, CT allows to identify pneumonia as well as obstructive CAD and PE (dual or triple rule in/out) (Fig. 5 ) [47]. Moreover, as a result of the potential advantages provided by different CT applications in comprehensive myocardial function assessment, prescription of CMR could be limited to COVID-19 patients in which initial assessment with echocardiography and CT did not provide a definitive diagnosis, mainly patients with persisting left ventricular systolic dysfunction after the acute phase (Fig. 6 ).
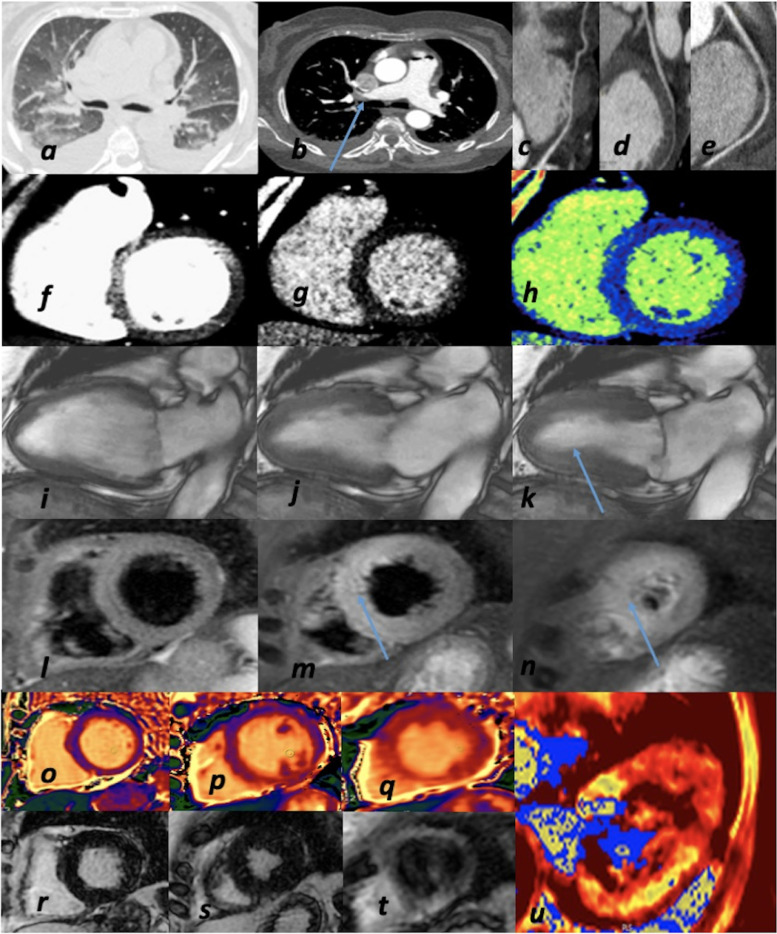
Fig. 5.
Comprehensive and advanced imaging in a COVID-19 patient. Severe acute respiratory distress in 66-year-old woman. (a) Chest CT: bilateral pleural effusion and common pattern of pulmonary edema and interstitial pneumonia; (b) CTPA: bilateral PE (arrow); (c-d-e) CCTA: coronary arteries: absence of obstructive CAD (LAD-c; LCx-d; RCA-e); f) CT: contrast-enhanced first pass myocardial perfusion imaging-left ventricle SAx view; (g) CT-late scan with color-map (h): after seven minutes absence of myocardial delayed enhancement); (i-j-k) CMR-functional CINE sequences: mild hypokinesis of LV apex (arrow) with improved LV EF (47%) as compared to TTE at 1st day; (l-m-n) CMR-TIR T2 sequences: hyperintense signal> mid-apical edema (arrow); (o-p-q) CMR-native T1 mapping: normal T1 mapping values; (r-s-t) CMR-post- contrast sequences: no LGE (late gadolinium enhancement); (u) CMR-T2 mapping: increased T2 values of LV apex (myocardial edema). Diagnosis: pulmonary edema due to TTS in concomitant PE.CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CMR, cardiac magnetic resonance; CT, computed tomography; CTPA, computed tomography pulmonary angiography; EF, ejection fraction; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LV, left ventricular; LGE, late gadolinium enhancement; MI, myocardial infarction; PE, pulmonary embolism; RCA, right coronary artery; SAx, short axis; TIR T2, triple inversion recovery T2-weighted; TTE, transthoracic echocardiography; TTS, takotsubo syndrome.
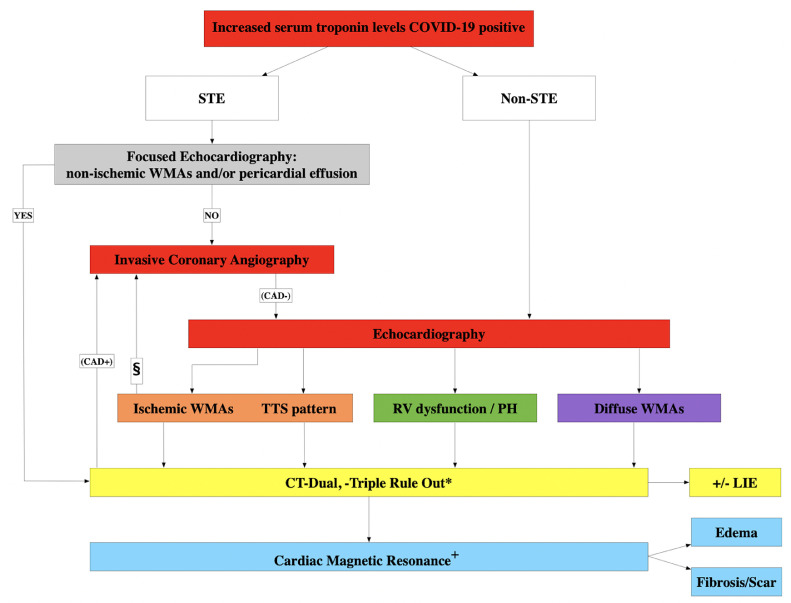
Fig. 6.
Proposed diagnostic algorithm for patients with COVID-19 and increased serum troponin level. § only in case of non-STE patients. *Dual rule out: pneumonia and CAD (low-pre-test, Age<65); Triple rule out: pneumonia, CAD (low-pre-test, Age<65) and pulmonary embolism. + Function and tissue characterization (edema & fibrosis). CAD, coronary artery disease; CT, computed tomography; ECG, electrocardiographic; LIE, late iodine enhancement; PH, pulmonary hypertension; RV, right ventricular; STE, ST-segment elevation; TTS, takotsubo syndrome; WMAs, wall motion abnormalities.
Table 3 summarizes the diagnostic role of different imaging modalities in COVID-19 patients with suspected cardiovascular involvement.
Table 3.
Role of imaging techniques in differential diagnosis of various cardiovascular complications in patients with COVID-19.
| Clinical scenario | Imaging modalities |
||||
|---|---|---|---|---|---|
| TTE | CT | CMR | ICA | ||
| Suspected ACS | STE | + | - | - | ++ |
| non-STE | ++ | ++*/⁎⁎ | - | + | |
| Suspected myocarditis | ++ | +⁎⁎ | ++ | - | |
| Suspected pericarditis | ++ | + | + | - | |
| Suspected decompensated chronic heart failure | ++ | - | - | - | |
| Suspected PE | + | ++⁎⁎⁎ | - | - | |
| Suspected TTS | ++ | +* | ++ | + | |
ACS, acute coronary syndrome; CMR, cardiac magnetic resonance; CT, computed tomography; ICA, invasive coronary angiography; PE, pulmonary embolism; STE, ST-segment elevation; TTE, transthoracic echocardiography; TTS, takotsubo syndrome.
ACS, acute coronary syndrome; PE, pulmonary embolism; STE, ST-segment elevation; TTE, transthoracic echocardiography; TTS, takotsubo syndrome.
Modified from Cosyns B et al. Eur Heart J Cardiovasc Imaging 2020;21:709-714.
CCTA should be performed to rule in/out coronary artery disease and schedule subsequent invasive coronary angiography;
CT with late iodine enhancement may be useful to detect areas of myocardial fibrosis;
CTPA is the gold standard for the assessment of pulmonary artery thrombi.
Conclusions
Echocardiography or point of care ultrasound are the first-line cardiac imaging modalities for patients with COVID-19 and suspected cardiac disease. Although the current challenging clinical scenario makes serial invasive and non-invasive diagnostic testing difficult and undesirable, advanced imaging modalities, mostly CT, should be considered if the initial echocardiogram is not fully diagnostic, in order to distinguish cardiac injury form myocardial infarction, myocarditis, TTS and PE, and to establish the most appropriate therapeutic strategy. While serial echocardiograms during follow-up are desirable for monitoring cardiac disease recovery, more research is needed in this regard to properly determine the clinical value and safety.
Ethical statement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that all authors are responsible for the content and have read and approved the manuscript; and that the manuscript conforms to the Uniform Requirements for Manuscripts Submitted to Biomedical Journals published in Annals in Internal Medicine
We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
References
- 1.Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the Cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5(5):518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. European journal of heart failure. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England). 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F. Association of cardiac injury with mortality in hospitalized patients With COVID-19 in Wuhan. China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS. COVID-19: Myocardial injury in survivors. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manka R, Karolyi M, Polacin M, Holy EW, Nemeth J, Steiger P. Myocardial edema in COVID-19 on cardiac MRI. J Heart Lung Transplant. 2020;39(7):730–732. doi: 10.1016/j.healun.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua A, O'Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41(22):2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, arrhythmic risk, and inflammation: mind the gap! Circulation. 2020;142(1):7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 18.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B. ST-Segment Elevation in Patients with Covid-19 - A Case Series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 20.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis: JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Wang X, Zhang S, Lin B, Wu X, Wang Y. Characteristics of acute pulmonary embolism in patients With COVID-19 associated pneumonia from the city of Wuhan. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng JJ, Choong A. Thromboembolic events in patients with SARS-CoV-2. J Vasc Surg. 2020;72(2):760–761. doi: 10.1016/j.jvs.2020.04.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Failure. 2016;18(1):8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 24.Meyer P, Degrauwe S, Van Delden C, Ghadri JR, Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41(19):1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scally C, Rudd A, Mezincescu A, Wilson H, Srivanasan J, Horgan G. Persistent long-term structural, functional, and metabolic changes after stress-induced (Takotsubo) Cardiomyopathy. Circulation. 2018;137(10):1039–1048. doi: 10.1161/CIRCULATIONAHA.117.031841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2020;33(6):648–653. doi: 10.1016/j.echo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo GD, Donal E. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21(6):592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganatra S, Dani SS, Shah S, Asnani A, Neilan TG, Lenihan D. Management of cardiovascular disease during coronavirus disease (COVID-19) pandemic. Trends Cardiovasc Med. 2020;30(6):315–325. doi: 10.1016/j.tcm.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Wang B, Zhou J, Kirkpatrick J, Xie M, Johri AM. Bedside focused cardiac ultrasound in COVID-19 from the Wuhan Epicenter: the role of cardiac point-of-care ultrasound, limited transthoracic echocardiography, and critical care echocardiography. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2020;33(6):676–682. doi: 10.1016/j.echo.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang G, Vengerovsky A, Morris A, Town J, Carlbom D, Kwon Y. Development of a COVID-19 point-of-care ultrasound protocol. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2020;33(7):903–905. doi: 10.1016/j.echo.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard MH, Weiner RB. Echocardiography in the time of COVID-19. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2020;33(6):674–675. doi: 10.1016/j.echo.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake DH, De Bonis M, Covella M, Agricola E, Zangrillo A, Zimmerman KG. Echocardiography in pandemic: front-line perspective, expanding role of ultrasound, and ethics of resource allocation. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2020;33(6):683–689. doi: 10.1016/j.echo.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo V, Di Maio M, Mottola F, Pagnano G, Attena E, Verde N, et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: a multicenter observational study. Eur J Clin Invest. 2020:e13387. [DOI] [PMC free article] [PubMed]
- 34.Cosyns B, Lochy S, Luchian ML, Gimelli A, Pontone G, Allard SD. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc Imaging. 2020;21(7):709–714. doi: 10.1093/ehjci/jeaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirtane AJ, Bangalore S. Why Fibrinolytic Therapy for ST-Segment-Elevation Myocardial Infarction in the COVID-19 pandemic is not your new best friend. Circul Cardiovasc Qual Outcomes. 2020;13(6) doi: 10.1161/CIRCOUTCOMES.120.006885. [DOI] [PubMed] [Google Scholar]
- 36.Citro R, Pontone G, Pace L, Zito C, Silverio A, Bossone E. Contemporary Imaging in Takotsubo Syndrome. Heart Failure Clin. 2016;12(4):559–575. doi: 10.1016/j.hfc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Citro R, Lyon AR, Meimoun P, Omerovic E, Redfors B, Buck T. Standard and advanced echocardiography in takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2015;28(1):57–74. doi: 10.1016/j.echo.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Citro R, Radano I, Parodi G, Di Vece D, Zito C, Novo G. Long-term outcome in patients with Takotsubo syndrome presenting with severely reduced left ventricular ejection fraction. Eur J Heart Failure. 2019 doi: 10.1002/ejhf.1373. [DOI] [PubMed] [Google Scholar]
- 39.Agricola E, Beneduce A, Esposito A, Ingallina G, Palumbo D, Palmisano A. Heart and lung multimodality imaging in COVID-19. JACC Cardiovasc Imaging. 2020;13(8):1792–1808. doi: 10.1016/j.jcmg.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudski L, Januzzi JL, Rigolin VH, Bohula EA, Blankstein R, Patel AR. Multimodality imaging in evaluation of cardiovascular complications in patients with COVID-19. J Am College Cardiol. 2020 doi: 10.1016/j.jacc.2020.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu ZW, Tang JJ, Chai XP, Fang ZF, Liu QM, Hu XQ. [Comparison of heart failure and 2019 novel coronavirus pneumonia in chest CT features and clinical characteristics] Zhonghua xin xue guan bing za zhi. 2020;48(0):E007. doi: 10.3760/cma.j.cn112148-20200218-00093. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Li H, Zhu S, Xie Y, Wang B, He L. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurnicka K, Lichodziejewska B, Goliszek S, Dzikowska-Diduch O, Zdończyk O, Kozłowska M. Echocardiographic pattern of acute pulmonary embolism: analysis of 511 consecutive patients. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2016;29(9):907–913. doi: 10.1016/j.echo.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Motwani M, Kidambi A, Greenwood JP, Plein S. Advances in cardiovascular magnetic resonance in ischaemic heart disease and non-ischaemic cardiomyopathies. Heart (British Cardiac Society) 2014;100(21):1722–1733. doi: 10.1136/heartjnl-2013-304680. [DOI] [PubMed] [Google Scholar]
- 46.Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113(6):823–833. doi: 10.1161/CIRCULATIONAHA.104.529511. [DOI] [PubMed] [Google Scholar]
- 47.Pontone G, Baggiano A, Conte E, Teruzzi G, Cosentino N, Campodonico J. "Quadruple Rule-Out" with computed tomography in a COVID-19 patient with equivocal acute coronary syndrome presentation. JACC Cardiovasc Imaging. 2020;13(8):1854–1856. doi: 10.1016/j.jcmg.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brett NJ, Strugnell WE, Slaughter RE. Acute myocarditis demonstrated on CT coronary angiography with MRI correlation. Circul Cardiovasc Imaging. 2011;4(3):e5–e6. doi: 10.1161/CIRCIMAGING.110.957779. [DOI] [PubMed] [Google Scholar]
- 49.Dambrin G, Laissy JP, Serfaty JM, Caussin C, Lancelin B, Paul JF. Diagnostic value of ECG-gated multidetector computed tomography in the early phase of suspected acute myocarditis. A preliminary comparative study with cardiac MRI. Eur Radiol. 2007;17(2):331–338. doi: 10.1007/s00330-006-0391-2. [DOI] [PubMed] [Google Scholar]
- 50.Hendren NS, Drazner MH, Bozkurt B, Cooper LT., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]