BACKGROUND:
A number of recent studies have reported an association between intraoperative burst suppression and postoperative delirium. These studies suggest that anesthesia-induced burst suppression may be an indicator of underlying brain vulnerability. A prominent feature of electroencephalogram (EEG) under propofol and sevoflurane anesthesia is the frontal alpha oscillation. This frontal alpha oscillation is known to decline significantly during aging and is generated by prefrontal brain regions that are particularly prone to age-related neurodegeneration. Given that burst suppression and frontal alpha oscillations are both associated with brain vulnerability, we hypothesized that anesthesia-induced frontal alpha power could also be associated with burst suppression.
METHODS:
We analyzed EEG data from a previously reported cohort in which 155 patients received propofol (n = 60) or sevoflurane (n = 95) as the primary anesthetic. We computed the EEG spectrum during stable anesthetic maintenance and identified whether or not burst suppression occurred during the anesthetic. We characterized the relationship between burst suppression and alpha power using logistic regression. We proposed 5 different models consisting of different combinations of potential contributing factors associated with burst suppression: (1) a Base Model consisting of alpha power; (2) an Extended Mechanistic Model consisting of alpha power, age, and drug dosing information; (3) a Clinical Confounding Factors Model consisting of alpha power, hypotension, and other confounds; (4) a Simplified Model consisting only of alpha power and propofol bolus administration; and (5) a Full Model consisting of all of these variables to control for as much confounding as possible.
RESULTS:
All models show a consistent significant association between alpha power and burst suppression while adjusting for different sets of covariates, all with consistent effect size estimates. Using the Simplified Model, we found that for each decibel decrease in alpha power, the odds of experiencing burst suppression increased by 1.33-fold.
CONCLUSIONS:
In this study, we show how a decrease in anesthesia-induced frontal alpha power is associated with an increased propensity for burst suppression, in a manner that captures individualized information above and beyond a patient’s chronological age. Lower frontal alpha band power is strongly associated with higher propensity for burst suppression and, therefore, potentially higher risk of postoperative neurocognitive disorders. We hypothesize that low frontal alpha power and increased propensity for burst suppression together characterize a “vulnerable brain” phenotype under anesthesia that could be mechanistically linked to brain metabolism, cognition, and brain aging.
KEY POINTS.
Question: Is a patient’s propensity for burst suppression during general anesthesia associated with other features in the electroencephalogram (EEG), namely alpha wave power?
Findings: Lower frontal alpha power is significantly associated with a higher propensity for burst suppression, independent of age and other factors.
Meaning: Low frontal alpha power and increased propensity for burst suppression under anesthesia could be signatures of a “vulnerable brain” phenotype.
Each year >19 million patients ≥65 years of age undergo anesthesia and surgery.1 Neurocognitive disorders (NCDs) are common after surgery, particularly in older patients.2 Delirium is an acute confusional state with fluctuating changes in attention, mental status, and level of consciousness. Postoperative delirium (POD) refers to delirium that occurs up to 1 week after surgery or until discharge.2,3 POD affects up to 70% of patients >60 years of age undergoing major inpatient surgeries and is associated with increased mortality, persistent cognitive decline, and prolonged intensive care and hospital length of stay. After hospital discharge, overall cognitive recovery may also take longer than expected, up to 30 days, referred to as delayed neurocognitive recovery. Postoperative cognitive decline may develop over a longer period after surgery, up to 12 months, referred to as a postoperative NCD.3 The existence and prevalence of postoperative cognitive disorders, and the extent to which anesthesia and surgery contribute to cognitive decline, remains a controversial subject because it is difficult to distinguish postoperative cognitive effects from ongoing cognitive decline in elderly patients.4 Both POD and postoperative NCDs are components of a broader phenomenon of perioperative NCDs that include NCDs that are discovered preoperatively.3 POD may be an independent risk factor for postoperative NCDs.5
The potential causes and predisposing factors for NCDs in the postoperative period (ie, POD, delayed neurocognitive recovery, and postoperative NCDs) are the focus of intense ongoing investigation. One possibility is that procedural factors such as postsurgical inflammation or anesthetic exposure could contribute to the development of an NCD in the postoperative period. Another possibility is that the risk of developing NCDs could come from a preexisting sensitivity due to poor brain health or brain vulnerability. It is also possible that a combination of these factors could contribute to the overall risk of NCDs after surgery. Recently, an association between intraoperative burst suppression and POD has been reported by a number of groups.6,7 Burst suppression is a state of profound brain inactivation, which can be induced at high anesthetic doses, beyond what is required to maintain unconsciousness during general anesthesia.8 Although susceptibility to anesthetic-induced burst suppression increases with age,9 high interindividual variability exists,10 suggesting other contributing factors such as variability in pharmacodynamics. Most recently, Fritz et al11 have reported that patients who enter burst suppression at low anesthetic gas levels are more likely to have POD than other patients. This suggests an underlying state of brain vulnerability that is unmasked by the anesthesia-induced state.
We wondered whether other aspects of anesthesia-induced brain activity might be related to postoperative neurocognitive impairment. Such markers could be used to identify patients who might have lower anesthetic requirements or who might have a higher likelihood of POD and postoperative NCDs requiring specialized care. Human and animal recordings as well as modeling studies over the past several years have shown that general anesthetic and sedative drugs induce highly structured oscillations within brain systems and circuits that are readily observed in the electroencephalogram (EEG). A prominent feature of EEG under propofol and sevoflurane anesthesia is the frontal alpha (8–12 Hz) oscillation.12 We hypothesize that the alpha oscillation could be a potential biomarker for brain vulnerability for the following reasons. First, high-density EEG source localization analysis13 and neuroimaging studies14 demonstrate that the putative prefrontal cortical generators for the propofol-induced frontal alpha oscillation appear to be susceptible to cortical thinning in both aging and Alzheimer disease. Second, propofol- and sevoflurane-induced frontal alpha power declines with increasing age,8 even when age-adjusted anesthetic doses are used.15 Given the putative thalamocortical mechanisms for the anesthesia-induced frontal alpha oscillations,16 alpha oscillations might therefore provide a marker of age-dependent decline in prefrontal thalamocortical circuit function. Third, another form of thalamocortical oscillation, the spontaneous occipital alpha wave during eye closure, is known to decline in amplitude with increasing severity of dementia.17 Fourth, intraoperative frontal alpha power correlates with preoperative cognitive function in older adults,18 further suggesting a link between alpha oscillations and brain vulnerability. Finally, recent work has shown that the absence of frontal alpha power during general anesthesia is strongly associated with postanesthesia care unit (PACU) delirium.19
Given the relationship between intraoperative burst suppression and POD,6,7 particularly when burst suppression occurs at low anesthetic concentrations,11 and the relationship between cognitive decline and alpha waves,17,18 and the relationship between alpha waves and PACU delirium,19 we hypothesize that the anesthesia-induced frontal alpha wave could be related to the propensity for intraoperative burst suppression.
METHODS
Patient Selection and Data Collection
The Human Research Committee at Partners HealthCare approved this retrospective observational study. Informed consent was waived by the institutional review board (IRB) because EEG is routinely recorded in our institution during procedures requiring general anesthesia and because analyses of these data pose minimal risk. We analyzed EEG data from a previously reported cohort in which a total of 155 patients received propofol (n = 60) or sevoflurane (n = 95) as the primary anesthetic.8 We reviewed our database of 627 patients who underwent general anesthesia and simultaneous EEG recordings collected between September 1, 2011, and May 1, 2014. We identified 328 patients for whom either propofol (n = 118) or sevoflurane (n = 210) was administered as the primary anesthetic. From these, we excluded 46 patients <18 years of age, 36 instances in which patients received either neuraxial or peripheral nerve block, and 19 patients who were not subjected to mechanical ventilation. Of the remaining 227 patients, we identified 81 patients with propofol and 146 patients with sevoflurane as the sole hypnotic. We excluded patients with improperly fitted EEG electrodes resulting in poor data quality, artifacts, and patients who were undergoing a procedure that interfered with electrode placement. Ultimately, 155 patients (92 women, 18–89 years old, mean: 48.69 ± 18.57) were regarded as suitable for analysis (95 for sevoflurane and 60 for propofol).
Frontal EEG data were recorded using the SedLine Brain Function Monitor (Masimo Corporation, Irvine, CA). The standard SedLine SEDTrace electrode array records from electrodes located approximately at positions Fp1, Fp2, F7, and F8, with ground electrode at Fpz and reference electrode ≈1 cm above Fpz. Electrode impedance was <5 kΩ for each electrode. Anesthetics were titrated based solely on the clinical judgment of the anesthesiologists. The rates of propofol infusion during analysis epochs were 119.3 ± 25.7 µg/kg/min (minimum: 75 µg/kg/min, maximum: 200 µg/kg/min), and the age-adjusted minimal alveolar concentration (MAC) values of sevoflurane during analysis epochs were 1.01 ± 0.23 (minimum: 0.39, maximum: 1.55)
Drug concentrations were captured automatically by the anesthesia machine for sevoflurane, manually for other administered drugs, and saved in the electronic medical record. Sevoflurane end-tidal concentrations were converted to age-adjusted MAC equivalent values. The duration and the extent of intraoperative hypotension were quantified by area under the curve (AUC) for mean arterial pressure (MAP) <75 mm Hg during the surgical procedure.
Spectral Analysis and Burst Suppression Analysis
We selected EEG data segments using information from both the electronic anesthesia record (Metavision, Dedham, MA) and EEG spectral analysis. For each subject, we selected a contiguous 2-minute window of EEG for analysis, beginning ≈10 minutes after the start of surgery. We visually inspected the EEG spectrogram to ensure that the analysis windows were free of artifacts, did not contain burst suppression, and that EEG dynamics were stable (ie, not transitioning to burst suppression or emergence). We computed the power spectra and spectrogram for each subject using multitaper spectral estimation methods implemented in the Chronux toolbox (http://chronux.org/) in Matlab (MathWorks, Natick, MA). The spectrogram is a time-varying version of the power spectrum, estimated using consecutive windows of EEG data. To obtain estimates of power spectra, we calculated an EEG derivation that equally weighted the signals obtained from Fp1, Fp2, F7, and F8. We estimated the spectra and spectrograms using the following parameters: window length (T) = 2 seconds with no overlap, time–bandwidth product (TW) = 3, number of tapers (K) = 5, and spectral resolution of 2W = 3 Hz. We computed the power in the alpha band by summation of the multitaper spectrum from 8 to 12 Hz.
We visually scored each record for episodes of burst suppression, as previously described in Purdon et al.8 We defined burst suppression as periods of bursting activity alternating with periods of isoelectricity or electrical silence, as visualized using the EEG spectrogram. Each EEG recording was inspected by at least 2 independent observers experienced in EEG interpretation for the occurrence of burst suppression. For each subject, we identified a postinduction period beginning ≈10 minutes after induction and concluding at the end of the procedure. We then visually analyzed the spectrogram for periods of burst suppression, defined operationally by the presence of at least 3 consecutive suppression events within a 1-minute period. Patients showing burst suppression during the postinduction period were graded with a “1,” and patients who did not were graded with a “0.”
Statistical Modeling and Analysis
We characterized the relationship between burst suppression and various potential predicting/confounding factors using logistic regression analyses. We considered 5 models consisting of different combinations of covariates to assess the association of the alpha power with burst suppression. We examined a small number of models with a limited number of covariates in each model according to clinical theory:
a “Base Model,” consisting of a single independent variable, alpha power, expressing the primary hypothesis that alpha power is associated with the propensity for intraoperative burst suppression;
an “Extended Mechanistic Model,” consisting of
alpha power,
age, the type of anesthetic, the standardized maintenance dose, an interaction term between the type and maintenance dose of the primary anesthetic drug, and the rate of intraoperative propofol boluses, calculated by dividing the cumulative amount of propofol boluses by the time interval from the start to end of the surgery; this model would allow us to characterize the association of other relevant physiological or pharmacological factors with burst suppression;
a “Clinical Confounding Factors Model,” consisting of alpha power, the sex of the patient, intraoperative hypotension, and perioperative use of midazolam, all of which could be potential confounding factors to the primary hypothesis;
a “Simplified Model,” consisting of alpha power and propofol bolus rate, which expresses the primary hypothesis as well as the clinical observation that episodes of burst suppression frequently occur after propofol boluses are administered; and
a “Full Model,” consisting of all the variables to control for as much confounding as possible.
In addition to age and anesthetic dose, which are directly related to propensity for burst suppression,8 we included intraoperative hypotension as a variable in our Clinical Confounding Factors Model, to account for the fact that EEG activity is sensitive to changes in cerebral perfusion. For instance, intraoperative continuous EEG is used during procedures such as carotid endarterectomy to monitor changes in cerebral perfusion. A decrease in cerebral perfusion is reflected in the EEG as a decrease in amplitude and/or as an attenuation of higher frequency activity20 and thus could result in apparent “suppression” of the EEG. In this way, MAP could be related to burst suppression. We included sex as another potential contributing factor to our Clinical Confounding Factors Model under the rationale that there might be sex-related differences in drug requirements that could in turn result in a difference in the propensity for burst suppression. We conducted a sparse data bias check to explore whether there were enough outcome cases for the full confounding variables set. Although our event rate was not extremely low, 24.5% (38 of 155 patients experienced burst suppression), our study data could have been subject to sparse data bias if the full confounding variable set were used. For this reason, we did not use the full model to illustrate quantitatively the relationship between alpha power and burst suppression.
We used R software (version 3.6.1; R Core Team, Vienna, Austria) for our statistical analysis. We interpreted P values <.05 as a significant level of association between each variable and the outcome. We had very limited missing values (2/155, 1.2%) for the MAP variable. We conducted a single missing data imputation using chain equation methods. We performed the Hosmer–Lemeshow test on all the models to characterize the model calibration for each model. We calculated the area under the receiver operating characteristic (ROC) curve and the Akaike information criterion (AIC) for each model to compare their performance. AIC estimates the Kullback–Leibler information distance between the model and the underlying data generating process, and its value represents a tradeoff between the goodness of fit of the model and the complexity of the model. A lower AIC value indicates a better model.
Utilizing a 2-tailed logistic regression, our study sample of N = 155 with binary primary outcome observed events = 117 would achieve a 80% power to detect a change in probability of outcome from the value of 0.75 at an average alpha power level to 0.64 when alpha power value is increased to 1 standard deviation above the mean. This change corresponds to an odds ratio (OR) of 0.60. The number of patients with burst suppression (the event rate) was 38 of 155 patients (24.5%). This observed sample N = 155 will yield a power = 0.8 to detect an effect size of mean difference of alpha power as small as 2.6 dB between participants with versus without the primary outcome events utilizing a 2-sided independent sample t test. This effect size equates to an alpha power of 5.4 in the burst suppression group versus an alpha power of 8 in the nonevent group assuming a standard deviation of 5.
RESULTS
In all of the proposed models, alpha power was significantly associated with burst suppression. In the Base Model, in which alpha power is the only independent variable, alpha power was significantly associated with burst suppression (estimated OR = 0.75 in reference to a 1 dB increase in alpha power, with a 95% confidence interval [CI], 0.67–0.84, P < .001). Figure 1 illustrates the fit of the Base Model, demonstrating the inverse relationship between alpha power and the probability of burst suppression. The empirical probabilities of burst suppression were estimated as a function of alpha power by calculating the proportions of subjects showing burst suppression in 20 equally spaced bins across the range of observed alpha power. Some alpha power bins did not have corresponding observations, so Figure 1 displays 18 pooled empirical estimates. We see that as alpha power decreases from >15 to <−5 dB, the probability of burst suppression increases from close to 0 to near 1 (Figure 1). This relationship is further illustrated by example spectrograms showing one subject with high alpha power and no burst suppression and another subject with low alpha power and sustained burst suppression (Figure 2).
Figure 1.
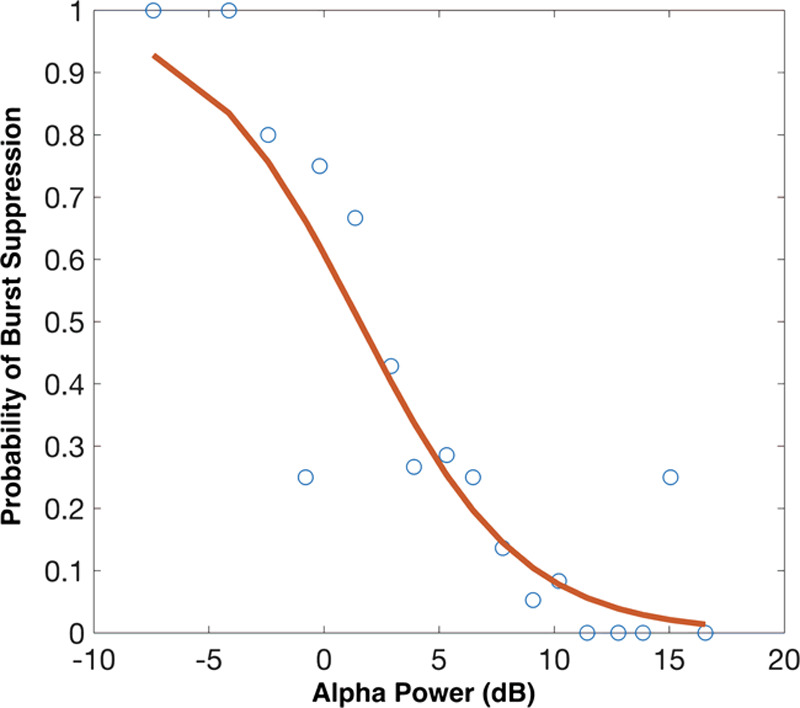
Logistic regression fit for the Base Model. Alpha power is strongly associated with burst suppression. The empirical probabilities of burst suppression were estimated as a function of alpha power by calculating the proportions of subjects showing burst suppression in 20 equally spaced bins across the range of observed alpha power. As alpha power decreases from >15 to <−5 dB, the probability of burst suppression increases from close to 0 to near 1.
Figure 2.
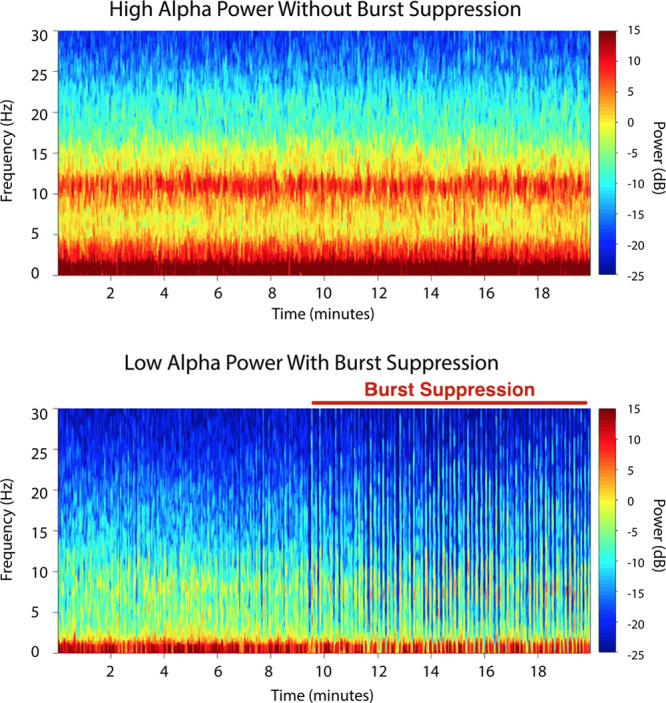
Electroencephalogram spectrograms from individual patients illustrating the relationship between power in anesthesia-induced alpha band activity and burst suppression. Top, The spectrogram shows an example of a patient with high alpha power and no burst suppression. Bottom, The spectrogram shows an example of a different patient with low alpha power and prolonged burst suppression.
For the Extended Mechanistic Model, alpha power (OR = 0.80; 95% CI, 0.69–0.92; P = .0017) and propofol bolus rate (OR = 1.02; 95% CI, 1–1.03; P = .0165) were significantly associated with burst suppression. The other variables in this model, most notably age, were not significantly associated with burst suppression (P > .05 in all cases). From previous studies, we know that there is a substantial decrease in alpha power with increasing age.8 However, there is also substantial between-subject variability in alpha power at any age, which appears to associate strongly with burst suppression. An example of this age-independent variability is shown in Figure 3.
Figure 3.
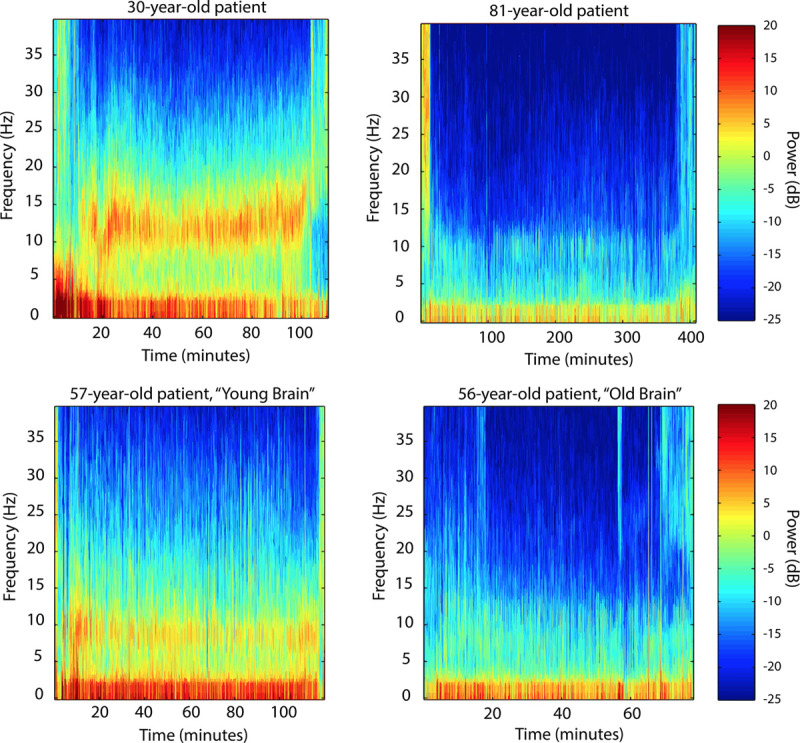
Electroencephalogram spectrograms from individual patients illustrating the variation in power in anesthesia-induced alpha band activity spanning young, middle-aged, and older adults. Top, There is an overall decreasing trend in alpha power with increasing age, comparing a 30-year-old patient and an 81-year-old patient. Bottom, On the other hand, at a given age, there can also be significant variation in frontal alpha power, comparing 2 middle-aged patients of comparable age.
In the Clinical Confounding Factors Model, only alpha power was significantly associated with the probability of burst suppression (OR = 0.75; 95% CI, 0.67–0.85; P < .001).
In the Simplified Model, both alpha power (OR = 0.75; 95% CI, 0.67–0.84; P < .001) and the propofol bolus rate (OR = 1.01; 95% CI, 1.00–1.03; P = .0142) were both significantly associated with the probability of burst suppression.
Finally, in the Full Model, only alpha power (OR = 0.81; 95% CI, 0.70–0.94; P = .0051) and the propofol bolus rate (OR = 1.02; 95% CI, 1.00–1.03; P = .0118) remained significantly associated with the probability of burst suppression.
Using the Simplified Model, we can obtain a quantitative understanding of the relationship between alpha power and burst suppression. After adjusting for propofol bolus rate, for each 1 dB decrease in alpha power, the odds of experiencing burst suppression increase by 1.33-fold (95% CI, 1.19–1.49) (Figure 4). This result from the Simplified Model was consistent with the results of the other candidate models (ie, the Base [1.33 (1.19–1.49)], Extended Mechanistic [1.25 (1.09–1.45)], Clinical Confounding Factors [1.33 (1.18–1.49)], and Full Models [1.23 (1.06–1.43)]).
Figure 4.
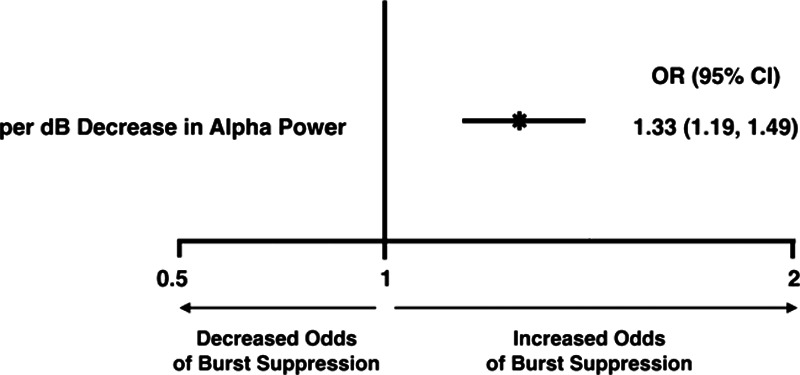
Odds ratios for burst suppression with different levels of alpha power, based on the Simplified Model. After adjusting for propofol bolus rate, for each decibel decrease in alpha power, the odds of experiencing burst suppression increase by 1.33-fold (95% CI, 1.19–1.49). CI indicates confidence interval.
The expected and observed event rates for burst suppression did not deviate significantly from one another under the Hosmer–Lemeshow test (P >.05 in all cases), suggesting that the models were all reasonably calibrated (Supplemental Digital Content, Figure 1, http://links.lww.com/AA/D56). The ROC curves suggest a good discriminative ability of the models (Figure 5). All models show a consistent significant association between alpha power and burst suppression while adjusting for different sets of covariates, with consistent effect size estimates.
Figure 5.
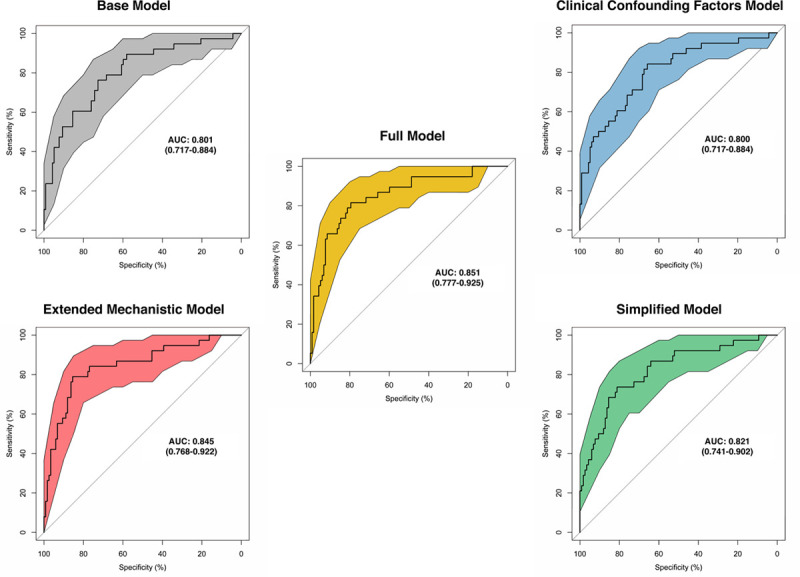
The receiving operating characteristic curves for the Base Model, the Extended Mechanistic Model, the Clinical Confounding Factors Model, the Simplified Model, and the Full Model. All of the models have comparable AUCs (0.801, 0.845, 0.800, 0.821, and 0.851 respectively). The colored area around each curve represents the 95% confidence interval. AUC indicates area under the curve.
The aforementioned results are summarized in Supplemental Digital Content, Table 1, http://links.lww.com/AA/D56.
DISCUSSION
In this study, we show how a decrease in anesthesia-induced frontal alpha power is associated with an increased probability of burst suppression, in a manner that appears to capture individualized information above and beyond a patient’s chronological age. Burst suppression has been identified as an independent predictor of poor postoperative cognitive outcomes.6,7 Fritz et al11 showed that even a few minutes of burst suppression during an hour-long surgery was associated with an increased likelihood of POD. In a more recent study, Fritz et al11 identified a group of patients who exhibits a phenotype of anesthetic sensitivity with suppression at lower anesthetic concentrations and alongside increased incidence of POD. Therefore, considering the potential predictive value of the anesthesia-induced frontal alpha power for burst suppression, we hypothesize that alpha oscillations could serve as a neurophysiological biomarker for brain vulnerability and may help identify patients with lower anesthetic requirements and higher risk of postoperative neurocognitive impairment.
Age has a unique effect on alpha power; generally, alpha power decreases with increasing age; however, there is a significant amount of variability in alpha power across individuals. Therefore, we think that the effect of age on the propensity for burst suppression is 2 layered: (1) entering the state of burst suppression becomes more probable toward the end of the age spectrum and (2) however, if an individual’s alpha power is low, they are more likely to go into burst suppression than their chronological age might indicate.
Our work, in combination with others,6,7,11,18,19 suggests an association among anesthesia-induced alpha oscillations, anesthesia-induced burst suppression, baseline cognition, and postoperative NCDs. We wondered if there might be a central mechanism that could explain the association among these phenomena? We propose that one potential mechanism could be the patients’ underlying brain metabolism. Figure 6 summarizes the proposed mechanistic model. Changes in brain metabolism are believed to underlie changes in brain function and cognition during aging.21 Human brain metabolism is highly glucose dependent. Most of this glucose is consumed via oxidative pathways. However, 10%–15% of glucose metabolism occurs via a nonoxidative pathway, even when adequate oxygen is available. This nonoxidative metabolism in the presence of adequate oxygen is referred to as aerobic glycolysis (AG).22,a AG has been associated with biosynthesis and neuroprotection.23 It is well known that astrocytes are the mediators of brain metabolism.24 AG happens predominantly in astrocytes, supplementing neurons with lactate to fuel oxidative phosphorylation as well as providing adenosine triphosphate (ATP) for the active recycling of glutamate at synapses.25
Figure 6.
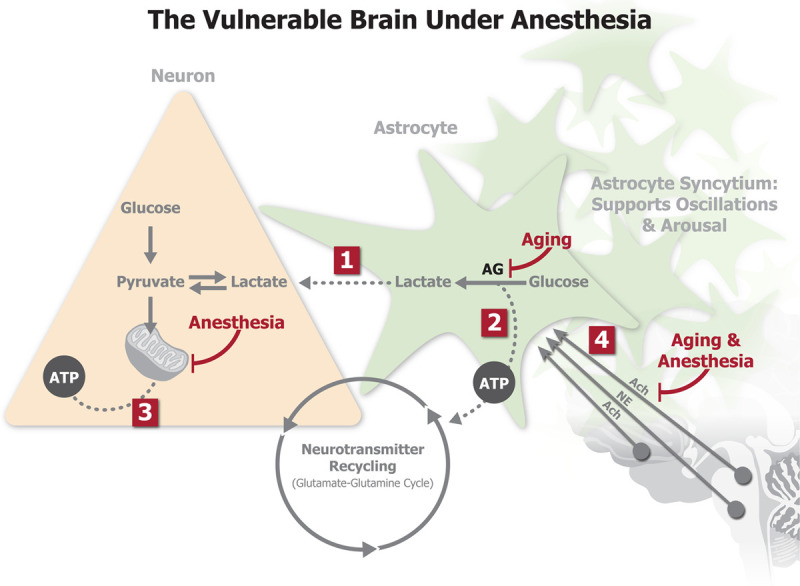
The “vulnerable brain” under anesthesia: a hypothesis linking metabolism, brain oscillations, burst suppression, and cognitive decline. Decreased astrocytic AG in prefrontal cortex fails to provide adequate metabolic support for neuronal oxidative phosphorylation (1) and sustained synaptic neurotransmission (2). Burst suppression is thought to occur when the brain has an inadequate supply of ATP. If metabolism is compromised as in (1) and (2), further depression of brain metabolism by anesthetic drugs via impaired mitochondrial function (3) results in a higher propensity for burst suppression. Astrocytes support brain metabolism, but are also thought to support brain oscillations through their highly connected syncytial networks. In the aging brain with preexisting neuromodulatory deficits, general anesthesia further inhibits subcortical neuromodulatory inputs on astrocyte syncytial networks (4) and suppresses astrocyte–neuron metabolic interactions, leading to less robust brain oscillations. Ach indicates acetylcholine; AG, aerobic glycolysis; ATP, adenosine triphosphate; NE, norepinephrine.
Reduced cerebral metabolic rate is thought to be a key mechanism for burst suppression.26 The characteristic neuronal silent periods that underlie EEG suppression are thought to occur when brain metabolism drops below a certain level required to maintain adequate neuronal ATP levels.26 Accordingly, the suppression periods are thought to increase in duration as metabolic rate continues to decrease.26 Age-related decreases in brain metabolism are believed to be driven by decreases in AG.27 There is evidence that anesthetic drugs impair mitochondrial function,28 shifting brain metabolism away from oxidative phosphorylation and toward glycolysis.29 Therefore, when AG is impaired, anesthesia could more easily lead to burst suppression.
Recent evidence suggests that astrocytes also play a causal role in supporting brain oscillations.30 Neural circuit function and states of arousal are thought to be signaled within astrocyte syncytial networks and through extensive expression of neuromodulator receptors.31,32 The spatial extent of these astrocyte syncytial networks might provide the structural connections necessary to support oscillations across large-scale neural circuits. Metabolic astrocyte–neuron interactions, particularly astrocyte AG and neuron–astrocyte lactate shuttling, are suppressed in brain states associated with decreased levels of subcortical neuromodulation.22,33 Therefore, in an aging brain where there are already preexisting neuromodulatory deficits, inhibition of subcortical neuromodulatory inputs by general anesthesia could further depress astrocyte–neuron metabolic interactions,34 which would lead to weaker oscillations and a greater tendency for burst suppression.
Brain regions with the highest aerobic glycolytic capacity in young adulthood show the most rapid loss of this capacity during aging.35 These brain regions include the prefrontal cortex and the precuneus, both of which are important players in arousal and cognition.36 In a recent study, Arenaza-Urquijo et al37 identified the metabolic profile of medial prefrontal regions as a signature of cognitive resilience in the elderly population. The medial prefrontal cortex is also one of the generators for the anesthesia-induced frontal alpha rhythm.36 Thus, we hypothesize that patients with compromised brain metabolism would be expected to have decreased cognitive ability, would tend to be older in age, would tend to have less robust brain oscillations particularly in prefrontal regions, and would enter burst suppression more readily, all of which are consistent with our findings and with other studies.6,7,11,18,19
This study has several limitations. We performed a retrospective analysis of previously collected data, from a heterogenous study population whose anesthetic regimens were not controlled. However, we still found a significant correlation between EEG power in the alpha band and the probability of burst suppression. This association remained even after controlling for a number of potential confounds. These analyses suggest that alpha power may be a strong predictor of burst suppression, independent of other potential predictor variables including chronological age. Measurements of preoperative delirium and POD and cognition, as well as baseline EEG, would have made our study more complete; however, such measurements will no doubt be featured in future studies.
In this article, we have proposed that EEG activity during general anesthesia, specifically frontal alpha power, could be viewed as a “trait” indicative of underlying brain physiology. Giattino et al18 have taken a similar approach in characterizing the relationship between preoperative cognition and alpha power. However, variations in frontal alpha power have also been proposed as a means to track nociception during general anesthesia, in essence, an instantaneous “state.”15,38,39 These 2 approaches are not mutually exclusive and in fact allude to the richness of information in the EEG. In an ongoing clinical trial, Gaskell et al40 hypothesize that frontal alpha power can be actively modulated by titration of anesthetic and analgesic drugs, and that maximizing the alpha power during anesthetic maintenance and emergence may improve postoperative neurocognitive recovery.
In summary, in this work, we illustrate how brain oscillations measured in the EEG are significantly associated with the propensity for burst suppression and propose a potential mechanistic link among brain oscillations, brain metabolism, cognition, and brain aging. Based on this and other work,11,17,18 we propose the existence of a vulnerable brain phenotype under anesthesia consisting of low frontal alpha power, combined with a propensity for burst suppression at lower-than-expected drug concentrations, even after age adjustment.8,11
In anesthesiology, the EEG is viewed in a highly reductive manner, most often as a single processed number between 0 and 100. In neurology, the EEG is still interpreted through visual scoring, and little is made of the potential mechanisms underlying the waveforms. In contrast, our work suggests that the EEG could contain important information about brain function and health that goes beyond visually scored waveforms or a single processed number.
DISCLOSURES
Name: Yu Raymond Shao, MD, PhD.
Contribution: This author helped design the study, derived the models, analyzed the data, and drafted the manuscript.
Conflicts of Interest: Y. R. Shao is an inventor on patents pending on brain monitoring technologies assigned to Massachusetts General Hospital.
Name: Pegah Kahali, MD.
Contribution: This author performed the data analysis, wrote the manuscript, and developed the mechanisms to interpret the results.
Conflicts of Interest: None.
Name: Timothy T. Houle, PhD.
Contribution: This author performed data analysis.
Conflicts of Interest: None.
Name: Hao Deng, MD, MPH.
Contribution: This author performed data analysis.
Conflicts of Interest: None.
Name: Christopher Colvin, MHSc.
Contribution: This author contributed to the development of mechanisms to interpret the results.
Conflicts of Interest: None.
Name: Bradford C. Dickerson, MD, PhD.
Contribution: This author helped interpret the results.
Conflicts of Interest: None.
Name: Emery N. Brown, MD, PhD.
Contribution: This author helped interpret the results.
Conflicts of Interest: E. N. Brown is an inventor on patents pending on brain monitoring technologies assigned to Massachusetts General Hospital; inventor on a patent assigned to Massachusetts General Hospital and licensed nonexclusively to Masimo Corporation; and is a cofounder of PASCALL Systems, Inc, a startup company developing closed-loop physiological control systems.
Name: Patrick L. Purdon, PhD.
Contribution: This author designed the study, analyzed the data, wrote and revised the manuscript, contributed to and supervised the development of mechanisms to interpret the results, and supervised the overall project.
Conflicts of Interest: P. L. Purdon is an inventor on patents pending on brain monitoring technologies assigned to Massachusetts General Hospital; inventor on a patent assigned to Massachusetts General Hospital and licensed nonexclusively to Masimo Corporation; received speaker's honoraria from Masimo Corporation; and is a cofounder of PASCALL Systems, Inc, a startup company developing closed-loop physiological control systems.
This manuscript was handled by: Maxime Cannesson, MD, PhD.
Supplementary Material
FOOTNOTES
Aerobic glycolysis can be defined as the disproportionate upregulation of the cerebral rate of glucose utilization (CMRglc) compared with oxygen consumption (CMRO2) even though blood oxygen level and delivery are sufficient to satisfy demand.
GLOSSARY
- Ach =
- acetylcholine
- AG =
- aerobic glycolysis
- AIC =
- Akaike information criterion
- ATP =
- adenosine triphosphate
- AUC =
- area under the curve
- CI =
- confidence interval
- EEG =
- electroencephalogram
- IRB =
- institutional review board
- K =
- number of tapers
- MAC =
- minimal alveolar concentration
- MAP =
- mean arterial pressure
- NCD =
- neurocognitive disorder
- NE =
- norepinephrine
- OR =
- odds ratio
- PACU =
- postanesthesia care unit
- POD =
- postoperative delirium
- ROC =
- receiver operating characteristic
- T =
- window length
- TW =
- time–bandwidth product
- W =
- bandwidth
Published ahead of print 14 May 2020.
Funding: This work has been supported by National Institute of Health (NIH) grants R01AG056015 and R01AG054081 and the Aging Brain Initiative at Massachusetts Institute of Technology.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Y. R. Shao and P. Kahali contributed equally and share first authorship.
Reprints will not be available from the authors.
REFERENCES
- 1.Doshi A, Cabeza R, Berger M. Reves JG, Barnett SR, McSwain JR, Rooke GA. Geriatric anesthesia: age-dependent changes in the central and peripheral nervous systems. In: Geriatric Anesthesiology. 2018Cham, Switzerland: Springer International Publishing; 145–160. [Google Scholar]
- 2.Hshieh TT, Inouye SK, Oh ES. Delirium in the elderly. Psychiatr Clin North Am. 2018;41:1–17. [DOI] [PubMed] [Google Scholar]
- 3.Evered L, Silbert B, Knopman DS, et al. ; Nomenclature Consensus Working Group. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avidan MS, Evers AS. Review of clinical evidence for persistent cognitive decline or incident dementia attributable to surgery or general anesthesia. J Alzheimers Dis. 2011;24:201–216. [DOI] [PubMed] [Google Scholar]
- 5.Aranake-Chrisinger A, Cheng JZ, Muench MR. Ability of postoperative delirium to predict intermediate-term postoperative cognitive function in patients undergoing elective surgery at an academic medical centre: protocol for a prospective cohort study. BMJ Open. 2018;8:e017079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol. 2015;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritz BA, Kalarickal PL, Maybrier HR. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg. 2016;122:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purdon PL, Pavone KJ, Akeju O, et al. The ageing brain: age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth. 2015;115suppl 1i46–i57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz AE, Tuttle RH, Poppers PJ. Electroencephalographic burst suppression in elderly and young patients anesthetized with isoflurane. Anesth Analg. 1989;68:9–12. [PubMed] [Google Scholar]
- 10.Thomsen CE, Prior PF. Quantitative EEG in assessment of anaesthetic depth: comparative study of methodology. Br J Anaesth. 1996;77:172–178. [DOI] [PubMed] [Google Scholar]
- 11.Fritz BA, Maybrier HR, Avidan MS. Intraoperative electroencephalogram suppression at lower volatile anaesthetic concentrations predicts postoperative delirium occurring in the intensive care unit. Br J Anaesth. 2018;121:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purdon PL, Pierce ET, Mukamel EA. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–E1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukamel EA, Pirondini E, Babadi B. A transition in brain state during propofol-induced unconsciousness. J Neurosci. 2014;34:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakkour A, Morris JC, Wolk DA, Dickerson BC. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 2013;76:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hight D, Voss LJ, Garcia PS, Sleigh J. Changes in alpha frequency and power of the electroencephalogram during volatile-based general anesthesia. Front Syst Neurosci. 2017;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babiloni C, Carducci F, Lizio R, et al. Resting state cortical electroencephalographic rhythms are related to gray matter volume in subjects with mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2013;34:1427–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giattino CM, Gardner JE, Sbahi FM, et al. ; MADCO-PC Investigators. Intraoperative frontal alpha-band power correlates with preoperative neurocognitive function in older adults. Front Syst Neurosci. 2017;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hesse S, Kreuzer M, Hight D, et al. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: an early sign of postoperative complications. Br J Anaesth. 2019;122:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuwer MR. Intraoperative electroencephalography. J Clin Neurophysiol. 1993;10:437–444. [DOI] [PubMed] [Google Scholar]
- 21.Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143:418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dienel GA, Cruz NF. Aerobic glycolysis during brain activation: adrenergic regulation and influence of norepinephrine on astrocytic metabolism. J Neurochem. 2016;138:14–52. [DOI] [PubMed] [Google Scholar]
- 23.Raichle ME. The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. [DOI] [PubMed] [Google Scholar]
- 25.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological–metabolic model for burst suppression. Proc Natl Acad Sci. 2012;109:3095–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goyal MS, Vlassenko AG, Blazey TM. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 2017;26:353.e3–360.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen PJ. Effect of anesthetics on mitochondrial function. Anesthesiology. 1973;39:153–164. [DOI] [PubMed] [Google Scholar]
- 29.Jacob Zvi MD, Li Haifang PD, Makaryus Rany MD, et al. Metabolomic profiling of children’s brains undergoing general anesthesia with sevoflurane and propofol. Anesthesiol J Am Soc Anesthesiol. 2012;117:1062–1071. [DOI] [PubMed] [Google Scholar]
- 30.Lee HS, Ghetti A, Pinto-Duarte A. Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A. 2014;111:E3343–E3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haydon PG, Nedergaard M. How do astrocytes participate in neural plasticity?. Cold Spring Harb Perspect Biol. 2014;7:a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Z, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature. 2016;539:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiNuzzo M, Nedergaard M. Brain energetics during the sleep-wake cycle. Curr Opin Neurobiol. 2017;47:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leanza G, Gulino R, Zorec R. Noradrenergic hypothesis linking neurodegeneration-based cognitive decline and astroglia. Front Mol Neurosci. 2018;11:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blazey T, Snyder AZ, Su Y, et al. Quantitative positron emission tomography reveals regional differences in aerobic glycolysis within the human brain. J Cereb Blood Flow Metab. 2019;39:2096–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flores FJ, Hartnack KE, Fath AB. Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2017;114:E6660–E6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arenaza-Urquijo EM, Przybelski SA, Lesnick TL. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain. 2019;142:1134–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacKay EC, Sleigh JW, Voss LJ, Barnard JP. Episodic waveforms in the electroencephalogram during general anaesthesia: a study of patterns of response to noxious stimuli. Anaesth Intensive Care. 2010;38:102–112. [DOI] [PubMed] [Google Scholar]
- 39.Hight DF, Gaskell AL, Kreuzer M, Voss LJ, García PS, Sleigh JW. Transient electroencephalographic alpha power loss during maintenance of general anaesthesia. Br J Anaesth. 2019;122:635–642. [DOI] [PubMed] [Google Scholar]
- 40.Gaskell A, Pullon R, Hight D. Modulation of frontal EEG alpha oscillations during maintenance and emergence phases of general anaesthesia to improve early neurocognitive recovery in older patients: protocol for a randomised controlled trial. Trials. 2019;20:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


