Abstract
Purpose
The Pheochromocytoma of the Adrenal Gland Scaled Score (PASS) and the Grading System for Adrenal Pheochromocytoma and Paraganglioma (GAPP) are scoring systems to predict metastatic potential in pheochromocytomas (PCC) and paragangliomas (PGLs). The goal of this study is to assess PASS and GAPP as metastatic predictors and to correlate with survival outcomes.
Methods
The cohort included PCC/PGL with ≥5 years of follow-up or known metastases. Surgical pathology slides were rereviewed. PASS and GAPP scores were assigned. Univariable and multivariable logistic regression, Kaplan–Meier survival analysis, and Cox proportional hazards were performed to assess recurrence-free survival (RFS) and disease-specific survival (DSS).
Results
From 143 subjects, 106 tumors were PCC and 37 were PGL. Metastases developed in 24%. The median PASS score was 6.5 (interquartile range [IQR]: 4.0-8.0) and median GAPP score was 3.0 (IQR: 2.0-4.0). Interrater reliability was low–moderate for PASS (intraclass correlation coefficient [ICC]: 0.6082) and good for GAPP (ICC 0.7921). Older age (OR: 0.969, P = .0170) was associated with longer RFS. SDHB germline pathogenic variant (OR: 8.205, P = .0049), extra-adrenal tumor (OR: 6.357, P < .0001), Ki-67 index 1% to 3% (OR: 4.810, P = .0477), and higher GAPP score (OR: 1.537, P = .0047) were associated with shorter RFS. PASS score was not associated with RFS (P = .1779). On Cox regression, a GAPP score in the moderately differentiated range was significantly associated with disease recurrence (HR: 3.367, P = .0184) compared with well-differentiated score.
Conclusion
Higher GAPP scores were associated with aggressive PCC/PGL. PASS score was not associated with metastases and demonstrated significant interobserver variability. Scoring systems for predicting metastatic PCC/PGL may be improved by incorporation of histopathology, clinical data, and germline and somatic tumor markers.
Keywords: PASS, GAPP, pheochromocytoma, paraganglioma, malignancy, histopathology
Pheochromocytomas (PCCs) and paragangliomas (PGLs) are neuroendocrine tumors derived from the adrenal medulla and extra-adrenal ganglia, respectively. Many PCCs/PGLs are associated with catecholamine hypersecretion, which alter cardiovascular physiology and cause morbidity and mortality. Approximately 15% to 25% of all PCCs/PGLs develop metastatic disease, defined as the presence of tumor in nonchromaffin-derived tissue (1, 2). For patients with metastatic PCC/PGL, treatment is essentially palliative and 5-year survival ranges from 37% to 77% (3-6). There are no reliable predictors of metastatic potential. As a result, patients with PCCs/PGLs require lifelong surveillance, as the latency for metastases can be 20 years or more (7). Some studies propose that large tumor size, extra-adrenal origin, and secretion of methoxytyramine are associated with increased risk of metastatic disease (1, 8). The presence of a germline pathogenic variant in Succinate Dehydrogenase Subunit B (SDHB) is a well-established risk factor for metastases, but only about half of patients with metastatic disease have an inherited SDHB pathogenic variant (9).
To address the need for predictive markers of metastatic potential, several histopathologic scoring systems have been developed. The first system introduced was Pheochromocytoma of the Adrenal Gland Scaled Score (PASS). Developed by Thompson in 2002, PASS is a composite score incorporating histologic features such as capsular or vascular invasion, mitotic rate, and necrosis (10). In the derivation cohort of 100 adrenal PCC, a PASS of <4 was predictive of benign clinical behavior. Most academic centers do not use the PASS due to wide intra- and interobserver variability (11). Several groups have proposed variations to the PASS, including the Grading system for Adrenal Pheochromocytoma and Paraganglioma (GAPP), which combines histopathologic and clinical data (12). The GAPP score includes overlapping features with the PASS score, but was developed to assess PGL as well as PCC (13). The GAPP score has yet to be validated in a US cohort. The goal of this study was therefore to evaluate the PASS and GAPP scores and correlate with survival outcomes in a large patient cohort of PCC/PGL patients with longitudinal follow-up.
Material and Methods
Study population
A retrospective cohort study was conducted with Institutional Review Board approval from the University of Pennsylvania (Protocol # 828891). The study cohort included all primary adrenal PCCs and thoracic, abdominal, and pelvic PGLs resected at the Hospital of the University of Pennsylvania between September 1997 and December 2012, to allow for adequate longitudinal follow-up postresection. Clinical data collection end date was December 2017. Primary adrenal PCC/PGL resected between 2012 and 2017 with known distant metastases were also included. Head and neck PGLs were excluded, as these tumors were not included in either PASS or GAPP scoring systems. One tumor per study subject was included, either the primary tumor in the case of single tumors, or the dominant tumor by size in the case of multiple primary tumors.
Clinical data
Chart review was performed and clinical data were abstracted including age, gender, self-reported race, germline genetic testing, biochemical testing, surgical pathology, radiologic results, and clinical follow-up and survival data. Date of primary surgery was used to approximate date of primary diagnosis. If primary surgery was performed at another health system and original records were unavailable to obtain the exact date of surgery, the following assumptions were made: for missing month, month was assigned as June to approximate mid-year; for missing day, day was assigned as the 15th, to approximate mid-month. Duration of follow-up was defined as time from first surgery to last clinical follow-up or date of death.
Genetic testing
Our institutional practice is to refer all subjects with PCC/PGL for germline genetic testing. Our current standard PCC/PGL susceptibility gene testing includes NF1, MAX, RET, SDHA, SDHAF2, SDHB, SDHC, SDHD, TMEM127, and VHL; the majority of patients are tested with the 10 gene PGLNext Assay (Ambry Genetics, Aliso Viejo, CA) which utilizes next-generation sequencing with reflex Sanger sequencing for regions with missing or insufficient read depth coverage. Suspect variant calls other than those classified as “benign” or “likely benign” are verified bidirectionally via Sanger sequencing. Gross duplications and deletions for all 10 genes are assessed utilizing a targeted chromosomal microarray. Depending on the testing date, a subset of the 10 genes may have been tested based on best clinical practice at that time.
Survival outcomes
Recurrence-free survival (RFS) was defined as time from surgery to clinical evidence of metastatic or recurrent disease (MRD) by elevation in catecholamine/metanephrines or definitive radiologic evidence of disease, as determined at the time of clinical evaluation. Clinical evidence of MRD was further categorized as follows: locoregional recurrence (disease in nonchromaffin-derived tissues in the area of primary tumor resection, ie, lymph nodes in the adrenal resection bed); distant metastases (disease in nonchromaffin-derived tissues excluding the area of primary tumor, ie, osseous metastases); or new primary/indeterminate (disease in chromaffin-derived tissues not in the area of primary tumor, ie, new contralateral PCC). Disease-specific survival (DSS) was defined as time from surgery to time of demise from PCC/PGL. Date of death was identified in clinical records and confirmed using the Social Security Death Index.
Histopathologic scoring
Formalin-fixed paraffin-embedded tissue blocks from PCC/PGL tumors underwent formal histopathologic rereview. Hematoxylin and eosin staining and Ki-67 (clone MIB-1, DAKO, #IR626, Santa Clara, CA) immunostaining were performed on selected tumor blocks. Regions of interest were hand-selected utilizing whole slide scanned images. Assessments of tumor cellularity and Ki-67 proliferative index in the most proliferative foci, scoring at least 500 cells, were performed using QuPath, an open-source digital pathology image analysis software program (14).
For PCCs, 2 independent surgical pathologists blinded to the clinical outcomes assessed PASS based on published criteria (Table 1) (10). PASS was categorized as <4 or ≥4, consistent with the established cut-point for risk of metastatic disease. For PCCs/PGLs, surgical pathologists blinded to the clinical outcomes assessed the histopathologic criteria for the GAPP score including Ki-67 staining; dominant tumor secreting catecholamine data were incorporated to generate a final GAPP score for each tumor (Table 1) (12). GAPP score was categorized as 0-2: well differentiated (WD); 3-6: moderately differentiated (MD); and 7-10: poorly differentiated (PD), based on published cut-points for risk stratification. In the subset of subjects with germline genetic testing (n = 87), modified GAPP (M-GAPP) was scored by published histopathologic and clinical criteria, incorporating SDHB germline genetic testing result (positive or negative) (15). M-GAPP was categorized as <3 or ≥3 based on published cut-points.
Table 1.
Pheochromocytoma of the Adrenal Gland Scaled Score (PASS)(10) and Grading System for Adrenal Pheochromocytoma and Paraganglioma (GAPP) (12) Scoring Criteria
| Characteristic | Score |
|---|---|
| PASS | |
| Large nests or diffuse growth | 2 |
| Central or confluent tumor necrosis | 2 |
| High cellularity | 2 |
| Cellular monotony | 2 |
| Tumor cell spindling | 2 |
| Mitotic figures >3/10 HPF | 2 |
| Atypical mitotic figure(s) | 2 |
| Extension into adipose tissue | 2 |
| Vascular invasion | 1 |
| Capsular invasion | 1 |
| Profound nuclear pleomorphism | 1 |
| Nuclear hyperchromasia | 1 |
| Total | 20 |
| GAPP | |
| Pattern | |
| Zellballen | 0 |
| Large and irregular-sized nest | 1 |
| Pseudorosette forming | 1 |
| Cellularity | |
| Low (<150/62.5 mm2) | 0 |
| Moderate (150-250/62.5 mm2) | 1 |
| High (>250/62.5 mm2) | 2 |
| Coagulation necrosis | |
| Presence | 2 |
| Absence | 0 |
| Vascular/capsular invasion | |
| Presence | 2 |
| Absence | 0 |
| Ki-67 immunoreactivity | |
| >3% or 50 cells/MPF | 2 |
| >1% or 20 cells/MPF | 1 |
| Few cells | 0 |
| Types of catecholamine | |
| Norepinephrine | 1 |
| Epinephrine | 0 |
| Nonfunctioning | 0 |
| Total | 10 |
Abbreviations: HPF, High-powered field; MPF, medium-powered field (magnification at 20× with objective lens and 10× ocular lens).
Statistical analysis
Data was reported as mean ± standard deviation (SD), median with interquartile range (IQR) or percentage. The Fisher’s exact test or chi-squared test was utilized as appropriate for categorical variable comparisons, and the Student’s T test was used for continuous variable comparisons.
Univariate analysis was performed to identify covariates associated with metastatic disease. Covariates meeting nominal significance and covariates of clinical interest were incorporated into multivariable logistic regression models. Backward stepwise selection was used to select the final multivariable logistic regression model. A P value <.05 was considered statistically significant.
To assess the relationship between PASS and metastatic disease and GAPP score and metastatic disease, receiver operator characteristic (ROC) curves were generated and the area under the curve (AUC) was calculated. For PASS and GAPP score, interrater reliability between 2 surgical pathologists was assessed utilizing the intraclass correlation coefficient (ICC), where ICC < 0.5 indicates poor reliability, ICC 0.5-0.75 indicates moderate reliability, ICC 0.75-0.9 indicates good reliability, and ICC ≥ 0.9 indicates excellent reliability (16). Assessment of interrater reliability was restricted to tumors for which original diagnostic slides and blocks were retrievable. Tumors for which original diagnostic slides and blocks were unable to be retrieved, or where original diagnostic blocks were of insufficient quality to be recut, were excluded. Of 104 PCCs evaluated by PASS score, a total of 102 tumors were evaluated by 2 independent pathologists, 2 tumors were unable to be scored for interrater reliability for PASS. Of 132 tumors evaluable by GAPP score, a total of 129 tumors evaluated by 2 independent pathologists, 3 tumors were unable to be scored for interrater reliability for GAPP. To evaluate survival outcomes, Kaplan–Meier survival curves were generated, and Cox proportional hazard regression models were developed to assess predictors of RFS and DSS. Statistical analysis was performed in SAS (version 9.4).
Results
Study population
We identified 143 subjects meeting inclusion criteria for the study. Of these, 74% (n = 106) were PCCs and 26% (n = 37) were PGLs (Table 2). The majority (57%, n = 82) of subjects were female, and the mean age in the cohort was 47.6 ± 15.4 years. Self-reported race was African American in 25% (n = 35), Caucasian in 56% (n = 80), and other in 20% (n = 28). Germline susceptibility gene testing was performed in 65% of subjects. The most common germline pathogenic variants were found in Succinate Dehydrogenase Subunit B (SDHB) in 7.7%, followed by Neurofibromatosis 1 (NF1) in 6.3%, and von Hippel Lindau (VHL) in 4.9%. Thirty-seven percent of subjects tested had no germline susceptibility gene mutation identified. The dominant secreted catecholamine was norepinephrine in the majority (62%) of tumors, with 32% of tumors being epinephrine dominant and 6% being unknown secretion. Ki-67 proliferation index was <1% in 94% of tumors (n = 129), 1% to 3% in 5% (n = 7) and >3% in 1% (n = 1). MRD was identified in 24% (n = 35) of subjects, and death due to disease was observed in 14% (n = 18) of subjects with complete data on survival outcomes (n = 133).
Table 2.
Characteristics of 143 patients with pheochromocytoma/paraganglioma (PCC/PGL)
| Characteristics | N, or mean |
|---|---|
| Mean age, years ± SD | 47.6 ± 15.4 |
| Gender, n (%) | |
| Female | 82 (57.3) |
| Male | 61 (42.7) |
| Race, n (%) | |
| African American | 35 (24.5) |
| Caucasian | 80 (55.9) |
| Other | 28 (19.6) |
| Germline susceptibility gene mutation, n (%) | |
| No mutation | 53 (37.1) |
| Not tested | 50 (35.0) |
| NF1 | 9 (6.3) |
| RET | 6 (4.2) |
| SDHB | 11 (7.7) |
| SDHD | 5 (3.5) |
| Variant of undetermined significancea | 2 (1.4) |
| VHL | 7 (4.9) |
| Tumor type, n (%) | |
| Pheochromocytoma | 106 (74.1%) |
| Paraganglioma | 37 (25.9%) |
| Dominant secreted catecholamine, n (%) | |
| Epinephrine | 46 (32.2%) |
| Norepinephrine | 89 (62.2%) |
| Unknown | 8 (5.6%) |
Abbreviations: SD, standard deviation; NF1, Neurofibromatosis 1; RET, rearranged during transfection; SDHB, Succinate dehydrogenase subunit B; SDHD, Succinate dehydrogenase subunit D; VHL, von Hippel Lindau.
a SDHD c.T320C; p.L107P; TMEM127 c0.409 + 7C>T.
In comparison with patients with PCC, patients with PGL were less frequently female (41% versus 63%, P = .0164) but had higher rates of germline susceptibility gene pathogenic variants (P < .0010). Of note, SDHB germline pathogenic variants were uniformly associated with PGL (Table 3). PGL tumors were more frequently norepinephrine secreting (78% versus 56%, P = .0373) than PCC tumors. There was no statistically significant difference between PCC and PGL subjects in age, race, or tumor size. The median PASS score in the PCC group was 6.5 (IQR: 4.0-8.0). The median GAPP score was 3.0 in both groups, but the range was significantly lower in PCC (P = 0.038) (Table 3).
Table 3.
Characteristics of 143 patients with pheochromocytoma/paraganglioma (PCC/PGL)
| Characteristics | Paraganglioma N = 37 | Pheochromocytoma N = 106 | P value |
|---|---|---|---|
| Mean age, years ± SD | 45.1 ± 15.5 | 48.5 ± 15.3 | .2516 |
| Female sex, n (%) | 22 (59.5) | 67 (63.2) | .0164 |
| Race, n (%) | .2955 | ||
| African American | 10 (27.0) | 25 (23.6) | |
| Caucasian | 23 (62.2) | 57 (53.8) | |
| Other | 4 (10.8) | 24 (22.6) | |
| Germline susceptibility gene mutation, n (%) | <.0001 | ||
| No mutation | 12 (32.4) | 41 (38.7) | |
| Not tested | 7 (18.9) | 43 (40.6) | |
| NF1 | 0 (0) | 9 (8.5) | |
| RET | 1 (2.7) | 5 (4.7) | |
| SDHB | 11 (29.7) | 0 (0) | |
| SDHD | 4 (10.8) | 1 (0.9) | |
| Variant of undetermined significance | 1 (2.7)a | 1 (0.9)b | |
| VHL | 1 (2.7) | 6 (5.7) | |
| Dominant secreted catecholamine, n (%) | .0373 | ||
| Epinephrine | 6 (16.2) | 36 (34.0) | |
| Norepinephrine | 29 (78.4) | 59 (55.7) | |
| Unknown | 1 (2.7) | 9 (8.5) | |
| Median tumor size, cm (IQR) | 4.5 (4.0, 5.8) | 4.6 (3.2, 7.5) | .4546 |
| Median PASS score (IQR) | — | 6.5 (4.0, 8.0) | — |
| Median GAPP score (IQR) N = 132 | 3.0 (3.0, 4.0) | 3.0 (2.0, 4.0) | .038 |
Bolded values indicate P ≤ 0.05.Abbreviations: SD, standard deviation; IQR, interquartile range; NF1, Neurofibromatosis 1; RET, Rearranged during transfection; SDHB, Succinate dehydrogenase subunit B; SDHD, Succinate dehydrogenase subunit D; VHL, von Hippel Lindau.
a SDHD c.T320C; p.L107P.
b TMEM127 c0.409 + 7C>T.
Survival outcomes
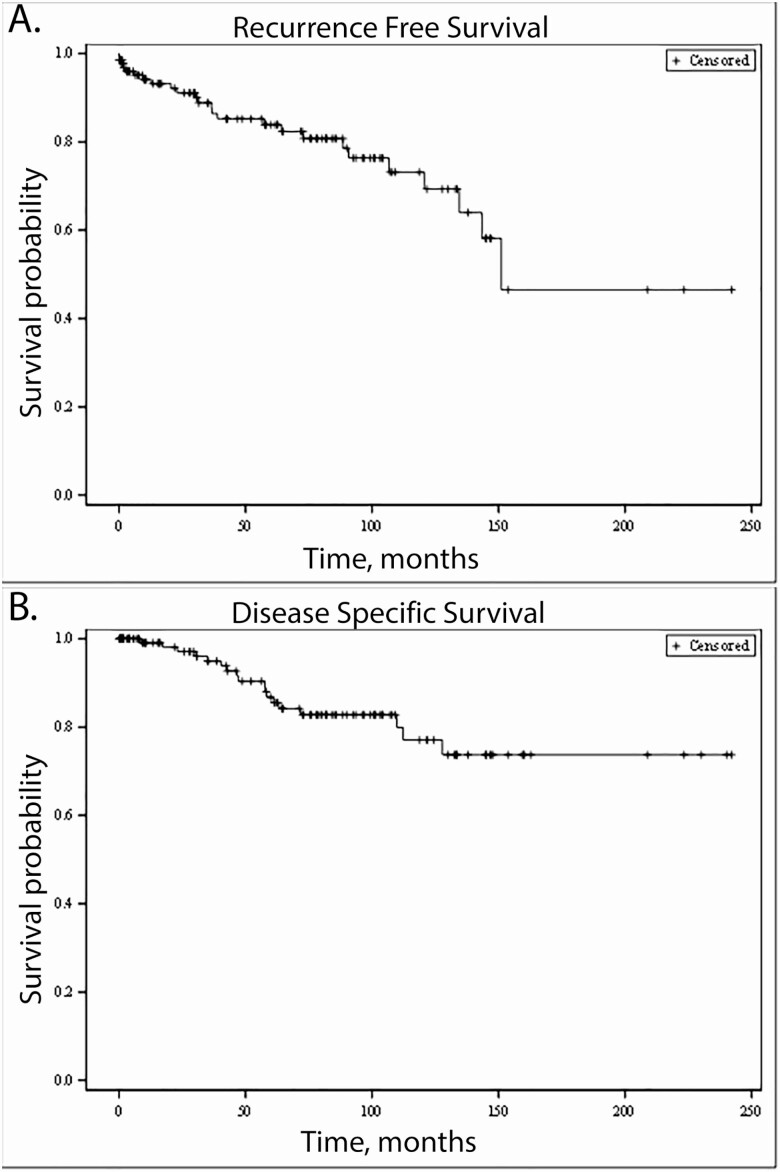
The median follow-up for the cohort was 62 months (IQR 17-104). Median RFS was 48.7 months (IQR 9.7-92.5). Median DSS was 62.7 months (IQR 16.2-103.9). Kaplan–Meier survival curves were generated for RFS (Fig. 1A) and DSS (Fig. 1B).
Figure 1.
Cohort survival. (A) Recurrence-free survival (RFS). (B) Disease-specific survival (DSS).
Univariate logistic regression was performed to identify covariates associated with MRD (Table 4). Longer RFS was associated with older age at diagnosis (OR 0.969, P = .0170) and with subjects who did not have clinical genetic testing performed (OR 0.128, P = .0092). Shorter RFS was associated with germline SDHB pathogenic variant (OR 8.205, P = .0049), PGL (OR 6.357, P < .0001), Ki67 index of 1% to 3% (OR 4.810, P = .0477) and higher GAPP score (OR 1.537, P = .0047). PASS score was not significantly associated with RFS (P = .1779).
Table 4.
Univariate logistic regression analysis of predictors of recurrent pheochromocytoma/paraganglioma (PCC/PGL)
| Characteristics | Odds Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Age | 0.969 | 0.945-0.994 | .0170 |
| Female sex | 1.601 | 0.744-3.445 | .2292 |
| Race | |||
| African-American | 3.819 | 0.947-15.393 | .0596 |
| Caucasian | 2.966 | 0.811-10.848 | .1004 |
| Other | Reference | — | — |
| Germline susceptibility gene mutation | |||
| No mutation | Reference | — | — |
| Not tested | 0.128 | 0.027-0.602 | .0092 |
| NF1 | <0.001 | <0.001-999.999 | .9665 |
| RET | 1.538 | 0.252-9.392 | .6407 |
| SDHB | 8.205 | 1.892-35.581 | .0049 |
| SDHD | >999.9999 | <0.001-999.999 | .9758 |
| Variant of undetermined significance | 3.077 | 0.179-52.746 | .4382 |
| VHL | 4.103 | 0.810-20.782 | .0881 |
| Dominant secreted catecholamine | |||
| Epinephrine | Reference | — | —- |
| Norepinephrine | 2.516 | 0.946-6.691 | .0645 |
| Unknown | 1.500 | 0.254-8.843 | .6542 |
| Tumor type | |||
| Pheochromocytoma | Reference | — | — |
| Paraganglioma | 6.357 | 2.736-14.767 | <.0001 |
| Tumor size, cm | 1.005 | 0.883-1.143 | .9410 |
| Ki-67 index (%) | |||
| <1 | Reference | — | — |
| 1-3 | 4.810 | 1.016-22.759 | .0477 |
| >3 | <0.001 | <0.001-999.999 | .9891 |
| PASS score | 1.128 | 0.947-1.343 | .1779 |
| GAPP score | 1.537 | 1.141-2.070 | .0047 |
Bolded values indicate P ≤ 0.05.Abbreviations: NF1, Neurofibromatosis 1; RET, Rearranged during transfection; SDHB, Succinate dehydrogenase subunit B; SDHD, Succinate dehydrogenase subunit D; VHL, von Hippel Lindau.
Multivariable logistic regression models were developed separately for PASS and for GAPP. On final multivariable modeling, PASS score (OR 1.124, 95% CI 0.938-1.346, P = .2064) was not associated with MRD. On final multivariable modeling using GAPP as a histopathologic predictor, GAPP was the only covariate associated with MRD (OR 1.497, 95% CI 1.103-2.030, P = .0095) after controlling for age.
Outcomes by PASS score
Of 106 PCC included in the study, 104 tumors were evaluable by PASS score. The median PASS score was 6.5 (IQR 4.0, 8.0). On ROC analysis, the AUC of the PASS score was 0.6033 for MRD. MRD was identified in 16 subjects. MRD was classified as locoregional recurrence in 13 subjects, distant metastases in 8 subjects, and new primary/indeterminate in 5 subjects; categories were not mutually exclusive. Median time to development of locoregional recurrence was 90.7 months (IQR 36.7, 124.4), to distant metastases was 72.7 months (IQR 36.7, 141.8), and to new primary/indeterminate tumor was 61.4 months (IQR 46.3, 124.4).
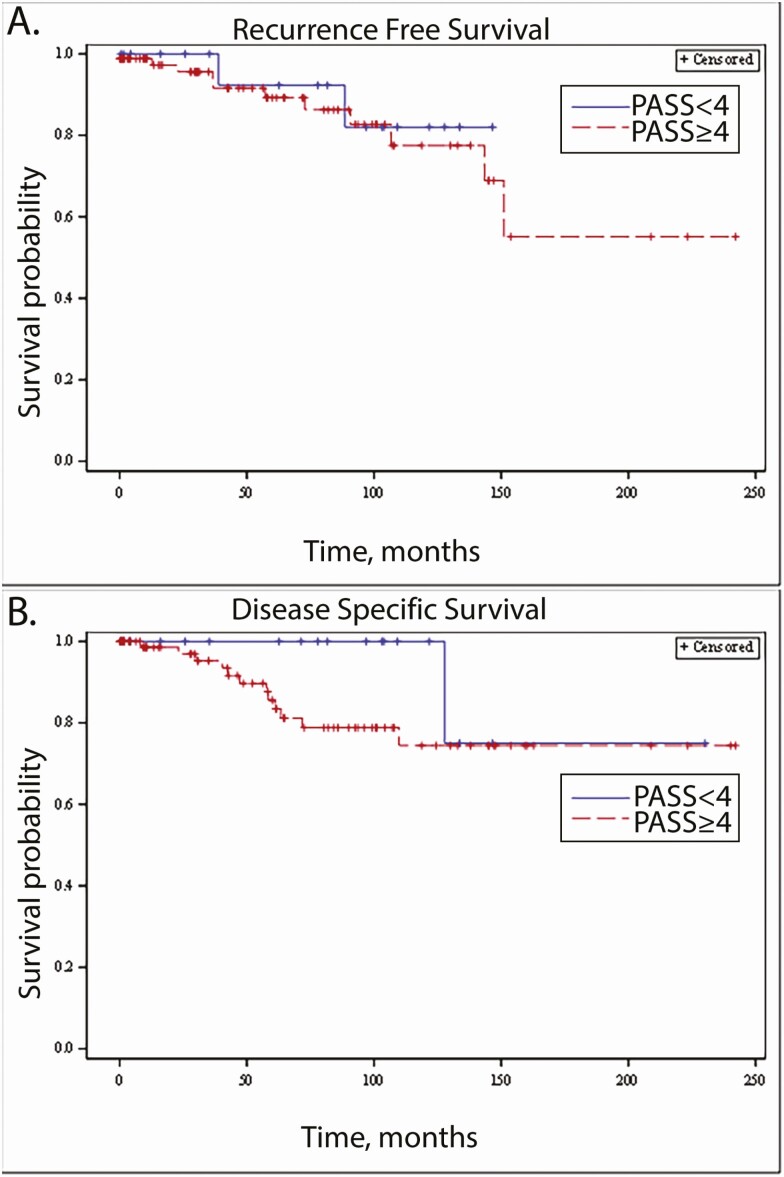
Tumors were categorized by PASS score as <4 or ≥4, consistent with the published cut-point for risk stratification. A PASS score of <4 was observed in 16% (n = 16), while 84% (n = 86) had a PASS score ≥4. MRD was identified in 2/16 (13%) subjects with tumors with a PASS score <4, and in 11/86 (13%) of subjects with tumors with a PASS score ≥4. When analyzed by MRD category, subjects with PASS <4 had locoregional disease in 1/16, distant metastases in 0/16, and new primary tumor/indeterminate in 1/16. Subjects with PASS ≥4 had locoregional disease in 12/86, distant metastases in 8/86, and new primary/indeterminate in 4/86. Disease-related mortality was identified in 1 (5%) subject with a tumor with a PASS score <4, and in 11 (13%) subjects with tumors with a PASS score ≥4, with an additional death due to undetermined cause, suspected to be metastatic PCC/PGL, in a 12th patient. Median RFS was 81.7 months (IQR 25.7, 109.2) in the PASS <4 group, versus 46.8 months (IQR 10.8, 93.6) in the PASS ≥4 group. Median DSS was 81.7 months (IQR 25.7, 121.7) in the PASS <4 group, versus 58.2 months (IQR 13.3, 101.2) in the PASS ≥4 group. Kaplan–Meier survival curves were generated for RFS (Fig. 2A) and DSS (Fig. 2B) by PASS score. On Cox proportional hazard regression analysis, a PASS cut-off of 4 was not significantly associated with either RFS (P = .6754) or DSS (P = .2478).
Figure 2.
Association between survival outcomes and PASS score of <4. (A) Recurrence-free survival (RFS), by PASS score <4 versus PASS score ≥4. (B) Disease specific survival (DSS), by PASS score <4 versus PASS score ≥4.
One hundred and two tumors were scored for PASS by 2 independent pathologists; interrater reliability was in the low moderate range for the PASS score (ICC 0.6082). Two tumors were unable to be scored for interrater reliability.
Outcomes by GAPP score
Of 143 tumors, 132 were evaluable by GAPP score. The median GAPP score was 3.0 (IQR 2.0, 4.0). MRD was identified in 31 subjects. On ROC analysis, the AUC of the GAPP score was 0.6619 for MRD. MRD was categorized as locoregional recurrence in 25 subjects, distant metastases in 19 subjects, and new primary tumor/indeterminate in 12 subjects, with 23 subjects having more than 1 type of MRD. Median time to development of locoregional recurrence was 71.3 months (IQR 20.5, 137.0), to distant metastasis was 73.0 months (IQR 31.3, 160.2), and to new primary/indeterminate tumor was 75.1 months (IQR 44.6, 160.2).
Subjects were categorized by GAPP score as WD, MD, or PD. The majority of subjects had MD tumors (61%, n = 75), followed by WD (37%, n = 46), while only 3 subjects (2%) had PD. Metastatic disease was identified in 5/46 (10%) subjects with WD, 18/75 (24%) of subjects with MD, and in 0/3 subjects with PD. Median follow-up time in the PD group was 13 months, compared with 62 months in the subjects with either WD or MD. Disease related mortality was recorded in 7/46 (15%) of WD, 8/75 (11%) MD, and 0/3 PD subjects. However, the death 1 (33%) PD subject was of undocumented cause, but suspected to be due to PCC/PGL.
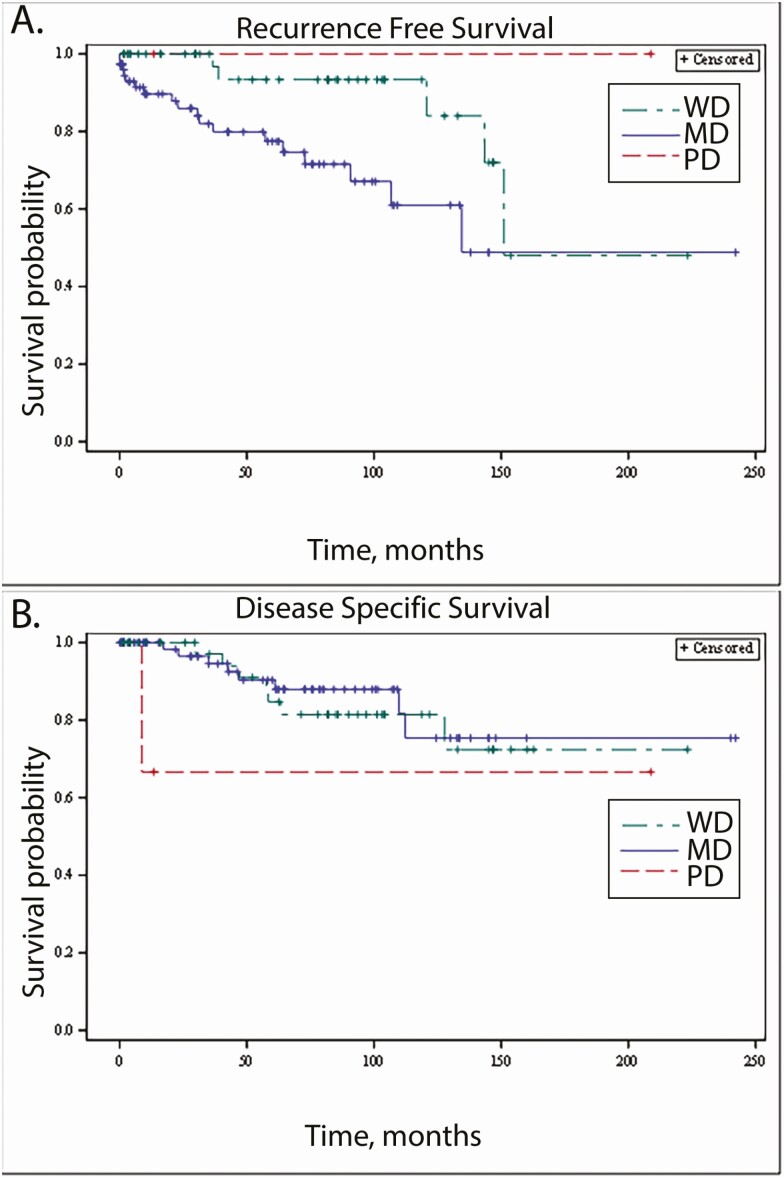
Median RFS was 79.8 months (IQR 29.6, 104.3) in WD, 42.4 months (IQR 8.0, 80.2) in MD, and 13.3 months (IQR 3.0, 208.8) in PD tumors. The median DSS was 79.8 months (IQR 29.6, 104.3) in WD, 61.1 months (IQR 17.3, 100.5) in MD, and 13.3 months (IQR 8.8, 208.8) in PD tumors, respectively. Kaplan–Meier survival curves were generated for RFS (Fig. 3A) and DSS (Fig. 3B). On Cox proportional hazard modeling, GAPP score verged on statistically significant association with RFS (P = .0622). GAPP score was significantly associated with distant metastatic disease (P = .003). Compared with a WD GAPP score, a MD GAPP score was significantly associated with increased risk of MRD (HR 3.367, P = .0184). GAPP scores in the PD range were not significantly associated with metastatic/recurrent disease (P = .9918). GAPP score was not significantly associated with death from disease (P = .4658).
Figure 3.
Association between survival outcomes and GAPP score of 3 categories: well differentiated (WD): 1-2; moderately differentiated (MD): 3-6; poorly differentiated (PD): 7-10. (A) Recurrence-free survival (RFS), by GAPP score. (B) Disease-specific survival (DSS), by GAPP score.
Of 132 tumors initially assessed for GAPP, 129 were scored for interrater reliability. ICC was 0.7921, indicating good reliability between observers. Three tumors were unable to be scored for interrater reliability.
Comparison of PASS, GAPP and M-GAPP
To determine the impact of SDH mutational status on metastatic potential, subgroup analysis was performed for subjects with complete germline testing of all SDH subunits (n = 87). MRD was stratified by recurrence category (locoregional recurrence, distant metastases, and new primary tumor/indeterminate). Higher PASS score was not significantly associated with higher risk of MRD (OR 1.114, P = .101), or with locoregional recurrence (OR 0.985, P = .901) but was significantly associated with distant metastatic disease (OR 1.340, P = .038). Higher PASS score was significantly associated with lower risk of new primary (OR 0.565, P = 0.0115). On ROC analysis, the AUC for PASS score and distant metastatic disease was 0.7399.
Higher GAPP score was significantly associated with MRD (OR 1.539, P = .011), but GAPP score was not associated with risk of locoregional recurrent disease (OR 1.161, P = .674). Higher GAPP score had the strongest association of all 3 scoring systems for distant metastatic disease (OR 3.012, P = .0140). Higher GAPP score was significantly correlated with decreased risk of new primary tumor (OR 0.216, P < .001). On ROC analysis, higher GAPP score correlated well with development of distant metastatic disease, with AUC 0.8182.
Higher M-GAPP score was significantly associated with MRD (OR 1.623, P = .013) but not with locoregional metastatic disease (OR 0.951, P = .895). Higher M-GAPP score was significantly associated with distant metastatic disease (OR 2.901, P = .0212). Higher M-GAPP score was associated with lower risk of new primary tumor (OR 0.347, P < .001). For distant metastatic disease, the AUC for M-GAPP was 0.7753, intermediate between PASS and GAPP.
Discussion
In this largest cohort to validate the PASS and GAPP scores, we found that a higher GAPP score is associated with metastatic PCC/PGL. In particular, a GAPP score in the MD range is associated with increased risk of metastases. The PASS score was not associated with metastatic PCC/PGL, and also demonstrated significant interobserver variability. Neither PASS score nor GAPP score was associated with mortality from PCC/PGL.
A “rule out” strategy has typically been utilized for both PASS and GAPP, as low scores in both systems demonstrate negative predictive value, but higher scores have low specificity for metastatic disease, with an advantage to GAPP over PASS (17). Although a low PASS score did not rule out recurrent disease, no subject with a PASS of <4 developed distant metastatic disease, which is consistent with prior studies. The relatively high interobserver variability inherent in the PASS score has been raised as a concern in prior studies (11). In our cohort, PASS demonstrated low–moderate reliability between observers, while GAPP score had good concordance, and significantly less interobserver variability than PASS score. This is likely because many of the criteria in the GAPP score are more objectively defined (ie, proliferation index, catecholamine secretion). In order to minimize subjectivity, our study utilized automated Ki-67 index profiling, which eliminates the manual counting employed in prior reports (12, 15). The GAPP score has been critiqued for incorporating catecholamine data directly into the scoring system, making it impossible to score tumors where the hormone data are incomplete (18). In our study cohort, 7 tumors were unable to be assigned GAPP scores due to missing catecholamine data.
Our findings regarding the predictive value of the GAPP score are consistent with those of Koh et al. In a cohort of 72 Korean subjects with PCC/PGL, they found that GAPP performed significantly better than PASS in predicting metastatic potential (15). Interestingly, we found that a GAPP score in the PD range was associated with better outcomes than a WD score. This is consistent with other studies which have noted that both PASS and GAPP are most effective for the negative predictive value of a low score (17). However, in our study cohort these data may also be limited by the small number of subjects (n = 3) with a GAPP score in the PD range, and a relatively short median follow-up time in this group of only 13 months.
One proposed modification of the GAPP score (M-GAPP) incorporates loss of SDHB immunohistochemistry (IHC) staining as a surrogate for SDHB expression, as SDHB germline mutation is a known risk factor for aggressive disease. Although the incorporation of genetic testing is of clear value, the rationale for using IHC rather than genetic sequencing is a choice of expedience rather than accuracy. IHC may also be a reasonable choice where cost or access to care limits genetic testing, particularly in underserved areas. Loss of SDHB expression by IHC is observed in all SDHx mutated tumors and is therefore not specific to SDHB germline mutation (19-21). One recent multi-institutional study found that the positive predictive value of SDHB IHC ranged from 67% to 93% when discriminating between SDHx mutated and non-SDHx mutated tumors (22). Even among expert endocrine pathologists, concordance on SDHB IHC was imperfect, with consensus reached in only 90% of cases. Some studies have suggested M-GAPP has both lower positive predictive value and lower negative predictive value than GAPP, we posit that this variability may be related to the incorporation of IHC (23). Therefore, in order to assess the M-GAPP system while minimizing the variability of SDHB IHC, we performed a subgroup analysis comparing PASS, GAPP, and M-GAPP in patients with germline genetic testing for SDHx, which was the majority of our cohort. More than 25% of patients in our study cohort had confirmed germline pathogenic variants associated with PCC/PGL, including 8% with SDHB germline mutations. In our analysis, we found that GAPP had the best predictive performance of the 3 scoring systems, and higher GAPP score was significantly associated with higher risk of recurrent disease overall, distant metastatic disease, and inversely associated with risk of new primary tumors. None of the 3 scoring systems were significantly associated with risk of locoregional recurrence, leading us to hypothesize that local recurrence may be related to completeness of surgical resection. For distant metastases, which are of heightened clinical interest, GAPP predicted metastatic disease well, with an AUC of 0.8182.
Clinically we follow patients with annual life-long biochemical screening, with structured imaging protocols for those with known germline pathogenic variants. It is our practice to recommend germline genetic testing in all subjects with PCC/PGL, as genetic testing informs surveillance. Our institutional practice has historically been to utilize the PASS score cut-off of 4 to counsel patients on risk of recurrence, while recognizing the limitations of the system. We are now incorporating the GAPP score into clinical practice due to the interreader reliability and the alignment with RFS. This current study would suggest that a shift from PASS to GAPP scores in conjunction with germline mutation testing will provide more accurate prognostication for our patients.
In addition to germline mutations, there is mounting evidence for performing somatic mutation profiling in PCC/PGL. Somatic mutations in HRAS, as well as known somatic mutations in susceptibility genes NF1, VHL, and RET, are associated with sporadic PCC/PGL with a more indolent phenotype (24-27). Conversely, somatic driver mutations in ATRX and CSDE1 along with MAML3 fusions and telomerase alterations may be associated with aggressive disease (28-30). Further comprehensive molecular investigations of germline genetics and somatic tumor markers could inform this promising area of inquiry. As genomic characterization of PCC/PGL continues to expand, molecular profiling of tumors will likely be incorporated with histopathological and genetic data for both prognostication and treatment.
Our study has some limitations. The number of metastatic PCC/PGL was relatively small. Nevertheless, it was within the expected range for PCC/PGL and larger than any prior cohorts. In addition, our median follow-up time was only 62 months. Metastatic disease can occur up to 20 years after initial diagnosis, so we may be missing some tumors which will eventually develop metastases. Additionally, germline genetic testing was performed for only two-thirds of our cohort. Although our institutional policy is to offer genetic testing to all patients with PCC/PGL, genetic testing was not available for those diagnosed in the earlier years of this cohort, and some patients declined testing. We also recognize that our pathologists are expert surgical pathologists at a referral center for neuroendocrine tumors, making the results less generalizable. However, the computer automated Ki-67 scoring methodology utilized in this study relies upon open source software, and therefore is readily replicable. This automated method may facilitate assessment of GAPP scores.
In summary, our data presented here suggest that histopathologic scores alone are not reliable enough to make clinical decisions at this time. The combination of these scores with somatic tumor profiling and germline genetic testing has great promise for future prognostication in PCC/PGL.
Acknowledgments
The authors gratefully acknowledge the contributions of Xiaoyan Han, BA, to data analysis, of Jae P. Ermer, BS, to database support, and of Julia Maisel, MD, to histopathologic review.
Financial Support: Support for LF from the American Cancer Society Mentored Research Scholar Grant MRSG-15-063-01-TBG. Support for HW from the National Center for Advancing Translational Sciences of the National Institutes of Health, KL2-TR001879.
Glossary
Abbreviations
- AUC
area under the curve
- DSS
disease-specific survival
- GAPP
Grading System for Adrenal Pheochromocytoma and Paraganglioma
- ICC
intraclass correlation coefficient
- IHC
immunohistochemistry
- MRD
metastatic or recurrent disease
- PASS
Pheochromocytoma of the Adrenal Gland Scaled Score
- PCC
pheochromocytoma
- PGL
paraganglioma
- RFS
recurrence-free survival
- ROC
receiver operator characteristic
- SD
standard deviation
Additional Information
Disclosure Summary: The authors have no disclosures.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Ayala-Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96(3):717-725. [DOI] [PubMed] [Google Scholar]
- 2. Dahia PLM, Clifton-Bligh R, Gimenez-Roqueplo AP, Robledo M, Jimenez C. Hereditary endocrine tumours: current state-of-the-art and research opportunities: metastatic pheochromocytomas and paragangliomas: proceedings of the MEN2019 workshop. Endocr Relat Cancer. 2020;27(8):T41-T52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007;14(3):569-585. [DOI] [PubMed] [Google Scholar]
- 4. Plouin PF, Fitzgerald P, Rich T, et al. Metastatic pheochromocytoma and paraganglioma: focus on therapeutics. Horm Metab Res. 2012;44(5):390-399. [DOI] [PubMed] [Google Scholar]
- 5. Fishbein L, Ben-Maimon S, Keefe S, et al. SDHB mutation carriers with malignant pheochromocytoma respond better to CVD. Endocr Relat Cancer. 2017;24(8):L51-L55. [DOI] [PubMed] [Google Scholar]
- 6. Hamidi O, Young WF Jr, Gruber L, et al. Outcomes of patients with metastatic phaeochromocytoma and paraganglioma: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2017;87(5):440-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lenders JW, Duh QY, Eisenhofer G, et al. ; Endocrine Society . Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. [DOI] [PubMed] [Google Scholar]
- 8. Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48(11):1739-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol. 2013;20(5):1444-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26(5):551-566. [DOI] [PubMed] [Google Scholar]
- 11. Wu D, Tischler AS, Lloyd RV, et al. Observer variation in the application of the pheochromocytoma of the adrenal gland scaled score. Am J Surg Pathol. 2009;33(4):599-608. [DOI] [PubMed] [Google Scholar]
- 12. Kimura N, Takayanagi R, Takizawa N, et al. ; Phaeochromocytoma Study Group in Japan . Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21(3):405-414. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. In: Lloyd RV, Osamura RY, Klöppel G, Rosai J, eds. WHO Classification of Tumours of Endocrine Organs. Vol. 10. 4th ed. Lyon, France: International Agency for Research on Cancer. [Google Scholar]
- 14. Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koh JM, Ahn SH, Kim H, et al. Validation of pathological grading systems for predicting metastatic potential in pheochromocytoma and paraganglioma. PLoS One. 2017;12(11):e0187398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: measures of agreement. Perspect Clin Res. 2017;8(4):187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stenman A, Zedenius J, Juhlin CC. The value of histological algorithms to predict the malignancy potential of pheochromocytomas and abdominal paragangliomas-a meta-analysis and systematic review of the literature. Cancers (Basel). 2019;11(2):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenhofer G, Tischler AS. Neuroendocrine cancer. Closing the GAPP on predicting metastases. Nat Rev Endocrinol. 2014;10(6):315-316. [DOI] [PubMed] [Google Scholar]
- 19. Castelblanco E, Santacana M, Valls J, et al. Usefulness of negative and weak-diffuse pattern of SDHB immunostaining in assessment of SDH mutations in paragangliomas and pheochromocytomas. Endocr Pathol. 2013;24(4):199-205. [DOI] [PubMed] [Google Scholar]
- 20. Gill AJ, Benn DE, Chou A, et al. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum Pathol. 2010;41(6):805-814. [DOI] [PubMed] [Google Scholar]
- 21. Menara M, Oudijk L, Badoual C, et al. SDHD immunohistochemistry: a new tool to validate SDHx mutations in pheochromocytoma/paraganglioma. J Clin Endocrinol Metab. 2015;100(2):E287-E291. [DOI] [PubMed] [Google Scholar]
- 22. Papathomas TG, Oudijk L, Persu A, et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod Pathol. 2015;28(6):807-821. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Li M, Deng H, Pang Y, Liu L, Guan X. The systems of metastatic potential prediction in pheochromocytoma and paraganglioma. Am J Cancer Res. 2020;10(3):769-780. [PMC free article] [PubMed] [Google Scholar]
- 24. Crona J, Delgado Verdugo A, Maharjan R, et al. Somatic mutations in H-RAS in sporadic pheochromocytoma and paraganglioma identified by exome sequencing. J Clin Endocrinol Metab. 2013;98(7):E1266-E1271. [DOI] [PubMed] [Google Scholar]
- 25. Burnichon N, Buffet A, Parfait B, et al. Somatic NF1 inactivation is a frequent event in sporadic pheochromocytoma. Hum Mol Genet. 2012;21(26):5397-5405. [DOI] [PubMed] [Google Scholar]
- 26. Burnichon N, Vescovo L, Amar L, et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974-3985. [DOI] [PubMed] [Google Scholar]
- 27. Welander J, Larsson C, Bäckdahl M, et al. Integrative genomics reveals frequent somatic NF1 mutations in sporadic pheochromocytomas. Hum Mol Genet. 2012;21(26):5406-5416. [DOI] [PubMed] [Google Scholar]
- 28. Fishbein L, Khare S, Wubbenhorst B, et al. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fishbein L, Leshchiner I, Walter V, et al. ; Cancer Genome Atlas Research Network . Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31(2):181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Job S, Draskovic I, Burnichon N, et al. Telomerase activation and ATRX mutations are independent risk factors for metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2019;25(2):760-770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.