Abstract
Cystic echinococcosis (CE) is a zoonotic helminthiasis caused by different species of the genus Echinococcus, and is a major economic and public health concern worldwide. Synthetic anthelmintics are most commonly used to control CE, however, prolonged use of these drugs may result in many adverse effects. This study aims to discuss the in vitro/in vivo scolicidal efficacy of different medicinal plants and their components used against Echinococcus granulosus. Google Scholar, ScienceDirect, PubMed and Scopus were used to retrieve the published literature from 2000–2020. A total of 62 published articles met the eligibility criteria and were reviewed. A total of 52 plant species belonging to 22 families have been reported to be evaluated as scolicidal agents against E. granulosus worldwide. Most extensively used medicinal plants against E. granulosus belong to the family Lamiaceae (25.0%) followed by Apiaceae (11.3%). Among various plant parts, leaves (36.0%) were most commonly used. Essential oils of Zataria multiflora and Ferula asafetida at a concentration of 0.02, and 0.06 mg/ml showed 100% in vitro scolicidal activity after 10 min post application, respectively. Z. multiflora also depicted high in vivo efficacy by decreasing weight and size while also causing extensive damage to the germinal layer of the cysts. Plant-based compounds like berberine, thymol, and thymoquinone have shown high efficacy against E. granulosus. These plant species and compounds could be potentially used for the development of an effective drug against E. granulosus, if further investigated for in vivo efficacy, toxicity, and mechanism of drug action in future research.
Introduction
Most helminth parasites are broadly categorized into two main phyla, namely, nematodes (roundworms) including intestinal and filarial worms, and the Platyhelminthes (flatworms) including the flukes (trematodes) and tapeworms (cestodes) [1]. Helminthic parasite infections receive less than one percent of global research funding and therefore, are considered as neglected tropical diseases [2]. About 1/3 of the 3 billion people in the developing regions of the Americas, sub-Saharan Africa, and Asia living in low socioeconomic conditions are infected with one or more helminths [1].
Cystic echinococcosis is a zoonotic disease caused by the larval stages of the taeniid helminth Echinococcus granulosus [3] and is still a major economic and public health concern in several countries around the world [4]. CE is characterized by the long-lasting growth of hydatid cysts in the viscera of intermediate hosts such as sheep, cattle, goats, and humans [3], and can pose a serious health threat to humans depending upon the stage and location of the cyst. Usually, E. granulosus causes infection by forming cysts in the lungs, liver, brain or other vital organs [5]. CE is especially predominant in sheep and cattle raising regions of the world, including South and Central America, the Middle East, and the Mediterranean [6]. CE causes financial losses to the livestock industry in the form of condemnation of the infested meat [7], increased mortality, and weight loss as well as decreased milk production, decreased hide value and fecundity [8]. In addition, CE also results in morbidity and mortality in humans [4].
Treatment of the disease depends on stage, size, location, and complications of the cysts. At present, four treatment modalities are in practice for CE: surgery (the only treatment until the 1980s), chemotherapy with synthetic drugs like benzimidazole compounds, puncture aspiration injection and re-aspiration (PAIR), and the watch and wait method for clinically silent and inactive cysts [9]. However, these treatment methods have significant limitations. Some of the chemotherapeutic drugs used against CE are only used for inoperable cysts, though 20–40% cases do not respond favorably to those drugs and there are many related adverse effects [10]. During surgical practices, there is a high risk of intraoperative release of cystic fluids that subsequently results in secondary infection and relapse of hydatid cysts in approximately 10% of the cases [4]. To minimize the risk of recurrence the use of active scolicidal agents is indispensable [10]. Recently, it has been shown that existing scolicidal agents like cetrimide, ethanol, hypertonic saline, silver nitrate and others are related to severe side effects such as sclerosing cholangitis [11]. In traditional and rural settings, natural compounds from medicinal plants are being used as a remedy against CE because they are easily available and are thought to be efficacious while presenting fewer adverse side effects [12]. There are indeed a large number of medicinal plants whose scolicidal activity has been demonstrated against the protoscoleces of E. granulosus, however, there are many more plants which have not been explored yet.
We gathered the published literature on plants with anthelmintic/scolicidal activity against protoscoleces of E. granulosus. The purpose of this review is to better understand the current trends in research addressing the development of new scolicidal agents from plant sources. The findings of this review could help to provide up to date knowledge concerning scolicidal potential of medicinal plants and to exploit existing knowledge gaps to improve future research by recognizing areas where more focus should be given.
Methodology
Study design
This systematic review was designed and conducted according to the provided guidelines of Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) [13]. There is no specific protocol for conducting this systematic review. The PRISMA check list S1 Table is provided in the supporting information section.
Search strategy
The search engines used for retrieving published data (from 2000 to 2020) include universally recognized databases, specifically, Scopus, PubMed, ScienceDirect, and Google Scholar. The search strategy was to download and retrieve published literature dealing with medicinal plants and compounds having scolicidal activity against E. granulosus. Specific keywords such as “scolicidal agents”, “medicinal plants used against E. granulosus”, “scolicidal activities of plant based compounds”, “in vitro or in vivo activity of plants against echinococcosis”, “natural products against protoscoleces”, “Natural scolicidal AND protoscolicidal”, and “scolicidal AND antihydatid NOT synthetic” were used.
Inclusion/Exclusion criteria
Studies reporting in vitro/in vivo scolicidal efficacy of medicinal plants against protoscoleces of E. granulosus were included in this review. Moreover, studies reporting scolicidal activity of plant based pure compounds and studies with available full text were also considered for the current review. Studies reporting in vitro/in vivo anthelmintic activity of helminth parasites other than E. granulosus, studies concerning synthetic scolicidal agents against E. granulosus, studies concerning nanoparticles and invertebrates as scolicidal agents, epidemiological and molecular studies, and studies published in languages other than English were excluded.
Study selection
Endnote (Thomson Reuters, San Francisco, CA, USA) was used to compile the articles. Initially, two investigators (RA and MR) assessed titles and abstracts of the retrieved articles for eligibility criteria. Then, the relevant full text published articles were reviewed by three investigators (RA, MR, and MK). In case of any controversy a fourth investigator (MA) was invited to discuss the article. Information including species of plant used, habitat, part(s) used, compound(s) used, concentration/dose, exposure time, scolicidal efficacy, and the name of the country in which the experimental work was performed was considered in the selection process. “Plant list” (http://www.theplantlist.org) and “Tropicos” (http://www.tropicos.org) were referenced for the standardization of plant scientific names, synonyms and families. PubChem (https://pubchem.ncbi.nlm.nih.gov) was also used to attain the IUPAC name (s) of pure compounds isolated from different plants. The summary measures were descriptive.
The software “MarvinSketch (18.24.0)” and “Inkscape (0.92)” were used to draw chemical structures of the compounds and figures/ illustrations, respectively.
Results
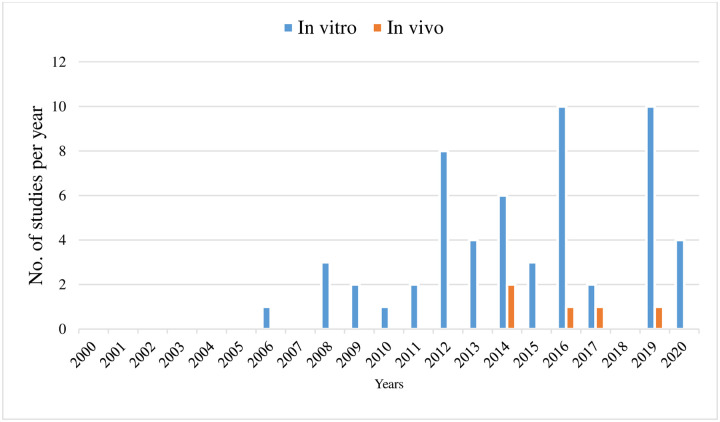
Medicinal plants with reliable therapeutic effects are valuable for modern systems of herbal and natural drug discovery. Plants could serve as a direct source of bioactive or therapeutic agents, and these bioactive ingredients act as a raw material for the development of more complex semisynthetic chemical compounds. Isolated compounds of medicinal plants can lead to the discovery of new drugs, and finally, plants can be used as bioactive markers for spectroscopic and chromatographic analyses along with the discovery of new compounds [14] (Fig 1). This review was designed to discuss those medicinal plants and their compounds which have proven scolicidal activity against the protoscoleces of E. granulosus. We identified a total of 188 published articles through literature search. After removing duplicates and irrelevant articles, a total of 62 articles were selected for this review. Among the 62 studies, 53 studies evaluated in vitro activity of medicinal plants, while 5 studies evaluated in vivo activity, and 9 studies discussed plant compound activity against E. granulosus (Figs 2 and 3).
Fig 1. Strategy for drug development from medicinal plants.
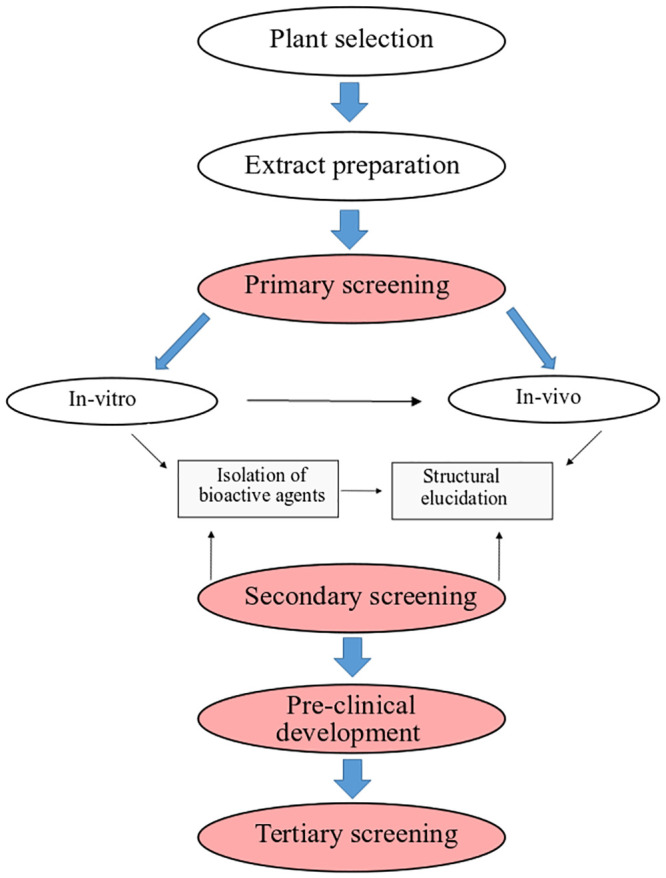
Fig 2. Flow chart of screening process for this review.
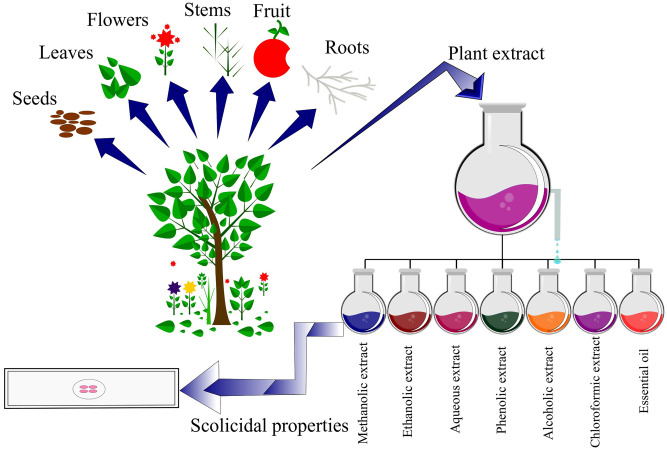
Fig 3. Schematic representation of medicinal plants and their extracts of various parts used against protoscoleces of E. granulosus.
Scolicidal medicinal plants, their families, and habitat
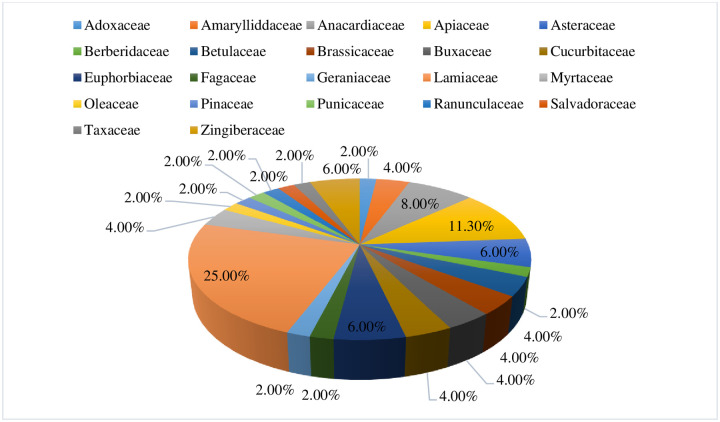
We reported a total of 52 plant species, belonging to 44 genera and 22 families, which have pharmacological validation and scolicidal activity against protoscoleces of E. granulosus. Plant families including Lamiaceae (n = 13, 25.0%), Apiaceae (n = 5, 11.3%), Anacardiaceae, Myrtaceae (n = 4, 8.0% each), and Euphorbiaceae (n = 3, 6.0%) were among the most commonly used plant families (Fig 4). The plant family Lamiaceae, Apiaceae, Anacardiaceae, and Myrtaceae were predominantly used in Iran, while Euphorbiaceae was reported to be used in India and Yemen. Herbs (n = 27, 61.4%) were most often used followed by trees (n = 11, 25.0%), and shrubs (n = 9, 28.0%).
Fig 4. Percentage of plant families evaluated for scolicidal activity.
Most of the studies describing scolicidal activities of medicinal plants were reported to be carried out in Iran (n = 43, 63.3%), Egypt (n = 4, 6.3%), and Argentina (n = 3, 5.0%). Tow studies each were carried out in India, Iraq, Saudi Arabia and Yamen, and the countries of Algeria, Pakistan, China, Turkey, and Switzerland each reported only a single study.
Parts used and herbal formulation of medicinal plants used as scolicidal agent
Leaves were found to be the most frequently used, being the part used in 36.0% of the studies, followed by seeds (16.0%), fruit (14.3%), aerial parts (7.1%), and roots (5.3%). Other parts used included cloves, flowers, stems, whole plants, and latex. Results of this review revealed that essential oil was the preferred method of herbal formulation was used in 28 different recipes, while methanolic extract (in 23 recipes) was the second most used herbal formulation (Table 1). All formulations were made from only a single species of the plant, and no polyherbal formulation was reported in this review against protoscoleces of E. granulosus.
Table 1. In vitro scolicidal efficacy of various medicinal plants and their parts used against protoscoleces of E. granulosus.
| Plant | Part used/ Location | Extract | Major phytochemical components (%) | Minimum concentration (mg/ml) | Exposure Time (min) | Maximum scolicidal efficacy (%) | Ethnomedicinal/ pharmacological uses | Citations | |
|---|---|---|---|---|---|---|---|---|---|
| Family | Botanical name/ Common name/Habit | ||||||||
| Adoxaceae | Sambucus ebulus L. (= Sambucus humilis Mill.)/ Elderberry/ Herb | Fruit/ Iran | Methanolic | Flavonoids, steroids, tannins, caffeic acid, ebulitins, α-triterpenes | 100 | 60 | 98.6 | Anti-inflammatory, anti-nociceptive, anti-cancer, anti-angiogenic, anti-oxidative | [15] |
| Amaryllidaceae | Allium sativum L./ Garlic/ Bulb | Cloves/ Iran | Aqueous | N/A | 200 | 60 | 42.3 | Antiviral, antibacterial, antifungal, antitumor, antioxidant, antihelminthic, antiprotozoal | [16] |
| Hydro-alcohol | 60 | 71.4 | |||||||
| Chloroform | 30 | 99.5 | |||||||
| Methanolic | Alkaloids (2.56), saponin (4.60), flavonoids (1.16), steroids (0.04), cardenolides (0.20) | 50 | 10 | 100 | [17] | ||||
| Chloroformic | N/A | 50 | 60 | 98 | [18] | ||||
| Hydro-alcoholic | 60 | 92 | |||||||
| Flowers/ Iran | Ultrasonic | N/A | 100 | 180 | 98 | [19] | |||
| Allium cepa L. (= Allium angolense Baker)/ N/A/ Bulb | Root/ Iran | Methanolic | Tannins, flavonoids, alkaloids | 100 | 60 | 21.8 | Anti-bacterial, anti-parasitic, anti-microbial, anti-ascorbic | [20] | |
| Anacardiaceae | Pistacia atlantica Desf. (= Pistacia mutica Fisch. & C.A.Mey.) / N/A/ Tree | Fruit/ Iran | Methanolic | β-myrcene (41.4%), α-pinene (32.48%), limonene (4.66%) | 50 | 10 | 100 | Anti-inflammatory, Anti-oxidant, anti-tumor, anti-asthmatic, anti-microbial | [21] |
| Pistacia khinjuk/ N/A/ Tree | Leaves/ Iran | Essential oil | Spathulenol (20.87), Germacrene B (9.53), β-pinene (1.49), Myrcene (2.85), α-pinene (2.11) | 0.512 | N/A | Strong | Gastralgia, dyspepsia, peptic ulcer, anti-inflammatory, antipyretic, antibacterial, antiviral | [22] | |
| Ethyl acetate | Weak | ||||||||
| Ethyl alcohol | Weak | ||||||||
| Chloroform | N/A | Weak | |||||||
| Pistacia vera L./ N/A/ Tree | Branch/stems/ Iran | Essential oil | Limonene (26.21), α-Pinene (18.07), α-Thujene (9.31), α-Terpinolene (9.28), Camphene (4.41), β-Pinene (3.06), | 200 | 5 | 100 | Anti-bacterial, anti-viral, anti-fungal, anti-parasitic, anti-inflammatory, anti-nociceptive, anti-athero-genic, anti-diabetic | [23] | |
| Rhus coriaria L. (= Toxicodendron coriaria (L.) Kuntze)/ Sumac/ Shrub | Fruit/ Iran | Methanolic | Tannins, flavonoids, terpenoids | 50 | 10 | 100 | Anti-oxidant, anti-fibrogenic, anti-bacterial, anti-diabetic, anti-tumorigenic, hypoglycemic | [24] | |
| Apiaceae (= Umbelliferae) | Bunium persicum (Boiss.) B.Fedtsch (= Carum persicum Boiss.) / Zireh Siah/ Herb | Seeds/ Iran | Essential oil | γ-Terpinene (46.1), cuminaldehyde (15.5), cuminyl alcohol (7.4), ρ-Cymene (6.7), β-Caryophyllene (0.2) | 25 | 5 | 100 | Anti-spasmodic, antimicrobial, antioxidant, anti-inflammatory | [25] |
| Ferula assafoetida L. (= Ferula foetida St.-Lag.) / Angozeh/ Herb | Latex/ Iran | Essential oil | α-Pinene (0.6), β-Myrcene (0.6), Decane (0.6) | 0.06 | 10 | 100 | Analgesic, anthelminitic, antiseptic, sedative, expectorant | [26] | |
| Latex/ India | Methanolic | Terpenoids, sulphide derivatives, phenols and minerals | 30 | 60 | 93.70 | Anthelmintic, antibiotic, antimicrobial, antifungal, anticancer, anti-diabetic and therapeutic properties | [27] | ||
| Ferula gummosa Boiss./ N/A/ Herb | Leaves/ Iran | Essential oil | N/A | 50ug/ml | 60 | 100 | N/A | [28] | |
| Foeniculum vulgare Mill. (= Anethum dulce DC.) / Fennel/ Herb | Seeds/ Iran | Essential oil | trans-anethole (36), α-pinene (20), limonene (13), methyle chavicol (88) | 1 | 5 | 100 | Anti-oxidant, cytotoxic, anti-inflammatory, anti-microbial, anti-mutagenic, anti-thrombotic, diuretic | [29] | |
| Trachyspermum ammi (L.) Sprague (Ammi copticum L.)/ Ajowan/ Herb | Fruit/ Iran | Essential oil | Thymol (50.07), γ-Terpinene (23.92), ρ-Cymene (22.9), Linalool (0.01) | 5 | 60 | 100 | Anthelminthic, insecticidal | [30] | |
| Latex/ India | Methanolic | Thymol, γ-terpinene, p-cymene | 20 | 60 | 93.69 | Anthelmintic, insecticidal and antiseptic properties | [27] | ||
| Coriandrum sativum L./ N/A/ Herb | Seeds/ Iran | Phenolic | N/A | 750 | 4,320 | 100 | Anti-bacterial, anti-parasitic, anti-fungal | [31] | |
| Asteraceae (= Compositae) | Artemisia aucheri Boiss./ Artemisia/ Herb | Fruit/ Iran | Methanolic | Linalool (27.1), Borneol (7.8), Decane (5.4), Lavandulol (4.1) | 100 | 60 | 17.4 | Anti-bacterial, ant-ileishmanial, anti-parasitic, antioxidant | [32] |
| Artemisia sieberi Besser/N/A/ Herb | N/A/ Iran | Aqueous | N/A | 75 | 10 | 92.6 | spasmolytic, wormicide, anti-inflammatory, anti-oxidant, antifungal, antimicrobial, anti-tumors | [33] | |
| Tripleurospermum disciforme (C.A.Mey.) Sch.Bip./N/A/ Herb | Leaves/ Iran | Methanolic | N/A | 50 | 10 | 100 | Anti-inflammatory, anti-spasmodic, Anti-septic, antibacterial, | [34] | |
| Berberidaceae | Berberis vulgaris L. (= Berberis abortiva P.Renault)/ Barberry, Zark/ Shrub | Fruit/ Iran | Aqueous | N/A | 4 | 5 | 100 | Anti-bacterial, anti-parasitic, anti-fungal | [35] |
| Hydro-alcoholic | 2 | ||||||||
| Root/ Iran | Methanolic | Isoquinoline alkaloid, carotenoid, flavonoid, tannin, flavonol, triterpene | 2 | 10 | 100 | [36] | |||
| Arial parts/ Pakistan | Ethanolic | N/A | 50 | 50 | 65 | Antimicrobial, antipyretic, antipruritic, antimetic and cholagogue actions, jaundice, dysentery, choleocystitis, leishmaniasis, gall stones and choleothiasis | [37] | ||
| Betulaceae | Corylus spp/ Hazel | Seeds/ Iran | Chloroformic | N/A | 50 | 60 | 36 | N/A | [18] |
| Hydro-alcoholic | 60 | 33 | |||||||
| Brassicaceae | Cardaria draba (L.) Desv. (= Lepidium draba L.) / Whitetop/ hoary cress/ Herb | Leaves/ Iran | Ethanolic | Isorhamnetin (13.8), Quercetin (12.9), Caffeic acid (7.2), Sinapic acid (6.7), Vanillin (6.4) | 10 | 60 | 67.6 | Anti-oxidant, anti-inflammatory, anti-parassitic, anti-bacterial | [38] |
| Seeds/ Iran | Ethanolic | Caffeic acid (13.3), Sinapic acid (7.9), Quercetin (7.8), Vanillin (6.7), Isorhamnetin (6.4) | 10 | 60 | 66.3 | [38] | |||
| Lepidium sativum L. (= Cardamon sativum (L.) Fourr.)/ Garden cress/ Herb | N/A | Essential oil | Thujene (88.86%), Myrcene (2.9%), ρ-cymene (1.67%) | 10 | 30 | 100 | Dysentery, diarrhea, skin diseases, diuretic, leprosy, asthma | [39] | |
| Buxaceae | Buxus wallichiana Baill./ Shamshad/ Shrub | Arial parts/ Pakistan | Ethanolic | N/A | 50 | 50 | 69.07 | Bittertonic, diaphoretic, vermifuge, antihelmentic, antireumatic, analgesic, antiepileptic, antileprotic and in hemorrhoids | [37] |
| Cucurbitaceae | Cucurbita spp (= Cucurbits)/ N/A | Edible part/ Iran | Chloroformic | N/A | 50 | 60 | 47 | N/A | [18] |
| Hydro-alcoholic | 60 | 44 | |||||||
| Dendrosicyos socotrana Balf. f./ N/A/ Tree | Leaves/ Yemen | Methanolic | Triterpenoids | 5 | 21,600 | 100 | Anti-malarial, anti-viral, urinary retention, cystitis, symptoms of diabetes, problems with the liver and burns, constipation | [40] | |
| Aqueous | 24, 480 | 100 | |||||||
| Euphorbiaceae | Euphorbia heliscopia/ Gandi Booti/ Herb | Arial parts/ Pakistan | Ethanolic | N/A | 50 | 50 | 62.24 | Edema, ascites, pulmonary tuberculosis, tinea, febrifuge, cathoratic, antihelminthic and purgative | [37] |
| Jatropha unicostata Balf. f./ N/A/ Tree | Leaves/ Yemen | Methanolic | Flavonoids, terpenoids, fatty acids | 5 | 17,280 | 100 | Anti-viral activity against influenza type A, Herpes simplex 1 | [40] | |
| Aqueous | |||||||||
| Mallotus philippinensis/ Shaandendri/ Tree | Fruit/ India | Methanolic | N/A | 2 | 60 | 99.2 | Anti-bacterial, anti-retroviral, anti-viral, anti-oxidant, anti-inflammatory, anti-parasitic | [41] | |
| Fagaceae | Quercus brantii Lindl. (= Qurecus persica Jaub. & Spach)/ Oak/ Tree | Stem/ Iraq | Aqueous | Tannins, flavonoids, and phenolic | 5 | 15,840 | 100 | [42] | |
| 10 | 14,400 | 100 | |||||||
| 15 | 12,960 | 100 | |||||||
| Alakloid | 5 | 14,400 | 100 | ||||||
| 10 | 12,960 | 100 | |||||||
| 15 | 11,520 | 100 | |||||||
| Phenolic | 5 | 8,640 | 100 | ||||||
| 10 | 8,640 | 100 | |||||||
| 15 | 7,200 | 100 | |||||||
| Geraniaceae | Pelargonium roseum/N/A/Shrub | Leaves/ Iran | Essential oil | N/A | 0.05 | 60 | 100 | Anti-trichomonal and insect Repellence | [28] |
| Lamiaceae | Hymenocarter longiflorus/ N/A/ Herb | Leaves/ Iran | Essential oil | α-Terpinene (0.11), Linalool (2.98), ρ-Cymene (0.2), trans-Caryophyllene (2.29) | N/A | N/A | Methanolic and essential oil extract showed significant scolicidal efficacy against E granulosus with LC50 values of 135.88 and 79.68 μm/ml respectively. | Antimicrobial, anti-mosquito agent, larvicidal | [22] |
| Stems, inflorescences/ Iran | Methanolic | ||||||||
| Mentha piperita L. (= Mentha × adspersa Moench)/ Peppermint/ Herb | Leaves/ Argentina | Essential oil | N/A | 0.01 | 34,560 | 50 | Anti-microbial, anti-viral, anti-oxidant, anti-allergic, anti-tumor | [43] | |
| N/A/ Argentina | Essential oil | N/A | 0.01 | 10,080 | 77 | [44] | |||
| Mentha pulegium L. (= Calamintha fenzlii Vis.)/ Pennyroyal/ Herb | Leaves/ Argentina | Essential oil | α-Pinene (24.7), Linalool (12.6), Myrtenyl acetate (8.3), α-Terpineol (6.1), Linalyl Acetate (5.9), α-Terpinyl acetate (3.8) | 0.01 | 25,920 | 100 | Anti-inflammatory, antioxidant, anti-nociceptive, neuroprotective, anti-hepatic ischemia, anti-microbial | [43] | |
| N/A/ Argentina | Essential oil | 0.01 | 10,080 | 82 | [44] | ||||
| Ocimum bacilicum/ Sweet basil/ Herb | Leaves/ Iran | Methanolic | N/A | 100 | 60 | 24.1 | Anti-bacterial, anti-fungal and in treatment of splenomegaly | [20] | |
| Origanum vulgare L. (= Origanum albiflorum K.Koch)/ Oregano/ Herb | Leaves/ Argentina | Essential oil | Thymol (19,71), Carvacrol (5.4), γ-Terpinene (12.77) | N/A | 86,400 | 23.5 | Anti-bacterial, Anti-parasitic, anti-fungal | [45] | |
| Salvia rosmarinus Spenn. (= Rosmarinus officinalis L.)/ Rosemary/ Herb | N/A/ Argentina | Essential oil | Diterpenes, triterpenes, flavonoids, | 0.01 | 10,080 | 71 | Anti-oxidant, anti-inflammatory, anti-proliferative, anti-cancer | [44] | |
| Salvia officinalis L. (= Salvia cretica L.)/ Sage/ Shrub | Aerial parts/ Egypt | Alcoholic | Apigenin-7-O-glucoside, Apigenin-acetylglucoside, sorhamnetin-luteolin, Apigenin | 2.5 | 8,640 | 100 | Anti-bacterial, anti-microbial, anti-oxidant | [46] | |
| Satureja hortensis L./N//A/ Herb | Arial part/ Iran | Essential oil | N/A | 2 | 20 | 100 | Antimicrobial, Antibacterial Antioxidant, and antifungal | [47] | |
| Satureja khuzestanica/ Jamzad/ Herb | Leaves/ Iran | Ethanolic | Thymol (t), Carvacrol (94.9), Thymol acetate (t), γ-Terpinene (0.49), ρ-Cymene (0.55), α –Terpinen (0.26) | 1 | 30 | 100 | Antidiarrheal, antispasmodic, anti-inflammatory, vasodilator, antioxidant, antibacterial, antiviral, antifungal | [48] | |
| Leaves/ Iran | Essential oil | N/A | 10 | 10 | 100 | [49] | |||
| Flowers/ Iran | |||||||||
| Thymus vulgaris L. (= Origanum thymus Kuntze)/ Thyme/ Shrub | Leaves/ Argentina | Essential oil | N/A | N/A | 86,400 | 38.1 | Anti-microbial, anti-inflammatory, anti-amoebic, anti-fungal, anti-parasitic, anti-bacterial | [45] | |
| Aerial parts/ Egypt | Alcoholic | Thymol (65.4), Carvacrol (5.4), Borneol (0.7), Borrnyl acetate (0.1) | N/A | 103,680 | 100 | [46] | |||
| Zataria multiflora Boiss. (= Zataria bracteata Boiss.) / Avishan Sherazi/ Herb | Leaves/ Iran | Essential oil | Thymol (40.8), Carvacrol (27.8), β-Caryophyllene (2.0), Linalool (1.7), α-Terpinolene (1.3), ρ-Cymene (8.4), Thymol acetate (0.5) | 0.02 | 10 | 100 | Analgesic, antiseptic, antibacterial, antifungal, antiprotozoal, anti-inflammatory, antioxidant, immunostimulant, pain-relieving | [26] | |
| 12.5 | 5 | 100 | [50] | ||||||
| Methanolic | N/A | 25 | 1 | 100 | [51] | ||||
| 20 | 10 | 100 | [50] | ||||||
| Essential oil | N/A | 2 | 10 | 100 | [52] | ||||
| Essential oil | N/A | 20 | 15 | 100 | [53] | ||||
| Ziziphora tenuior L. (= Faldermannia parviflora Trautv.)/ N/A/ Herb | Aerial parts/ Iran | Total extract | Polegon (87), thymol (3.4), piperitenone (12.19), mentha-2-ethanol (31.5), carvacrol (10.5), menthone (46.4), neomenthol (78.4) | 25 | 10 | 25 mg/ml concentration exhibited the highest scolicidal activity after 10 minutes of post-incubation. Considering the effect of different fractions Fof Z. tenuior against protoscoleces, the ethanolic fraction showed the highest effect followed by ethyl acetate, petroleum ether, and chloroform, respectively. | Antiseptic, antifungal, antibacterial, used in uterine diseases and dysentery | [54] | |
| Myrtaceae | Eucalyptus globulus Labill. (= Eucalyptus gigantea Dehnh.)/ Eucalyptus/ Tree | Leaves/ Iran | Methanolic | Alkaloids, glycosides, terpenoids, steroids, flavonoids, tannins | 50 | 40 | 100 | Antiseptic, antimicrobial | [32] |
| Myrtus communis L. (= Myrtus acuta Mill.)/ Myrtle/ Shrub | Leaves/ Iran | Essential oil | α-Pinene (24.7), 1,8- Cineole (19.6), Linalool (12.6), Myrtenyl acetate (8.3), α-Terpineol (6.1), Linalyl Acetate (5.9) | 100 | 5 | 100 | Anti-inflammatory, anti-nociceptive, anti-oxidant, anti-microbial | [55] | |
| Leaves/ Iran | Methanolic | N/A | 50 | 10 | 100 | Anti-fungal and Antibacteria | [34] | ||
| Oleaceae | Olea europaea L./ Olive/ Tree | Leaves/ Iran | Ethanolic | Flavonols, caffeic acid, gallic acid, oleuropein | 1 | 120 | 96.7 | Anti-oxidant, anti-allergic, anti-inflammatory, anti-microbial, anti-tumor, anti-hypersensitivity, anti-anthrogenic | [20] |
| Pinaceae | Pinus nigra Arn. subsp. pallasiana (Lamb.) Holmboe /N/A/ Tree | Fresh needle/ Turkey | Essential oil | N/A | 50 | 60 | 100 | wound healing, Hemorrhoids, diabetes, liver diseases, cold, bronchitis, stomachache, and fungal infections on the skin | [56] |
| Punicaceae | Punica granatum L. (= Punica nana L.)/ Pomegranate/ Shrub | Fruit peel/ Algeria | Aqueous | Triterpenoids, Steroids, Glycosides, Flavonoids, Tannins | 16 | 66,240 | 100 | Anti-coccidial, anthelmintic, anti-bacterial, anti-oxidant, anti-inflammatory, anti-microbial | [57] |
| Ranunculaceae | Nigella sativa L. (Nigella cretica Mill.)/ Black cumin seed/ Herb | Seeds/ Iran | Essential oil | Thymoquinone (42.4), ρ-Cymene (14.1), carvacrol (10.3), longifolene (6.1), 4-terpineol (5.1), t-anethole (2.3), limonene (1.7), thymol (1.2) | 10 | 10 | 100 | Anti-inflammatory, cough, bronchitis, eczema, influenza, anti-microbial, anti-cancer, anti-oxidant, anti-bacterial, anti-viral, anti-fungal, anti-parasitic | [58] |
| Methanolic | N/A | 50 | 10 | 100 | [59] | ||||
| Aqueous | 30 | 100 | |||||||
| Seeds/Iraq | Aqueous | N/A | 25 | 10,080 | 62.3 | [60] | |||
| Seed/ Egypt | Essential oil | N/A | 100 | 120 | 100 | [61] | |||
| Salvadoraceae | Salvandora persica/ Arak/ Shrub | Roots/ Saudi Arabia | Ethanolic | Alkaloids, tannins, saponins, flavonoids, sterols, terpenoids | 50 | 20 | 100 | Anti-inflammatory, analgesic, anti-microbial, anti-bacterial, anti-plaque | [62] |
| Taxaceae | Taxus baccata L./ N/A/ Tree | Gum resin/ Iran | Ethanolic | N/A | 150 | 60 | 66.6 | N/A | [63] |
| Zingiberaceae | Curcuma longa L. (= Amomum curcuma Jacq.)/ Turmeric/ Herb | N/A/ Saudi Arabia | Ethanolic | Alkaloids, saponins, flavonoids, terpenes, steroids | 50 | 30 | 100 | Anorexia, biliary disorders, cough, coryza, sinusitis, rheumatism, anti-inflammatory | [64] |
| Curcuma zadoaria L./N/A/ Herb | Rhizome/ Iran | Aqueous | oxygenated monoterpenes, sesquiterpene hydrocarbons and oxygenated sesquiterpenes; | 75 | 40 | 100 | Hepatoprotective, anti-cancer, anti-analgesic, anti-allergen, and antimicrobial | [65] | |
| Zingiber officinale Roscoe (= Amomum zingiber L.)/ Ginger/ Herb | Rhizome/ Iran | Methanolic | Camphene (15.9), α–terpineol (8.8), farnesene (8.8), p-cineole (8.4), zingiberene (7.5), β-mycrene (7.7) | 100 | 40 | 100 | Arthritis, atherosclerosis, migraine headaches, rheumatoid arthritis, high cholesterol, ulcers, depression, impotence, common cold, antioxidant, antimicrobial, anti-inflammatory, antifungal | [32] | |
| Crude Methanol | N/A | 25 | 60 | 100 | [66] | ||||
| N/A/ Saudi Arabia | Ethanolic | N/A | 50 | 10 | 100 | [64] | |||
| N/A/ Iran | Ethanolic | N/A | 150 | 60 | 92.3 | [67] | |||
N/A indicates data not available
In vitro scolicidal activities of medicinal plants
We reported a total of 52 plant species of (22 families), used in vitro against the protoscoleces of E. granulosus (Fig 5). Plant species (n = 13) of the family Lamiaceae were predominantly used in vitro against larvae of E. granulosus. Leaves among plant parts, herbs among plant life forms, and essential oil among extracts were dominant in in vitro studies (Table 1). Among the reported antihydatid medicinal plants, Zataria multiflora Boiss, Ferula asafetida L., and Foeniculum vulgare Mill. were found to be highly effective in vitro. Essential oil of Z. multiflora at a concentration of 0.02 mg/ml after exposure for 10 min caused 100% mortality of the protoscoleces. Similarly, F. assafoetida and F. vulgare essential oil at 0.06 and 1 mg/ml concentration were 100% effective after 10 and 5 min, respectively.
Fig 5. Year-wise comparison of in vitro and in vivo studies.
In vivo scolicidal activities of medicinal plants
Several medicinal plants and pure compounds were reported that are being investigated for preventive and therapeutic activities against E. granulosus to underpin new alternative treatment for CE with fewer or less severe side effects. A total of 2 plant species, namely T. vulgaris and Z. multiflora of the family Lamiaceae (22 families reported against E. granulosus in this review) were potentially used in in vivo scientific validation of medicinal plants against the protoscoleces of E. granulosus (Fig 4). Extracts of leaves and whole plants were tested using animal models to validate the scolicidal efficacies in vivo. Mice (Mus musculus) were reported to be used as the animal model in in vivo studies (Table 2).
Table 2. In vivo scolicidal efficacy of medicinal plants against E. granulosus.
| Plant | Part used/ location | Extract | Compound used | Concentration (mg/kg) | Time (days) | Animal model | Effect | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Family | Botanical name/ common name | ||||||||
| Lamiaceae | T. vulgaris/Thyme/ Shrub | -/Argentina | Olive oil | Carvacrol* | 40 | 20 | Female CF-1 mice | Cysts were reduced in size and germinal layer of cysts lost their multicellular structure feature. | [68] |
| Z. multiflora Boiss/ Avishan Sherazi/ Herb | Leaves/ Iran | Methanolic | - | 4 | 243 | Balb/C mice | Proved preventive and therapeutic efficacy. Moreover, weight and size of the cysts decreased and the germinal layer was completely damaged. | [69] | |
| 8 | 30 | ||||||||
| Whole plant/ Iran | Nano emulsion Essential oil | - | 20 | 60 | Female mice (Mus musculus) | Recovered cysts were significantly reduced in size and number. | [52] | ||
| Aerial parts/ Iran | Aromatic water | - | 20000 | 243 | Balb/C mice | Showed preventive and therapeutic effects and the germinal layer of hydatid cysts were completely damaged | [70] | ||
| 40000 | 30 | ||||||||
| Aerial parts/ Iran | Aromatic water | - | 50 | 60 | Female mice (Mus musculus | Z. multiflora AW in combination with Albendazole significantly reduce the size and weight of cysts. | [71] | ||
Key: DMSO = dimethyl sulphoxide;
*purchased from Sigma-Aldrich
Scolicidal activities of active phyto-compounds
A total of 8 active compounds, which were isolated from different medicinal plants, are documented in this review article. Of the reported active compounds, thymol, carvacrol, menthol, berberine, genistein, thymoquinone, ampelopsin, and gallic acid are included. All of these 7 compounds were investigated for in vitro efficacy against the protoscoleces, while only 2 compounds (thymol and carvacrol) were used in in vivo assays. Thymol, berberine, and thymoquinone revealed significant in vitro scolicidal activity at concentration of 0.1, 0.5, and 1 mg/ml after 5, 10, and 1 minute of exposure (Table 3). Thymol and carvacrol also showed promising scolicidal activity in in vivo assays (Table 2).
Table 3. Various plant-based compounds protoscolicidal efficacy used against E. granulosus.
| Compound | IUPAC name | Chemical structure | Solvent used | Minimum concentration (mg/ml) | Time exposure (min) | Maximum efficacy (%) | Product company | Plant species | References |
|---|---|---|---|---|---|---|---|---|---|
| Thymol | 2-isopropyl-5-methylphenol | 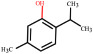 |
Dimethyl sulphoxide | 0.01 | 115,200 | 100 | Sigma Aldrich (USA) | T. vulgaris | [72] |
| Dimethyl sulphoxide | 0.005 | 10,080 | 63 | Sigma Aldrich (USA) | O. vulgare | [44] | |||
| Distilled water | 0.05 | 8,640 | 100 | BDH Chemicals LTD (Poole, UK) | Z. multiflora | [46] | |||
| Normal saline plus Tween 80 | 0.1 | 5 | 100 | Sigma-Aldrich (St. Louis, MO) | [73] | ||||
| Menthol | 2-Isopropyl-5-methylcyclohexanol | 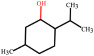 |
Distilled water | 0.05 | 4,320 | 100 | El-Nasr Company for Pharmaceuticals and Chemicals (Cairo, Egypt). | T. vulgaris | [46] |
| S. officinalis | |||||||||
| Berberine | 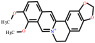 |
Dimethyl sulfoxide | 0.5 | 10 | 100 | Sigma-Aldrich, (St. Louis, MO, USA) | B. vulgaris | [36] | |
| Thymoquinone | 2-Isopropyl-5-methyl-1,4-benzoquinone | 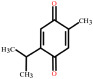 |
Dimethyl sulfoxide (DMSO) | 1 | 1 | 100 | Sigma-Aldrich (St. Louis, Missouri, USA) | N. sativa | [58] |
| Gallic acid | 3,4,5- trihydroxybenzoic acid | 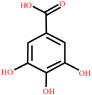 |
Distilled waster | 35 | 3 | 100 | Sigma-Aldrich (St. Louis, MO) | [74] | |
| Carvacrol | 5-Isopropyl-2-methylphenol | 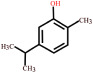 |
Normal saline plus Tween 20 | 100 | 5 | 100 | Sigma-Aldrich (St. Louis, MO) | Z. multiflora | [73] |
| Genistein | 4’,5,7-Trihydroxyisoflavone | 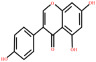 |
Dimethyl sulfoxide | 0.01 | 5,760 | 60 | Synthesized at the Department of Chemistry, University of Liverpool | [75] | |
| Ampelopsin | (2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one | 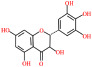 |
Dimethyl sulphoxide | 160 μM | 10080 | 100 | - | Ampelopsis grossedentata | [76] |
Toxicity of scolicidal medicinal plants and compounds
Generally, toxicity tests are performed for the purpose to evaluate the toxicity levels of herbal formulations. Optimal scolicidal agents are those that are nontoxic and destroy the protoscoleces in short period of time and at minimum concentration [77]. Results of the review revealed that the only one plant species, Z. multiflora, has been evaluated for toxicity along with 2 compounds, viz. thymol and carvacrol. In a study, the extract of Z. multiflora was proven to be safe and have no toxic effects when used in pregnant “Blab/C mice” [69, 70]. Similarly, oral administration of 40 mg/kg of thymol and carvacrol revealed no toxic effects in the CF-1 mice during the course of the experiment [68, 78].
Discussion
A total of 52 plant species were reported to be pharmacologically evaluated for their scolicidal activity against protoscoleces of E. granulosus in this review, most of which belong to the families Lamiaceae, Apiaceae, Anacardiaceae, Myrtaceae, and Euphorbiaceae. The traditional beliefs of herbalists, high abundance of these plant families, and the presence of phenolic compounds, essential oil, and saponins in Lamiaceae [79], polyacetylenes in Apiaceae [80], and terpenoids and alkaloids in Euphorbiaceae family [81] could be possible reasons behind such an extensive use and anthelmintic activity of these plant families. Plants of the family Lamiaceae could be easily cultivated and propagated. Moreover, they are extensively used for their strong aroma and ability to survive in severe hot weather because of their essential oils (122). The extensive use of the Euphorbiaceae family for various medicinal purposes may be attributed to its global distribution and mode of adaptation in dry conditions because of the succulent nature of its species and CAP pathway ability. Plant species of this family possess a wide array of secondary metabolites and tendency of mutation load due to their exposure to a wide range of environmental conditions (126). Herbs were reported to be the most frequently used form of plant life used against helminth parasites as compared to shrubs and trees. The dominancy of herbs over other forms of life could be attributed to their easy availability and high efficacy against different ailments as compared to shrubs and trees [82]. Tariq et al. (2017) reported that herbs are widely used in folk medicines all over the globe and contain a large number of active compounds which are responsible for their high efficacy and make them the first choice for scientists and traditional healers [83]. Our results showed that trees are the least commonly utilized plant life form when compared to herbs and shrub, which is possibly due to threats to biodiversity loss and ecosystem consequences. According to Sadia et al. (2018) several tree species have been placed under key protection due to over harvesting. In these situations, modern techniques i.e. cloning, callus cultivation, cultivation in nature, genetically transformed cultures and propagation should be used to obtain the chemical constituents of medicinal importance and to overcome the supply demand imbalance [84].
It was worth mentioning that most (68.3%) studies were conducted in Iran only and the remaining (32.0%) studies in other countries of the world including Argentina, Pakistan, Turkey, Saudi Arabia, Yemen, India, Iraq, Algeria, Egypt and Switzerland. The reason behind such an exceptional number of studies on control of CE in Iran could be the fact that agriculture is the main economic sector that contributes more than 25% of GDP and 1/3 of total employment in Iran. About 90% of the population food requirements are covered by domestic production and domestic supplies cover 95% of agro-industry needs [85]. The livestock sector plays an essential role in the livelihood of the rural population that is mainly dependent on livestock by providing about 80% of their income and on average, 31.8% of the gross value of agriculture production is attributed to livestock production. By-products of livestock e.g. hair, hides, intestines, milk, and red meat are among the major sources of the country’s economic activity. Sheep and goats solely produce about 53% of the red meat while the production of red meat and milk increased during the last decade by 3.14% and 7.19% annually [86]. Other countries such as Argentina, Pakistan, Turkey, Saudi Arabia, Yemen, India, Iraq, Algeria, Egypt and Switzerland contributed to the studies on pharmacological validation of medicinal plants against E. granulosus but their contributions are less in number. This supports the notion that CE is a highly underappreciated threat to human health along with other neglected helminthic infections all over the world. There is a dire need of research on CE and researchers are invited from all over the world to explore the existing knowledge gaps, as there are many medicinal plants and their compounds which are still unexplored that may prove useful in future research.
Among all the plants parts, leaves were most frequently reported to be used during pharmacological validation of medicinal plants against larvae of the helminth parasite E. granulosus. According to Moshi et al. (2012) leaves are preferred by herbalists because they prefer a sustainable supply of raw materials [87]. Moreover, leaves can be easily harvested without extensively harming plants and this could be the possible reason that leaves are the most utilized plant part [88]. Tariq et al. (2017) reported that leaves contain different bioactive compounds which cause a variety of medicinal effects [83]. In contrast, Albuquerque (2006) found that such an exceptional use of leaves in herbal medicines could possibly slow down the process of plant growth, which would lead to infrequent plant recipes [89]. Seeds of plants contain flavonoids, saponins, and tannins etc. and it seems that these phytochemical compounds play key roles in the bioactivity of medicinal plants [90]. Roots act as storing organs of various nutrients for plants, which could explain why they are extensively used in herbal medicines [91]. However, root collection usually results in the death of the plant and can pose serious threats to conservation [79]. Similarly, harvesting of whole plants for evaluation of their anti-parasitic activities is also problematic from a conservation point of view [92]. Extensive use of essential oil and methanolic extract emphasize the role of solvents in extraction of potential bioactive compounds from different plants and their parts as well. Methanol, due to its polar nature, is extremely effective in extracting bioactive compounds from plants [93], and this could be the possible reason of such an extensive of this solvent is herbal formulations. On the other hand, essential oils have proven anthelmintic activity [94], moreover they are comprised of terpenes (secondary metabolites) that interfere with biochemical and physiological functions of parasites. No polyherbal formulation was reported from the pharmacological validations of medicinal plants against protoscoleces of E. granulosus, even though combination of different plants and extracts are commonly more effective than sole plant/extract [95]. This is a clear gap and in future research polyherbal formulations should be evaluated to obtain promising results.
In vitro confirmation of the mentioned medicinal plants reveals the proof of reliability of the plants against the protoscoleces of E. granulosus. Lamiaceae family among plant families, leaves among plant parts, while herbs among plant life forms were predominant in in vitro evaluation of the medicinal plants against larvae of E. granulosus. In vitro studies mostly utilized essential oils for evaluation of scolicidal activity. This could be attributed to the fact that secondary metabolites present in the essential oils inhibit parasite’s biochemical and physiological functions and thus causing death of the cells [96, 97]. To obtained promising results, from a plant’s active compounds, are mainly depend upon the solvent used in the herbal formulation.
From the results it is evident that most of the studies are focused on in vitro rather than in vivo evaluation of plants against the protoscoleces of E. granulosus. This could be attributed to the fact that in vitro screening of plants is cost effective, less time consuming, and quick turnover of results, which allow plants screening on a large scale. In addition, these tests measured the effect of anthelmintic activity directly on the process of hatching, development, and motility of parasites without interfering the internal physiological functions of the hosts [98]. Another advantage of in vitro studies is that after getting reliable results, then the extract/compound could further be evaluated in vivo [99]. However, compounds/extracts that are effective in vitro may or may not active in vivo to the same extent [100]. This type of discrepancy in the activity of testing new anthelmintic drugs is relatively common which may be attributed to several factors such as (a) bioavailability and intrinsic pharmacology of the compound tested (b) the possible destruction or insolubility of the compounds in the rumen of animal (c) and additional protection of the parasite [101]. This limitation/gap signifies the importance of pharmacokinetics and pharmacodynamics studies for the industrial development of new anthelmintic products against E. granulosus.
Essential oils of Z. multiflora Boiss depicted promising antihydatid activity at the possible low concentration (0.02 mg/ml) and minimum time exposure, this potent activity of Zataria oil probably be due to the major phenolic monoterpenes components. Phenolic monoterpenes antimicrobial activity could be related to its intrinsic hydrophobicity and in addition the presence of a hydroxyl group; thus these compounds cause cells disruption by crossing the cell membrane [102, 103]. Mechanism of action of phenolic monoterpenes is not evaluated against protoscoleces yet, however, studies on other eukaryotic cells revealed that phenolic monoterpenoids mainly act on the plasma and mitochondrial membranes and induce cell apoptosis. They diffuse through membrane, disturb lipid bilayer structure, and hence change cell permeability, which in turn enhances the leakage of ions and reduces membrane electric potential. This disturbance in electric potential of plasma membrane eventually leads to the leakage of ATPs, amino acids, proteins, and ions especially potassium and calcium, which induce membrane damage and cell death [104–106]. Similarly, altering the biochemical structure of mitochondrial membrane results in the leakage of proteins, radicals, calcium, and cytochrome c, which cause cell death by apoptosis [107–109].
F. assafoetida and F. vulgare essential oil was relatively more effective in terms of efficacy and time exposure than other plants reported in this review. The essential oils from these plants bearing disulphide compounds, tested against various eukaryotic cancerous cells for their cytotoxic activity, which are also taught to be responsible for scolicidal activity [110, 111].
Mechanisms of drug action of many antihydatid plants are not evaluated and there is a dire need of research on this aspect to provide a detailed information on the scientific background of ethnomedicinal plants for the development of a novel scolicidal agent.
In vivo studies are desirable to assess the pharmacokinetics/pharmacodynamics of the target extract/compound, in vivo efficacy, host immune response to the target extract/compound, and toxicity levels. Besides a number of advantages of in vivo studies, there are also some disadvantages as well. In vivo studies would be more accurate and precise as compared to in vitro, but more timing consuming, costly, and difficult to reproduce due to the inter animal and pharmacodynamics in the host [112]. In short, both of the techniques have vital roles to play and the one will not exclude the other [113].
The two plant species T. vulgaris and Z. multiflora Boiss of the Lamiaceae family were used in vivo against protoscoleces. Use of the family Lamiaceae and plant part leaves was in accordance with ethnomedicinal use, where this family and leaves were extensively used. This shows the reliability and beliefs of modern science on ethnomedicines. Mouse is the most commonly used model for in vivo echinococcosis studies. The reason could be the high similarity of its genome with that of humans. Moreover, its small size, short generation time, and easy to breed, makes it an efficient cost effective model for in vivo studies and to get functional information about the human health and diseases [114, 115].
We reported a total of 8 compounds, which were isolated and evaluated for scolicidal activity against the protoscoleces. Plants have biologically active compounds in the form of primary and secondary metabolites. Among these, chlorophyll, proteins, and sugars are included in primary, while flavonoids, alkaloids, terpenoids, and phenols are the secondary compounds [116]. These different bio-active compounds work synergistically in combination to produce a pharmacological effect [117]. The efficacy of plant compounds may be attributed to the fact that they inhibit or retard the growth, maturation damage, suppress appetite or reduce procreative ability, which are all the causes of mortality. Moreover, the considerable activity of plants extracts may be due to the additive or synergistic relationship among different major components which can interact with multiple molecular targets in various developmental stages of the parasite [118]. Thymol, berberine, and thymoquinone revealed significant in vitro scolicidal activity at concentration of 0.1, 0.5, and 1 mg/ml after 5, 10, and 1 minute of exposure [36, 72]. Thymol effects were promising that could be explained by the fact that, it induces shrinkage of the soma region, loss of turgidity, rostellar disorganization, hooks loss, formation of blebs on the tegument, and destruction of microtriches, which finally lead to death of the protoscoleces [72]. This could be attributed to the fact that any damage to hooks and blebs formation on the tegument of protoscoleces are generally stress responses which are brought about by different harmful conditions. Moreover, microtriches destruction could affect the absorption of nutrients in protoscoleces and cause starvation and finally death of the larvae [119].
Berberine is an alkaloid broadly used in ethnomedicinal systems of Ayurveda and Traditional Chinese Medicines. Berberine is reported to inhibit growth and induce morphological changes which could be the possible reason of the mortality of the parasites [120]. Thymoquinone is the main component of essential oil of Nigella sativa, the exact mechanism of its effects in not evident, however, studies suggest that it can inhibit DNA synthesis by inhibiting histone deacetylase (HDAC) enzyme interacting with the chromosomes [121].
Thymol at concentration of 40 mg/kg showed both therapeutic and preventive effects. Ultrastructural observation revealed that the germinal layer was highly damaged and this could be attributed to the fact that the drug enters the hydatid cyst to cause the effect [122].
Moreover, studies concerning isolation and purification of plant compounds responsible for anti-parasitic activities are very scarce and insufficient. Most of the studies reported the presence of key components of the plants which do not provide information about the effective anti-parasitic compounds and their mechanism. Therefore, more in depth studies are required to evaluate mostly used plants of the aforementioned plant families.
Safety issues of herbal medicines have been remained a big question and scientists are taking keen interests in herbal medicines for a decade. The notion that ‘natural’ equals ‘safe’ is apparently deceptive, since natural products comprise pharmacologically active compounds which, when taken in high doses or in specific conditions, can be detrimental to health [83].
The in vitro and in vivo cytotoxicity of thymol was assessed by Robledo et al., 2005 [123]. The cytotoxic effect was observed to be 400 ± 0 μg/ml in U-937 human promonocytic cells. An oral dose of 40 mg/kg of body weight/day, thymol was not toxic to Golden hamsters based on corporal weight, behavior and serum levels of bilirubin, uric acid, and glucose [123]. Moreover, the toxicity evaluation of thymol in mice was also reported to safe and non-toxic, no changes in mice behavior, mobility, and feeding habits were observed [122]. Berberine at the tested clinical doses is not cytotoxic and mutagenic, whereas, the adverse effects can be pertained to dose enhancement [124]. Similarly, thymoquinone administration in drinking water at concentration of 0.1, 0.2, 0.3 mg/ml for 3 months to mice revealed no mortality and toxicity and were proved to be safe [125].
In this review, we reported that inadequate literature is available concerning the toxicology and pharmacology of different medicinal plants and their compounds assessed as scolicidal agents against protoscoleces of E. granulosus. Further research should be conducted to evaluate the toxicology and pharmacology of those plants and compounds possessing promising scolicidal activity.
Conclusion and recommendations
Recently, research has been flourished and researchers are constantly working on plant extracts and essential oils to find out compounds with high scolicidal efficacies that could be used for the treatment of CE either in combination with or as a replacement for synthetic drugs. Firstly, the benefit of using natural compounds instead of synthetic is that there are fewer chances to develop resistance because there is commonly a mixture of various active compounds having different mechanisms of action. Secondly, due to anthelmintics resistance, the subsequent development of new anthelmintics is very time consuming, and requires a tremendous effort and money. Though, mostly research conducted to control CE via natural products comes to an end in the laboratory because it is very difficult to obtained the same efficacy in the field. Other major obstacles in commercializing an active compound are safety for humans, development of resistance, stability, the probability of synthesis at a reasonable cost as well as environmental safety. It is also concluded from the study that the market for plant-based scolicidal agents is very promising, particularly if the increasing number of side effects of synthetic scolicidal agents are considered. Based on the findings in this review, the following suggestions are recommended.
Among all plant parts, leaves are widely studied for scolicidal activities, other plant parts should also be investigated for active compounds against CE.
In future active phytochemicals of plants with high efficiency against E. granulosus should be investigated separately, that would be helpful to identify and quantify the efficacy of each and every individual compound.
Among the pure compounds isolated from plants reported in this review, thymoquinone showed a remarkable in vitro scolicidal activity with a minimum concentration of 1 mg/ml and 1 min of exposure time. However, its toxicity levels and in vivo efficacy has not been documented yet. It is highly recommended to further exploit the efficacy of this compound using in vivo models and its toxicity levels in the future.
The mechanisms of action of plant-based compounds should be investigated in order to thoroughly understand and make improvements in the pharmacological and therapeutic properties of these compounds.
Most of the plant’s scolicidal activities have been tested by in vitro studies, only few studies reported the in vivo efficacy of medicinal plants, it is recommended to explore the in vivo biological activities of different plants against E. granulosus to understand more in-depth scolicidal efficacy.
The knowledge about the toxicity of the plants reviewed in the article is very scarce, hence, further research should be carried out to exploit the toxicity levels of various plants and their active components.
Most of the medicinal plants and their compounds are evaluated against the common G1 genotype of E. granulosus focus should be given to other genotypes of E. granulosus in order to thoroughly understand the efficacy of medicinal plants against other genotypes.
More focus should be given on parasitocidal rather than parasitotatic (or anti-parasitic) activities of medicinal plants and their constituents.
More than 68.3% of pharmacological studies are carried out in Iran while the remaining 32.0% in other countries of the world, hence researchers are invited from all over the world to explore the medicinal plants against E. granulosus for the development of novel and cost effective scolicidal agent to control this zoonotic helminthiasis.
Supporting information
(DOCX)
Acknowledgments
We are grateful to Ethan Jensen at Eureka! Writing, LLC for providing technical writing support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118(4):1311–21. 10.1172/JCI34261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez PJ. Forgotten people, forgotten diseases. George Washington University and Sabin Vaccine Institute ASM Press, Washington, DC. 2008.
- 3.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16(1):18–36. 10.1128/cmr.16.1.18-36.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17(1):107–35. 10.1128/cmr.17.1.107-135.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koshki MHK, Nourian A, Rahimi MT, Daryani A, Spotin A, Ahmadpour E. Natural products applied against hydatid cyst protoscolices: a review of past to present. Acta Trop. 2017. [DOI] [PubMed] [Google Scholar]
- 6.Lahmar S, Sarciron M-E, Rouiss M, Mensi M. Echinococcus granulosus and other intestinal helminths in semi-stray dogs in Tunisia: infection and re-infection rates. Tunis Med. 2008;86(7):657–64. [PubMed] [Google Scholar]
- 7.Bekele J, Butako B. Occurrence and financial loss assessment of cystic echinococcosis (hydatidosis) in cattle slaughtered at Wolayita Sodo municipal abattoir, Southern Ethiopia. Trop Anim Health Prod. 2011;43(1):221–8. 10.1007/s11250-010-9680-5 [DOI] [PubMed] [Google Scholar]
- 8.Tasawar Z, Naz F, Lashari MH. The Prevalence of Hydatidosis in Sheep and Buffaloes at Multan, Punjab, Pakistan. Glob Vet. 2014;12(3):332–5. [Google Scholar]
- 9.Stojkovic M, Zwahlen M, Teggi A, Vutova K, Cretu CM, Virdone R, et al. Treatment response of cystic echinococcosis to benzimidazoles: a systematic review. PLoS Negl Trop Dis. 2009;3(9):e524 10.1371/journal.pntd.0000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. The Lancet. 2003;362(9392):1295–304. [DOI] [PubMed] [Google Scholar]
- 11.Rajabi MA. Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst. Surgical Pract. 2009;13(1):2–7. [Google Scholar]
- 12.Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol. 2006;106(3):290–302. 10.1016/j.jep.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 14.Koparde AA, Doijad RC, Magdum CS. Natural products in drug discovery Pharmacognosy-Medicinal Plants: IntechOpen; 2019. [Google Scholar]
- 15.Gholami S, Rahimi-Esboei B, Ebrahimzadeh M, Pourhajibagher M. In vitro effect of Sambucus ebulus on scolices of Hydatid cysts. Eur Rev Med Pharmacol Sci. 2013;17(13):1760–5. [PubMed] [Google Scholar]
- 16.Sadjjadi SM, Zoharizadeh MR, Panjeshahin MR. In vitro screening of different Allium sativum extracts on hydatid cysts protoscoleces. J Invest Surg. 2008;21(6):318–22. 10.1080/08941930802348261 [DOI] [PubMed] [Google Scholar]
- 17.Moazeni M, Nazer A. In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. World J Surg. 2010;34(11):2677–81. 10.1007/s00268-010-0718-7 [DOI] [PubMed] [Google Scholar]
- 18.Eskandarian AA. Scolicidal effects of squash (Corylus spp) seeds, hazel (Curcurbia spp) nut and garlic (Allium sativum) extracts on hydatid cyst protoscolices. J Res Med Sci. 2012;17(11):1011 [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimi-Esboei B, Ebrahimzadeh M, Fathi H, Anzahaei FR. Scolicidal effect of Allium sativum flowers on hydatid cyst protoscolices. Eur Rev Med Pharmacol Sci. 2016;20(1):129–32. [PubMed] [Google Scholar]
- 20.Haghani A, Roozitalab A, Safi SN. Low scolicidal effect of Ocimum bacilicum and Allium cepa on protoccoleces of hydatid cyst: an in vitro study. Comp Clin Path. 2014;23(4):847–53. [Google Scholar]
- 21.Mahmoudvand H, Kheirandish F, Ghasemi Kia M, Tavakoli Kareshk A, Yarahmadi M. Chemical composition, protoscolicidal effects and acute toxicity of Pistacia atlantica Desf. fruit extract. Nat Prod Res. 2016;30(10):1208–11. 10.1080/14786419.2015.1046868 [DOI] [PubMed] [Google Scholar]
- 22.Taran M, Azizi E, Shikhvaisi A, Asadi N. The anthelmintic effect of Pistacia khinjuk against protoscoleces of Echinococcus granulosus. World J Zool. 2009;4(4):291–5. [Google Scholar]
- 23.Mahmoudvand H, Kheirandish F, Dezaki ES, Shamsaddini S, Harandi MF. Chemical composition, efficacy and safety of Pistacia vera (var. Fandoghi) to inactivate protoscoleces during hydatid cyst surgery. Biomed Pharmacother. 2016;82:393–8. 10.1016/j.biopha.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 24.Moazeni M, Mohseni M. Sumac (Rhus coriaria L.): scolicidal activity on hydatid cyst protoscolices. Surgi Sci. 2012;3(09):452. [Google Scholar]
- 25.Mahmoudvand H, Tavakoli Oliaei R, Mirbadie SR, Kheirandish F, Tavakoli Kareshk A, Ezatpour B, et al. Efficacy and safety of Bunium persicum (Boiss) to inactivate protoscoleces during hydatid cyst operations. Surg Infect (Larchmt). 2016;17(6):713–9. [DOI] [PubMed] [Google Scholar]
- 26.Kavoosi G, Purfard AM. Scolicidal effectiveness of essential oil from Zataria multiflora and Ferula assafoetida: disparity between phenolic monoterpenes and disulphide compounds. Comp Clin Path. 2013;22(5):999–1005. [Google Scholar]
- 27.Moudgil AD, Moudgil P, Sharma D, Daundkar PS, Agnihotri R. In vitro protoscolicidal efficacy appraisal of methanolic herbal extracts against hydatid cysts. Vet Arh. 2020;90(2):197–204. [Google Scholar]
- 28.Tabari MA, Youssefi MR, Nasiri M, Hamidi M, Kiani K, Samakkhah SA, et al. Towards green drugs against cestodes: Effectiveness of Pelargonium roseum and Ferula gummosa essential oils and their main component on Echinococcus granulosus protoscoleces. Vet Parasitol. 2019;266:84–7. 10.1016/j.vetpar.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 29.Lashkarizadeh MR, Asgaripour K, Dezaki ES, Harandi MF. Comparison of scolicidal effects of amphotricin B, silver nanoparticles, and Foeniculum vulgare Mill on hydatid cysts protoscoleces. Iran J Parasitol. 2015;10(2):206 [PMC free article] [PubMed] [Google Scholar]
- 30.Moazeni M, Saharkhiz MJ, Hosseini AA. In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet Parasitol. 2012;187(1–2):203–8. 10.1016/j.vetpar.2011.12.025 [DOI] [PubMed] [Google Scholar]
- 31.Al-Maliki ADM. Investigation of biochemical effect of phenols extract isolated from Coriandrum sativum seeds against Echinococcus granulosus parasite in vitro. J Thi-Qar Sci. 2008;1(1):2–9. [Google Scholar]
- 32.Faizei F, Maghsood AH, Parandin F, Matini M, Moradkhani S, Fallah M. Antiprotoscolices effect of methanolic extract of Zingiber officinale, Artemisia aucheri and Eucalyptus globulus against Echinococcus granulosus in vitro. Iran J Pharmacol Therapeut. 2015;14(1):7–11. [Google Scholar]
- 33.Vakili Z, Radfar MH, Bakhshaei F, Sakhaee E. In vitro effects of Artemisia sieberi on Echinococcus granulosus protoscolices. Exp Parasitol. 2019;197:65–7. 10.1016/j.exppara.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 34.Amiri K, Nasibi S, Mehrabani M, Nematollahi MH, Harandi MF. In vitro evaluation on the scolicidal effect of Myrtus communis L. and Tripleurospermum disciforme L. methanolic extracts. Exp Parasitol. 2019;199:111–5. 10.1016/j.exppara.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 35.Rouhani S, Salehi N, Kamalinejad M, Zayeri F. Efficacy of Berberis vulgaris aqueous extract on viability of Echinococcus granulosus protoscolices. J Invest Surg. 2013;26(6):347–51. 10.3109/08941939.2013.818746 [DOI] [PubMed] [Google Scholar]
- 36.Mahmoudvand H, Dezaki ES, Sharififar F, Ezatpour B, Jahanbakhsh S, Harandi MF. Protoscolecidal effect of Berberis vulgaris root extract and its main compound, berberine in cystic echinococcosis. Iran J Parasitol. 2014;9(4):503 [PMC free article] [PubMed] [Google Scholar]
- 37.Haleem S, Niaz S, Qureshi NA, Ullah R, Mahmood HM, Shahat AA. Phytochemical analysis, Antioxidant and Antiprotoscolices potential of ethanol extracts of selected plants species against Echinococcus granulosus: In-vitro study. Open Chem. 2019;17(1):874–83. [Google Scholar]
- 38.Sharifi-Rad J, Hoseini-Alfatemi SM, Sharifi-Rad M, da Silva JAT, Rokni M, Sharifi-Rad M. Evaluation of biological activity and phenolic compounds of Cardaria draba (L.) extracts. J Biol Today’s World. 2015;4(9):180–9. [Google Scholar]
- 39.Bahrami S, Razi Jalali M, Ramezani Z, Pourmehdi Boroujeni M, Toeimepour F. In vitro Scolicidal Effect of Lepidium sativum Essential Oil. J Ardabil Univ Med Sci. 2016;15(4):395–403. [Google Scholar]
- 40.Barzinji R, Kadir A, Mothana RA, Nasher AK. Effect of leaf extracts of Dendrosicyos socotrana and Jatropha unicostata on the viability of Echinococcus granulosus protoscoleces. Eurasia J Biosci. 2009;3:122–9. [Google Scholar]
- 41.Gangwar M, Verma VC, Singh TD, Singh SK, Goel R, Nath G. In-vitro scolicidal activity of Mallotus philippinensis (Lam.) Muell Arg. fruit glandular hair extract against hydatid cyst Echinococcus granulosus. Asian Pac J Trop Med. 2013;6(8):595–601. 10.1016/S1995-7645(13)60103-0 [DOI] [PubMed] [Google Scholar]
- 42.Hamady Obeid Al-Taei A, Omran AM, Alyasari HF, Al-taei MH. The effect of the activity of Qurecus persica oak plant secondary compound extracts on the viability of protoscolices for Echinococcus granulosus parasite in vitro. Plant Arch. 2019;19(2):1085–91. [Google Scholar]
- 43.Maggiore MA, Albanese AA, Gende LB, Eguaras MJ, Denegri GM, Elissondo MC. Anthelmintic effect of Mentha spp. essential oils on Echinococcus granulosus protoscoleces and metacestodes. Parasitol Res. 2012;110(3):1103–12. 10.1007/s00436-011-2595-x [DOI] [PubMed] [Google Scholar]
- 44.Albani CM, Denegri GM, Elissondo MC. Effect of different terpene-containing essential oils on the proliferation of Echinococcus granulosus larval cells. Interdiscip Perspect Infect Dis. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pensel P, Maggiore M, Gende L, Eguaras M, Denegri M, Elissondo M. Efficacy of essential oils of Thymus vulgaris and Origanum vulgare on Echinococcus granulosus. Interdiscip Perspect Infect Dis. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yones DA, Taher GA, Ibraheim ZZ. In vitro effects of some herbs used in Egyptian traditional medicine on viability of protoscolices of hydatid cysts. Korean J Parasitol. 2011;49(3):255 10.3347/kjp.2011.49.3.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moazeni M, Hosseini S, Al-Qanbar M, Alavi A, Khazraei H. In vitro evaluation of the protoscolicidal effect of Eucalyptus globulus essential oil on protoscolices of hydatid cyst compared with hypertonic saline, povidone iodine and silver nitrate. J Visc Surg. 2019;156(4):291–5. 10.1016/j.jviscsurg.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 48.Zibaei M, Sarlak A, Delfan B, Ezatpour B, Azargoon A. Scolicidal effects of Olea europaea and Satureja khuzestanica extracts on protoscolices of hydatid cysts. Korean J Parasitol. 2012;50(1):53 10.3347/kjp.2012.50.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moazeni M, Saharkhiz MJ, Hoseini AA, Alavi AM. In vitro scolicidal effect of Satureja khuzistanica (Jamzad) essential oil. Asian Pac J Trop Biomed. 2012;2(8):616–20. 10.1016/S2221-1691(12)60107-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jahanbakhsh S, Azadpour M, Kareshk AT, Keyhani A, Mahmoudvand H. Zataria multiflora Bioss: lethal effects of methanolic extract against protoscoleces of Echinococcus granulosus. J Parasit Dis. 2016;40(4):1289–92. 10.1007/s12639-015-0670-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moazeni M, Roozitalab A. High scolicidal effect of Zataria multiflora on protoccoleces of hydatid cyst: an in vitro study. Comp Clin Path. 2012;21(1):99–104. [Google Scholar]
- 52.Moazeni M, Borji H, Darbandi MS, Saharkhiz MJ. In vitro and in vivo antihydatid activity of a nano emulsion of Zataria multiflora essential oil. Res Vet Sci. 2017;114:308–12. 10.1016/j.rvsc.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 53.Yazdi MK, Haniloo A, Ghaffari A, Torabi N. Antiparasitic effects of Zataria multiflora essential oil nano-emulsion on larval stages of Echinococcus granulosus. J Parasit Dis. 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahnazi M, Azadmehr A, Andalibian A, Hajiaghaee R, Saraei M, Alipour M. Protoscolicidal and immunomodulatory activity of Ziziphora tenuior extract and its fractions. Asian Pac J Trop Med. 2016;9(11):1062–8. 10.1016/j.apjtm.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 55.Mahmoudvand H, Fallahi S, Mahmoudvand H, Shakibaie M, Harandi MF, Dezaki ES. Efficacy of Myrtus communis L. to inactivate the hydatid cyst protoscoleces. J Invest Surg. 2016;29(3):137–43. 10.3109/08941939.2015.1088601 [DOI] [PubMed] [Google Scholar]
- 56.Kozan E, Ilhan M, Tümen I, Akkol EK. The scolicidal activity of the essential oil obtained from the needles of Pinus nigra Arn. subsp. pallasiana (Lamb.) Holmboe on hydatid cyst. J Ethnopharmacol. 2019;235:243–7. 10.1016/j.jep.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 57.Labsi M, Khelifi L, Mezioug D, Soufli I, Touil-Boukoffa C. Antihydatic and immunomodulatory effects of Punica granatum peel aqueous extract in a murine model of echinococcosis. Asian Pac J Trop Med. 2016;9(3):211–20. 10.1016/j.apjtm.2016.01.038 [DOI] [PubMed] [Google Scholar]
- 58.Mahmoudvand H, Dezaki ES, Kheirandish F, Ezatpour B, Jahanbakhsh S, Harandi MF. Scolicidal effects of black cumin seed (Nigella sativa) essential oil on hydatid cysts. Korean J Parasitol. 2014;52(6):653 10.3347/kjp.2014.52.6.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahmoudvand H, Asadi A, Harandi MF, Sharififar F, Jahanbakhsh S, Dezaki ES. In vitro lethal effects of various extracts of Nigella sativa seed on hydatid cyst protoscoleces. Iran J Basic Med Sci. 2014;17(12):1001 [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Mayah KS, Al-Bashir NM, Al-Azzawi BM. In vivo efficacy of Nigella sativa aqueous seed extract against metacestode of Echinococcus granulosus. Med J Babylon. 2012;9(1):140–51. [Google Scholar]
- 61.El-Bahy NM, Abdelaziz AR, Khalafalla RE. In-vitro evaluation of Nigella sativa and Punica granatum effect on protoscolices of hydatid cysts. Rev Bras Parasitol Vet. 2019;28(2):210–4. 10.1590/S1984-29612019019 [DOI] [PubMed] [Google Scholar]
- 62.Abdel-Baki A-AS, Almalki E, Mansour L, Al-Quarishy S. In vitro scolicidal effects of Salvadora persica root extract against protoscolices of Echinococcus granulosus. Korean J Parasitol. 2016;54(1):61 10.3347/kjp.2016.54.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norouzi R, Hejazy M, Azizi D, Ataei A. The effect of Taxus baccata L. extract on hydatid cyst protoscolices In vitro. Arch Razi Inst. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Almalki E, Al-Shaebi EM, Al-Quarishy S, El-Matbouli M, Abdel-Baki A-AS. In vitro effectiveness of Curcuma longa and Zingiber officinale extracts on Echinococcus protoscoleces. Saudi J Biol Sci. 2017;24(1):90–4. 10.1016/j.sjbs.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahmoudvand H, Pakravanan M, Kheirandish F, Jahanbakhsh S, Sepahvand M, Niazi M, et al. Efficacy and Safety Curcuma zadoaria L. to Inactivate the Hydatid Cyst Protoscoleces. Curr Clin Pharmacol. 2020;15(1):64–71. 10.2174/1574884714666190918155147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moazeni M, Nazer A. In vitro lethal effect of Zingiber officinale R. on protoscolices of hydatid cyst from sheep liver. Microbiol Res (Pavia). 2011;2(2):25. [Google Scholar]
- 67.Houshmand E, Kamalifar HS, Elmi H. In vitro scolicidal effect of ginger (Zingiber officinale roscoe) ethanolic extract against protoscolices of hydatid cyst. Iran J Vet Res. 2019;13(1):87–99. [Google Scholar]
- 68.Fabbri J, Maggiore MA, Pensel PE, Denegri GM, Gende LB, Elissondo MC. In vitro and in vivo efficacy of carvacrol against Echinococcus granulosus. Acta Trop. 2016;164:272–9. 10.1016/j.actatropica.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 69.Moazeni M, Larki S, Oryan A, Saharkhiz MJ. Preventive and therapeutic effects of Zataria multiflora methanolic extract on hydatid cyst: An in vivo study. Vet Parasitol. 2014;205(1–2):107–12. 10.1016/j.vetpar.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 70.Moazeni M, Larki S, Saharkhiz MJ, Oryan A, Lari MA, Alavi AM. Efficacy of the aromatic water of Zataria multiflora on hydatid cysts: an In vivo study. Antimicrob Agents Chemother. 2014:AAC. 02963–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moazeni M, Borji H, Saboor Darbandi M. Enhancement of the Therapeutic Effect of Albendazole on Cystic Echinococcosis using a Herbal Product. J Invest Surg. 2019;32(2):103–10. 10.1080/08941939.2017.1380089 [DOI] [PubMed] [Google Scholar]
- 72.Elissondo MC, Albani CM, Gende L, Eguaras M, Denegri G. Efficacy of thymol against Echinococcus granulosus protoscoleces. Parasitol Int. 2008;57(2):185–90. 10.1016/j.parint.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 73.Mahmoudvand H, Mirbadie SR, Sadooghian S, Harandi MF, Jahanbakhsh S, Saedi Dezaki E. Chemical composition and scolicidal activity of Zataria multiflora Boiss essential oil. J Essent Oil Res. 2017;29(1):42–7. [Google Scholar]
- 74.Larki S, Jalali MHR, Goodarzi S. Scolicidal effects of gallic acid, one of the major compounds of plants, on protoscolices of hydatid cyst. Zahedan J Res Med Sci. 2017;19(5). [Google Scholar]
- 75.Naguleswaran A, Spicher M, Vonlaufen N, Ortega-Mora LM, Torgerson P, Gottstein B, et al. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob Agents Chemother. 2006;50(11):3770–8. 10.1128/AAC.00578-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xin Q, Yuan M, Li H, Lu J, Song X, Jing T. In vitro efficacy of ampelopsin against Echinococcus granulosus and Echinococcus multilocularis. J Vet Med Sci. 2019:19–0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahin M, Eryilmaz R, Bulbuloglu E. The effect of scolicidal agents on liver and biliary tree (experimental study). J Invest Surg. 2004;17(6):323–6. 10.1080/08941930490524363 [DOI] [PubMed] [Google Scholar]
- 78.Albani CM, Pensel PE, Elissondo N, Gambino G, Elissondo MC. In vivo activity of albendazole in combination with thymol against Echinococcus multilocularis. Vet Parasitol. 2015;212(3–4):193–9. 10.1016/j.vetpar.2015.06.030 [DOI] [PubMed] [Google Scholar]
- 79.Raja RR. Medicinally potential plants of Labiatae (Lamiaceae) family: an overview. Res J Med Plant. 2012;6(3):203–13. [Google Scholar]
- 80.Christensen LP, Brandt K. Bioactive polyacetylenes in food plants of the Apiaceae family: occurrence, bioactivity and analysis. J Pharm Biomed Anal. 2006;41(3):683–93. 10.1016/j.jpba.2006.01.057 [DOI] [PubMed] [Google Scholar]
- 81.Mwine JT, Van Damme P. Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. J Med Plant Res. 2011;5(5):652–62. [Google Scholar]
- 82.Ahmad H, Khan SM, Ghafoor S, Ali N. Ethnobotanical study of upper Siran. J Herbs, Spices Med Plants. 2009;15(1):86–97. [Google Scholar]
- 83.Tariq A, Sadia S, Pan K, Ullah I, Mussarat S, Sun F, et al. A systematic review on ethnomedicines of anti-cancer plants. Phytother Res. 2017;31(2):202–64. 10.1002/ptr.5751 [DOI] [PubMed] [Google Scholar]
- 84.Sadia S, Tariq A, Shaheen S, Malik K, Ahmad M, Qureshi H, et al. Ethnopharmacological profile of anti-arthritic plants of Asia-a systematic review. J Herb Med. 2018. [Google Scholar]
- 85.Kamalzadeh A, Rajabbaigy M, Kiasat A. Livestock production systems and trends in livestock industry in Iran. J Agri Soc Sci. 2008;4:183–88. [Google Scholar]
- 86.Kamalzadeh A, Shabani A. Maintenance and growth requirements for energy and nitrogen of Baluchi sheep. Int J Agric Biol. 2007. [Google Scholar]
- 87.Moshi MJ, Otieno DF, Weisheit A. Ethnomedicine of the Kagera Region, north western Tanzania. Part 3: plants used in traditional medicine in Kikuku village, Muleba District. J Ethnobiol Ethnomed. 2012;8(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhat JA, Kumar M, Bussmann RW. Ecological status and traditional knowledge of medicinal plants in Kedarnath Wildlife Sanctuary of Garhwal Himalaya, India. J Ethnobiol Ethnomed. 2013;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Albuquerque UP. Re-examining hypotheses concerning the use and knowledge of medicinal plants: a study in the Caatinga vegetation of NE Brazil. J Ethnobiol Ethnomed. 2006;2(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adegboye M, Akinpelu D, Okoh A. The bioactive and phytochemical properties of Garcinia kola (Heckel) seed extract on some pathogens. Afr J Biotechnol. 2008;7(21). [Google Scholar]
- 91.Adnan M, Ullah I, Tariq A, Murad W, Azizullah A, Khan AL, et al. Ethnomedicine use in the war affected region of northwest Pakistan. J Ethnobiol Ethnomed. 2014;10(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amber R, Adnan M, Tariq A, Mussarat S. A review on antiviral activity of the Himalayan medicinal plants traditionally used to treat bronchitis and related symptoms. J Pharm Pharmacol. 2017;69(2):109–22. 10.1111/jphp.12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Altemimi A, Lakhssassi N, Baharlouei A, Watson D, Lightfoot D. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ribeiro J, Ribeiro W, Camurça-Vasconcelos A, Macedo I, Santos J, Paula H, et al. Efficacy of free and nanoencapsulated Eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Vet Parasitol. 2014;204(3–4):243–8. 10.1016/j.vetpar.2014.05.026 [DOI] [PubMed] [Google Scholar]
- 95.Xue L, Zhang H, Qin L, Wang X, Wang L. Effect of chuanwu and baishao used separately or in combination on adjuvant arthritis in rats. China Journal of Chinese Materia Medica. 2000;25(3):175–8. [PubMed] [Google Scholar]
- 96.Kaplan R, Storey B, Vidyashankar A, Bissinger B, Mitchell S, Howell S, et al. Antiparasitic efficacy of a novel plant-based functional food using an Ascaris suum model in pigs. Acta Trop. 2014;139:15–22. 10.1016/j.actatropica.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 97.Nordi E, Costa R, David C, Parren G, Freitas A, Lameirinha L, et al. Supplementation of moist and dehydrated citrus pulp in the diets of sheep artificially and naturally infected with gastrointestinal nematodes on the parasitological parameters and performance. Vet Parasitol. 2014;205(3–4):532–9. 10.1016/j.vetpar.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 98.Al-Shaibani I, Phulan M, Arijo A, Qureshi T. Ovicidal and larvicidal properties of Adhatoda vasica (L.) extracts against gastrointestinal nematodes of sheep in vitro. Pak Vet J. 2008;28(2):79–83. [Google Scholar]
- 99.Zips D, Thames HD, Baumann M. New anticancer agents: in vitro and in vivo evaluation. In Vivo. 2005;19(1):1–7. [PubMed] [Google Scholar]
- 100.Sangster N, Gill J. Pharmacology of anthelmintic resistance. Parasitol Today. 1999;15(4):141–6. 10.1016/s0169-4758(99)01413-1 [DOI] [PubMed] [Google Scholar]
- 101.Buttle DJ, Behnke JM, Bartley Y, Elsheikha HM, Bartley DJ, Garnett MC, et al. Oral dosing with papaya latex is an effective anthelmintic treatment for sheep infected with Haemonchus contortus. Parasite Vector. 2011;4(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dorman H, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308–16. 10.1046/j.1365-2672.2000.00969.x [DOI] [PubMed] [Google Scholar]
- 103.Griffin SG, Wyllie SG, Markham JL, Leach DN. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragrance J. 1999;14(5):322–32. [Google Scholar]
- 104.Deb DD, Parimala G, Devi SS, Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem-Biol Interact. 2011;193(1):97–106. 10.1016/j.cbi.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 105.Díaz C, Quesada S, Brenes O, Aguilar G, Ciccio JF. Chemical composition of Schinus molle essential oil and its cytotoxic activity on tumour cell lines. Nat Prod Res. 2008;22(17):1521–34. 10.1080/14786410701848154 [DOI] [PubMed] [Google Scholar]
- 106.Péres V, Moura D, Sperotto A, Damasceno F, Caramão E, Zini C, et al. Chemical composition and cytotoxic, mutagenic and genotoxic activities of the essential oil from Pipergaudichaudianum Kunth leaves. Food Chem Toxicol. 2009;47(9):2389–95. 10.1016/j.fct.2009.06.035 [DOI] [PubMed] [Google Scholar]
- 107.Chang H-T, Hsu S-S, Chou C-T, Cheng J-S, Wang J-L, Lin K-L, et al. Effect of thymol on Ca2+ homeostasis and viability in MG63 human osteosarcoma cells. Pharmacology. 2011;88(3–4):201–12. 10.1159/000331864 [DOI] [PubMed] [Google Scholar]
- 108.Hsu S-S, Lin K-L, Chou C-T, Chiang A-J, Liang W-Z, Chang H-T, et al. Effect of thymol on Ca2+ homeostasis and viability in human glioblastoma cells. Eur J Pharmacol. 2011;670(1):85–91. 10.1016/j.ejphar.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 109.Kumar A, Malik F, Bhushan S, Sethi VK, Shahi AK, Taneja SC, et al. An essential oil and its major constituent isointermedeol induce apoptosis by increased expression of mitochondrial cytochrome c and apical death receptors in human leukaemia HL-60 cells. Chem-Biol Interact. 2008;171(3):332–47. 10.1016/j.cbi.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 110.Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 2010;48(3):242–6. 10.3109/13880200903081796 [DOI] [PubMed] [Google Scholar]
- 111.Kuete V, Wiench B, Hegazy M-EF, Mohamed TA, Fankam AG, Shahat AA, et al. Antibacterial activity and cytotoxicity of selected Egyptian medicinal plants. Planta Med. 2012;78(02):193–9. [DOI] [PubMed] [Google Scholar]
- 112.Lacey E, Redwin J, Gill J, Demargheriti V, Waller P. A larval development assay for the simultaneous detection of broad spectrum anthelmintic resistance. 1990. [Google Scholar]
- 113.Qi H, Wang W, Dai J, Zhu L. In vitro anthelmintic activity of Zanthoxylum simulans essential oil against Haemonchus contortus. Vet Parasitol. 2015;211(3–4):223–7. 10.1016/j.vetpar.2015.05.029 [DOI] [PubMed] [Google Scholar]
- 114.Nguyen D, Xu T. The expanding role of mouse genetics for understanding human biology and disease. Dis Model Mech. 2008;1(1):56–66. 10.1242/dmm.000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bedell MA, Jenkins NA, Copeland NG. Mouse models of human disease. Part I: techniques and resources for genetic analysis in mice. Genes Dev. 1997;11(1):1–10. 10.1101/gad.11.1.1 [DOI] [PubMed] [Google Scholar]
- 116.Wadood A, Ghufran M, Jamal SB, Naeem M, Khan A, Ghaffar R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem. 2013;2(4):1–4. [Google Scholar]
- 117.Meena AK, Bansal P, Kumar S. Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J Tradit Med. 2009;4(4):152–70. [Google Scholar]
- 118.Marie-Magdeleine C, Hoste H, Mahieu M, Varo H, Archimede H. In vitro effects of Cucurbita moschata seed extracts on Haemonchus contortus. Vet Parasitol. 2009;161(1–2):99–105. 10.1016/j.vetpar.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 119.Pérez-Serrano J, Casado N, Rodriguez-Caabeiro F. The effects of albendazole and albendazole sulphoxide combination-therapy on Echinococcus granulosus in vitro. Int J Parasitol. 1994;24(2):219–24. 10.1016/0020-7519(94)90029-9 [DOI] [PubMed] [Google Scholar]
- 120.Amritpal S, Sanjiv D, Navpreet K, Jaswinder S. Berberine: alkaloid with wide spectrum of pharmacological activities. J Nat Prod. 2010;3:64–75. [Google Scholar]
- 121.Suthar M, Patel P, Shah T, Patel R. In vitro screening of Nigella sativa seeds for antifungal activity. Int J Pharm App Sci. 2010;1(1):84–91. [Google Scholar]
- 122.Maggiore M, Pensel P, Denegri G, Elissondo M. Chemoprophylactic and therapeutic efficacy of thymol in murine cystic echinococcosis. Parasitol Int. 2015;64(5):435–40. 10.1016/j.parint.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 123.Robledo S, Osorio E, Munoz D, Jaramillo LM, Restrepo A, Arango G, et al. In vitro and in vivo cytotoxicities and antileishmanial activities of thymol and hemisynthetic derivatives. Antimicrob Agents Chemother. 2005;49(4):1652–5. 10.1128/AAC.49.4.1652-1655.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin CC, Ng LT, Hsu FF, Shieh DE, Chiang LC. Cytotoxic effects of Coptis chinensis and Epimedium sagittatum extracts and their major constituents (berberine, coptisine and icariin) on hepatoma and leukaemia cell growth. Clin Exp Pharmacol Physiol. 2004;31(1-2):65–9. 10.1111/j.1440-1681.2004.03951.x [DOI] [PubMed] [Google Scholar]
- 125.Badary OA, Al-Shabanah OA, Nagi MN, Al-Bekairi AM, Elmazar M. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res. 1998;44(2-3):56–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.