Abstract
Background
In this large-scale cluster-randomized controlled trial (cRCT) we sought to assess the effectiveness of facemasks against viral respiratory infections.
Methods and results
Over three consecutive Hajj seasons (2013, 2014, 2015) pilgrims’ tents in Makkah were allocated to ‘facemask’ or ‘no facemask’ group. Fifty facemasks were offered to participants in intervention tents, to be worn over four days, and none were offered to participants in control tents. All participants recorded facemask use and respiratory symptoms in health diaries. Nasal swabs were collected from the symptomatic for virus detection by reverse transcription polymerase chain reaction. Clinical symptoms and laboratory results were analyzed by ‘intention- to-treat’ and ‘per-protocol’. A total of 7687 adult participants from 318 tents were randomized: 3864 from 149 tents to the intervention group, and 3823 from 169 tents to the control group. Participants were aged 18 to 95 (median 34, mean 37) years, with a male to female ratio of 1:1.2. Overall, respiratory viruses were detected in 277 of 650 (43%) nasal/pharyngeal swabs collected from symptomatic pilgrims. Common viruses were rhinovirus (35.1%), influenza (4.5%) and parainfluenza (1.7%). In the intervention arm, respectively 954 (24.7%) and 1842 (47.7%) participants used facemasks daily and intermittently, while in the control arm, respectively 546 (14.3%) and 1334 (34.9%) used facemasks daily and intermittently. By intention-to-treat analysis, facemask use did not seem to be effective against laboratory-confirmed viral respiratory infections (odds ratio [OR], 1.4; 95% confidence interval [CI], 0.9 to 2.1, p = 0.18) nor against clinical respiratory infection (OR, 1.1; 95% CI, 0.9 to 1.4, p = 0.40). Similarly, in a per-protocol analysis, facemask use did not seem to be effective against laboratory-confirmed viral respiratory infections (OR 1.2, 95% CI 0.9–1.7, p = 0.26) nor against clinical respiratory infection (OR 1.3, 95% CI 1.0–1.8, p = 0.06).
Conclusion
This trial was unable to provide conclusive evidence on facemask efficacy against viral respiratory infections most likely due to poor adherence to protocol.
Introduction
Viral respiratory infections are a major public health burden, causing serious disease especially in vulnerable populations. Influenza-associated lower respiratory tract disease alone causes over 54 million infections per year, eight million cases of severe illness, and 145,000 deaths across all age groups [1]. Ever-increasing and faster international travel intensifies the transmission of respiratory infections, especially in the setting of mass gatherings such as Hajj pilgrimage in Makkah [2]. The rites of Hajj are performed over five or six days, beginning on the eighth day and ending on the thirteenth day of the last month of the Islamic calendar. Coming from over 180 countries pilgrims converge on Makkah to join a procession of two to three million people who perform a series of physically demanding rituals. Such religious and other mass gatherings amplify the transmission of respiratory viruses by up to eight times [3] and may even accelerate the progression of a pandemic as occurred during the 2009 influenza A(H1N1) pandemic following the Iztapalapa Passion Play mass gathering in Mexico in April 2009 [4]. The current outbreak of coronavirus disease 2019 (COVID-19) is an example of how travel accelerates the spread of respiratory viral infection [5].
Non-pharmacological interventions, such as facemask use, and hand washing have been used to complement pharmacological measures in the prevention and control of viral respiratory infections at mass gatherings with no documented efficacy [6]. There is clinical and experimental evidence that surgical masks and respirators reduce transmission of drug-resistant tuberculosis and influenza from infected patients [7, 8], but randomized trials examining the effectiveness of facemasks against viral respiratory infections in household, community or healthcare settings have been either conflicting or inconclusive [9–15], though at least one randomized controlled trial has suggested protection against influenza by facemasks and hand washing, if applied early after exposure [13].
Inadequate sample size is thought to be an important cause of this discrepancy [16–18]. Therefore we designed a large cluster-randomized controlled trial (cRCT) over three years among pilgrims at Hajj to evaluate the efficacy of facemasks against laboratory-confirmed viral respiratory infections. The rationale of the cluster design was to increase administrative efficiency.
Methods
Trial design
Our study was an open label cRCT conducted during Hajj in Mina, Greater Makkah, Saudi Arabia among pilgrims from Saudi Arabia, Australia and Qatar over three Hajj seasons, 2013 to 2015. Mina is an uninhabited valley at the outskirts of Makkah and has about 30,000 tents to accommodate pilgrims for up to five days as part of Hajj rituals. Generally, 50 to 150 pilgrims occupy each large tent, allocated by gender and country of origin, but tents with a much smaller number also exist. Pilgrims in each tent sleep close to each other, head-to-head, have meals and perform rites together hence are considered a cluster. A pilot trial was conducted among Australian pilgrims in 2011 to examine the feasibility of such a study and inform power calculations [19]. Following the Consolidated Standards of Reporting Trials (CONSORT) guidelines (S1 Checklist) for cRCTs participants in respective tents were allocated to intervention or control group as per the trial protocol (S2 Appendix) [20, 21]. Our null hypothesis was that facemask use, according to protocol, does not protect from viral respiratory diseases.
Ethical approval for this study was obtained in Saudi Arabia from the Institutional Review Board of King Abdullah Medical City, Makkah, (IRB Ref. No.: 15–205), in Australia from the Hunter New England Human Research Ethics Committee (Reference No: 13/07/17/3.04), and in Qatar from the Joint Institutional Review Board of Hamad Medical Corporation/Weill Cornell Medical College (Ref: 13–00039).
Procedure
Hajj pilgrims aged ≥18 years from participating countries, staying in allocated tents and able to provide signed informed consent were included. Pilgrims aged <18 years, or ≥18 who had a known contraindication to mask use, had participated in another randomized trial investigating a medical intervention, refused or were unable to sign the consent were excluded.
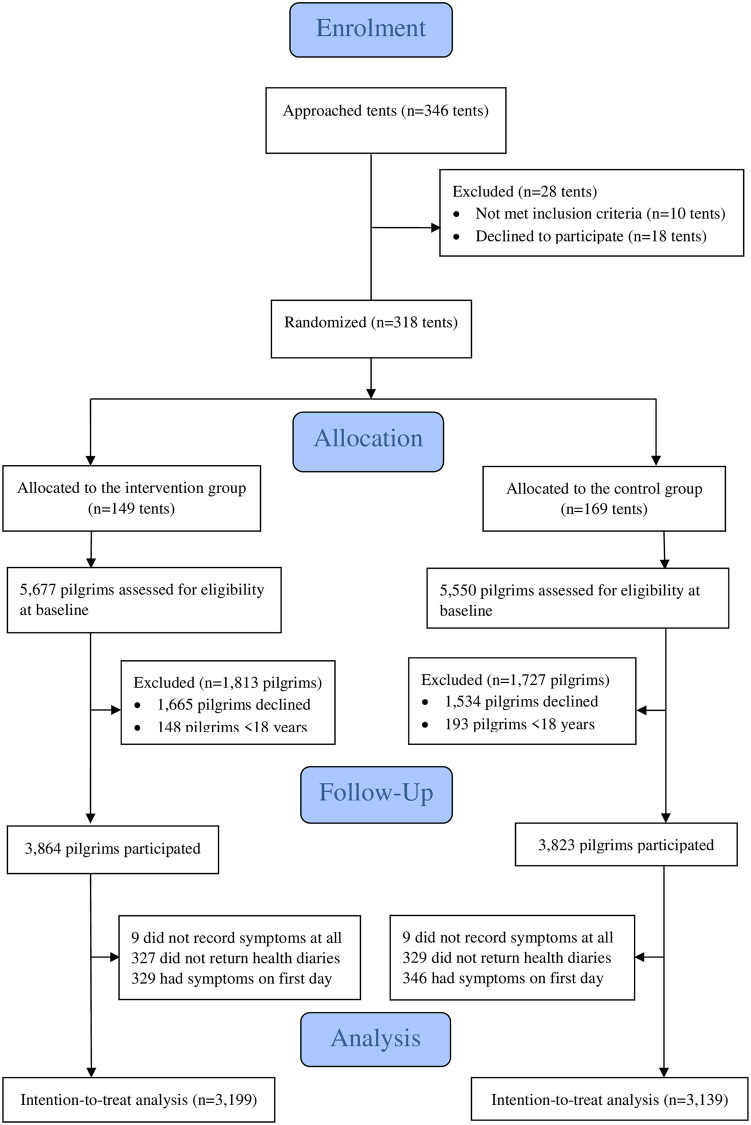
Agreement was secured from 318 Hajj tour group leaders for 346 tents, occupied by pilgrims from Saudi Arabia, Qatar and Australia to facilitate the study. The randomization unit of this trial was the accommodation tent. We planned to stratify the randomization by country and gender. Although per protocol computer-generated random number allocation by an offsite research coordinator had been planned, this proved impractical in this field study due to poor internet/mobile phone network at the study sites. Because real-time, effective and smooth communication with the offsite research coordinator responsible for random allocation generation was not possible, coin-tossing by an individual who was not a member of the research team (i.e., a fellow pilgrim who was not a participant in the trial, a tour operator or a medical volunteer at Hajj who was not a study team member) was used. As the intervention of wearing a facemask was visible to participants and investigators, the trial could not be blinded; laboratory staff could be, and were blinded to the intervention.
Trained research team members approached adult pilgrims aged 18 years or older in their assigned tents and explained the study in detail on the first day of each Hajj (October 13th 2013, October 2nd 2014 and September 22nd 2015). Individual research team members were assigned about 15 participants from the first day of Hajj. The researchers gave pilgrims an information sheet and answered their queries. Written informed consent was obtained from pilgrims who agreed to participate in the study. As per the study design, no minor, i.e., person under 18 years of age, was recruited in this study. All participants were asked to complete a baseline questionnaire and were provided with a health diary (S1 Appendix) in their preferred language (Arabic or English) which they were to fill out daily during the trial. Each participant was identified with a unique barcode number on their consent form, baseline questionnaire, health diary and any clinical specimens taken. A post-Hajj diary (S1 Appendix) for an additional three days was planned but a negligible return rate prevented this information being included in the analysis.
The consent forms and the baseline questionnaires were collected on day one, whereas participants retained diaries for completion over the next four days of Hajj rituals while they were being actively followed (Fig 1).
Fig 1. Overall trial flow.
Each participant in the intervention group was provided with 50 surgical facemasks (3M™ Standard Tie-On surgical mask, Cat No: 1816) as well as verbal and printed instructions and demonstration of appropriate facemask usage (S1 Appendix). Pilgrims in the control group were not provided with facemasks and instructions but could use their own masks if they chose to do so. All pilgrims in both study arms were asked to record their facemask usage (including number of masks used and hours worn each study day) in their health diary daily for four consecutive days. Although facemasks were to be worn for 24 hours daily per protocol if possible, for the analysis, pilgrims who used at least one facemask each day during Hajj were considered to have used a facemask during that day, counter to the planned design.
Measures
As the primary objectives of our trial were to assess the role of facemasks in preventing the acquisition of laboratory-confirmed viral respiratory infections and symptomatic respiratory infection, first primary endpoint was the efficacy of facemasks against laboratory-confirmed viral respiratory infections, and the second primary endpoint was the efficacy of facemasks against clinical respiratory infections in participants.
A total of 464 volunteer researchers were trained by the principal investigators before the study period. Training activities included how to approach pilgrims, the trial processes, explanation and demonstration of facemask use, data collection, follow-up, and sample collection and storage. Study team members were oriented to good clinical practice guidance for the conduct of clinical trials according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.
The research team visited the study tents twice daily during the study period to ask if the participants developed respiratory or systemic symptoms and collected a nasal swab (FLOQSwabs™; COPAN Diagnostics Inc., Murrieta, CA) from those who developed subjective fever together with one respiratory symptom, or two or more respiratory symptoms without fever. Swabs were placed it into UTM™ (COPAN) viral transport media. Swabs labelled with the participant’s unique barcode number were stored in an icebox at –20°C before being re-stored by day’s end in a –80°C freezer at the laboratory of the Hajj Research Center at Umm Al-Qura University, Makkah. After Hajj, these swabs were shipped in refrigerated or cold containers to the Centre for Infectious Disease and Microbiology Laboratory Services, Westmead Hospital, NSW, Australia. There, nucleic acid was extracted with the Qiagen bioROBOT EZ instrument (Qiagen, Valencia, CA), and amplification was performed using the Roche LC 480 (Roche Diagnostics GmbH, Mannheim, Germany) instrument. Respiratory viruses were detected using a real-time, multiplex reverse transcription polymerase chain reaction assay targeting human coronaviruses (OC43, 229E and NL63), influenza A and B viruses, respiratory syncytial virus (RSV), parainfluenza viruses 1–3, human metapneumovirus, rhinovirus, enterovirus and adenovirus as described elsewhere [22, 23]. Middle East respiratory syndrome coronavirus (MERS-CoV) assay targeting the upstream region of the E gene (upE) was also performed as described previously [24].
Symptomatic pilgrims were given generic medications for fever and pain, usually acetaminophen.
Statistical analysis and power calculation
Data from baseline questionnaires and health diaries were entered by trained research staff into customized web-based forms (WUFOO®, https://www.wufoo.com), and extracted into Excel sheets. Data checking against paper records was undertaken by four dedicated researchers (M.A., O.B., A.-M.B., M.T).
Statistical analysis was performed using the SPSS Statistics® v25 (IBM, Chicago, IL, USA) and checked by a statistician using SAS V.9.3.
Assuming that the prevalence of symptomatic viral respiratory infections is 30% in controls and the prevalence of laboratory-confirmed viral respiratory infections in controls is approximately 12%, the intervention was considered clinically worthwhile if it reduced the prevalence of clinical respiratory infection or laboratory-confirmed viral respiratory infections by 50%.
Assuming a moderate intra-cluster correlation of 0.1 and a mean of 75 participants per cluster (tent), and inflating the sample by a design effect of 8.4 to account for clustering [25], the sample size required for a cRCT to detect a reduction from 12% to 6% with 80% power at 5% significance is 2976 per arm. An additional inflation factor of 1.2 was included to allow for up to 15% loss to follow-up or incomplete outcome data. This resulted in a sample size of approximately 3500 participants per arm, making a total of 7000.
A descriptive analysis compared the characteristics of participants in the two arms (intervention and control), both at tent level and at individual participant level, as appropriate. Categorical variables were described using frequencies and percentages, and were compared, where appropriate, by using the Chi-squared test. Continuous data were described using the mean and standard deviation, and were compared by the Student’s t-test. The number or proportion of participants with missing data were reported for all variables, but comparisons between groups only included known values, except where otherwise specified. P values and 95% confidence intervals (CIs) were presented without adjustment.
The first and second primary endpoints were analyzed by intention-to-treat analysis according to the participants’ randomized treatment group regardless of treatment actually received. Outcomes were analyzed using a generalized estimating equation statistical model that accounted for the binary distribution of the data and the correlation between participants in the same tent, assuming an exchangeable correlation structure.
Exploratory multivariable analyses examined the effect of randomized treatment on outcomes in models adjusted for demographic factors, facemask usage and compliance with treatment. Subgroup analyses were conducted to compare the effect of treatment between groups of participants: male vs. female, those with known risk factors vs. those without risk factors or risk status unknown for viral respiratory infections, vaccinated against influenza vs. unvaccinated (or vaccination status reported as unknown), smoker vs. non-smoker, compliance with daily facemask use vs. non-compliance, and by pilgrim’s country of origin.
Subgroup analyses used the same statistical model as the primary outcomes and included an interaction term between randomized treatment and subgroup. If the p value for the interaction term was < 0.05, the effect of treatment was described separately within each subgroup. Primary analysis included all participants who reported symptoms at any time during the study period. Eighteen participants who failed to report symptoms were excluded, but those (n = 675) who reported symptoms only at baseline (i.e., on day one) were included. A per-protocol analysis was undertaken amongst participants who were compliant with instructions and reported symptoms daily after the baseline time point. An analysis was performed on participants who were symptom-free at baseline and who completed symptom reports at least once after baseline (i.e., on day one).
For the primary analysis, pilgrims who did not report symptoms daily were assumed to have no change in their symptoms compared to the most recent reporting day. Those who reported no symptom on any study day were assumed to have never developed symptoms while those who reported symptoms on any day were considered symptomatic.
Results
Participants
A total of 318 tents that housed 11,227 pilgrims were recruited in both study arms on the first day of each Hajj over three years (13th October in 2013, 2nd October in 2014 and 22nd September in 2015) and followed for the next four days. The number of occupants in each tent varied according to the size of the tent, ranging from 6 to 150 pilgrims per tent. The total number of participants across all study years was 7,687 with an average participation rate of 68.5% (7687 of 11,227) (ranged from 10 to 100%). Of the total 7687 participants, 3864 from 149 tents were assigned to the intervention and 3,823 from 169 tents were assigned to the control group, with an overall participation rate of respectively 68% (3864 of 5686) and 69% (3823 of 5541). Their age ranged from 18 to 95 years (median, 34; mean, 37; standard deviation, 12.3 years), with 53.9% female. Of all participants, 6998 (91%) were from Saudi Arabia and Qatar, and the rest were from Australia. A large proportion of pilgrims 57.6% (4428 of 7687) were recruited in the third year, 2015. The baseline characteristics are shown in Table 1.
Table 1. Baseline characteristics of participants and their compliance with facemask and hand hygiene across the study arms.
| Intervention n (%) | Control n (%) | |
|---|---|---|
| Tents | ||
| Total number of recruited tents | 149 | 169 |
| Gulf | 137 (91.9) | 151 (89.3) |
| Australia | 12 (8.1) | 18 (10.7) |
| 2013 | 26 (17.4) | 22 (13) |
| 2014 | 46 (30.9) | 55 (32.5) |
| 2015 | 77 (51.7) | 92 (54.4) |
| Male tent | 71 (47.7) | 72 (42.6) |
| Participants | ||
| Total number of participants | 3,864 | 3,823 |
| Gulf countries | 3,575 (92.5) | 3,423 (89.5) |
| Australia | 289 (7.5) | 400 (10.5) |
| 2013 | 551 (14.3) | 474 (12.4) |
| 2014 | 1,106 (28.6) | 1,128 (29.5) |
| 2015 | 2,207 (57.1) | 2,221 (58.1) |
| Male | 1,891 (48.9) | 1,651 (43.2) |
| Mean (standard deviation) age | 36.9 (12.1) | 37.2 (12.5) |
| Median (range) age | 34 (18–95) | 35 (18–95) |
| With any risk factor | 741 (19.2) | 715 (18.7) |
| Smoking as the single risk factor | 401 (10.4) | 355 (9.3) |
| Pregnant among women | 32 (1.6) | 31 (1.4) |
| Influenza vaccine uptake | 1,929 (49.9) | 1,887 (49.4) |
| Facemask use before recruitment | 1,057 (27.4) | 924 (24.2) |
| Daily use of facemask | 954 (24.7) | 545 (14.3) |
| Intermittent usage | 1,842 (47.7) | 1,333 (34.9) |
| Did not use facemask | 808 (20.9) | 1,672 (43.7) |
| Used antiseptic/hand rub | 1,818 (47) | 1,720 (45) |
| Washed hands frequently (>2 times a day) | 2670 (69.1) | 2520 (65.9) |
| Rarely washed hands (1–2 times a day) | 578 (15) | 619 (16.2) |
| Never washed their hands | 140 (3.6) | 178 (4.5) |
Overall facemask use was low, even in the intervention tents, with only 24.7% of participants using facemasks daily. Conversely, in the control tents 14.3% participants used facemasks daily. More participants in the intervention group had used a facemask anytime in the weeks before the actual Hajj compared to those assigned in the control group (27.4% vs. 24.2%, p < 0.01).
Slightly more pilgrims in the intervention group than in the control group reported frequent hand washing during Hajj, including their ritual ablutions (69.1% vs. 65.9%, p < 0.01).
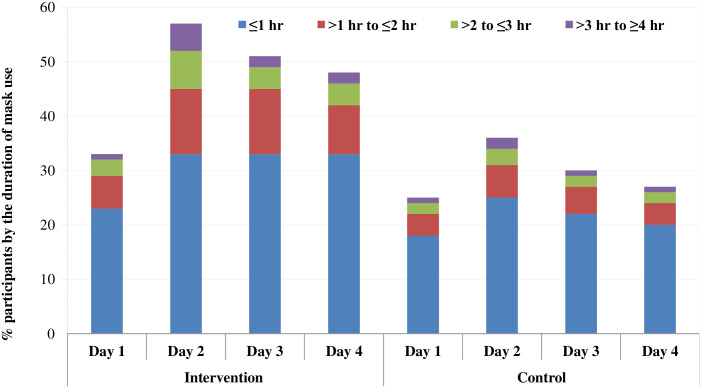
The proportion of pilgrims who participated in the study and used facemasks ranged between 0 and 50% per tent and the proportion of pilgrims who reported developing clinical respiratory infection in each tent ranged between 0 and 46%. However, in the intervention group, the number by subgroup of recorded time of daily facemask use for at least 4 hours was consistently greater than in the control group (Fig 2).
Fig 2. Proportion of facemask using participants by the duration of mask use across the study arms.
(This stacked bar chart shows the proportion by subgroup of recorded time of daily facemask use in the intervention group was consistently greater than in the control group).
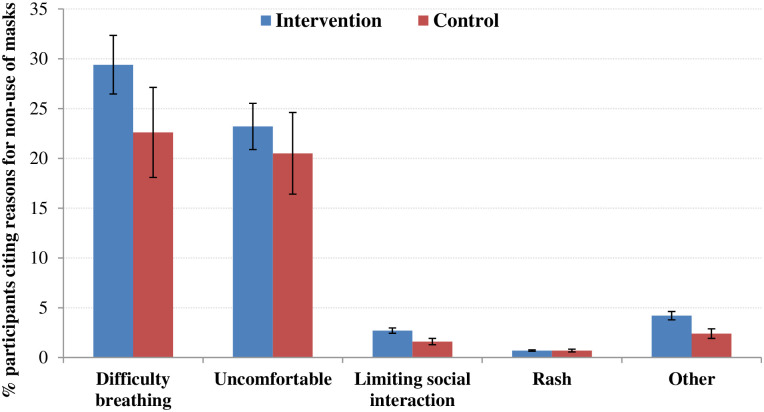
The most common side effects of using facemask were difficulty in breathing (26.2%) and discomfort (22%); a small minority (3%) reported feeling hot, sweating, a bad smell or blurred vision with eyeglasses (Fig 3).
Fig 3. Reasons for not using facemasks across the study arms.
A total of 650 nasal swabs were collected from symptomatic pilgrims in both groups. Overall, one or more respiratory viruses were detected in 277 (42.6%) of samples tested. The most prevalent viruses were rhinovirus (35.1%), influenza, including A/H1N1 and A/H3N2 (4.5%), and parainfluenza (1.7%), and 2% had dual infections (Table 2).
Table 2. The attack rate of viral respiratory infections during Hajj in both arms.
| Total | Intervention | Control | |
|---|---|---|---|
| N = 650 | N = 358 | N = 292 | |
| n (%) | n (%) | n (%) | |
| Positive | 277 (42.6) | 149 (41.6) | 128 (43.8) |
| Rhinoviruses | 228 (35.1) | 121 (33.8) | 107 (36.6) |
| Influenza A | 29 (4.5) | 17 (4.7) | 12 (4.1) |
| Influenza A/H1N1 | 9 (1.4) | 5 (1.4) | 4 (1.4) |
| Influenza A/H3N2 | 14 (2.2) | 9 (2.5) | 5 (1.7) |
| Influenza (subtype undetermined) | 6 (0.9) | 3 (0.8) | 3 (1) |
| Influenza B | 1 (0.2) | 0 (0) | 1 (0.3) |
| Enteroviruses | 10 (1.5) | 5 (1.4) | 5 (1.7) |
| Parainfluenza virus 1 | 5 (0.8) | 3 (0.8) | 2 (0.7) |
| Parainfluenza virus 2 | 1 (0.2) | 0 (0) | 1 (0.3) |
| Parainfluenza virus 3 | 5 (0.8) | 3 (0.8) | 2 (0.7) |
| hMPV | 2 (0.3) | 2 (0.6) | 0 (0) |
| Human coronaviruses | 3 (0.5) | 2 (0.6) | 1 (0.3) |
| Adenoviruses | 3 (0.5) | 1 (0.3) | 2 (0.7) |
| RSV | 2 (0.3) | 2 (0.6) | 0 (0) |
| MERS-CoV | 0 (0) | 0 (0) | 0 (0) |
| Dual infection | 13 (2) | 8 (2.2) | 5 (1.7) |
hMPV: Human metapneumovirus.
In the intention-to-treat analysis, allocation to facemask use was not associated with reduced laboratory-confirmed viral respiratory infections (odds ratio [OR], 1.4; 95% CI, 0.9 to 2.1; p = 0.18) or clinical respiratory infection (OR, 1.1; 95% CI 0.9 to 1.4; p = 0.40) (Table 3).
Table 3. Primary and subgroup analyses by intention to treat.
| Intervention n/N (%) | Control n/N (%) | OR (95% CI) | Interaction p value | |
|---|---|---|---|---|
| Clinical respiratory infection | 354/3,199 (11.1) | 322/3,139 (10.3) | 1.1 (0.9–1.4) | |
| Influenza vaccinated | 160/1,677 (9.5) | 181/1,631 (11.1) | 0.8 (0.7–1.1) | < 0.01 |
| Not vaccinated or vaccination status reported as unknown | 176/1,361 (12.9) | 131/1,381 (9.5) | 1.4 (1.0–2.0) | |
| At increased risk | 86/621 (13.8) | 69/615 (11.2) | 1.3 (0.9–1.8) | 0.28 |
| Not at increased risk | 254/2,439 (10.4) | 239/2,399 (10) | 1.1 (0.8–1.4) | |
| Smoking as the single risk factor | 40/350 (11.4) | 30/311 (9.6) | 1.2 (0.7–2) | 0.86 |
| Non-smoking | 303/2,721 (11.1) | 279/2,725 (10.2) | 1.1 (0.8–1.4) | |
| Male | 148/1,576 (9.4) | 120/1,354 (8.9) | 1.1 (0.8–1.5) | 0.73 |
| Female | 206/1,623 (12.7) | 202/1,785 (11.3) | 1.2 (0.9–1.6) | |
| Gulf | 332/3,043 (10.9) | 287/2,954 (9.7) | 1.2 (0.9–1.5) | 0.13 |
| Australia | 22/156 (14.1) | 35/185 (18.9) | 0.7 (0.4–1.3) | |
| Laboratory-confirmed viral respiratory infection | 96/218 (44) | 60/161 (37.3) | 1.4 (0.9–2.1) | - |
| Influenza vaccinated | 44/106 (41.5) | 37/102 (36.3) | 1.3 (0.7–2.2) | 0.85 |
| Not vaccinated or vaccination status reported as unknown | 54/95 (56.8) | 21/53 (39.6) | 1.3 (0.7–2.2) | |
| At increased risk | 20/42 (47.6) | 12/31 (38.7) | 3 (1.9–4.9) | 0.71 |
| Not at increased risk | 71/164 (43.3) | 45/114 (39.5) | 1.2 (0.7–2.1) | |
| Smokers | 11/28 (39.3) | 5/9 (55.6) | 0.5 (0.1–2.8) | 0.33 |
| Not smokers | 80/179 (44.7) | 53/138 (38.4) | 1.3 (0.8–2) | |
| Male | 45/101 (44.6) | 28/50 (56) | 0.7 (0.3–1.4) | 0.02 |
| Female | 51/117 (43.6) | 32/111 (28.8) | 1.9 (1.2–3) | |
| Gulf countries | 15/35 (42.9) | 17/48 (35.4) | 1.4 (0.6–3.4) | 0.96 |
| Australia | 81/183 (44.3) | 43/113/ (38.1) | 1.3 (0.8–2.2) |
In a per-protocol analysis (including only participants allocated to the intervention group who used facemasks daily, and participants allocated to the control group who never used any facemasks) there was no benefit of facemask in preventing laboratory-confirmed viral respiratory infections (OR, 1.2; 95% CI 0.9 to 1.7; p = 0.26) or clinical respiratory infection (OR, 1.3; 95% CI 1.0 to 1.8; p = 0.06) (Table 4).
Table 4. Per-protocol analysis: Effect of facemasks against clinical and laboratory-confirmed viral respiratory infections.
| Respiratory infection | Intervention n (%) | Control n (%) | ORa (95% CI; p) | |
|---|---|---|---|---|
| Clinical respiratory infection | Did not use facemask | 1.3a (1.0–1.8; 0.06) | ||
| Symptoms present | 55 (8) | 141 (9) | ||
| Symptoms absent | 648 (92) | 1356 (91) | ||
| Used facemask | ||||
| Symptoms present | 97 (12) | 38 (8) | ||
| Symptoms absent | 731 (88) | 425 (92) | ||
| Laboratory-confirmed viral respiratory infections based on facemask use | Did not use facemask | 1.2b (0.9–1.7; 0.26) | ||
| Positive | 29 (45) | 50 (41) | ||
| Negative | 35 (55) | 72 (59) | ||
| Used facemask daily | ||||
| Positive | 46 (50) | 20 (53) | ||
| Negative | 47 (50) | 18 (47) | ||
aAnalysis includes only participants from intervention group who used facemasks daily (n = 828) and those from control group who did not use facemasks (n = 1497).
bAnalysis includes only participants from intervention group who used facemasks daily (n = 93) and those from control group who did not use facemasks (n = 122).
Discussion
This randomized trial, like other smaller trials [9–15], failed to provide conclusive evidence on facemask efficacy against laboratory-confirmed or clinical respiratory infections. Inconclusiveness of this and previous studies might be attributed in part to respiratory pathogens having multiple routes of transmission including contact with contaminated surface [26, 27] and fecal-oral transmission of some respiratory viruses [28].
The large sample size in our cRCT enabled the comparison of a much larger number of clinical infections (intervention: control = 354: 322) and many more laboratory-confirmed infections (intervention: control = 96: 60) with higher power than the other randomized trials combined. Although unvaccinated pilgrims in the intervention group had a higher rate of clinical respiratory infection than their counterpart in the control group (13% vs. 10%, p = 0.03), this difference is unexplained. However, in previous studies, the prevalence of influenza-like illness among Hajj pilgrims was inversely proportional to their influenza vaccination uptake [29], and vaccinated Hajj pilgrims had 43% reduction in the probability of proven influenza infection [30]. A meta-analysis of six observational studies has shown influenza vaccine to be significantly protective against laboratory-confirmed influenza (relative risk, 0.56; 95% CI, 0.41 to 0.75) [31].
Females in the intervention group of our cRCT were at higher risk of acquiring laboratory-confirmed viral respiratory infections than in the control group (44% vs. 29%; p < 0.01). The reason is unclear, though one possible explanation is Muslim women prefer a loose face cover to a fitted facemask. Over 70% female pilgrims use a face veil during Hajj: one fifth of them use both face veil and mask [32]. Female pilgrims who used a face cover only occasionally (43.2%) tended to have higher rates of clinical respiratory infection compared to those who used it most of the time (36%) [33]. Although we did not assess face cover use by female pilgrims in our study, given that most pilgrims used facemasks only occasionally, the higher rate of viral respiratory infections among women might be due to intermittent use of face cover or even contamination of their masks [9, 33]. When they become wet, facemasks may even increase the transmission of infection, either through becoming more porous or by allowing passage of virus through the mask which is transmitted when the mask is handled [34].
The detection rate of respiratory viruses (43%) in our trial was higher than that reported in other studies (4 to 15%) [35], possibly due to the active case ascertainment strategy employed, including close follow-up of the symptomatic participants. However, the distribution of the viruses was similar to that in other studies: i.e., predominance of rhinovirus, followed by influenza and parainfluenza virus, these three viruses accounting for 97% (269/277) of viruses detected in our study.
No MERS-CoV was detected among the studied participants. Since the emergence of MERS-CoV in Saudi Arabia in 2013, multiple surveillance studies among >10,000 pilgrims from various countries have been undertaken without identifying a Hajj-related case [36, 37]. Now that there is a grave fear of massive acceleration of COVID-19 spread via large population movements [38], other preventive measures including hand and food hygiene, safe drinking water and physical distancing should be encouraged in addition to facemasks [39, 40]. We note that 0.5% of the symptomatic pilgrims tested positive for seasonal coronavirus indicating coronavirus transmission may occur in this setting and that, without the possibility of cross-protection, it is imperative that the Hajj should be scaled-down until the COVID-19 pandemic ends.
Findings of systematic reviews have been conflicting [17, 18, 39, 41–45], the most recent showing protective effects of masks against respiratory viral infection including pandemic coronaviruses across different populations but with low certainty [39, 41]. An observational study conducted at Hajj over four consecutive years (2014 to 2017) found pilgrims who reported using facemasks had higher likelihood of suffering from influenza-like illness symptoms (adjusted relative risk, 1.42; 95% CI, 1.10 to 1.82) and acquiring rhinovirus infection (adjusted relative risk, 1.30; 95% CI, 1.03 to 1.65) [46]. In an earlier study, 20.7% pilgrims who used a facemask reported fever compared with 15.6% who did not (p < 0.01) [47].
The most important limitation of this RCT was that despite much effort to encourage adherence with our protocol, compliance was limited. On the other hand many pilgrims randomized to the control group used facemasks, contrary to the research protocol. The Saudi Arabian Ministry of Health had issued advice to pilgrims to use facemasks while MERS-CoV was circulating in Saudi Arabia during our study period [48], though this was without definitive advice on how to wear masks and the duration of their use. It would have been unethical to counter the Saudi authority’s official advice on facemask use. Our trial does indicate, however, that as a public health intervention, facemask use is not practicable. Another important limitation of the study is that nasal swabs were not performed on the first day when subjects were enrolled. We depended on the reporting of clinical symptoms from day one and followed up directly for four days but did not validate the asymptomatic state with virological testing i.e., some asymptomatic pilgrims could have been virus positive. Longer follow-up was attempted through post-Hajj surveillance, but the low compliance precluded any meaningful analysis. Pilgrims moving from place to place to accomplish Hajj rites made it difficult for researchers to follow them as well as planned. While our study protocol required a clinical sample to be collected in participants with clinical respiratory infection, sampling was performed in some who did not meet the clinical respiratory infection definition whilst others were not swabbed when symptomatic. Though a cRCT design, complying as far as possible with CONSORT guidance, not all occupants in the selected tents participated. On average 69% of tent occupants participated in the trial, ranging widely from 10 to 100%, with possible dilution of the effect of facemask use. The trial was conducted over three years, with uneven recruitment over the years, and most participants (58%) recruited in the final year (2015). The rate of clinical respiratory infection over the study years was 15.5% in 2013, 8.3% in 2014, and 10.7% in 2015, reflecting the known seasonal variability of respiratory viruses, which may have affected the outcome of our study. About 9% participants in each study arm failed to return their diaries or returned dairies without recording symptoms, and another 9% in each group were excluded for being symptomatic on day one of our trial.
Another limitation is that participating tents varied between the study arms (intervention: control = 149: 169) because some tents, although designated as separate units, were later found to be parts of a larger tent. This was more common when several small tour groups were managed under a large tour operator and communal activities (e.g., meals, congregational prayers, sermons) were combined in one large tent. It is also possible that some of these tents were allocated to both intervention and control arms and may have further contributed to dilution of the magnitude of the effect or cluster contamination.
The failure of post-Hajj reporting was another limitation of our study. However, the median incubation periods of the three most common viruses (rhino, influenza and parainfluenza) are <3 days [49], indicating that the majority of the detected infections were acquired after enrolment. Though dangerous to presume very little symptomatic disease post-Hajj this is one explanation for non-adherence with post-Hajj follow-up.
Our cRCT, which was a field study in real time, was unable to refute our null hypothesis. Lack of facemask efficacy observed in this trial could be attributed to limited facemask use by participants (only 24.7% used daily and 47.7% used intermittently in the intervention group), the substantial proportion of participants in the control group who used facemasks, the inability to follow participants after Hajj or the likely contamination of masks [9, 26]. Though more in the intervention group consistently wore masks for defined periods daily, facemask use by controls further reduced the ability of the study to detect differences in infection rates between the study arms.
Due to lack of blinding pilgrims in control tents reporting even mild illness may have reported symptoms because they knew that they had not received the intervention. Also research team members may have been biased towards swabbing such participants assuming them to be less protected, which could have led to an overestimate of the effect of intervention.
The high rate of facemask use (almost 80%) observed among French Hajj pilgrims during the 2009 influenza pandemic year [50], compared to about 55% in a non-pandemic year [3], and in pilgrims from Southeast Asia (e.g., 73% among Malaysian pilgrims in 2007) seem to be due to cultural differences and heightened awareness during a pandemic [51]. The lower uptake of facemasks among participants in our cRCT is similar to the uptake among Saudi Arabian (35 to 57%) and Australian pilgrims (53 to 57%) observed in previous surveys [31, 32, 52, 53], while uptake as low as 0.02% was also recorded among some international pilgrims [54]. Although 78% in the intervention group of our cRCT used facemasks, only a quarter used them regularly. The most common reasons for non-compliance, difficulty in breathing and feeling of discomfort, found also in previous surveys among Hajj pilgrims [52], limited the use of facemasks in this cRCT. These might be strong and important limitations to effective facemask use against respiratory infections, since, in a mass gathering, close contact setting, around the clock protection would be necessary.
Given the number of people who attended Hajj and the close proximity within and among the living quarters, contact transmission from direct contact and indirect contact with contaminated surfaces may have been important and perhaps another reason why these results should be considered inconclusive. There is also the possibility of exposure and infection during travel and prior to Hajj itself. This RCT demonstrates the difficulties of participants’ adherence to instructions and protocol even with active supervision.
Several previous observational studies at Hajj have failed to show the effectiveness of facemasks in preventing respiratory infections [50–52], possibly due also to poor adherence to instructions, although a recent study showed that changing facemask every four hourly reduced the chance of upper respiratory tract infections among Hajj pilgrims (adjusted OR 0.56; 95% CI 0.34 to 0.92; p = 0.02) [55]. In our cRCT, though pilgrims in both intervention and control groups were close to each other day and night, none wore a mask for 24 hours as advised. This may have been an unrealistic expectation. Mask wearing during the COVID-19 pandemic has highlighted the importance of effective and realistic health messaging.
Additional studies with an even larger sample size and more intense supervision would better test the efficacy of facemasks and the role of other interventions, such as hand hygiene, in a mass gathering setting. These will be especially important to evaluate such interventions during the COVID-19 pandemic.
Conclusion
This trial failed to provide definitive evidence for the effectiveness of facemasks during the Hajj. This was likely due to poor compliance with facemask use. We report difficulties in implementing a large cRCT, evaluating the effectiveness of facemasks against viral respiratory infections including participants’ poor compliance with the protocol, despite active explanation and support.
Supporting information
(DOC)
(DOCX)
(DOC)
Acknowledgments
The authors acknowledge the help and support of: the Royal Embassy of Saudi Arabia in Canberra; Saudi Arabian Cultural Mission, Canberra; Ministry of Higher Education, Riyadh; Ministry of Health, Riyadh; Ministry of Hajj (Deputy Minister’s Office), Makkah; the Custodian of the two Holy Mosques Institute for Hajj and Umrah Research (Dr. Abdulaziz Seroji and Dr. Turki Habeebullah), Makkah. The authors also thank Dr Anisul Awal of Chittagong Medical College, for his valuable comments on study design, and Mr Saad Tamimi, Hamad Medical Corporation for his help with grant management.
Other members of the Hajj Research Team: Nedal Almasri (email: dr.nedal@gmail.com), Jassir Alshehri, Ghassan Matbouly, Jamil Samkari, Nadeen Kalantan, Mohammed Alhefzi, Hisham Alqari, Mukhtaar Sayid, Bayan Hariri, Moataz Fakeerah, Daniah Bondagji, Mohammed Alluhidan, Sami Mushta, Saeed Alsharif, Mohammed G Asiri, Rakan Ikram, Ibtihal Malawi, Ebtehal Matar, Atheer Alshareif, Israa Kalantan, Eatimad Alalawi, Afnan AlGhamdi, Amani Koshak, Ameerah Alkhaldi, Inaam Al-Nami, Anwar Howsawi, Bashaier Fairaq, Bushra Maghrabi, Tafaol Murad, Hanan Alzahrani, Kholood Almehmadi, Doaa Milibari, Rehab Hafiz, Rawdhah Kalantan, Shahad Al-Ansari, Aeshah Rajab, Anood Alfahmy, Ghaida Ali, Fatimah Abu naji, Lujin Hassan, Lulwah Althumali, Layla Farhat, Najlaa Baddour, Hibh Alandanusi, Waad Alqurashi, Sumayyah Fallata, Azhar Alharbi, Joud Bahakeem, Abrar Alshareef, Badr Rawa, Ahmed Alghamdi, Ahmed Muqadimi, Osama Alamri, Jehad Qutub, Abdulrahman Al-Ghamdi, Abdurrahman Mirza, Abdulghafur Alandijani, Omar Qoqandi, Faisal Mandourah, Muhammad Alghamdi, Mohammed Mahboob, Mohannad Alsulami, Moayyd Hinnawi, Naif Hawsawi, Nawaf Dhabab, Ahmed Balamash, Mohammed Bawazir, Raif Nassir, Mohammed AlAsmari, Faisal Alzahrani, Abdulrahman Alomari, Ahmad Makeen, Ibraheem Almani, Ahmed Baabdullah, Osama Alamoudi, Ahmed Alzhrani, Ahmed Bagabas, Ahmad Ahmad, Anwar Alammari, Ayman Alghamdi, Badr Alaifan, Badr Al Dahlawi, Turki Almalki, Thamer Zoghbi, Hussam Patwa, Hasan Ghannam, Hussien Alkully, Samir Alsulaimani, Samee Al Heraki, Saad AlGhamdi, Sultan AlBalawi, Sultan Albukhari, Saleh Algamdi, Abdululah Alsolami, Abdullah Alghanmi, Abdulellah Alturkistani, Abdullrhman Alayad, Abdulrahman Althagafi, Abdulrahman Makki, Abdulrahman Khinkar, Abdulaziz Alalawi, Abdulaziz Alhoqail, Abdulaziz Alshoaibi. Abdullah Aldour, Abdulhadi Towairqi, Ali Ali, Ali Alshubaily, Firas Atwah, Majed Daqeeq, Mohammed Aljunaid, Mohammed Alghamdi, Mohammed Alsefri, Mohammed Alamoudi, Mohammed Alghamdi, Mohammad Melibary, Mohammad Bakhaidar, Mohammad Albogami, Mohammed Almoflihi, Muaath AlGhamdi, Mutaz Abdulhaq, Monther Farghali, Mohannad Khyyat, Moayad Banjar, Wael Almaghthawi, Wael Khalifa, Yasser Halabi, Mohanad Aljohani, Riyadh Alharbi, Moayad Sumnudi, Sultan Al Jaid, Rayan Makeen, Mahmoud Eid, Mohammed Alaryni, Abdulrahman Qahtani, Saud Bakhsh, Turki Alkharji, Ahmed Qadah, Albraa Kashegari, Ahmad Alabbasi, Abdulmohsen Al-Sofi, Meshary Alhassni, Nawaf Alharbi, Ahmad Al Ahdal, Abdulghani Alserafi, Ibrahim Alomry, Mohammed Kadi, Abdulrahman Almalki, Bassel Katib, Ibrahim Sameer, Fares Alnajjar, Mohammed Hawsawi, Rayan Mohammad, Ebtihal Turkistani, Abrar Tawakoul, Arwa Bajabaa, Areej Alzaidi, Ashar Almusallam, Asraa Turkistani, Asmaa Alattas, Alshaima Alghamdi, Esraa Kashkari, Elaf Altwairqi, Elaf Alrehaili, Elaf Khalifa, Inas Magharbil, Abrar Salloma, Arwa Alzaidi, Arej Fadel, Afnan AL Gothami, Amal Al-Saedi, Alaa Binsalman, Aya Kutbi, Baraah Tatwany, Basmah Fallata, Bashayer Al Mutairi, Bashaer Alrefaie, Bashayer Al-huthali, Bashayer Alsaati, Bashaer ALzahrani, Bushra Fallatah, Bushra Alattas, Bushra Alhajjaji, Banan Almalki, Bayan Zamil, Tamador Alghamdi, Tahani Al-Ghamdi, Jenan Jawi, Haneen Sibieh, Kholoud Natto, Duaa Eid, Reem Alamoodi, Sumaia Felimban, Etaf Kassem, Faten Althobaiti, Fadya Althobaiti, Maria AL-Jehani, Muneerah Al-youbi, Nadeen Bugis, Duaa Aiash, Duaa Assaqaf, Duaa Almouallimi, Rania Iraqi, Rasha Qurashi, Rasha Baqis, Raghad Jamal Aldeen, Ruqaiah Baharoon, Ranad Medhir, Renad Gashlan, Renad Aljohani, Randa Al-Bloushy, Raneen Abu Saadah, Rahaf Shafi, Rawan Gaafar, Reem Alshareef, Zahra Othman, Sara Aljuaid, Sara Al-Ghfari, Salma Sait, Samaa Sangouf, Samar Alsubhi, Sahar Alharbi, Samar Al-harbi, Sana’a Kelantan, Shahad Aldor, Shahd Alshareef, Shaimaa Halabi, Shaimaa Hawsawi, Seba AlHarbi, Azzah Azzouz, Alyaa Idris, Fatimah Alosaimi, Fatimah Alsomali, Lujain Al-Thakafi, Lujain Abdalwassie, Lama Alarabi, Lina Alsaiari, Majedah Alshammari, Mahacen Alnadwi, Mada Abdulhaq, Mada Al Zahrani, Maradi Murad, Maram AlShareef, Marwah Hadidi, Nojoud Benhli, Najwa Mohammad, Nada Almuqati, Noor Alessa, Noura Bakhsh, Nuran Sultan, Norah Alotaibi, Heba Waez, Heba Al-Qethami, Heba Alsheikh, Hebah Alwafi, Hoda Al-Sayid, Hadeel Khoj, Wejdan Makeen, Woud AlMusallam, Waed Yaseen, Wafa Sohail, Sara Fallata, Abdel Mejid Mohamed, Abdulkarim Al-Sabyani, Abdullah Elhosiny, Abdullah Alsayed, Abdullah Nawab, Abdullah Alharbi, Abdulraheem Al-Sadat, Abdulrahman Aldarkhbani, Abdulrahman Alnaser, Abdulrahman Allahyani, Abdulrahman Bazaid, Abdulrhman Al-Malki, Abdulrhman Kinsara, Abrar Khalil, Abrar Ainousa, Abrar Ghulam, Afaf Ebraheem Mas, Ahmad Charbatji, Ahmad Altalhi, Ahmad Bimah, Ahmad Maqadmi, Ahmad Albeshri, Ahmed Sindi, Ahmed Aljuhani, Ahmed Almikhlafi, Aisha Muhammed Memon, Alaa Khoja, Alaa Habib, Alhassan Alhasani, Ali Medher, Ali Alkhathami, Ali Al-Attas, Abdullah Alqarni, Amal Faheem, Amal Alsaedi, Amani Hussin, Amir Khogeer, Ammar Alfattni, Ammar Almaghrabi, Ammar Alaaddin, Anas Salman, Anwar Alharbi, Aqeel Alkhiri, Areej Abdul- Gader, Arwa Shaheen, Arwa Alasmari, Asma Mansoor, Asmah Aldobashi, Ataa Mesbah, Awnallah Al-Otaibi, Ayah Istanbouli, Badriah Aldeaiq, Banan Bawazeer, Bandar Ghonaim, Bashayer Alsaadi, Bassam Elkhouly, Bassil Aladani, Bayan Fatani, Bshaer Badakhan, Bushra Al-Harbi, Dania Shaikh, Daniah Alnemari, Deemah Alindonosi, Elaf Tayeb, Elaf Albasheri, Emad Alharbi, Eman Al Hindi, Eman Almarwani, Ensaf Fatani, Eyaad Ghallab, Faisal Alterazi, Faisal Almaabadi, Faisal Mahmood, Ghadah Althbiti, Ghadi Alotaibi, Ghaliah Al-Haqas, Hadeel Alhassani, Hamad Alhilabi, Hamis Alalhareth, Hanadi Al-Thobaiti, Hashem Moafa, Hassan Almalki, Hayaa Zaki, Heba Aziza, Hind Alrefai, Hisham Alkhuzaei, Horia Abou Shousha, Huda Mansoor, Hussain Jammal, Hussam Rawas, Jawaher Alqurashi, Jomana Ajawi, Jumana Melebari, Jumanah Al-Saedi, Khadeejah Aljifri, Khalid Alsubaie, Leenah Abdulgader, Lin Charbatji, Linah Zamzami, Maan Alraddadi, Mahmood Yasawy, Mahmoud Chaker, Majed Alamoudi, Majid Jawa, Malek Alsairafi, Maram Albarakati, Marium Iqbal, Marwa Hawsawi, Masheal Bawahhab, Mohammad Alzhrani, Mohammad Shaheen, Mohammed Algarni, Mohammed Alzahrani, Mohammed Al-Fageeh, Mohammed Alaamri, Mohammed Al-Jeddawi, Mohammed Alshreef, Mohammed Alsaggaf, Mohammed Dumyati, Mohammed Al- Mikhlafi, Mohsen Alzamanan, Mona Alghamdi, Muhab Hindi, Muteb Almarwani, Nada Telmesani, Nada Mohammed, Naif Alhowaiti, Nasser Alshehri, Nedaa Karami, Neveen Awad, Nizar Almaghrabi, Nojoud Hli, Nuha Jazzar, Omar Alzhrani, Omar Alotaibi, Osama Alharbi, Qutaibah Aldurrah, Raghad Namnqani, Rahma Al-Ghamdi, Raid Alghamdi, Rana Almimoni, Rana Abbas, Raneem Rawa, Rayyan Alqurayyan, Razan Melibari, Reem Altaifi, Reem Bajunaid, Reem Altowairqi, Reem Alenazi, Reem Alghamdi, Refal Aziz Al-Rahman, Reham Bin Hassan, Roaa Khan, Rowaynah Aziz Al-Rahman, Ruba Alshaikh, Saad Algarni, Saadiah Balkhy, Saeed Balubaid, Safa’A Al-Hasani, Sahar Futtiny, Salman Melhem, Salwa Alotaibi, Samah Alqurashi, Samaher Melybari, Sana Nargis, Sara Alshehtha, Sarah Radwan, Saud Bakhsh, Shahad Bamani, Shahad Alharbi, Shahd Hafiz, Shoroug Alkhabiry, Sofana Alqawsi, Sultan Alzahrani, Sultan Shaqra, Thamer Alanazi, Wafa Alkhuzaie, Wafa Sidiqqi, Wafaa Altaezi, Wafaa Alharbi, Walid Almutairi, Wasaif Aljuhany, Wed Jawa, Yaser Badawood, Yasser Hadi, Yousef Alzahrani, Abaad Al-Mutairi, Abdulaziz Ajaj, Abdullah Tai, Abdullah Ashour, Abdullah Aljohni, Abdullah Alshehri, Abdullateef Allebdi, Abdullateef Alzhrani, Abdulmajeed Alzahrani, Abrar Alnami, Adel Almaymuni, Afnan Joudah, Ali Fadel, Ali Alshehri, Ali Alelyani, Ali Alkhulaifi, Amal Alnakhli, Amani Alharbi, Anas Heji, Arwa Badakhan, Asal Arbaeen, Asmaa Nassir, Basim Almutairi, Atrab Bayazeed, Bushra Alahmadi, Dania Almunami. Dareen Alsaidalany, Ebtehal Yamani, Ebtesam Alghamdi, Eman Alharbi, Eman Kotbi, Fahad Altowairqi, Faisal Althobaiti, Faisal Alsobyani, Farraj Al-Zahrani, Ghadah Althbiti, Ghadah Alshehri, Hadeel Alqahtani, Hanan Mughallis, Haneen Taher, Heba Bayoumi, Hesham Essa, Hussein Alshamrani, Khalil Alghamdi, Malak Alshammari, Maram Albarakati, Marwah Bin-Garhom, Marwan Albeshri, Mohamed Bayoumi, Mohammad Althobaiti, Mohammed Toras, Mohammed Al Thebyani, Naif Alzahrani, Nasheal Bawahhab, Noura Al-Zahrani, Ohoud Alharbi, Radwan Badr, Rahma Aljedaani, Rakan Alnefaie, Rawa’A Al- Maghrabi, Rehab Alshamrani, Reham Abdulgader, Riyadh Alharbi, Sara Al-Ghfari, Sarah Aljoudi, Saud Alzhrani, Somayah Alsolami, Suliman Badi, Sultan Alghamdi, Tawfiq Al Laylah, Wasfi Almusaddi, Wijdan Alzhrani.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This report was made possible by a National Priorities Research Program grant (NPRP 6- 1505-3-358) from the Qatar National Research Fund, a member of Qatar Foundation. The grant was offered to HR, RB, EAH, HE-B, OB, GJW, DED, ECH, and LGH. This body was not involved in the study design study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7(1):69–89. 10.1016/S2213-2600(18)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Memish ZA, Steffen R, White P, Dar O, Azhar EI, Sharma A, et al. Mass gatherings medicine: public health issues arising from mass gathering religious and sporting events. Lancet. 2019;393(10185):2073–84. 10.1016/S0140-6736(19)30501-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benkouiten S, Charrel R, Belhouchat K, Drali T, Salez N, Nougairede A, et al. Circulation of respiratory viruses among pilgrims during the 2012 Hajj pilgrimage. Clin Infect Dis. 2013;57(7):992–1000. 10.1093/cid/cit446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zepeda-Lopez HM, Perea-Araujo L, Miliar-García A, Dominguez-López A, Xoconostle-Cázarez B, Lara-Padilla E, et al. Inside the outbreak of the 2009 influenza A (H1N1)v virus in Mexico. PLoS One. 2010;5(10):e13256 10.1371/journal.pone.0013256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajema KL, Oster AM, McGovern OL, Lindstrom S, Stenger MR, Anderson TC, et al. Persons Evaluated for 2019 Novel Coronavirus—United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(6):166–70. 10.15585/mmwr.mm6906e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haworth E, Barasheed O, Memish ZA, Rashid H, Booy R. Prevention of influenza at Hajj: applications for mass gatherings. J R Soc Med. 2013;106(6):215–23. 10.1258/jrsm.2012.120170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharmadhikari AS, Mphahlele M, Stoltz A, Venter K, Mathebula R, Masotla T, et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward. Am J Respir Crit Care Med. 2012;185(10):1104–9. 10.1164/rccm.201107-1190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis. 2009;49(2):275–7. 10.1086/600041 [DOI] [PubMed] [Google Scholar]
- 9.MacIntyre CR, Seale H, Dung TC, Hien NT, Nga PT, Chughtai AA, et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5(4):e006577 10.1136/bmjopen-2014-006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs JL, Ohde S, Takahashi O, Tokuda Y, Omata F, Fukui T. Use of surgical face masks to reduce the incidence of the common cold among health care workers in Japan: a randomized controlled trial. Am J Infect Control. 2009;37(5):417–9. 10.1016/j.ajic.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 11.Cowling BJ, Fung RO, Cheng CK, Fang VJ, Chan KH, Seto WH, et al. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS One. 2008;3(5):0002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiello AE, Murray GF, Perez V, Coulborn RM, Davis BM, Uddin M, et al. Mask use, hand hygiene, and seasonal influenza-like illness among young adults: a randomized intervention trial. J Infect Dis. 2010;201(4):491–8. 10.1086/650396 [DOI] [PubMed] [Google Scholar]
- 13.Suess T, Remschmidt C, Schink SB, Schweiger B, Nitsche A, Schroeder K, et al. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009–2011. BMC Infect Dis. 2012;12:26 10.1186/1471-2334-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacIntyre CR, Cauchemez S, Dwyer DE, Seale H, Cheung P, Browne G, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. 2009;15(2):233–41. 10.3201/eid1502.081167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canini L, Andreoletti L, Ferrari P, D’Angelo R, Blanchon T, Lemaitre M, et al. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PLoS One. 2010;5(11): e13998 10.1371/journal.pone.0013998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid H, Booy R, Heron L, Memish ZA, Nguyen-Van-Tam J, Barasheed O, et al. Unmasking masks in Makkah: preventing influenza at Hajj: Clin Infect Dis. 2012;54(1):151–3. 10.1093/cid/cir826 [DOI] [PubMed] [Google Scholar]
- 17.Cowling BJ, Zhou Y, Ip DK, Leung GM, Aiello AE. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138(4):449–56. 10.1017/S0950268809991658 [DOI] [PubMed] [Google Scholar]
- 18.Bin-Reza F, Chavarrias VL, Nicoll A, Chamberland ME. The use of masks and respirators to prevent transmission of influenza: a systematic review of the scientific evidence. Influenza Other Respi Viruses. 2012. l;6(4):257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barasheed O, Almasri N, Badahdah AM, Heron L, Taylor J, McPhee K, et al. Pilot Randomised Controlled Trial to Test Effectiveness of Facemasks in Preventing Influenza-like Illness Transmission among Australian Hajj Pilgrims in 2011. Infect Disord Drug Targets. 2014;14(2):110–6. 10.2174/1871526514666141021112855 [DOI] [PubMed] [Google Scholar]
- 20.Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Barasheed O, Rashid H, Booy R, El Bashir H, Haworth E, et al. A cluster-randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims. J Epidemiol Glob Health. 2015;5(2):181–9. 10.1016/j.jegh.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratnamohan VM, Taylor J, Zeng F, McPhie K, Blyth CC, Adamson S, et al. Pandemic clinical case definitions are non-specific: multiple respiratory viruses circulating in the early phases of the 2009 influenza pandemic in New South Wales, Australia. Virol J. 2014;11:113 10.1186/1743-422X-11-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010;48(8):2940–7. 10.1128/JCM.00636-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corman VM, Olschlager S, Wendtner CM, Drexler JF, Hess M, Drosten C. Performance and clinical validation of the RealStar MERS-CoV Kit for detection of Middle East respiratory syndrome coronavirus RNA. J Clin Virol. 2014;60(2):168–71. 10.1016/j.jcv.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machin DC, M J, Tan SB, Tan SH. Sample size tables for clinical studies. 3rd ed: Wiley-Blackwell; 2009. [Google Scholar]
- 26.Hoang VT, Sow D, Belhouchat K, Dao TL, Ly TDA, Fenollar F, et al. Environmental investigation of respiratory pathogens during the Hajj 2016 and 2018. Travel Med Infect Dis. 2020;33:101500 [Epub ahead of print]. 10.1016/j.tmaid.2019.101500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killingley B, Nguyen-Van-Tam J. Routes of influenza transmission. Influenza Other Respir Viruses. 2013;7 Suppl 2:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfelali M, Barasheed O, Tashani M, Azeem MI, El Bashir H, Memish ZA, et al. Changes in the prevalence of influenza-like illness and influenza vaccine uptake among Hajj pilgrims: A 10-year retrospective analysis of data. Vaccine. 2015;33(22):2562–9. 10.1016/j.vaccine.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 30.Alfelali M, Barasheed O, Koul P, Badahdah AM, Bokhary H, Tashani M, et al. Influenza vaccine effectiveness among Hajj pilgrims: a test-negative case-control analysis of data from different Hajj years. Expert Rev Vaccines. 2019;18(10):1103–14. 10.1080/14760584.2019.1646130 [DOI] [PubMed] [Google Scholar]
- 31.Alqahtani AS, Rashid H, Heywood AE. Vaccinations against respiratory tract infections at Hajj. Clin Microbiol Infect. 2015;21(2):115–27. 10.1016/j.cmi.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 32.Alqahtani AS, Wiley KE, Tashani M, Willaby HW, Heywood AE, BinDhim NF, et al. Exploring barriers to and facilitators of preventive measures against infectious diseases among Australian Hajj pilgrims: cross-sectional studies before and after Hajj. Int J Infect Dis. 2016;47:53–9. 10.1016/j.ijid.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhry AJ, Al-Mudaimegh KS, Turkistani AM, Al-Hamdan NA. Hajj-associated acute respiratory infection among hajjis from Riyadh. East Mediterr Health J. 2006;12(3–4):300–9. [PubMed] [Google Scholar]
- 34.Isaacs D, Britton P, Howard-Jones A, Kesson A, Khatami A, Marais B, et al. Do facemasks protect against COVID-19? J Paediatr Child Health. 2020;56(6):976–7. 10.1111/jpc.14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautret P, Benkouiten S, Al-Tawfiq JA, Memish ZA. Hajj-associated viral respiratory infections: A systematic review. Travel Med Infect Dis. 2016;14(2):92–109. 10.1016/j.tmaid.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Tawfiq JA, Benkouiten S, Memish ZA. A systematic review of emerging respiratory viruses at the Hajj and possible coinfection with Streptococcus pneumoniae. Travel Med Infect Dis. 2018;23:6–13. 10.1016/j.tmaid.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rashid H, Azeem MI, Heron L, Haworth E, Booy R, Memish ZA. Has Hajj-associated Middle East Respiratory Syndrome Coronavirus transmission occurred? The case for effective post-Hajj surveillance for infection. Clin Microbiol Infect. 2014;20(4):273–6. 10.1111/1469-0691.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Yang J, Yang W, Wang C, Bärnighausen T. COVID-19 control in China during mass population movements at New Year. Lancet. 2020;395(10226):764–6. 10.1016/S0140-6736(20)30421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020; 395(10242):1973–1987. 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang M, Gao L, Cheng C, Zhou Q, Uy JP, Heiner K, et al. Efficacy of face mask in preventing respiratory virus transmission: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101751. 10.1016/j.tmaid.2020.101751 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020; 108: 103629 [Epub ahead of print]. 10.1016/j.ijnurstu.2020.103629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders-Hastings P, Crispo JAG, Sikora L, Krewski D. Effectiveness of personal protective measures in reducing pandemic influenza transmission: A systematic review and meta-analysis. Epidemics. 2017;20:1–20. 10.1016/j.epidem.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 44.Smith SM, Sonego S, Wallen GR, Waterer G, Cheng AC, Thompson P. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: A systematic review. Respirology. 2015;20(6):896–903. 10.1111/resp.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207 10.1002/14651858.CD006207.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoang VT, Ali-Salem S, Belhouchat K, Meftah M, Sow D, Dao TL, et al. Respiratory tract infections among French Hajj pilgrims from 2014 to 2017. Sci Rep. 2019;9(1):17771 10.1038/s41598-019-54370-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maslamani YAMC, A. J. Health related experiences among international pilgrims departing through King Abdul Aziz international airport, Jeddah, Saudi Arabia, Hajj 1431 H (201 0). Saudi Epidemiology Bulletin. 2011;18:42–4. [Google Scholar]
- 48.Algarni H, Memish ZA, Assiri AM. Health conditions for travellers to Saudi Arabia for the pilgrimage to Mecca (Hajj) - 2015. J Epidemiol Glob Health. 2016;6(1):7–9. 10.1016/j.jegh.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9(5):291–300. 10.1016/S1473-3099(09)70069-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautret P, Vu Hai V, Sani S, Doutchi M, Parola P, Brouqui P. Protective measures against acute respiratory symptoms in French pilgrims participating in the Hajj of 2009. J Travel Med. 2011;18(1):53–5. 10.1111/j.1708-8305.2010.00480.x [DOI] [PubMed] [Google Scholar]
- 51.Deris ZZ, Hasan H, Sulaiman SA, Wahab MS, Naing NN, Othman NH. The prevalence of acute respiratory symptoms and role of protective measures among Malaysian hajj pilgrims. J Travel Med. 2010;17(2):82–8. 10.1111/j.1708-8305.2009.00384.x [DOI] [PubMed] [Google Scholar]
- 52.Barasheed O, Alfelali M, Mushta S, Bokhary H, Alshehri J, Attar AA, et al. Uptake and effectiveness of facemask against respiratory infections at mass gatherings: a systematic review. Int J Infect Dis. 2016;47:105–11. 10.1016/j.ijid.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alqahtani AS, Althimiri NA, BinDhim NF. Saudi Hajj pilgrims’ preparation and uptake of health preventive measures during Hajj 2017. J Infect Public Health. 2019;12(6):772–6. 10.1016/j.jiph.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 54.Elachola H, Assiri AM, Memish ZA. Mass gathering-related mask use during 2009 pandemic influenza A (H1N1) and Middle East respiratory syndrome coronavirus. Int J Infect Dis. 2014;20:77–8. 10.1016/j.ijid.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alasmari AK, Edwards PJ, Assiri AM, Behrens RH, Bustinduy AL. Use of facemasks and other personal preventive measures by Hajj pilgrims and their impact on health problems during the Hajj. J Travel Med. 2020; 10.1093/jtm/taaa155 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.