Abstract
Purpose:
To review the clinical features of orbital and choroidal metastases from urothelial carcinomas of the urinary tract among cases reported in the literature, and to describe a case of orbital metastasis from bladder cancer presenting as apparent internuclear ophthalmoplegia.
Methods:
Case reports of orbital and choroidal metastases from urothelial carcinomas published in the literature from 1965 to 2018 were reviewed. Data collected included patient demographics, cancer stage and primary site, time to onset of ocular symptoms, length of presenting ocular symptoms, types of primary ocular symptoms, diagnostic imaging, histology, systemic and ocular treatments, and survival time.
Results:
Twenty-eight cases of urothelial carcinoma with metastasis to the orbit or choroid were reviewed. Men were significantly more likely to suffer from this condition than women (p = 0.011). The average age of presentation with orbital symptoms was 63 years, with an average time of 19 months between primary cancer diagnosis and onset of orbital symptoms. Twenty-two patients had metastasis to the orbit and 6 to the choroid. In 4 cases, ocular deficits secondary to orbital and/or choroidal metastases were the initial presenting symptoms in patients with previously undiagnosed urothelial carcinoma. The most commonly noted primary ocular symptoms and signs consisted of decreased visual acuity, decreased ocular motility, proptosis, and diplopia. Average survival from onset of ocular symptoms was 4.67 months.
Conclusions:
Urothelial carcinoma may metastasize to the orbit or choroid; furthermore, its presentation may mimic internuclear ophthalmoplegia. It is recommended that any patient with visual symptoms and known urothelial cancer should undergo expedited workup for metastatic disease.
Orbital metastases may occur in 2% to 3% of patients with cancer.1 Although this condition is rare, in 19% to 25% of cases patients may have no history of systemic cancer when presenting with ophthalmic symptoms.1,2 The most common primary tumors to metastasize to the orbit are breast, prostate, and lung.1 Prognosis is poor in this population given metastatic disease to other organs.
To date, there have been ~30 reported cases of urothelial carcinomas of the urinary tract with orbital or choroidal metastases.3–27 Here, the authors present the first case of urothelial carcinoma of the urinary bladder to present with acute symptoms consistent with internuclear ophthalmoplegia (INO) and presumed stroke. Previously, there was 1 reported case of colorectal carcinoma metastatic to the orbit presenting as apparent INO.28 The authors further provide a systematic review of published cases and analysis of the literature on urothelial carcinomas with orbital or choroidal metastasis.
CASE REPORT
Collection and evaluation of protected patient health information was compliant with the Health Insurance Portability and Accountability Act. A 65-year-old Caucasian male with a medical history of hepatitis B, 10 pack-year smoking history, and stage intravenous urothelial carcinoma of the bladder status-post radical cystectomy with pelvic and pulmonary recurrence, initially presented with fever and feculent drainage from the urethra secondary to urorectal fistula. The patient was hospitalized, started on intravenous antibiotics, and underwent transverse loop colostomy for persistent urethral drainage.
On postoperative day 2, a stroke code was called for acute onset left ophthalmoplegia, vision loss, and vomiting. The patient reported acute worsening of diplopia and “dimming vision in the left eye, starting in the center” that had started 2 to 3 weeks prior. Initial physical examination was significant for impaired adduction of the left eye on rightward gaze, horizontal nystagmus of the right eye, and preserved convergence. Examination also showed mild left exophthalmos, diplopia on rightward gaze, and tenderness to palpation in the left peri-orbital region. CT of the head showed no acute hemorrhage. On ophthalmology consultation, the patient denied eye pain, redness, tearing, flashes, floaters, and curtains. The patient endorsed chin numbness but denied numbness or paresthesias in the V2/V3 distribution. Visual acuity was OD 20/25, OS 20/200. Left afferent pupillary defect and mild proptosis were present. Ophthalmologic examination was significant for extra-ocular movements of OS −4 in adduction, −3.5 to −4 in supraduction, −2.5 in infraduction, and −1 in abduction. Extraocular movements were full OD. Left inferocentral and central scotomas were present with grossly intact periphery, and visual fields were full on the right. Sensation was decreased in CN V1 distribution, but all other cranial nerves were intact. Anterior segment examination was within normal limits. On dilated fundus examination, cup-to-disc ratio OS was 0.1 with 360° of optic nerve head edema and flame heme nasally. The cup-to-disc ratio OD was 0.2 and disc margins were sharp. The macula and blood vessels were within normal limits bilaterally. On examination of the retinal periphery, 1 flame hemorrhage was present in the periphery OS.
MRI brain and orbits with and without contrast demonstrated a 1.6 × 2.6 × 1.8 cm T2 hypointense and T1 hyper-intense infraorbital mass OS encasing the inferior rectus and abutting the medial rectus, elevating the optic nerve and extending posteriorly to the orbital apex. The patient subsequently underwent decompression of the orbital floor and excision of a large portion of the mass from the medial orbit. Pathologic finding was consistent with metastatic urothelial carcinoma by morphology and + p40 and GATA3 staining. The patient ultimately decided to pursue hospice care and died 3 weeks after the initial presentation of ocular symptoms.
METHODS
The authors reviewed case reports of orbital and choroidal metastases from urothelial carcinomas published in the literature from 1965 to 2018. Data collected included patient demographics, cancer stage and primary site, time to onset of ocular symptoms, length of presenting ocular symptoms, types of primary ocular symptoms, diagnostic imaging, histology, systemic and ocular treatments, and survival. This report adhered to the ethical principles outlined in the Declaration of Helsinki as amended in 2013. This research was compliant with the Health Insurance Portability and Accountability Act. Written consent for publication was obtained from the patient’s next of kin and is on file with the authors. Statistical analysis was performed using R to complete a binomial test (2 tailed).
RESULTS
In addition to the case described above, 28 cases of urothelial carcinoma with metastasis to the orbit or choroid were reviewed from the published literature (Table). There were 23 men and 5 women (ratio 4.6/1; p = 0.011). The average age at first presentation of orbital symptoms was 63 years (range 43–88, median 62). The average time between diagnosis of primary urothelial carcinoma and the onset of orbital symptoms was 19 months (range 0–132, median 8).
Reported cases of orbital and choroidal metastases from urothelial carcinoma
| Authors | Year | Age | Sex | Origin | TCC first | Site | Time from TCC to met | Laterality | Ocular symptoms | Abnormal findings | Ocular therapy | Systemic therapy | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sklar et al. | 2018 | 64 | M | B | Y | Orbit | 6 months | OS | D, M, Pain, Prop | MRI | Decompression, surgical debulking | Comfort | 3 weeks |
| Gene et al. | 2017 | 67 | M | B | N | Orbit | 0 day | OD | M, V | MRI | NS | Chemotherapy + RT | Unknown |
| Magrath et al. | 2015 | 57 | F | B | Y | Orbit | - | OD | M, Prop, V | CT | NS | NS | 3 weeks |
| Mitsui et al. | 2014 | 48 | M | B | Y | Choroid | 17 months | OD | V | OCT, US, MRI | NS | Chemotherapy | 5 months |
| SooHoo et al. | 2012 | 53 | F | B | Y | Orbit | - | OD | Pt | CT | NS | NS | 3 months |
| Wiltshire et al. | 2009 | 88 | M | B | Y | Choroid | - | OU | V | DFE, ultrasonography | RT bl | RT | 4+ years |
| Wettach and Steele | 2008 | 66 | M | B | N | Orbit | - | OD | D, M, Pain, Prop, Pup, V | CT | NS | NS | 1 month |
| Zwicker et al. | 2008 | 50 | M | B | Y | Orbit | - | OS | Ch, D, M, Prop | MRI, AS, DFE | RT | NS | 6 months |
| Lin et al. | 2007 | 60 | M | B | Y | Orbit | 8 months | OD | Ch, D, M, Prop, V | CT, DFE | NS | Chemotherapy | 6+ months |
| Shikishima et al. | 2006 | 74 | M | B | Y | Orbit | 3 years | OD | Ch, D, IOP, M, Pain, Palp, Prop, V | CT, DFE | NS | Chemotherapy + RT | 7 months |
| Souza Filho et al. | 2005 | 53 | M | B | Y | Orbit | 3 weeks | OS | Ch, D, M, Prop, V | MRI | NS | NS | 1 month |
| Chua et al. | 2004 | 78 | F | B | Y | Orbit | 4 years | OD | Ch, D, M, Palp, Prop, Pt, V, Pain | CT | NS | NS | Unknown |
| Amemiya et al. | 2003 | 55 | M | B | Y | Orbit | 5 months | OU | D, M, Pain, Prop | MRI | NS | NS | 1 month |
| Amemiya et al. | 2003 | 73 | M | B | Y | Orbit | 6 months | OD | M, V | MRI | NS | NS | 1 month |
| Fynn-Thompson et al. | 2003 | 68 | M | B | Y | Orbit | 11 years | OS | M, Prop, Pup, V | CT, DFE | NS | NS | 1 month |
| Nabi et al. | 2002 | 43 | F | B | Y | Orbit | 6 months | OS | Ch, Prop, V | CT, DFE | NS | Chemotherapy | 1 month |
| Nabi et al. | 2002 | 79 | M | B | Y | Intraocular | 8 months | OD | D, Ph, V | CT, DFE | NS | Chemotherapy | 3 weeks |
| Scott and Williams | 1995 | 75 | M | B | Y | Orbit | 3 years | OD | Ch, D, IOP, M, Pain, Prop, Pup, V | CT | NS | Steroids + abx | 13 days |
| Hugkulstone et al. | 1994 | 45 | M | B | N | Orbit | 0 days | OU | Ch, D, M, Palp | CT, DFE | NS | Chemotherapy + RT | 5 months |
| Angulo et al. | 1991 | 61 | M | B | Y | Orbit | 11 months | OD | D, Prop | CT | NS | NS | 4 weeks |
| Felip et al. | 1991 | 58 | M | B | Y | Orbit | 3 weeks | NS | M, Pt, T, V | CT | NS | NS | 3 months |
| Felip et al. | 1991 | 62 | M | B | Y | Orbit | 3 years | OS | D, M, Pain, Prop, V | CT, DFE | NS | Declined | Unknown |
| Prats et al. | 1989 | 58 | M | B | Y | Orbit | 3 weeks | OS | M, Prop, Pt, T, V | CT | Decompression | NS | 4 months |
| Pe’er and Zimmerman | 1984 | 69 | M | U | Y | Choroid, ON | 2.5 years | OS | IOP, Pain, V | AS, DFE | RT, enucleation | NS | Unknown |
| Atta | 1983 | 50 | F | RP | Y | Choroid | 2 years | OS | Ph | DFE, US, FA | NS | Chemotherapy + RT | 5 months |
| Krauss et al. | 1982 | 64 | F | B | Y | Orbit | 15 months | OD | C, D, M, Prop, Pt, Pup, T, V | CT | RT | NS | 1 month |
| Cieplinski et al. | 1982 | 57 | M | B | Y | Choroid | 6 months | OU | V | DFE | RT bl | Chemotherapy | Unknown |
| Gordon and Munro | 1974 | 65 | M | B | Y | Choroid | 2 years | OU | V | DFE | RT bl | NS | 1 month |
| Smiley | 1965 | 75 | M | B | N | Orbit | 0 day | OS | D, M, Palp, Prop, Pup, V | DFE, skull films | NS | NS | 8+ months |
AS, anterior segment; B, bladder; bl, bilateral; C, reduced color vision; Ch, Chemosis; D, diplopia; DFE, dilated fundus examination; FA, fluorescein angiography; IOP, change in intraocular pressure; M, motility defect; NS, not stated; OCT, optical coherence tomography; ON, optic nerve; Palp, palpable mass; Ph, photopsia; Prop, proptosis; Pt, ptosis; Pup, pupillary defect; RT, radiotherapy; RP, renal pelvis; T, trigeminal hypoesthesia; TCC, transitional cell carcinoma; U, ureter; US, ultrasound; V, decreased visual acuity.
There were 25 cases of urothelial carcinoma originating in the urinary bladder, 1 case in the renal pelvis, and 1 in the ureter. Twenty-two patients had metastases to the orbit, 6 to the choroid(s), and 1 nonspecific intraocular metastasis. There were 23 unilateral lesions (13 right, 10 left) and 5 bilateral (3 orbital and 2 choroidal). Metastases to other locations within the body were noted in 13 (49%) cases. Lung metastases were present in 7 (24%) of cases.
In 4 cases, ocular deficits secondary to orbital and/or choroidal metastases were the initial presenting symptoms in patients with previously undiagnosed urothelial carcinoma (14%). The average time the patient experienced ocular symptoms before diagnosis of metastasis was 28 days (range 0–112, median 18). Three patients received alternate presumed diagnoses prior to definitive diagnosis of metastatic disease, including stroke (presented case), infection, and central retinal vein occlusion.
The most commonly noted primary ocular symptoms and signs consisted of decreased visual acuity (22, 76%), decreased ocular motility (19, 66%), proptosis (17, 59%), diplopia (15, 52%), chemosis (8, 26%), pain (7, 24%), relative afferent pupillary defect (5, 17%), palpable mass (5, 17%), ptosis (4, 14%), change in intraocular pressure (2, 7%), photopsia (2, 7%), and reduced color vision (1, 3%). Twenty-two patients experienced visual loss, the visual acuities of whom were as follows: no light perception (4, 18%), hand motion (1, 4.5%), count fingers (2, 9%), and 20/200 (1, 4.5%). Of the 19 patients with decreased ocular motility, 10 had limitation of movement in all directions, 2 had CN VI palsy, 1 had CN intravenous nerve palsy, and 1 had INO (case described).
Abnormal findings were apparent on CT for 16 cases (55%), dilated fundus examination for 14 cases (48%), MRI for 5 cases (17%), ultrasound for 2 cases (7%), anterior segment examination for 2 cases (7%), optical coherence tomography for 1 case (3%), fluorescein angiography for 1 case (3%), and skull films for 1 case (3%). Ocular-directed treatment included decompression surgery for 2 cases and orbital radiotherapy for 6 cases, one of which was followed by enucleation. Systemic therapy typically included a combination of chemotherapy agents and local radiotherapy. Average survival from the time of ocular symptoms was 4.67 months (range 13–1460 days). Kaplan–Meier survival curve is found in the Figure.
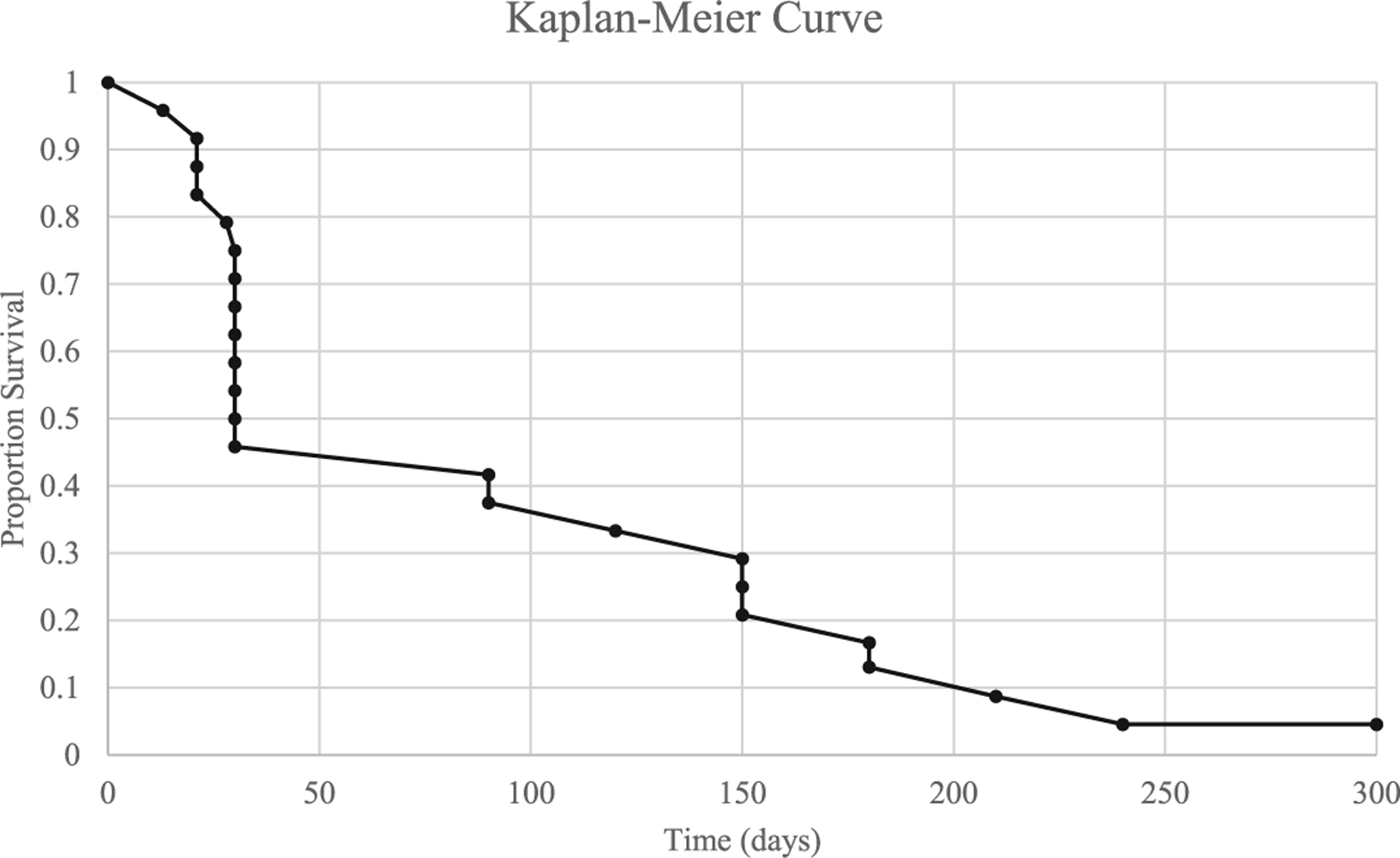
Kaplan-Meier curve showing proportion survival versus time (days). Five patients were excluded because no length of survival was reported. Of the excluded patients, 1 was described as alive at the time of publication while the status of the other 4 is unknown.
Two cases noted that the orbital lesion described was the singular site of metastasis. Three patients had a known smoking history, and 1 had a history of lead exposure.
DISCUSSION
The American Cancer Society estimates that in 2018 there will be 81,190 new cases of urothelial cancer of the urinary bladder and 17,240 related deaths in the United States.29 The most common sites of metastasis from urothelial carcinoma of the bladder include the lymph nodes, bones, lungs, liver, and peritoneum.30 In general, the presence of orbital metastasis indicates hematogenous spread of a primary cancer and is accompanied by poor prognosis.2
This is the first reported case of urothelial carcinoma of the bladder metastatic to the orbit presenting as apparent INO with subsequent stroke workup. Symptoms consistent with INO included paralysis of adduction in the left eye for conjugate eye movements, horizontal nystagmus in the right eye, and preservation of convergence. True INO is caused by a brainstem lesion involving the medial longitudinal fasciculus in the pons.31 In a case series investigating the etiology of INO in 410 inpatients over a 33-year period, 38% were due to infarction, 34% were due to multiple sclerosis, and 28% were due to “unusual causes” including tumors, trauma, and infection affecting the medial longitudinal fasciculus.32 One prior report documented apparent INO from orbital metastasis originating from a rectal adenocarcinoma, presenting with left adduction deficit.28
In this case, the patient’s symptoms included acute unilateral vision loss, ocular motility defects, and vomiting. The differential for these symptoms included a stroke in the setting of malignancy, pupil sparing ischemic third nerve palsy, lymphoma, and metastatic disease. Other possible etiologies of orbital masses include vascular lesions (cavernous hemangiomas, lymphangiomas), benign tumors (schwannomas, dermoid cysts), and malignancies (lymphoma, metastasis, rhabdomyosarcoma).33,34 One study of 2480 patients evaluated for an orbital mass found that orbital metastases accounted for 3.5% of all lesions and 11% of malignant lesions.34
Analysis from the literature review shows that orbital and choroidal metastases from urothelial carcinoma affect men significantly more frequently than women (ratio 4.6/1; p = 0.011). This is consistent with the incidence of primary urothelial carcinoma, which favors males. In Norway, the reported ratio of males to females with urothelial carcinoma was 3.2 in 2016, and the American Cancer Society estimates that the incidence of new cases will affect 3.3 males per female in 2018.29,35
Prognosis for patients with orbital metastases is poor. The average survival for patients with metastatic orbital tumors has been reported to be 15 months.1,36 Previous reviews of patients with orbital metastasis from urothelial carcinoma of the bladder found the average survival to be 3 months.11 The analysis of patients with orbital and choroidal metastases from urothelial carcinoma showed an average survival of 4.67 months.
In this review, decreased visual acuity, impaired ocular motility, proptosis, and diplopia were the most common symptoms of orbital metastasis. While orbital masses of various etiologies may produce these symptoms, it is important to consider metastatic disease in patients with known primary urothelial carcinoma. Furthermore, ocular symptoms preceded the diagnosis of primary bladder cancer in 4 cases; therefore, metastasis should be considered in the differential diagnosis for an orbital mass.
CONCLUSIONS
The findings in this literature review suggest that urologists, neurologists, hospitalists, and ophthalmologists should be aware that not only can urothelial carcinoma metastasize to the orbit or choroid but its presentation may mimic INO. Any patient with visual symptoms and known urothelial cancer should receive prompt workup for metastatic disease.
Footnotes
The authors have no financial or conflicts of interest to disclose.
REFERENCES
- 1.Shields JA, Shields CL, Brotman HK, et al. Cancer metastatic to the orbit: the 2000 Robert M. Curts Lecture. Ophthalmic Plast Reconstr Surg 2001;17:346–54. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad SM, Esmaeli B. Metastatic tumors of the orbit and ocular adnexa. Curr Opin Ophthalmol 2007;18:405–13. [DOI] [PubMed] [Google Scholar]
- 3.Genc AC, Hacioglu MB, Kostek O, Hacibekiroglu I. Metastasis of transitional cell carcinoma of the bladder to the orbit: a case report. Eurasian J Med Oncol 2017;1:47–8. [Google Scholar]
- 4.Magrath GN, Proctor CM, Reardon WA, et al. Esophageal adeno-carcinoma and urothelial carcinoma orbital metastases masquerading as infection. Orbit 2015;34:51–5. [DOI] [PubMed] [Google Scholar]
- 5.Mitsui Y, Arichi N, Inoue K, et al. Choroidal and cutaneous metastasis from urothelial carcinoma of the bladder after radical cystectomy: a case report and literature review. Case Rep Urol 2014;2014:491541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SooHoo JR, Gonzalez MO, Siomos VJ, et al. Urothelial carcinoma with orbital metastasis. Urology 2012;80:e45–6. [DOI] [PubMed] [Google Scholar]
- 7.Wiltshire KL, Laperriere N, Bristow RG. Prolonged survival in a patient with choroidal metastases from urothelial bladder cancer. Can Urol Assoc J 2009;3:E36–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wettach GR, Steele EA. Urothelial cell carcinoma of the bladder presenting as orbital metastasis. Arch Pathol Lab Med 2008;132:1224. [DOI] [PubMed] [Google Scholar]
- 9.Zwicker F, Herfarth K, Debus J. Palliative radiotherapy for a retrobulbar metastasis from a urinary bladder carcinoma. Ophthalmologe 2008;105:170–2. [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, Chang CH, Li WM, et al. Orbital metastasis from urothelial carcinoma of the urinary bladder. Kaohsiung J Med Sci 2007;23:84–8. [DOI] [PubMed] [Google Scholar]
- 11.Shikishima K, Miyake A, Ikemoto I, et al. Metastasis to the orbit from transitional cell carcinoma of the bladder. Jpn J Ophthalmol 2006;50:469–73. [DOI] [PubMed] [Google Scholar]
- 12.Souza Filho JP, Odashiro AN, Pereira PR, et al. Orbital metastasis of urinary bladder carcinoma: a clinicopathologic report and review of the literature. Orbit 2005;24:269–71. [DOI] [PubMed] [Google Scholar]
- 13.Chua WC, Martin PA, Kourt G. Orbital metastasis from transitional cell carcinoma of the bladder. Clin Exp Ophthalmol 2004;32:447–9. [DOI] [PubMed] [Google Scholar]
- 14.Amemiya T, Hayashida H, Dake Y. Metastatic orbital tumors in Japan: a review of the literature. Ophthalmic Epidemiol 2002; 9:35–47. [DOI] [PubMed] [Google Scholar]
- 15.Fynn-Thompson N, McKiernan JM, Fay A. Transitional cell carcinoma of the urinary bladder metastatic to the orbit. Ophthalmic Plast Reconstr Surg 2003;19:165–7. [DOI] [PubMed] [Google Scholar]
- 16.Nabi G, Dadeya S, Dogra PN, et al. Eye metastasis form urothelial tumours. Int Urol Nephrol 2002;34:51–4. [DOI] [PubMed] [Google Scholar]
- 17.Scott JA, Williams R. Transitional cell carcinoma of the bladder metastatic to the orbit. Eye (Lond) 1995;9(pt 5):664–6. [DOI] [PubMed] [Google Scholar]
- 18.Hugkulstone CE, Winder S, Sokal M. Bilateral orbital metastases from transitional cell carcinoma of the bladder. Eye (Lond) 1994;8(pt 5):580–2. [DOI] [PubMed] [Google Scholar]
- 19.Angulo JC, Lopez JI, Larrinaga JR, et al. Metastasising carcinoma of the urinary bladder presenting as a retro-orbital mass. Case report. Scand J Urol Nephrol 1991;25:83–4. [DOI] [PubMed] [Google Scholar]
- 20.Felip E, Rovirosa MA, Salud A, et al. Orbital metastases from transitional-cell cancer of the urinary bladder. Urol Int 1991;46:82–4. [DOI] [PubMed] [Google Scholar]
- 21.Prats J, Bellmunt J, Calvo MA, et al. Orbital metastasis, by transitional cell carcinoma of the bladder. Int Urol Nephrol 1989;21:389–92. [DOI] [PubMed] [Google Scholar]
- 22.Atta HR. Presumed metastatic transitional cell carcinoma of the choroids. Br J Ophthalmol 1983;67:830–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krauss HR, Slamovits TL, Sibony PA, et al. Orbital metastasis of bladder carcinoma. Am J Ophthalmol 1982;94:265–7. [DOI] [PubMed] [Google Scholar]
- 24.Cieplinski W, Ciesielski TE, Haine C, et al. Choroid metastases from transitional cell carcinoma of the bladder: a case report and a review of the literature. Cancer 1982;50:1596–600. [DOI] [PubMed] [Google Scholar]
- 25.Gordon HL, Munro R. Ocular metastasis of bladder cancer. South Med J 1974;67:745–6. [DOI] [PubMed] [Google Scholar]
- 26.Pe’er J, Zimmerman LE. Ocular metastases from transitional cell carcinoma of the urinary tract. Graefes Arch Clin Exp Ophthalmol 1984;221:137–41. [DOI] [PubMed] [Google Scholar]
- 27.Smiley SS. An orbital metastasis from the urinary bladder. Arch Ophthalmol 1965;74:809–10. [DOI] [PubMed] [Google Scholar]
- 28.Lin WV, Chevez-Barrios P, Sadaka A, Lee AG. Orbital metastasis mimicking internuclear ophthalmoplegia: A case report and review. Can J Ophthalmol 2017;52:e149–51 [DOI] [PubMed] [Google Scholar]
- 29.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 30.Shinagare AB, Ramaiya NH, Jagannathan JP, et al. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol 2011;196:117–22. [DOI] [PubMed] [Google Scholar]
- 31.Nuclear Pierrot-Deseilligny C., internuclear, and supranuclear ocular motor disorders. Handb Clin Neurol 2011;102:319–31. [DOI] [PubMed] [Google Scholar]
- 32.Keane JR. Internuclear ophthalmoplegia: unusual causes in 114 of 410 patients. Arch Neurol 2005;62:714–7. [DOI] [PubMed] [Google Scholar]
- 33.Khan SN, Sepahdari AR. Orbital masses: CT and MRI of common vascular lesions, benign tumors, and malignancies. Saudi J Ophthalmol 2012;26:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonavolonta G, Strianese D, Grassi P, et al. An analysis of 2,480 space-occupying lesions of the orbit from 1976 to 2011. Ophthalmic Plast Reconstr Surg 2013;29:79–86. [DOI] [PubMed] [Google Scholar]
- 35.Andreassen BK, Aagnes B, Gislefoss R, et al. Incidence and survival of urothelial carcinoma of the urinary bladder in Norway 1981–2014. BMC Cancer 2016;16:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland D, Maune S, Kovács G, et al. Metastatic tumors of the orbit: a retrospective study. Orbit 2003;22:15–24. [DOI] [PubMed] [Google Scholar]


