Abstract
While early studies reported superior survival for cancer patients enrolled on clinical trials, recent findings are inconclusive. We investigated the association between enrollment on contemporary trials and event-free survival (EFS) in pediatric B-cell acute lymphoblastic leukemia (B-ALL). In a retrospective cohort of 274 children (1-21 years) treated for B-ALL from 2008 to 2015, 55.5% enrolled with no disparity in enrollment by age, sex, or ethnicity. Three-year EFS was similar for enrolled and not enrolled patients (90.1% [95% CI, 82.5-94.5] versus 86.5% [95% CI, 77.7-92.0]). Clinical trial enrollment did not affect pediatric B-ALL survival, albeit in a limited-size cohort treated at a single academic institution.
Keywords: clinical trial enrollment, clinical trial participation, disparities, pediatric B-cell acute lymphoblastic leukemia, survival
1 |. INTRODUCTION
A series of population-based studies from 1970s to 1990s reported that children with leukemia enrolled on a clinical trial experienced greater survival than children not enrolled on a trial.1–3 Advances in treatment for childhood leukemia have substantially improved survival. Control arms on recently completed pediatric B-cell acute lymphoblastic leukemia (B-ALL) trials from the Children’s Oncology Group (COG) and other consortia report 5-year event-free survival (EFS) of 75–94% compared to 19–60% in earlier decades.4–7 Given these improvements, the continued association of trial enrollment with survival, especially in the era of minimal residual disease (MRD) based risk-stratified therapy, is unclear. Even with these treatment advances, survival for certain demographic subgroups lag behind, including adolescents and young adults (AYAs, aged ≥15–39 years8) and Hispanic patients.9,10 These subgroups are underrepresented on trials,11 a proposed contributing factor to their poorer survival.9 However, no reports have investigated whether enrollment influences survival for these subgroups. We hypothesized that in the contemporary treatment era of MRD risk-stratified B-ALL therapy, trial enrollment is not associated with EFS or overall survival (OS) and that poorer survival among AYAs and Hispanic patients is not due to lower enrollment.
2 |. RESULTS
We analyzed survival of a retrospective cohort of patients 1–21 years old with Philadelphia chromosome negative B-ALL, diagnosed between 2008 and 2015 at a single academic center, with available diagnostic flow cytometry. All flow cytometry data were independently reviewed (M.O.) to ensure a uniform cohort of confirmed B-ALL. During the assessment period, trials included AALL0331, AALL0932, AALL0232, and AALL1131. Patients not enrolled on study were treated on institutional protocols (not at the discretion of physicians) with modified regimens based on CCG1991, CCG1961, or AALL0232 (all studies available at https://clinicaltrials.gov, Supplementary Table S1 for treatment descriptions). Demographic, disease, and treatment data were extracted (Table 1). Endpoints included trial enrollment (yes/no), EFS (relapse, second malignancy, or death), and OS. Patients receiving hematopoietic cell transplant were censored at the time of transplant. Following standard analyses of distribution between groups, a multivariable logistic regression model was constructed using candidate predictors (Table 1) with trial enrollment as the endpoint. For survival analyses, Kaplan–Meier curves with associated log-rank tests were constructed. Following confirmation of the proportional hazards assumption via Schoenfeld residuals, multivariable Cox proportional hazard models were constructed incorporating enrollment status, MRD, and other known predictors of EFS/OS. The study was approved by the Institutional Review Board. All statistical tests were two-sided, with significance set at P ≤ 0.05 and performed with STATA software 14.2 (Stata statistical software, release 14; College Station, TX).
TABLE 1.
Description of cohort by trial enrollment status
| Overall |
Not enrolled |
Enrolled |
|||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | Pa | |
| Total cohort | 274 | 100 | 122 | 45.5 | 152 | 55.5 | - |
| Age (mean, median [range]) | 7.4, 5.2 [1.2–20.0] | 7.9, 5.6 [1.6–20.0] | 6.9, 4.8 [1.2–19.0] | 0.11 | |||
| Age <15 | 238 | 87.0 | 103 | 84.4 | 135 | 88.8 | 0.26 |
| Age ≥15 | 36 | 13.1 | 19 | 15.6 | 17 | 11.2 | |
| Sex | |||||||
| Female | 135 | 49.3 | 61 | 50.0 | 74 | 48.7 | 0.83 |
| Male | 139 | 50.7 | 61 | 50.0 | 78 | 51.3 | |
| Ethnicity | |||||||
| Not-Hispanic | 78 | 28.5 | 28 | 23.0 | 50 | 32.9 | 0.07 |
| Hispanic | 196 | 71.5 | 94 | 77.0 | 102 | 67.1 | |
| Down syndrome | |||||||
| Not Down syndrome | 267 | 97.4 | 119 | 97.5 | 148 | 97.4 | 1.00 |
| Down syndrome | 7 | 2.6 | 3 | 2.5 | 4 | 2.6 | |
| NCI/Rome risk | |||||||
| SR | 167 | 60.9 | 74 | 60.7 | 93 | 61.2 | 0.93 |
| HR | 107 | 39.1 | 48 | 39.3 | 59 | 38.8 | |
| Presenting WBC (max) | |||||||
| WBC (mean, median [range]) | 39.1, 9.0 [0.3–771.8] | 36.3, 8.3 [0.3–771.8] | 41.2, 11.5 [0.8–562] | 0.43 | |||
| WBC <50 K/μl | 222 | 81.0 | 100 | 82.0 | 122 | 80.3 | 0.72 |
| WBC ≥50 K/μl | 52 | 19.0 | 22 | 18.0 | 30 | 19.7 | |
| CNS status | |||||||
| CNS1 | 244 | 89.1 | 108 | 88.5 | 136 | 89.5 | 0.38 |
| CNS2 | 28 | 10.2 | 14 | 11.5 | 14 | 9.2 | |
| CNS3 | 2 | 0.7 | 0 | 0 | 2 | 1.3 | |
| Cytogenetic Categoryb | |||||||
| Average | 157 | 57.3 | 79 | 64.8 | 78 | 51.3 | 0.02 |
| Favorable | 113 | 41.2 | 43 | 35.2 | 70 | 46.1 | |
| Unfavorable | 4 | 1.5 | 0 | 0 | 4 | 2.6 | |
| EOI MRD | |||||||
| <0.01% | 165 | 60.2 | 66 | 54.1 | 99 | 65.1 | 0.15 |
| ≥0.01% | 40 | 14.6 | 22 | 18.0 | 18 | 11.8 | |
| Unknown | 69 | 25.2 | 34 | 27.9 | 35 | 22.7 | |
| For MRD+ (median, [range]) | 0.26 [0.012–36] | 0.22 [0.026–36] | 0.30 [0.012–22.4] | 0.91 | |||
NCI, National Cancer Institute; WBC, white blood cell; CNS, central nervous system; EOI MRD, end of induction minimal residual disease (bone marrow).
Pearson chi-square, Fisher exact test, or K-S test; significance set at P < 0.05 (bolded).
Favorable includes “double trisomy” 4+10 (n = 45) and ETV6-RUNX1 (n = 69). Unfavorable includes hypodiploid (<44 chromosomes, n = 1), rMLL (n = 3), iAMP21 (n = 0).
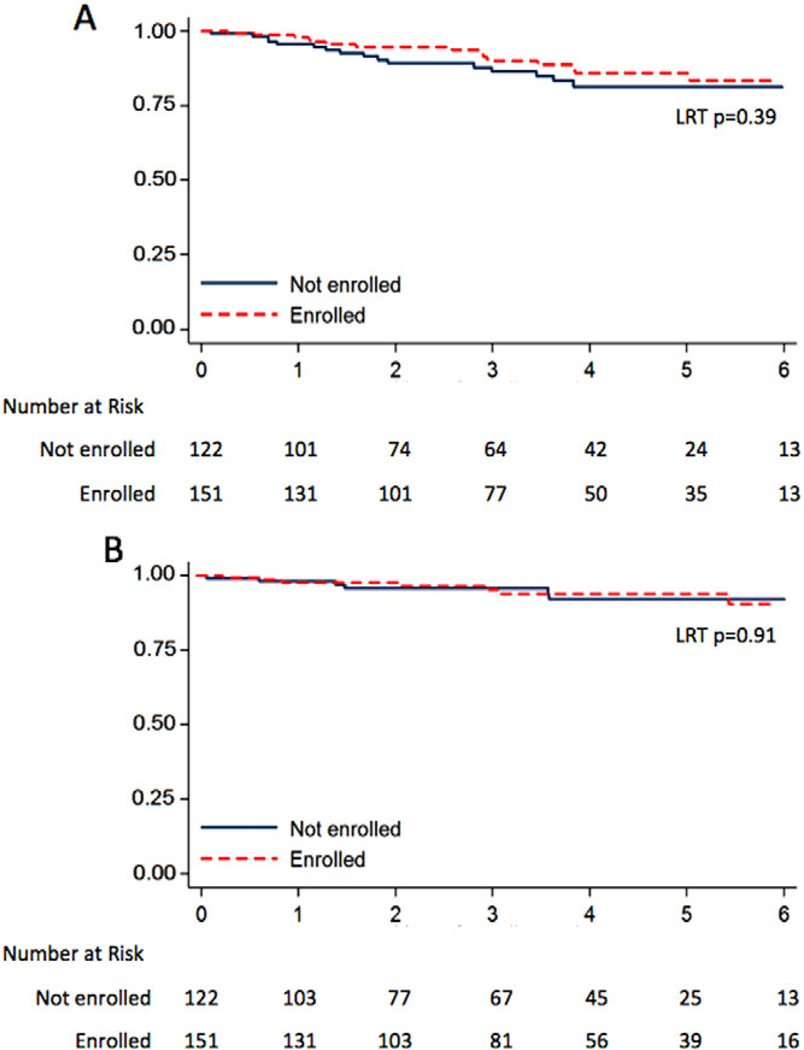
Of the 274 patients in the cohort, 152 (55.5%) enrolled on a trial. We found no significant differences between enrolled and not enrolled patients, except the cytogenetic classification (Table 1). In multivariable analysis, no candidate predictors, including AYA and Hispanic ethnicity, were significantly associated with enrollment (AYA odds ratio [OR] = 0.67, [95% confidence interval [95% CI] 0.29–1.54], P = 0.35; Hispanic OR = 0.61, [95% CI 0.35-1.06], P = 0.08). At a median follow-up of 3.1 years, trial enrollment was not associated with EFS (Fig. 1A, 3-year EFS enrolled 90.1% [95% CI 82.5-94.5] vs. not enrolled 86.5% [95% CI 77.7-92.0%]) or OS (Fig. 1B, 3-year OS enrolled 95.8% [95% CI 89.9-98.3] vs. not enrolled OS 96.2% [95% CI 90.1-98.6%]). In multivariable analysis of EFS, enrollment was not significantly associated with EFS (hazard ratio [HR] = 0.61, [95% CI 0.29-1.30], P = 0.20) (Supplementary Table S1). In models inclusive of AYA, ethnicity, and enrollment, only AYA was associated with poorer EFS (Supplementary Table S2).
FIGURE 1.
Kaplan–Meier survival curves of event-free survival (A) and overall survival (B) for not enrolled (blue solid line) and enrolled (red dashed line) patients. LRT, log-rank test P-value
3 |. DISCUSSION
While prior studies from the 1970s to 1990s showed superior survival for pediatric leukemia patients enrolled on clinical trials,1–3 recent studies have contradicted earlier results.12–14 The current study supports the absence of an association between trial enrollment and survival for pediatric ALL in the modern MRD risk-stratified treatment era and in the context of the most recent generation of trials. In this cohort, 55.5% of patients enrolled on trial, similar to 55.8% of pediatric ALL patients nationwide enrolled onto COG studies.4 In contrast to previous studies,11 we report similar trial enrollment among at-risk AYA and Hispanic subgroups. Our findings are the first to suggest that lower enrollment rates on trials do not contribute to poorer survival for AYA and Hispanic patients.
Several reasons could account for the differences between our and previously reported results. First, high survival rates with contemporary regimens limit the interventional arms’ potential effect sizes, making it less likely to observe a survival benefit. Second, the relationship between enrollment and survival in prior studies may be confounded by disproportionately greater enrollment at large cancer centers, potentially affecting delivery of care. Third, earlier studies may have found a survival benefit because of selection biases, with healthier patients meeting eligibility criteria for trial enrollment.14
The study’s principal strength is the investigation of a cohort of patients with a uniform diagnosis treated at a single institution, an approach that minimizes institutional and disease-specific confounders. The duration of follow-up encompasses a time frame within which most events in pediatric B-ALL have been shown to occur.15 Our findings are consistent with the lack of association between survival and enrollment reported in a previous study13 that largely included patients from an era predating the use of MRD for risk stratification. However, in contrast to previous reports, our study is unique in its incorporation of MRD in predictive models, its inclusion of the most recent clinical trials using MRD-based risk stratification, and its ethnic diversity allowing for a sizable Hispanic representation.
As a retrospective cohort, the study is limited by incomplete MRD results (no differences were detected in unknown MRD between enrolled and not enrolled groups, Table 1), a lower than expected representation of AYA patients and those with adverse cytogenetics, and no available documentation of reasons for patient nonenrollment. In addition, the sample size and follow-up time were not sufficient to detect small differences in rates of events. Nevertheless, the association between enrollment and survival for children with B-ALL with a focus on at-risk AYA and Hispanic subgroups is presented here for the first time. Given that this is a single academic institution study, caution is warranted in generalizing these results, especially in situations where MRD is not available as standard of care for patients not enrolled on a study.
In summary, we found that trial enrollment is not itself associated with improved survival using recent MRD risk-stratified approaches to pediatric B-ALL therapy. However, while enrollment itself might not confer a survival benefit, trial participation is critically important for continued improvement in therapy for childhood ALL. Our findings are thus highly relevant to inform provider discussions of trials with patients and families and to aid in the interpretation of the applicability of trial data to the general population. Additional studies are necessary to better understand barriers to enrollment and to prospectively validate these findings in diverse, multicenter cohorts of sufficient size to detect subtle differences in survival.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Stephanie Garcia, the clinical research coordinator, and the rest of the Clinical Trials Office staff for their assistance in compiling the data. We would also like to thank the many patients and families who volunteer to participate in research trials seeking to improve therapy. C.P. was supported by a St. Baldrick’s Foundation Scholar award and the Nautica Foundation.
Grant sponsor: St. Baldrick’s Foundation; Grant sponsor: Nautica Foundation.
Abbreviations:
- AYA
adolescent and young adult
- B-ALL
B-cell acute lymphoblastic leukemia
- COG
Children’s Oncology Group
- EFS
event-free survival
- MRD
minimal residual disease
- NCI
National Cancer Institute
- OS
overall survival
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Stiller CA, Draper GJ. Treatment centre size, entry to trials, and survival in acute lymphoblastic leukaemia. Arch Dis Child. 1989;64(5):657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiller CA, Eatock EM. Survival from acute non-lymphocytic leukaemia, 1971–88: a population based study. Arch Dis Child. 1994;70(3):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stiller CA, Eatock EM. Patterns of care and survival for children with acute lymphoblastic leukaemia diagnosed between 1980 and 1994. Arch Dis Child. 1999;81(3):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study—Dana-Farber Cancer Institute ALL Consortium Protocol 00–01. J Clin Oncol. 2013;31(9):1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pui C-H, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrappe M, Zimmermann M, Möricke A, et al. Dexamethasone in induction can eliminate one third of all relapses in childhood acute lymphoblastic leukemia (ALL): Results of an international randomized trial in 3655 patients (trial AIEOP-BFM ALL 2000). Blood. 2008;112(11):7–7.18574037 [Google Scholar]
- 8.Health NCIatNIo. Adolescents and young adults with cancer. 2015. http://www.cancer.gov/cancertopics/aya.
- 9.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107(7 Suppl):1645–1655. [DOI] [PubMed] [Google Scholar]
- 10.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2008–2014. [DOI] [PubMed] [Google Scholar]
- 11.Aristizabal P, Singer J, Cooper R, et al. Participation in pediatric oncology research protocols: racial/ethnic, language and age-based disparities. Pediatr Blood Cancer. 2015;62(8):1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jastaniah W, Elimam N, Abdalla K, Felimban S, Abrar MB. Comparison of clinical trial versus non-clinical trial treatment outcomes of childhood acute lymphoblastic leukemia using comparable regimens. Hematology. 2016;21(3):175–181. [DOI] [PubMed] [Google Scholar]
- 13.Koschmann C, Thomson B, Hawkins DS. No evidence of a trial effect in newly diagnosed pediatric acute lymphoblastic leukemia. Arch Pediatr Adolesc Med. 2010;164(3):214–217. [DOI] [PubMed] [Google Scholar]
- 14.Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363(9405):263–270. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22(12):2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.