Abstract
OBJECTIVE
To characterize contributing factors for ovarian conservation during surgical treatment for endometrial cancer and to examine the association of ovarian conservation on survival of young women with early-stage, low-grade tumors.
METHODS
This was a population-based study using the Surveillance, Epidemiology, and End Results program to identify surgically treated stage I type I (grade 1–2 endometrioid histology) endometrial cancer cases diagnosed between 1983 and 2012 (N=86,005). Multivariable models were used to identify independent factors for ovarian conservation. Survival outcomes and cause of death were examined for women aged younger than 50 with stage I type I endometrial cancer who underwent ovarian conservation (1,242 among 12,860 women [9.7%]).
RESULTS
On multivariable analysis, age younger than 50 years, grade 1 endometrioid histology, and tumor size 2.0 cm or less were noted to be independent factors for ovarian conservation (all, P<.001). For 9,110 women aged younger than 50 years with stage I grade 1 tumors, cause-specific survival was similar between ovarian conservation and oophorectomy cases (20-year rates 98.9% compared with 97.7%, P=.31), whereas overall survival was significantly higher in ovarian conservation cases than oophorectomy cases (88.8% compared with 82.0%, P=.011). On multivariable analysis, ovarian conservation remained an independent prognostic factor for improved overall survival (adjusted hazard ratio 0.73, 95% confidence interval [CI] 0.54–0.98, P=.036) and was independently associated with a lower cumulative risk of death resulting from cardiovascular disease compared with oophorectomy (20-year rates, 2.3% compared with 3.7%, adjusted hazard ratio 0.40, 95% CI 0.17–0.91, P=.029). Contrary, cause-specific survival (20-year rates 94.6% compared with 96.1%, P=.68) and overall survival (81.0% compared with 80.6%, P=.91) were similar between ovarian conservation and oophorectomy among 3,750 women aged younger than 50 years with stage I grade 2 tumors.
CONCLUSION
Ovarian conservation is performed in less than 10% of young women with stage I type I endometrial cancer. Ovarian conservation is associated with decreased mortality in young women with stage I grade 1 tumors.
Endometrial cancer remains the most common gynecologic malignancy in the United States in 2016.1 The majority of endometrial cancers are obesity-driven and estrogen-related tumors, so-called type I tumors.2 The mainstay of endometrial cancer treatment is surgery, including total hysterectomy and bilateral salpingo-oophorectomy.2 The rationale of oophorectomy in the surgical management is that endometrial cancer can metastasize to the ovary, women with endometrial cancer are at risk for synchronous and metachronous ovarian cancers, and that the source of estrogen can be eliminated by oophorectomy.3,4
Women with endometrial cancer who are premenopausal at the time of surgery will undergo an abrupt disruption in ovarian hormone levels after oophorectomy.5 Such women may not only experience menopausal symptoms that can compromise quality of life, but also develop a metabolic syndrome, which can lead to diabetes mellitus and hypercholesterolemia through nonalcoholic fatty liver disease resulting from lack of estrogen regulation of lipid metabolism in hepatocytes.5 These phenomena will increase the risk of future cardiovascular disease.6 Indeed, patients with endometrial cancer with early-stage tumors are more likely to die from cardiovascular disease than from endometrial cancer.7
Ovarian conservation at the time of surgery in premenopausal women has been considered to avoid the long-term effects of estrogen deprivation.8 However, it is not known whether ovarian conservation results in lower long-term cardiovascular mortality or other causes of death in patients with endometrial cancer.9 The objective of this study was to examine the patterns of death among young women with low-grade, early-stage endometrial cancer who underwent ovarian conservation.
MATERIALS AND METHODS
The Surveillance, Epidemiology, and End Results program is a population-based database launched in 1973, supported and managed by the National Cancer Institute in the United States.10 This database covers approximately 27.8% of the U.S. population from 11 states and seven areas and is publicly available and deidentified. The University of Southern California institutional review board exempted the use of this deidentified publicly available data for the study. The STrengthening the Reporting of OBservational studies in Epidemiology guidelines were consulted for performance of this observational study.
SEER*Stat 8.2.1 was used to extract the data set from SEER18 Regs Research Data+ Hurricane Katrina Impacted Louisiana Cases (1973–2012). Cases recorded in the section for “Corpus Uteri/Uterus NOS” limited to malignancy and female sex were generated. Within the extracted data set, patients with stage I type I endometrial cancer with known oophorectomy status who received no radiotherapy before the hysterectomy between 1983 and 2012 were included in the study. Data from 1973 to 1982 were removed as a result of lack of adequate information on the surgical procedure. Uterine sarcomas and metastatic tumors to the uterus were excluded. Variables ascertained from the database were patient demographics, tumor information, treatment patterns, and survival outcome.
Patient demographics included age at and year of diagnosis, ethnicity, marital status, and registration area. Tumor information included cancer stage, histologic subtype, tumor grade, tumor size, depth of myometrial invasion, cervical stromal involvement, and pelvic or paraaortic lymph node status. Recorded cancer stage was reclassified into the American Joint Committee on Cancer 7th surgical–pathologic staging classification schema. The International Classification of Diseases for Oncology, 3rd Revision site and histology validation list and World Health Organization histologic classification were used for grouping histologic subtypes, as shown in Appendix 1, available online at http://links.lww.com/AOG/A864. In this study, type I tumors were categorized into grade 1 endometrioid and grade 2 endometrioid histology.11
Treatment patterns included type of hysterectomy-based surgery and postoperative radiotherapy. Surgical codes including oophorectomy were considered as the oophorectomy group, and surgical codes without oophorectomy were considered as the ovarian conservation group in this study. If surgical codes did not specify oophorectomy status, these cases were considered as unknown oophorectomy status and excluded from the analysis. For survival outcome, cause-specific survival and all-cause mortality (overall survival) after the initial diagnosis of endometrial cancer were collected. Among deceased cases, causes of death were examined (endometrial cancer, other malignancy, cardiovascular disease, and other chronic disease) grouped per prior study.7,12
The primary outcome of the analysis was to examine characteristics and trends of ovarian conservation among women with stage I type I endometrial cancer. The secondary interest of analysis was to examine survival outcomes of women aged younger than 50 years with stage I grade 1 and 2 endometrioid histologic types of endometrial cancer who underwent ovarian conservation. This age cutoff was chosen based on mean age of spontaneous menopause in the North American population.13 Women who underwent oophorectomy were compared with those who had ovarian conservation. Statistical significance of age at diagnosis was assessed by Student t test. Ordinal and categorical variables were examined with the χ2 test. A binary logistic regression test was used for multivariable analysis to determine independent contributing factors for ovarian conservation. In this model, all patient characteristics and tumor factors were entered in the final model.
Joinpoint Regression Program 4.2.0.2 provided by the National Cancer Institute was used to examine temporal (calendar years) and age-related (year of age) trends in the utilization of ovarian conservation.14 In analyses of temporal trends, the frequency of ovarian conservation in each 6-month period (January 1 to June 30 and July 1 to December 31) was examined to identify the presence of changes in trend precisely.15 The presence of trends in annual and age-specific frequency of ovarian conservation were examined with a linear segmented regression test and log transformation was performed to determine annual and per-year-of-age percent change of the slope.
For evaluating survival and cumulative risk of other causes of death (cardiovascular disease, other chronic disease, and other malignancy) among women aged younger than 50 years with stage I grade 1 and 2 endometrioid endometrial cancer, the Kaplan-Meier method was used to construct survival and cumulative risk curves,16 and statistical significance between the curves was examined with log-rank test for univariable analysis. In addition, a Cox proportional hazard regression model for multivariable analysis was performed.17 Covariates entered in the final model were patient demographics, tumor factors, and treatment patterns. The variance inflation factor was determined among covariates in the multivariable analysis, and variance inflation factor 2.0 or greater was interpreted as multicollinearity. P<.05 was considered statistically significant (two-tailed). Statistical Package for Social Sciences 22.0 was used for the analysis.
RESULTS
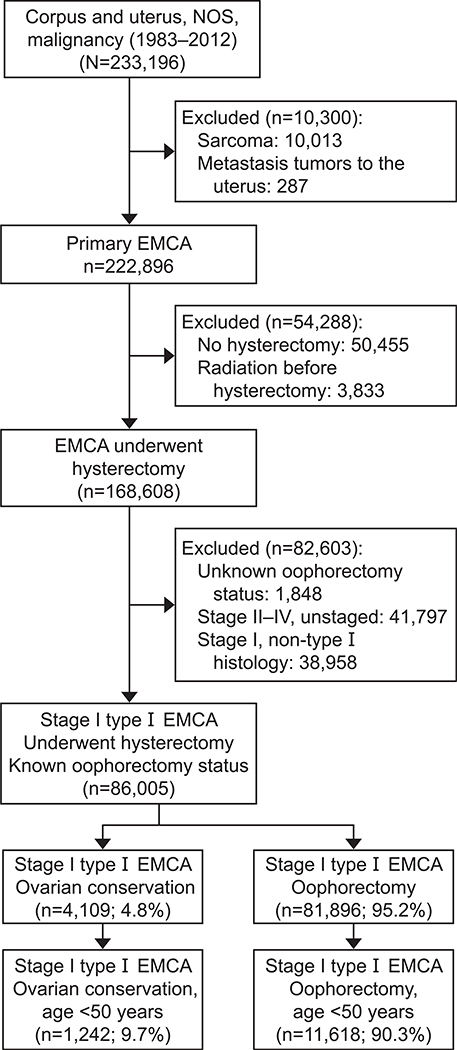
The patient selection criteria are shown in Figure 1. Among 233,196 cases identified in the database, there were 86,005 patients with stage I type I endometrial cancer who underwent hysterectomy with known oophorectomy status. Of those, 4,109 (4.8%) patients had ovarian conservation and 81,896 (95.2%) underwent oophorectomy. Characteristics associated with ovarian conservation are displayed in Table 1 and Appendix 2, available online at http://links.lww.com/AOG/A864.
Fig. 1.
Selection criteria. NOS, not otherwise specified; EMCA, endometrial cancer; type I, grade 1 and 2 endometrioid adenocarcinoma.
Matsuo. Ovarian Conservation in Endometrial Cancer. Obstet Gynecol 2016.
Table 1.
Patient Demographics and Contributing Factors for Ovarian Conservation (N=86,005)
| Ovarian Conservation | Oophorectomy | Adjusted OR* (95% CI) | P | |
|---|---|---|---|---|
| n | 4,109 (4.8) | 81,896 (95.2) | ||
| Age (y) | 58.4±15.0 | 61.6±11.6 | ||
| 50 or older | 2,867 (3.9) | 70,278 (96.1) | 1 | |
| 40–49 | 734 (7.5) | 9,116 (92.5) | 1.90 (1.75–2.08) | <.001 |
| Younger than 40 | 508 (16.9) | 2,502 (83.1) | 4.78 (4.29–5.33) | <.001 |
| Ethnicity | ||||
| White | 3,141 (4.6) | 65,732 (95.4) | 1 | |
| Black | 205 (5.2) | 3,762 (94.8) | 1.10 (0.95–1.28) | .20 |
| Hispanic | 437 (6.6) | 6,153 (93.4) | 1.16 (1.03–1.29) | .011 |
| Asian | 219 (4.5) | 4,673 (95.5) | 0.81 (0.70–0.94) | .006 |
| Others | 84 (5.9) | 1,329 (94.1) | 0.92 (0.73–1.16) | .48 |
| Unknown | 23 (8.5) | 247 (91.5) | 1.69 (1.09–2.62) | .018 |
| Marital status | ||||
| Single | 595 (4.8) | 11,909 (95.2) | 1 | |
| Others | 3,306 (4.7) | 66,372 (95.3) | 1.06 (0.97–1.16) | .18 |
| Unknown | 208 (5.4) | 3,615 (94.6) | 1.20 (1.01–1.43) | .042 |
| Registry area | ||||
| West | 2,212 (4.9) | 42,971 (95.1) | 1 | |
| Central | 863 (4.4) | 18,535 (95.6) | 0.89 (0.82–0.97) | .009 |
| East | 1,034 (4.8) | 20,390 (95.2) | 1.01 (0.93–1.09) | .88 |
| Year of diagnosis | ||||
| 1983–1989 | 435 (5.4) | 7,584 (94.6) | 1 | |
| 1990–1999 | 938 (4.9) | 18,037 (95.1) | 0.90 (0.80–1.02) | .10 |
| 2000–2009 | 2,067 (4.7) | 41,604 (95.3) | 0.84 (0.75–0.94) | .002 |
| 2010–2012 | 669 (4.4) | 14,671 (95.6) | 0.81 (0.71–0.93) | .002 |
| Stage | ||||
| IA | 3,315 (4.8) | 65,153 (95.2) | 0.94 (0.85–1.05) | .30 |
| IB | 390 (3.7) | 10,188 (96.3) | 1 | |
| I NOS | 404 (5.8) | 6,555 (94.2) | 1.01 (0.87–1.17) | .89 |
| Histology | ||||
| Grade 1 endometrioid | 3,045 (5.7) | 50,357 (94.3) | 1.62 (1.50–1.74) | <.001 |
| Grade 2 endometrioid | 1,064 (3.3) | 31,539 (96.7) | 1 | |
| Tumor size (cm) | ||||
| 2.0 or less | 974 (5.9) | 15,438 (94.1) | 1.96 (1.55–2.49) | <.001 |
| 2.1–4.0 | 534 (3.4) | 15,351 (96.6) | 1.23 (0.97–1.57) | .09 |
| 4.1–6.0 | 199 (3.1) | 6,317 (96.9) | 1.06 (0.82–1.39) | .65 |
| Greater than 6.0 | 80 (3.2) | 2,427 (96.8) | 1 | |
| Unknown | 2,322 (5.2) | 42,363 (94.8) | 1.71 (1.36–2.16) | <.001 |
OR, odds ratio; CI, confidence interval; NOS, not otherwise specified.
Data are n (%) or mean±standard deviation unless otherwise specified.
Percentages are shown per row. A binary logistic regression model was used for multivariable analysis. All patient factors and tumor characteristics were entered in the final model.
Bold indicates a significant P value.
OR for ovarian conservation compared with oophorectomy.
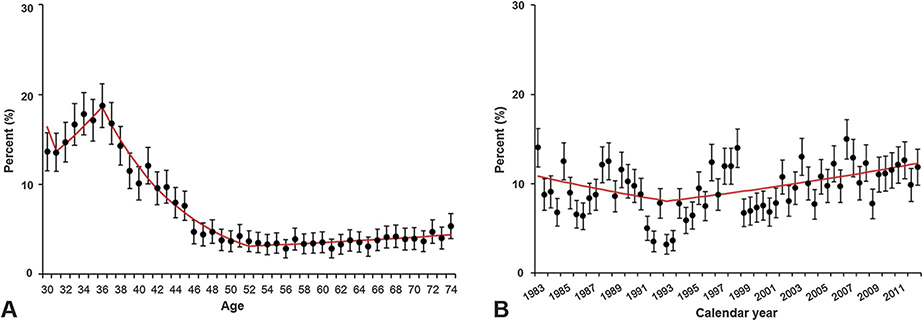
Patient age was significantly associated with ovarian conservation (age younger than 40, 16.9%; 40–49, 7.5%; and 50 years or older, 3.9%; P<.001). When a trend analysis was performed (Fig. 2A), the frequency of ovarian conservation declined significantly with each additional year of age after 37 years (percentage change per year 10.6, 95% confidence interval [CI] 9.4–11.7, P<.001). The age-dependent decline in the frequency of ovarian conservation reached a nadir at age 53 years. Over this age interval, the frequency of ovarian conservation declined from 18.8% (95% CI 16.4–21.2) at age 37 years to 3.7% (95% CI 2.5–4.9) at age 53 years (risk difference 15.1%, 95% CI 12.9–17.3). Temporal trends in the frequency of ovarian conservation among women aged younger than 50 years who had stage I grade 1 endometrioid adenocarcinoma were statistically significantly but of limited clinical significance. In this group, the frequency of ovarian conservation was noted to increase gradually in the frequency between 1993 and 2012 (annual percent change 1.1, 95% CI 0.4–1.8, P=.003), reaching 11.8% in year 2012 (Fig. 2B).
Fig. 2.
Temporal trend in frequency of ovarian conservation. A. Frequency of ovarian conservation by age at hysterectomy is shown for all cases (N=86,005). Between age 37 and 53 years, there was a statistically significant decline in the frequency of ovarian conservation with each subsequent year of age (percent change per year −10.6, 95% confidence interval [CI] −11.7 to −9.4, P<.001). B. Frequency of ovarian conservation at hysterectomy by calendar year is shown for women aged younger than 50 years with stage I grade 1 endometrioid endometrial cancer (n=9,110). Median frequency of ovarian conservation during the study period was 10.6%. There was a statistically significant increase in frequency of ovarian conservation between 1993 and 2012 (percent change per year 1.1, 95% CI 0.4–1.8, P=.003). Error bars represent 95% CI.
Matsuo. Ovarian Conservation in Endometrial Cancer. Obstet Gynecol 2016.
In a multivariable analysis of factors associated with ovarian conservation, women younger than 40 years of age were more likely to have ovarian conservation (adjusted odds ratio [OR] 4.78, 95% CI 4.29–5.33, P<.001) compared with women older than 50 years of age (Table 1). Among the histologic subtypes, women with grade 1 endometrioid histology were more likely to have ovarian conservation (adjusted OR 1.62, 95% CI 1.50–1.74, P<.001) compared with those with grade 2 endometrioid histology. Women with tumor size 2.0 cm or less were more likely to have ovarian conservation (adjusted OR 1.96, 95% CI 1.55–2.49, P<.001) compared with tumor size greater than 6.0 cm.
There were 12,860 (15.0%) women aged younger than 50 years with stage I type I endometrial cancer among the 86,005 cases, and ovarian conservation was performed in 1,242 (9.7%, 95% CI 9.1–10.2) patients in this population. Of those, there were 9,110 cases of grade 1 tumors with the ovarian conservation rate being 11.4% (95% CI 10.7–12.0) and 3,750 cases of the grade 2 tumors with the ovarian conservation rate being 5.5% (95% CI 4.8–6.3).
Patient demographics were compared between ovarian conservation and oophorectomy cases among women aged younger than 50 years with stage I grade 1 endometrioid tumors (n=9,110; Table 2). In this subgroup, the median follow-up was 7.8 years (7.1 years for the ovarian conservation patients). There were 3,427 (37.6%) women who had follow-up longer than 10 years. Women who underwent ovarian conservation were younger, had more frequently tumor size 2.0 cm or less, underwent supracervical hysterectomy more often, and were more likely to receive surgery alone without adjuvant therapy (all P<.01). Ovarian conservation rates were similar between stage IA and IB patients (P=.17).
Table 2.
Characteristics of Ovarian Conservation in Women Aged Younger Than 50 Years With Stage I Grade 1 Endometrioid Endometrial Cancer (n=9,110)
| Ovarian Conservation | Oophorectomy | P | |
|---|---|---|---|
| n | 1,034 (11.4) | 8,076 (88.6) | |
| Age (y) | 40.5±65.6 | 43.1±5.3 | <.001 |
| 40–49 | 616 (8.9) | 6,307 (91.1) | |
| 30–39 | 371 (18.7) | 1,609 (81.3) | |
| Younger than 30 | 47 (22.7) | 160 (77.3) | |
| Ethnicity | <.001 | ||
| White | 584 (10.0) | 5,240 (90.0) | |
| Black | 71 (14.9) | 407 (85.1) | |
| Hispanic | 223 (15.6) | 1,204 (84.4) | |
| Asian | 109 (11.5) | 841 (88.5) | |
| Others | 42 (10.9) | 345 (89.1) | |
| Unknown | 5 (11.4) | 39 (88.6) | |
| Marital status | .11 | ||
| Single | 303 (12.2) | 2,171 (87.8) | |
| Others | 684 (10.9) | 5,593 (89.1) | |
| Unknown | 47 (13.1) | 312 (86.9) | |
| Registry area | .001 | ||
| West | 602 (12.1) | 4,385 (87.9) | |
| Central | 182 (9.1) | 1,828 (90.9) | |
| East | 250 (11.8) | 1,863 (88.2) | |
| Year of diagnosis | .08 | ||
| 1983–1989 | 75 (11.7) | 566 (88.3) | |
| 1990–1999 | 177 (9.8) | 1,637 (90.2) | |
| 2000–2009 | 584 (11.5) | 4,486 (88.5) | |
| 2010–2012 | 198 (12.5) | 1,387 (87.5) | |
| Stage | .17 | ||
| IA | 901 (11.4) | 6,993 (88.6) | |
| IB | 20 (7.8) | 238 (92.2) | |
| I, NOS | 113 (11.8) | 845 (88.2) | |
| Tumor size (cm) | <.001 | ||
| 2.0 or less | 294 (14.4) | 1,746 (85.6) | |
| 2.1–4.0 | 91 (7.9) | 1,062 (92.1) | |
| 4.1–6.0 | 50 (8.7) | 526 (91.3) | |
| Greater than 6.0 | 20 (5.7) | 330 (94.3) | |
| Unknown | 579 (11.6) | 4,412 (88.4) | |
| Surgery type | <.001 | ||
| Total, pan, simple hysterectomy | 926 (11.4) | 7,221 (88.6) | |
| Supracervical, subtotal hysterectomy | 70 (46.1) | 82 (53.9) | |
| Others | 38 (4.7) | 773 (95.3) | |
| Adjuvant radiation | .004 | ||
| No | 1,005 (11.6) | 7,641 (88.4) | |
| Yes | 22 (5.5) | 377 (94.5) | |
| Unknown | 7 (10.8) | 58 (89.2) | |
NOS, not otherwise specified.
Data are n (%) or mean±standard deviation unless otherwise specified.
Percentages are shown per row. Univariable analysis with Student t test or χ2 test for P values.
Bold indicates a significant P value.
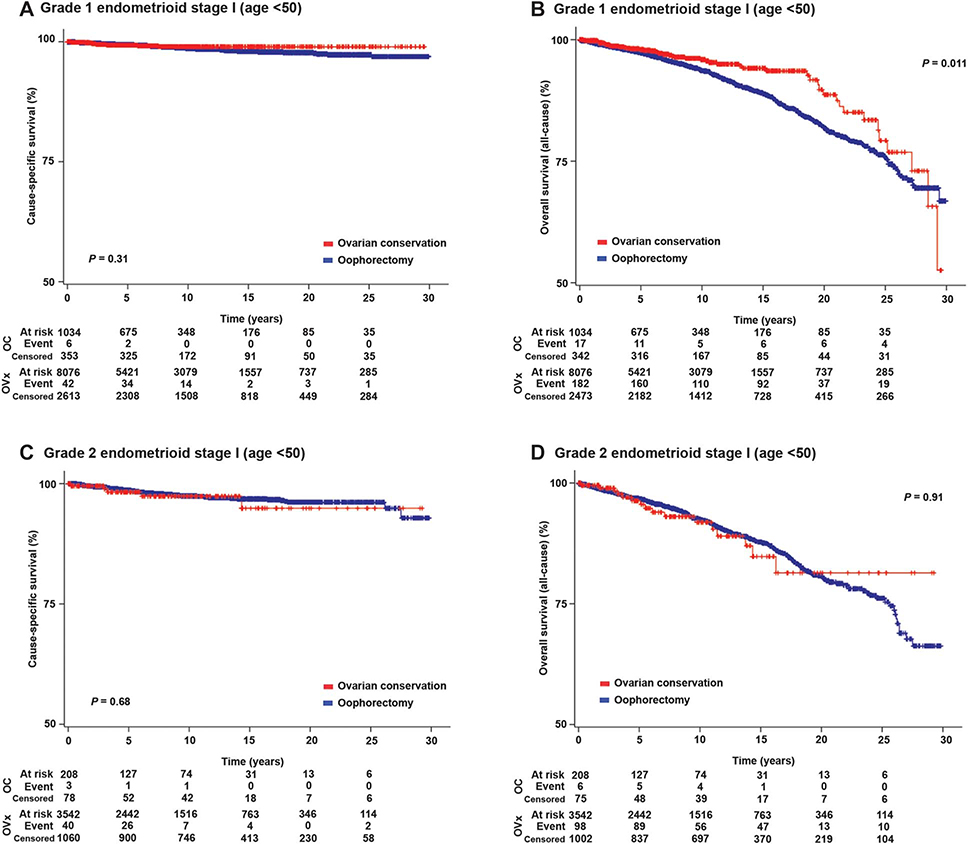
On univariable analysis, ovarian conservation was not associated with cause-specific survival (20-year rates 98.9% compared with 97.7%, P=.31; Fig. 3A). In contrast, ovarian conservation was significantly associated with improved overall survival (10-year rates 95.6% compared with 93.7%; and 20-year rates 88.8% compared with 82.0%; hazard ratio [HR] 0.73, 95% CI 0.54–0.98, P=.011; Fig. 3B). On multivariable analysis (Table 3), ovarian conservation remained an independent prognostic factor for improved overall survival among women aged younger than 50 years with stage I grade 1 endometrioid endometrial cancer (adjusted HR 0.73, 95% CI 0.54–0.98, P=.036).
Fig. 3.
Survival curves for women aged younger than 50 years with stage I grade 1 and 2 endometrioid endometrial cancer. Log-rank test for P value. Y-axis was truncated to 50–100%. Survival curves were constructed for: cause-specific survival for women aged younger than 50 years with stage I grade 1 endometrioid (A) and grade 2 endometrioid (C) endometrial cancer and overall survival for women aged younger than 50 years with stage I grade 1 endometrioid (B) and grade 2 endometrioid (D) endometrial cancer. OC, ovarian conservation; Ovx, oophorectomy; censored, no event during the time period.
Matsuo. Ovarian Conservation in Endometrial Cancer. Obstet Gynecol 2016.
Table 3.
Multivariable Analysis for Women Aged Younger Than 50 Years With Stage I Grade 1 Endometrioid Endometrial Cancer (n=9,110)
| Overall Survival |
Death From Cardiovascular Disease |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Multivariable |
Multivariable |
||||||||
| n | 10-y | 20-y | HR (95% CI) | P | 10-y | 20-y | HR (95% CI) | P | |
| Age (y) | |||||||||
| 40–49 | 6,923 | 93.8 | 82.3 | 1 | 1.0 | 3.4 | 1 | ||
| 30–39 | 1,980 | 94.1 | 84.0 | 0.80 (0.66–0.98) | .034 | 1.1 | 3.7 | 0.82 (0.52–1.32) | .42 |
| Younger than 30 | 207 | 98.3 | 86.1 | 0.60 (0.33–1.10) | .10 | 0.6 | 3.9 | 0.60 (0.14–2.50) | .48 |
| Ethnicity | |||||||||
| White | 5,824 | 94.2 | 83.7 | 1 | 1.0 | 3.4 | 1 | ||
| Black | 478 | 88.8 | 73.7 | 1.81 (1.32–2.48) | <.001 | 3.7 | 8.5 | 3.75 (2.05–6.83) | <.001 |
| Hispanic | 1,427 | 94.4 | 82.4 | 1.07 (0.82–1.40) | .62 | 0.6 | 1.7 | 0.66 (0.30–1.46) | .31 |
| Asian | 950 | 94.5 | 83.4 | 0.83 (0.61–1.13) | .25 | 0.7 | 3.5 | 0.65 (0.29–1.45) | .30 |
| Others | 387 | 94.5 | 68.6 | 1.80 (1.28–2.53) | .001 | 0.8 | 4.2 | 1.76 (0.78–3.97) | .17 |
| Unknown | 44 | 100 | 100 | NA | .88 | 0 | 0 | NA | .97 |
| Marital status | |||||||||
| Single | 2,474 | 91.4 | 78.8 | 1 | 1.4 | 4.8 | 1 | ||
| Others | 6,277 | 94.8 | 84.1 | 0.68 (0.57–0.81) | <.001 | 0.9 | 3.2 | 0.79 (0.52–1.20) | .27 |
| Unknown | 359 | 94.6 | 80.3 | 0.91 (0.59–1.39) | .65 | 1.4 | 1.4 | 0.71 (0.22–2.35) | .58 |
| Registry area | |||||||||
| West | 4,987 | 93.9 | 82.6 | 1 | 0.8 | 3.5 | 1 | ||
| Central | 2,010 | 93.6 | 82.5 | 1.05 (0.86–1.28) | .66 | 1.8 | 3.8 | 0.97 (0.62–1.52) | .89 |
| East | 2,113 | 94.3 | 83.2 | 0.89 (0.71–1.10) | .27 | 0.9 | 3.4 | 0.82 (0.50–1.36) | .44 |
| Year of diagnosis | |||||||||
| 1983–1989 | 641 | 95.6 | 83.4 | 1 | 1.0 | 3.7 | 1 | ||
| 1990–1999 | 1,814 | 93.8 | 83.3 | 1.05 (0.83–1.33) | .66 | 1.4 | 4.1 | 1.04 (0.63–1.72) | .88 |
| 2000–2009 | 5,070 | 93.4 | 92.4 | 1.09 (0.83–1.43) | .55 | 1.0 | NR | 0.56 (0.29–1.09) | .09 |
| 2010–2012 | 1,585 | NR | NR | 0.40 (0.16–1.02) | .06 | NR | NR | 0.43 (0.05–3.52) | .43 |
| Stage | |||||||||
| IA | 7,894 | 94.1 | 83.1 | 1 | 1.1 | 3.5 | 1 | ||
| IB | 258 | 88.5 | 78.2 | 1.40 (0.86–2.26) | .18 | 1.5 | 9.3 | 2.12 (0.74–6.07) | .16 |
| I NOS | 958 | 93.3 | 81.3 | 1.16 (0.94–1.44) | .17 | 1.0 | 3.2 | 1.11 (0.69–1.78) | .68 |
| Tumor size (cm) | |||||||||
| 2.0 or less | 2,040 | 94.7 | 86.5 | 0.72 (0.44–1.18) | .20 | 1.2 | 4.3 | 3.28 (0.43–23.9) | .25 |
| 2.1–4.0 | 1,153 | 94.7 | 79.7 | 0.78 (0.47–1.32) | .35 | 1.3 | 3.5 | 3.03 (0.39–23.4) | .29 |
| 4.1–6.0 | 576 | 94.4 | 71.8 | 0.87 (0.48–1.57) | .64 | 0.2 | 5.1 | 2.03 (0.21–19.6) | .54 |
| Greater than 6.0 | 350 | 90.2 | 70.4 | 1 | 0.3 | 0.3 | 1 | ||
| Unknown | 4,991 | 93.6 | 82.2 | 0.86 (0.54–1.37) | .51 | 1.0 | 3.2 | 2.58 (0.35–18.6) | .35 |
| Surgery type | |||||||||
| Total, pan, simple hysterectomy | 8,147 | 94.0 | 82.7 | 1 | 1.0 | 3.5 | 1 | ||
| Supracervical, subtotal hysterectomy | 811 | 93.2 | 82.5 | 0.98 (0.77–1.24) | .87 | 0.6 | 3.0 | 0.54 (0.28–1.04) | .07 |
| Others | 152 | 92.2 | NR | 1.37 (0.64–2.90) | .42 | 3.3 | NR | 3.95 (0.94–16.6) | .06 |
| Adjuvant radiation | |||||||||
| No | 8,646 | 94.0 | 82.8 | 1 | 1.1 | 3.6 | 1 | ||
| Yes | 399 | 92.3 | 80.6 | 1.22 (0.90–1.67) | .21 | 0.7 | 3.4 | 1.09 (0.53–2.24) | .81 |
| Unknown | 65 | 97.9 | 95.3 | 0.59 (0.18–1.89) | .37 | 0 | 0 | NA | .95 |
| Ovarian conservation | |||||||||
| No | 8,076 | 93.7 | 82.0 | 1 | 1.1 | 3.7 | 1 | ||
| Yes | 1,034 | 95.6 | 88.8 | 0.73 (0.54–0.98) | .036 | 0.2 | 2.3 | 0.40 (0.17–0.91) | .029 |
HR, hazard ratio; CI, confidence interval; NA, not available; NR, patients did not reach the 10-year and 20-year follow-ups; NOS, not otherwise specified.
Data are % unless otherwise specified.
Cox proportional hazard regression models for multivariable analysis adjusted for collected covariates. All patient factors, tumor characteristics, and treatment patterns were entered in the final model.
Bold indicates a significant P value.
Patterns of death were examined in women aged younger than 50 years with stage I grade 1 endometrioid endometrial cancer (Appendix 3, available online at http://links.lww.com/AOG/A864). A total of 649 (7.1%) deaths were recorded for this subgroup, with death resulting from cardiovascular disease being the most common cause of death (n=121 [18.6%]); endometrial cancer accounted for 104 (16.0%) deaths. Ovarian conservation was only associated with a decreased cumulative risk of death resulting from cardiovascular disease (10-year rates 0.2% compared with 1.1%, and 20-year rates 2.3% compared with 3. 7%, HR 0.44, 95% CI 0.19–0.99, P=.042; Appendix 4, part A [available online at http://links.lww.com/AOG/A864]) and was not associated with other causes of death. On multivariable analysis controlling for patient demographics, tumor factors, and treatment patterns (Table 3), ovarian conservation remained an independent predictor for decreased risk of death from cardiovascular disease among women aged younger than 50 years with stage I grade 1 endometrioid endometrial cancer (adjusted HR 0.40, 95% CI 0.17–0.91, P=.029).
Patient demographics were compared between ovarian conservation and oophorectomy cases among women aged younger than 50 years with stage I grade 2 endometrioid endometrial cancer (n=3,750; Appendix 5, available online at http://links.lww.com/AOG/A864). Median follow-up was 8.4 years (7.1 years for the ovarian conservation group). There were 1,590 (42.4%) women who had a follow-up longer than 10 years. There were 329 (20.7%) deaths recorded in this group. Women who underwent ovarian conservation were more likely to be younger, had tumor size 2.0 cm or less more often, and to undergo supracervical hysterectomy (all P<.01).
On univariable analysis, ovarian conservation was not associated with cause-specific survival (20-year rates 94.6% compared with 96.1%, P5.68; Fig. 3C), overall survival (20-year rates 81.0% compared with 80.6%, P=.91; Fig. 3D) or cumulative risk of cardiovascular death (P=.43; Appendix 4, part B [http://links.lww.com/AOG/A864]) in this subgroup. When the grade 1 patients and the grade 2 patients were compared, the grade 2 group was more likely to be older, have stage IB disease, and undergo radical hysterectomy (all P<.001; Appendix 6, available online at http://links.lww.com/AOG/A864).
DISCUSSION
The key finding of this study is that, in young women with low-grade, early-stage endometrial cancer, ovarian conservation was associated with decreased long-term all-cause mortality, specifically from cardiovascular disease.
Our study showed that the frequency of ovarian conservation in young women with low-grade, early-stage endometrial cancer has increased minimally during the study period as was reported in the prior 2009 study examining the same population.18 Our study also confirmed that the frequency of ovarian conservation is low even among young women with low-grade, early-stage endometrial cancer similar to what was reported in a prior study.8 Identification of risk factor for ovarian recurrence will be therefore useful to help surgeons identify ideal candidates for ovarian conservation.
One of the salient findings in this study was that ovarian conservation was associated with improved long-term overall survival in young women with stage I grade 1 endometrioid endometrial cancer but not in grade 2 tumors. This finding differs from prior studies, which showed similar overall survival between the ovarian conservation group and the oophorectomy group in stage I disease.8,18 However, these studies classified all grades of endometrioid cancers together in their analyses and included smaller sample sizes. By stratifying by tumor grade, we noted that the benefit of ovarian conservation was more evident in grade 1 endometrioid tumors.
It is possible that the difference in survival for patients with ovarian conservation was the result of other health factors. For instance, those women who had ovarian conservation are potentially healthier and may have had better performance status. Similarly, women who underwent ovarian conservation to retain ovarian function may be more proactive in general health aspects. However, this study lacked data on why the decision to perform oophorectomy or to offer ovarian conservation was made; thus, our findings are susceptible to selection bias. In addition, we are unable to perform complete risk adjustment. Numerous factors are associated with the development of cardiovascular disease that are not captured in this study.
Emerging data have noted the beneficial effects of ovarian conservation, particularly in younger women, on preventing cardiovascular disease.19–21 Our study partly supports these findings. A possible reason for this association may include the cardioprotective effect of estrogen. This theory is supported by a prior study that demonstrated increased risks of postoperative nonalcoholic fatty liver disease, a hepatic-based metabolic syndrome that was associated with postoperative development of diabetes mellitus and hypercholesterolemia after surgical menopause in young women with endometrial cancer.5 These conditions lead to subclinical atherosclerotic vascular changes resulting in increased risk of clinical cardiovascular disease in the long term.22 We were not able to obtain information from this database regarding the use of hormone therapy after oophorectomy in women with endometrial cancer; however, use of hormone therapy is generally rare in this disease population (less than 2%).23
Similar to other reports, our study showed that cause-specific survival rates were similar between the ovarian conservation and the oophorectomy groups.8,18 However, this result may not imply that recurrence-free survival rate is similar between the two groups. As a result of the lack of data on recurrence, our analysis was not able to assess the risk of ovarian recurrence after ovarian conservation. However, the chance of occult metastasis in a grossly normal-appearing ovary is generally low (0.8%).24
Our analysis showed that the effects of ovarian conservation on overall survival were different between the grade 1 and 2 cases among women aged younger than 50 with stage I endometrial cancer. It is speculated that the differences in patient characteristics, tumor factors, and treatment patterns between these two groups might lead to differences in ovarian function after surgery. For instance, grade 2 patients were more likely to have radical pelvic surgery and be older, both of which are more likely to diminish ovarian function sooner after surgery compared with the grade 1 patients.25,26
Strengths of this study are that this is a population-based study with a large sample size and long-term follow-up. This methodology was indeed useful because the effects of ovarian conservation on survival outcome were not apparent until 10 years after surgery. We recognize a number of important limitations as described previously. Also, we cannot exclude the possibility that a small number of women had previously undergone oophorectomy and thus may have been misclassified. However, given that our primary analysis was performed in women younger than 50 years of age, it is likely that few women would have previously undergone oophorectomy. Lastly, this study does not have information for potential confounders such as hysterectomy route, adnexal pathology, body habitus, and other important predictors of general health and life expectancy.
Supplementary Material
Acknowledgments
Supported by the Ensign Endowment for Gynecologic Cancer Research (Koji Matsuo).
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
From the Division of Gynecologic Oncology and the Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, Los Angeles County Medical Center, and the Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, California; the Department of Obstetrics and Gynecology, Brigham and Women’s Hospital and Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts; and the Department of Obstetrics and Gynecology, Columbia University College of Physicians and Surgeons, New York, New York.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet 2012;379:1352–60. [DOI] [PubMed] [Google Scholar]
- 3.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013;31:2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JD. Take ‘em or leave ‘em: management of the ovaries in young women with endometrial cancer. Gynecol Oncol 2013;131:287–8. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo K, Gualtieri MR, Cahoon SS, Jung CE, Paulson RJ, Shoupe D, et al. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause 2016;23:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Haas EC, Oosting SF, Lefrandt JD, Wolffenbuttel BH, Sleijfer DT, Gietema JA. The metabolic syndrome in cancer survivors. Lancet Oncol 2010;11:193–203. [DOI] [PubMed] [Google Scholar]
- 7.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol 2012;126: 176–9. [DOI] [PubMed] [Google Scholar]
- 8.Wright JD, Jorge S, Tergas AI, Hou JY, Burke WM, Huang Y, et al. Utilization and outcomes of ovarian conservation in premenopausal women with endometrial cancer. Obstet Gynecol 2016;127:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun C, Chen G, Yang Z, Jiang J, Yang X, Li N, et al. Safety of ovarian preservation in young patients with early-stage endometrial cancer: a retrospective study and meta-analysis. Fertil Steril 2013;100:782–7. [DOI] [PubMed] [Google Scholar]
- 10.Lyman GH, Khorana AA, Falanga A. Thrombosis and cancer: emerging data for the practicing oncologist. Am Soc Clin Oncol Educ Book 2013. doi: 10.1200/EdBook_AM.2013.33.e337. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, et al. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome. Obstet Gynecol 2015;125:424–33. [DOI] [PubMed] [Google Scholar]
- 12.Kyrle PA. Predicting recurrent venous thromboembolism in cancer: is it possible? Thromb Res 2014;133(suppl 2):S17–22. [DOI] [PubMed] [Google Scholar]
- 13.Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol 2015;126:859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005;105: 178–85. [DOI] [PubMed] [Google Scholar]
- 15.Melamed A, Rauh-Hain JA, Clemmer JT, Diver EJ, Hall TR, Clark RM, et al. Changing trends in lymphadenectomy for endometrioid adenocarcinoma of the endometrium. Obstet Gynecol 2015;126:815–22. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 17.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol 1972;34:187–220. [Google Scholar]
- 18.Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ. Safety of ovarian preservation in premenopausal women with endometrial cancer. J Clin Oncol 2009;27:1214–9. [DOI] [PubMed] [Google Scholar]
- 19.Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int 2008;14:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoupe D, Parker WH, Broder MS, Liu Z, Farquhar C, Berek JS. Elective oophorectomy for benign gynecological disorders. Menopause 2007;14:580–5. [DOI] [PubMed] [Google Scholar]
- 21.Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol 2013;121:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation 2009;119:382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo K, Gualtieri MR, Cahoon SS, Toboni MD, Machida H, Moeini A, et al. Contributing factors for menopausal symptoms after surgical staging for endometrial cancer. Menopause 2016; 23:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin KY, Miller DS, Bailey AA, Andrews SJ, Kehoe SM, Richardson DL, et al. Ovarian involvement in endometrioid adenocarcinoma of uterus. Gynecol Oncol 2015; 138:532–5. [DOI] [PubMed] [Google Scholar]
- 25.Ellsworth LR, Allen HH, Nisker JA. Ovarian function after radical hysterectomy for stage IB carcinoma of cervix. Am J Obstet Gynecol 1983;145:185–8. [DOI] [PubMed] [Google Scholar]
- 26.Muraji M, Sudo T, Iwasaki S, Ueno S, Wakahashi S, Yamaguchi S, et al. The effect of abdominal radical trachelectomy on ovarian reserve: serial changes in serum anti-müllerian hormone levels. J Cancer 2012;3:191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.