Abstract
Cancer metastases accounts for most cancer deaths. The secreting glycoprotein Wnt5a impairs tumor cell migration and reduces invasiveness and metastasis. High Wnt5a expression in tumor cells is correlated to better outcomes in patients with breast, prostate and epithelial ovarian cancer. We aimed to investigate the association between the Wnt5a expression and outcomes in patients with colon cancer (CC) stage II/III. We performed a retrospective single-center study evaluating 345 patients with radical resection for primary CC, stage II/III, who started 6 months of adjuvant chemotherapy with 5-FU or capecitabine ± oxaliplatin between 2001 and 2015. Archived formalin-fixed paraffin embedded tumor tissue from resection specimens were stained with Wnt5a antibody using immunohistochemistry. Cytoplasmatic Wnt5a staining was assessed according to intensity and percentage of stained cells. Patients were divided in groups depending on high (n = 230) or low (n = 115) Wnt5a expression. Disease free survival (DFS) and overall survival (OS) were analyzed for the two groups using Kaplan-Meier plots and Long rank test. Patients with Wnt5a-negative tumors had significantly poorer performance status (PS) than patients with high Wnt5a expression (p = 0.046). No significant difference was seen between patients with low and high Wnt5a expression in terms of 5-year DFS (p = 0.517) or 5-year OS (p = 0.415). Poor PS was associated with lower DFS (p = 0.002) and OS (p < 0.001). In conclusion, we found no significant difference in prognosis for patients with stage II/III CC depending on their Wnt5a expression. Patients with Wnt5a-negative tumors had significant poorer PS than patients with higher levels. Poor PS was associated with lower DFS and OS.
Keywords: Colon cancer, Disease free survival, Overall survival, Performance status, Wnt5a
Highlights
-
•
High expression of Wnt5a in tumor cells are correlated to significantly better outcomes in patients with different cancers.
-
•
We found no difference in survival among patients with colon cancer stage II-III depending on their Wnt5a expression.
-
•
Patients with low Wnt5a expression had significantly poor performance status than patients with high levels.
-
•
Poor performance status was shown to predict poorer outcomes.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide, with about 1.8 million new cases and almost 881.000 deaths in 2018 [1]. New surgical techniques, combination chemotherapy and biological therapy have improved survival in patients with CRC.
Primary treatment for colon cancer (CC) stages I, II and III is a radical resection. Depending on different surgical techniques, tumor stage and risk factors, 4-year disease-free survival (DFS) is 67.5–100% [2]. Adjuvant chemotherapy with 5-fluorouracil (5-FU) or capecitabine after surgery for CC has been used to reduce recurrence rate and improve overall survival (OS) for nearly 30 years [3,4]. During this period, the use of adjuvant chemotherapy has increased [5], and many studies have confirmed the beneficial effect in reducing recurrence rate and improving OS for CC stages II–III [6,7].
Cancer metastases is the major cause of cancer morbidity and mortality [8,9], and accounts for about 90% of cancer deaths. The metastatic process is complex involving multiple sequential steps including invasion, angiogenesis, cancer cell migration, homing, and growth [8,9].
Wnt5a is a member of the Wnt family of secreting glycoproteins, which affects surroundings through autocrine and paracrine routes [10]. The protein has been shown to play a role in cell adhesion, tumor development [11], and cell migration [12] and has potency as a novel therapeutic target in cancer treatment.
Low Wnt5a expression is associated with poor tumor cell differentiation and early relapse in invasive ductal breast carcinomas [13], whereas expression of Wnt5a is associated with better prognosis in this cancer type [14]. The better prognosis is probably due to the ability of Wnt5a to impair tumor cell migration and thus reduce invasiveness and metastasis [15]. High expression levels of Wnt5a are also shown to be correlated to significantly better outcomes in patients with localized low grade prostate cancer after radical prostatectomy [16,17]. In addition, loss of Wnt5a is correlated to higher tumor stage and to predict poor outcomes in patients with epithelial ovarian cancer [18].
One small study has investigated the association between expression of Wnt5a and prognosis in CC [15]. For patients with node-negative CC (Duke B), median survival time after diagnosis was 109 months for patients with Wnt5a-positive primary tumors compared to only 58 months for those with Wnt5a-negative primary tumors [15]. In colorectal cancer, Foxy 5, a pharmaceutical agent derived from the primary structure of the Wnt5a protein, has shown to impair (both β-catenin and PGE2) several steps in cell signaling pathway, thus impacting colonic cancer stem cells [19]. Mehdawi et al. found that Wnt5a acts by up-regulating the tumor suppressor 15-PGDH (prostaglandin dehydrogenase) which is down-regulated in cancer [20]. However, a potential clinical advantage for CC patients with high Wnt5a expression must be confirmed in a larger study. The aim of this study was to investigate the association between the Wnt5a expression and DFS and OS in CC stage II and III.
Material and methods
This was a retrospective single-center study evaluating the DFS and OS of patients with stage II–III CC depending on Wnt5a expression in the primary tumor.
Patients and setting
All patients had undergone radical resection for primary CC, stage II or III. They all started 6 months adjuvant chemotherapy with of 5-FU or capecitabine ± oxaliplatin at the Department of Oncology, Herlev and Gentofte Hospital, Denmark, from January 2001 to January 2015. Patients with available tumor samples were included in the study.
Patients were identified from the hospital records and clinical data was extracted from electronic medical records. Manual assessment of Wnt5a staining was performed by an experienced pathologist with no knowledge of the clinical outcome.
Data extraction
Baseline characteristics included age, sex, comorbidities, European Cooperative Oncology Group (ECOG) performance status (PS) [21], body mass index (BMI), tobacco use, and tumor characteristics.
WNT5a staining
To assess the Wnt5a expression, we retrieved archived formalin-fixed paraffin embedded tumor tissue from resection specimens of CC patients. After evaluating haematoxylin and eosin (H&E) stained slides the most representative tumor block from each CC specimen was selected. Immunohistochemical stains (IHC) were performed by the IHC-laboratory at the Department of Pathology at Herlev and Gentofte Hospital, Denmark, on a DAKO Autostainer (DAKO, Denmark). Each immunostain was performed with negative/positive tissue controls. The IHC-laboratory uses immunostains in routine diagnostics and follow the guidelines of Nordic immunohistochemical Quality Control (NordicQC).
From each tumor block 1–3 μm sections were stained with Wnt5a antibody (WNT-5a AF645 (polyclonal), R&D systems, Biotechne). The primary antibody was diluted in sterile PBS 1:100.
Cytoplasmatic staining of Wnt5a was assessed according to intensity (0: negative, 1: weak, 2: moderate, 3: strong) and percentage of stained cells (0: no stained cells, 1: 25% stained cells, 2: 25–50% stained cells, 3: 51–75% stained cells and 4: 76–100% stained cells). Intensity and maximum percentage of stained cells were multiplicated into a final score ranging from 0 to 12 (Supplementary Table 1). A score ≤ 2 was considered as low Wnt5a expression and a combined score >2 as high Wnt5a staining (For examples of different staining intensities see Fig. 1).
Fig. 1.
IHC staining of Wnt5a in colorectal cancer. A) 0, negative B) 1+, weak intensity C) 2+, moderate intensity and D) 3+, strong intensity. ×100 magnification, scale bar 100 μm.
Statistical methods
Power calculation was based on the assumption of risk of recurrence of 30% [22]. With inclusion of 350 patients a difference of hazard ratio (HR) 1.75 should be detected at a power of 80%. Categorical variables were analyzed using a Chi-squared test where appropriate; otherwise Fisher's exact test was used. In all analyses, patients were divided into two subgroups depending on the presence of Wnt5a in primary tumor.
DFS was defined as time from surgery to time of recurrence or death, whatever happened first. OS was defined as time from surgery to end of follow up or death of any cause. DFS and OS were analyzed by the Kaplan–Meier estimator and Log rank test. The difference in median DFS for patients with low and high Wnt5a expression was compared with Mann-Whitney U test.
Cause of death was categorized into CC mortality, other causes of death. Associations between PS, stage of disease, Wnt5a level, and disease-free survival, overall survival and cc mortality were analyzed with multivariate regression analyses.
Results
Totally 374 patients were assessed for eligibility for the present study. Of them 29 patients were excluded (missing clinical information (n = 12), no adenocarcinoma (n = 2), only metastatic tumor available (n = 4), not enough tumor material for Wnt5a staining (n = 5) and no tumor material (n = 6)). Totally 345 patients were included in the study; group 1 with absence or very low Wnt5a expression (a score of ≤2) (n = 115), and group 2 with medium to high Wnt5a expression (a score of >2) (n = 230). Baseline characteristics for all patients and for patients with low and high expression of Wnt5a in the primary tumor are presented in Table 1.
Table 1.
Baseline characteristics for all patients and for patients with low and high level of Wnt5a expression.
| Variable | All patients |
Patients with low and high Wnt5a |
p | ||
|---|---|---|---|---|---|
| All patients N = 345 N (%) |
Low Wnt5 (0) n = 115 n (%) |
High Wnt5 (1 + 2) n = 230 n (%) |
|||
| Age | Median (min–max) | 67 (23–88) | 66 (37–85) | 68 (23–88) | 0.573 |
| Sex | Female | 169 (49) | 56 (51) | 112 (49) | 1.000 |
| Male | 178 (51) | 59 (49) | 118 (51) | ||
| PS | 0 | 278 (80) | 85 (74) | 191 (83) | 0.046 |
| 1 | 59 (17) | 23 (20) | 36 (16) | ||
| 2+ | 10 (3.9) | 7 (6.1) | 3 (1.3) | ||
| Civil status | Single | 90 (26) | 33 (28.7) | 57 (25) | 0.391 |
| Living together | 245 (71) | 77 (67) | 166 (72) | ||
| NK | 12 (3.2) | 5 (4.3) | 7 (3.0) | ||
| BMI | Median (min–max) | 24.1 (16.3–43) | 24.1 (17.5–38.4) | 24.1 (16.3–43.0) | 0.599 |
| T | 1 | 5 (1.4) | 1 (0.9) | 4 (1.7) | 0.748 |
| 2 | 16 (4.6) | 7 (6.1) | 9 (3.9) | ||
| 3 | 209 (60) | 69 (60) | 138 (60) | ||
| 4 | 117 (34) | 38 (33) | 79 (34) | ||
| N | 0 | 90 (26) | 24 (21) | 65 (29) | 0.060 |
| 1 | 161 (46) | 50 (44) | 110 (48) | ||
| 2 | 94 (27) | 41 (36) | 53 (23) | ||
| Stage | II | 90 (26) | 24 (21) | 65 (29) | 0.189 |
| III | 255 (74) | 91 (79) | 163 (71) | ||
| MSI | MSI | 48 (14) | 88 (77) | 188 (82) | 0.319 |
| MSS | 278 (80) | 17 (15) | 31 (14) | ||
| NK | 21 (6.1) | 10 (8.7) | 11 (4.8) | ||
| Smoke | No | 139 (40) | 42 (37) | 96 (42) | 0.041 |
| Yes | 198 (57) | 71 (62) | 126 (55) | ||
| NK | 10 (2.9) | 2 (1.7) | 8 (3.5) | ||
| Start dose | Full dose | 275 (79) | 88 (77) | 185 (80) | 0.402 |
| PDR | 72 (21) | 27 (23) | 45 (20) | ||
| Adjuvant regimen | Capecitabine | 49 (14) | 18 (16) | 31 (14) | 0.327 |
| 5-FU | 9 (2.6) | 2 (1.7) | 7 (3.0) | ||
| Capeox | 114 (33) | 31 (27) | 82 (36) | ||
| Folfox | 175 (50) | 64 (56) | 110 (48) | ||
| Hypertension | No | 211 (61) | 65 (57) | 144 (63) | 0.275 |
| Yes | 136 (29) | 50 (44) | 86 (38) | ||
| Heart disease | No | 277 (80) | 21 (18) | 181 (79) | 0.554 |
| Yes | 70 (20) | 94 (82) | 49 (21) | ||
| hypercholesterolemia | No | 276 (80) | 90 (78) | 184 (80) | 0.706 |
| Yes | 71 (20) | 25 (22) | 46 (20) | ||
| Diabetes | No | 307 (88.5) | 97 (84) | 208 (90) | 0.096 |
| Yes | 40 (11.5) | 18 (16) | 22 (9.6) | ||
BMI = body mass index PDR = primary dose reduction PS = performance status.
The two groups were generally equal in baseline characteristics, however, patients with absence or low expression of Wnt5a had significantly poorer performance status (p = 0.046) than patients with higher Wnt5a expression, and more patients with low Wnt5a expression had a history as smokers (p = 0.041).
The relation between Wnt5a level and disease-free survival
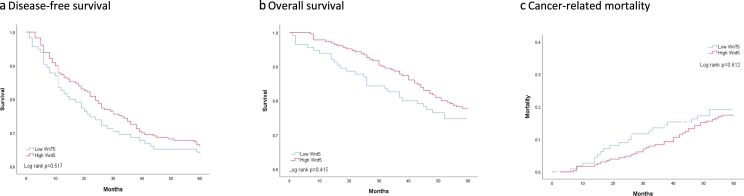
No significant difference was seen in 5-year DFS between patients with low and high Wnt5a expression (Log rank test, p = 0.517) (Fig. 2a) with median DFS of 84 months (range 1 to 158) for patients with low Wnt5a, and 74 months (range 3 to 157) for patients with high Wnt5a expression, with no significant difference between the two groups (p = 0.777). Also, when dividing patients into cancer stage II and III, no significant difference was seen in DFS between patients with low and high levels of Wnt5a (Supplementary Fig. 1a–b).
Fig. 2.
Prognosis for colon cancer patients with low and high Wnt5a expression.
DFS significantly depended on the patients´ PS, where poorer PS was associated with lower DFS, Log rank test (p = 0.002) (Fig. 3a).
Fig. 3.
Prognosis for patients depending on performance status.
PS = performance status.
Patients with absence or low expression of Wnt5a had significantly poorer PS (p = 0.046) than patients with higher Wnt5a expression. When dividing patients into sub-groups depending on PS 0, 1 or 2+ (Supplementary Fig. 2a–c) DFS seemed better for patients with higher Wnt5a expression compared with low expression for patients with PS 2+, however, this was not significant (p = 0.190). Poor PS but not high Wnt5a level or stage of disease were found to be associated with lower disease-free survival (Supplementary Table 2).
The relation between Wnt5a level and overall survival
Median follow-up for the study population was 84 months (range 1 to 158 months). There was no significant difference between patients with low and high Wnt5a expression in 5-year OS (median 85 months (range 1 to 158) for patients with low Wnt5a and 81.5 months (range 7 to 157) for patients with high Wnt5a expression (p = 0.761)). For Kaplan-Meier plot (Fig. 2b) (Log rank test, p = 0.415) no difference in OS was seen after dividing patients into subgroups depending on cancer stage (Supplementary Fig. 1c–d). OS significantly depended on the patients´ PS where poorer PS was associated with lower OS, Log rank test p < 0.001 (Fig. 3b). When dividing patients into sub-groups depending on PS 0, 1 or 2+ (Supplementary Fig. 2d–f) OS seemed better for patients with higher Wnt5a expression compared with low expression for patients with PS 2+, however, this was not significant (p = 0.190). Poor PS but not high Wnt5a level or stage of disease was found to be associated with lower overall survival (Supplementary Table 2).
The relation between Wnt5a level and colon cancer mortality
Only 60 patients in the cohort died from CC within 5 years. When analyzing the association between cancer-related death and Wnt5a expression, we found no significant difference between patients with low and high Wnt5a expression (Log rank test, p = 0.612) (Fig. 2c). Poor PS, but not high Wnt5a level or stage of disease were found to be associated with higher cc mortality (Supplementary Table 2).
Discussion
In this retrospective study of 345 patients who started adjuvant chemotherapy after surgery for stage II and III CC, we found no significant difference in DFS, OS or cancer-related mortality for patients with low compared with high grade of Wnt5a expression. However, there was generally a tendency towards better outcomes for patients with high Wnt5a levels compared with patients with absent or low levels, as shown in Fig. 2. The reason for this could be the relatively small difference in survival between patients with high and low Wnt5a levels, approximately 5–10%, which was lower than expected. Thus, the insignificant results of this study might be due to the relatively small sample size. Our insignificant findings are in contrary with previous findings of Dejmek et al. [15] who found a significant lower survival in patients with low Wnt5a expression compared with patients with higher grade of Wnt5a expression. The study was small with only 55 patients, all with node negative CC. In the present study, patients with lymph node metastases were also included. However, according to our sub-group analyses stratified on tumor stage there were no prognostic Wnt5a-related differences regardless of tumor stage group.
In the present study, patients with low Wnt5a expression had significant poorer PS, which indeed is known to have a negative impact on prognosis [23]. In the multivariate analyses we confirmed the association between poor PS and poorer outcomes. Furthermore, Wnt5a level was still not significantly associated with outcomes. Surprisingly, we found no significant association between stage of disease (stage II or III) in the multivariate analyses, which probably can be explained by the relatively small no of patients with stage II disease (26%), and the presence of high-risk factors which indicated adjuvant chemotherapy. In the study from Dejmek et al. [15] there was no information on patients´ PS, and results were not adjusted for this important confounder, why the significant findings in the study could be due to differences in PS. In our subgroup analyses, for patients with PS2+ there seemed to be a marked difference in DFS depending on Wnt5a level, however, the analysis did not meet statistical significance probably due to the very small sample size (n = 18). This is an interesting finding, identifying an additional factor that might have impact on prognosis in this already frail group with poor outcomes.
Further, data from the study of Dejmek et al. [15], did not reveal whether patients received adjuvant chemotherapy or not. Thus, it is unknown if the study population with poor PS was not offered chemotherapy, which further could have impact on the poorer survival found in patients with low Wnt5a. In the present study, there was no difference in received chemotherapy between the groups with low and high Wnt5a expression.
Wnt5a mRNA is expressed in most normal tissues including the colon, but is highly up-regulated in the progression from normal tissue to carcinomas [24]. The expression of Wnt5a protein, however, seems to be downregulated as it is frequently inactivated in CRC by tumor-specific methylation, and thus, could be a potential biomarker [25].
Wnt5a is suggested to act as a tumor suppressor for CRC by antagonizing the Wnt/beta-catenin signaling [25]. Dejmek et al. demonstrated that addition of recombinant Wnt5a to the human Wnt5a-negative CC cell line SW480, significantly reduced the migratory capacity of SW480 cells [15]. In addition, Ying et al. found that Wnt5a expression can be reactivated by pharmacologic or genetic demethylation, indicating that methylation directly mediates its silencing and is a plausible therapeutic target [25].
The present study has several strength and limitations. To our knowledge, this is the largest study investigating the prognostic value of Wnt5a expression in CC. Even though, the present study had lack of power to dedicate the relatively small difference found in survival. Tumor staining was combined with complete clinical data, which made it possible to address important confounders i.e. PS and received chemotherapy.
Many Wnt5a antibodies are available commercially for IHC. The antibody used for this study has proven to be suitable for both IHC and western blotting [26]. Using whole slide resection specimens instead of the tissue microarray (TMA) technique minimizes the risk of misinterpretation of Wnt5a expression due to tumor heterogeneity.
Previous studies have reported high non-specific background staining when using a polyclonal antibody and suggest the use of a secondary antibody to overcome the problem [26]. In the present study only 33% of patients had low or absent Wnt5a expression, which is lower than the study from Dejmek et al. who found Wnt5-negative tumors in 45% of patients. Although we did not encounter significant background staining, the possibility of cross-reactions adding to the high number of Wnt5a positive sample cannot be excluded.
For scoring the IHC stained slides we used a modified Allred scoring system combining the percentage of positive cells and the intensity of the reaction. Combined scoring is a widely used semiquantitative scoring method but is inaugurally prone to subjective interpretation.
Conclusions
We found no significant difference in prognosis for patients with stage II and III colon cancer depending on their levels of Wnt5a expression in tumor cells. Patients with low levels of Wnt5a expression had significant poorer PS than patients with higher levels. Poor PS was associated with lower DFS, OS and higher CC mortality.
The following are the supplementary data related to this article.
Disease free survival in patients with Colon cancer depending on Wnt5a expression and tumor stage.
Disease free survival in patients with Colon cancer depending on Wnt5a expression and performance status.
Assessment of cytoplamatic Wnt5a staining according to intensity and percentage of stained cells.
Multivariate analyses; association between baseline characteristics and outcome.
Ethical considerations
The study was approved by the local ethical committee (J no.: H-16016203), and Danish Data Protection Agency (J no.: HEH-2015-031, I-Suite no: 03621).
Funding
This study was supported by Eurostar [project number E7090].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Bertelsen C.A., Neuenschwander A.U., Jansen J.E. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161–168. doi: 10.1016/S1470-2045(14)71168-4. [DOI] [PubMed] [Google Scholar]
- 3.Laurie J.A., Moertel C.G., Fleming T.R. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J. Clin. Oncol. 1989;7:1447–1456. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- 4.Moertel C.G., Fleming T.R., Macdonald J.S. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 5.Jessup J.M., Stewart A., Greene F.L., Minsky B.D. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 6.Gill S., Loprinzi C.L., Sargent D.J. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J. Clin. Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 7.Gray R., Barnwell J., McConkey C. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 8.Seyfried T.N., Huysentruyt L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013;18:43–73. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Zijl F., Krupitza G., Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat. Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anastas J.N., Moon R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 11.Lustig B., Behrens J. The Wnt signaling pathway and its role in tumor development. J. Cancer Res. Clin. Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles R.H., van Es J.H., Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta. 1653;2003:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson M., Dejmek J., Bendahl P.O., Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409–416. [PubMed] [Google Scholar]
- 14.Dejmek J., Leandersson K., Manjer J. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin. Cancer Res. 2005;11:520–528. [PubMed] [Google Scholar]
- 15.Dejmek J., Dejmek A., Safholm A. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 2005;65:9142–9146. doi: 10.1158/0008-5472.CAN-05-1710. [DOI] [PubMed] [Google Scholar]
- 16.Syed Khaja A.S., Helczynski L., Edsjo A. Elevated level of Wnt5a protein in localized prostate cancer tissue is associated with better outcome. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khaja A.S., Egevad L., Helczynski L. Emphasizing the role of Wnt5a protein expression to predict favorable outcome after radical prostatectomy in patients with low-grade prostate cancer. Cancer Med. 2012;1:96–104. doi: 10.1002/cam4.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitler B.G., Nicodemus J.P., Li H. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res. 2011;71:6184–6194. doi: 10.1158/0008-5472.CAN-11-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osman J., Bellamkonda K., Liu Q. The WNT5A agonist Foxy5 reduces the number of colonic cancer stem cells in a xenograft mouse model of human colonic cancer. Anticancer Res. 2019;39:1719–1728. doi: 10.21873/anticanres.13278. [DOI] [PubMed] [Google Scholar]
- 20.Mehdawi L.M., Prasad C.P., Ehrnstrom R. Non-canonical WNT5A signaling up-regulates the expression of the tumor suppressor 15-PGDH and induces differentiation of colon cancer cells. Mol. Oncol. 2016;10:1415–1429. doi: 10.1016/j.molonc.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 22.Osterman E., Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire Swedish population. Dis. Colon Rectum. 2018;61:1016–1025. doi: 10.1097/DCR.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 23.Lund C.M., Nielsen D., Dehlendorff C. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colorectal cancer: the ACCORE study. ESMO Open. 2016;1 doi: 10.1136/esmoopen-2016-000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith K., Bui T.D., Poulsom R. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br. J. Cancer. 1999;81:496–502. doi: 10.1038/sj.bjc.6690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying J., Li H., Yu J. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin. Cancer Res. 2008;14:55–61. doi: 10.1158/1078-0432.CCR-07-1644. [DOI] [PubMed] [Google Scholar]
- 26.Prgomet Z., Andersson T., Lindberg P. Optimization, validation, and identification of two reliable antibodies for immunodetection of WNT5A. Biotech Histochem. 2017;92:46–58. doi: 10.1080/10520295.2016.1255995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disease free survival in patients with Colon cancer depending on Wnt5a expression and tumor stage.
Disease free survival in patients with Colon cancer depending on Wnt5a expression and performance status.
Assessment of cytoplamatic Wnt5a staining according to intensity and percentage of stained cells.
Multivariate analyses; association between baseline characteristics and outcome.