ABSTRACT
Immune checkpoint inhibitors (CPIs) have expanded treatment options for patients with solid tumors. Systemic corticosteroids (CSs) have an indispensable role in cancer care, but CS-related immunosuppression may counteract the CPI-driven antitumor immune response. This retrospective study investigated the association between baseline CS use (bCS; ≤14 days before, ≤30 days after CPI initiation) and clinical outcomes in patients with advanced non-small cell lung cancer (aNSCLC), melanoma (aMel), or urothelial carcinoma (aUC). We analyzed data from the Flatiron Health electronic health record–derived de-identified database for adults diagnosed with aNSCLC, aMel, or aUC between January 2011 and June 2017 who received ≥1 CPI monotherapy in any treatment line. Associations of bCS use with overall survival (OS) and time to next treatment (TTNT) were estimated using multivariable Cox proportional hazards models adjusting for demographic and clinical characteristics (i.e., ECOG performance status, site of metastases). In total, 2,213 patients were diagnosed with aNSCLC (n = 862), aMel (n = 742), or aUC (n = 609) and received ≥1 CPI administration. Most patients (67%-95%) received CSs, many during the baseline period (19%-30%). Patients with bCS use had shorter median OS than those with no bCS use for aNSCLC (6.6 vs 10.6 months; P= .00018), aMel (16.4 vs 21.5; P= .095), and aUC (4.1 vs 7.7; P= .0012). bCS use was associated with shorter OS (not significant for aMel) and TTNT in adjusted multivariable analyses, and clinical outcomes were not explained by prior CS use or other measured confounders. These findings suggest a potential association between bCS use and decreased CPI effectiveness, warranting further investigation.
KEYWORDS: Checkpoint inhibitors, corticosteroids, non-small cell lung cancer, melanoma, urothelial cancer
Introduction
Cancer immunotherapy has expanded the landscape of treatment options in the medical management of solid tumors. The anti-PD-L1/PD-1 (programmed death-ligand 1/programmed death-1 protein) immune checkpoint inhibitors (CPIs) atezolizumab, avelumab, cemiplimab, durvalumab, nivolumab, and pembrolizumab in particular have offered longer survival and improved clinical outcomes across several indications as monotherapies or in combination regimens (e.g., the CTLA-4 [cytotoxic T-lymphocyte-associated protein 4] inhibitor ipilimumab with nivolumab).1 Some of the earliest approved indications included advanced melanoma (aMel),2–5 advanced urothelial carcinoma (aUC),6–8 and certain types of advanced non-small cell lung cancer (aNSCLC).9–13
Systemic corticosteroids (CSs) are known for their anti-inflammatory, analgesic, and antiemetic properties, and as a result have an indispensable role in cancer care. CSs have the ability to reduce T-cell production, function, and migration in immune and inflammatory processes.14,15 In addition to alleviating cancer-related symptoms and treating underlying medical disorders,16 CSs are commonly used to manage immune-related adverse events associated with the enhanced immune response induced by CPIs, such as dermatologic, gastrointestinal, hepatic, renal, ocular, and musculoskeletal events among others.17,18 The immunosuppressive and anti-inflammatory effects of CSs can help to alleviate short- and long-term symptoms and complications with or without additional immunosuppressive therapy.17,18
The mechanisms of PD-L1/PD-1 inhibitors counteract immune suppression in the tumor microenvironment by restoring antitumor activity of T cells;19 immunosuppression from CSs administered prior to or at the start of CPI treatment, or “baseline” CS use (bCS), may counteract the CPI-driven antitumor immune response.20–22 On the other hand, CSs used to manage immune-mediated adverse events later in the course of CPI treatment may not hinder its efficacy.18,23–25 In fact, differences in survival outcomes and peripheral blood immune cells associated with CSs may be limited to use within the first 28 days of CPI treatment. 20, 22
Patients receiving bCSs may be limited or excluded from participation in CPI clinical trials due to the potential impact on CPI efficacy;20,21,26 therefore, clinical trials have not directly addressed the potential interactions between CSs and CPIs, which may vary by tumor site.18,27,28 Real-world data can provide insight into CS use and impact on CPI effectiveness in clinical practice, particularly across multiple indications. Recent observational studies have suggested a negative impact of bCS use on CPI effectiveness, but have been conducted predominantly in patients with aNSCLC; questions remain regarding the impact of early CS exposure, dosing, and duration on the effectiveness of CPI treatment.20,21,26,29,30 Given the increasing use of CPIs across tumor types, it is critical for clinicians and population health managers, and policymakers to understand the potential impact of CSs on CPI efficacy. This real-world study explored the association between bCS use and clinical outcomes including survival and time to next treatment (TTNT) in a large US database of abstracted medical records from patients receiving CPIs for aNSCLC, aMel, or aUC.
Materials and methods
Study design and patients
A retrospective observational study was conducted using the Flatiron Health electronic health record (EHR)–derived de-identified database, which contains structured and unstructured data curated via technology-enabled abstraction. The database is a longitudinal, de-identified database derived from EHRs from >265 cancer clinics representing >2 million patients with cancer in the United States. Institutional Review Board approval of the study protocol was obtained prior to study conduct, and included a waiver of informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Eligible adults (≥18 years) diagnosed with aNSCLC (International Classification of Diseases, Ninth Revision [ICD-9] 174.x or Tenth Revision [ICD-10] C34), aMel (ICD-9 172.x or ICD-10 C43x or D03x) or aUC (ICD-9 188x, 189.1, 189.2, 189.3, or ICD-10 C65x, C66x, C67x, C68.0) between January 1, 2011, and June 30, 2017, who received ≥1 administration of CPI only (or ipilimumab and nivolumab combination therapy for aMel) in any treatment line were included in the study; patients who received other concomitant targeted therapy (i.e., tyrosine kinase inhibitors) or chemotherapy were excluded. The database began on January 1, 2011, for all cancers, and ipilimumab was the first CPI approved by the US Food and Drug Administration in 2011, while other CPIs were approved from 2014 to 2017. The first CPI taken in any line of therapy was considered for this analysis for patients who received at least one administration and were exclusively treated with CPI in that line of treatment (aMel patients were also eligible if they received nivolumab and ipilimumab; for all others, CPI monotherapy). Of the CPIs with FDA approval, atezolizumab, nivolumab (and nivolumab-ipilimumab combination), and pembrolizumab were the only ones received by these patients during the study period. Follow-up information for outcomes was available through March 30, 2018. The analysis included all patients who were diagnosed with advanced-stage cancer on or after January 1, 2011. Patients with aNSCLC were diagnosed with stage IIIB or IV NSCLC at diagnosis or recurrent NSCLC; patients with aMel had evidence of pathologic stages III or IV or had developed first locoregional or distant recurrence; and patients with aUC had stage IV or evidence of advanced transitional cell (urothelial) cancer. To ensure that patient journeys were completely captured in the database, the analysis focused on patients who initiated first-line treatment within 90 days of their date of advanced diagnosis.
Systemic CSs that were administered via intravenous (IV), intramuscular (IM), or oral routes were evaluated, which included betamethasone, dexamethasone, hydrocortisone, fludrocortisone, methylprednisolone, prednisolone, prednisone, and triamcinolone; topical and ocular CSs were excluded. To standardize documented CS doses (when available), we calculated a prednisone-equivalent dose based on the published literature (Supplementary Table S3).31,32 Baseline CS use was defined as the presence of ≥1 IM or IV administration or a recorded order for oral CSs within 14 days before or within 30 days after the CPI start date. Prior CS use was defined as ≥1 administration or oral order of CSs > 14 days before the start of CPI treatment.
Measured outcomes and statistical analysis
Outcomes included overall survival (OS) and TTNT from the CPI start date (index date) to OS or TTNT event. Overall follow-up time was accrued from the index date until the date of death or last visit in the EHR database before March 30, 2018, whichever came earlier during the study period. OS was estimated based on a composite mortality variable generated using structured and unstructured EHR data, commercial sources (obituary data), and the Social Security Death Index, and validated against the National Death Index.33 This composite OS variable has a sensitivity of 90% for these indications (90.4% for aNSCLC, 89.5% for aMel, 90.2% for aUC).34 Mortality data were in month and year format and included deaths due to any cause. The last day of the month was imputed for the date of death. TTNT is used as a proxy for disease progression in real-world data and has been correlated with OS in observational settings.35 A TTNT event was defined as time from start of CPI treatment to initiation of a new line of non-maintenance anti-neoplastic systemic treatment or death within 60 days of the last CPI treatment during the index line of therapy if a subsequent line of treatment was not observed. Remaining patients without a subsequent treatment (or death) were censored at the latter of either their last CPI administration or last recorded visit in the database before March 30, 2018.
Descriptive statistics summarized demographic and clinical characteristics through the use of Chi-squared and t tests to compare categorical and continuous variables, respectively. Kaplan-Meier methods were employed for the univariate analysis of OS and TTNT between patients who did or did not receive bCSs (yes/no bCS) in each tumor type. Associations of bCSs with OS and with TTNT in each tumor type were estimated using Cox proportional hazards multivariate models adjusting for confounders and important predictors at baseline including sex, race/ethnicity (White, other, missing), stage at initial diagnosis (0-II, IIIA, IIIB, IV, missing), prior CS use (yes/no), age at start of CPI treatment (years), modified Charlson Comorbidity Index (CCI) score (0–1, 2+, Missing),36 Eastern Cooperative Oncology Group (ECOG) performance status as of the CPI index date (<2, ≥2, missing), brain metastases prior to or at CPI index date (yes/no), and line of therapy (first line, second or subsequent line). Persons with missing values (i.e., race/ethnicity, CCI) were modeled as a separate category and included in the final multivariable models. Other important indication-specific variables adjusted in final multivariate models included smoking status (ever/never for aNSCLC and aUC), histology (squamous, non-squamous, or not otherwise specified for aNSCLC), and tumor grade (low, high, missing for aUC). The CCI is a validated instrument used for predicting clinical prognosis and mortality, and it generates an index score based on a list of 15 conditions (e.g., congestive heart failure, chronic obstructive pulmonary disease, liver disease) weighted by the condition’s relative risk of 1-year mortality. The modified CCI excludes patients with cancer and weights each comorbid condition on a scale of 0 to 4, where a higher CCI score indicates a greater comorbidity burden (a score of 0 indicates none of the major conditions were present in that patient).36,37 The CCI is prevalent in real-world research because it can be calculated from information often available in observational data sources and it provides a single value that can be used as a model parameter. For this analysis, histology and smoking status were also included for patients with aNSCLC, and tumor grade and smoking status were included for patients with aUC. Sensitivity analyses were conducted to test the robustness of the primary findings, including further adjustment for chemotherapy prior to first CPI, a history of liver metastases at baseline, abnormal hemoglobin at baseline, abnormal alkaline phosphatase at baseline, and a subset analysis in patients with CPI treatment limited to early lines of therapy (first- or second-line) or patients with at least three cycles of CPI treatment.
Results
Study population
A total of 2,213 patients were diagnosed with aNSCLC (n = 862), aMel (n = 742), or aUC (n = 609), and received ≥1 CPI administration during the study period. Overall, most patients were men (1,436 of 2,213; 65%) and White (1,680 of 2,213; 76%); median age at CPI start date was 69 to 74 years across tumor types (Table 1). Median follow-up time from CPI start date to the date of death or last visit, whichever came earlier during the study period, was 5.0 months in patients with aUC (interquartile range [IQR], 2.4–9.9), 7.6 months with aNSCLC (IQR, 3.2–13.5), and 11.4 months with aMel (IQR, 4.7–19.8; Table 1). Patients with aMel most often received CPI during their first line of therapy (88%), while the majority of patients with aNSCLC or aUC received their first CPI during the second line of therapy (55% and 56%, respectively; Table 1). All patients received CPI with doses consistent with their labels during the baseline period (Table 1).
Table 1.
Demographic and clinical characteristics.
| Patients, n (%) unless noted |
aNSCLC (n = 862) |
aMel (n = 742) |
aUC (n = 609) |
|---|---|---|---|
| Age at CPI start date, median (IQR) | 69.0 (61.3–76.0) | 69.0 (59.0–77.0) | 74.0 (67.0–80.0) |
| Male | 466 (54) | 520 (70) | 450 (74) |
|
Smoking historya Yes No |
759 (88) 100 (12) |
N/A N/A |
436 (72) 163 (27) |
|
Race/ethnicity Non-Hispanic White Non-Hispanic Black or African American Non-Hispanic Asian or other Hispanic or Latino Missing |
604 (70) 59 (7) 85 (10) 28 (3) 86 (10) |
629 (85) 4 (1) 37 (5) 11 (2) 61 (8) |
447 (73) 26 (4) 46 (8) 29 (5) 61 (10) |
|
Practice typeb Academic Community |
88 (10) 774 (90) |
131 (18) 611 (82) |
31 (5) 578 (95) |
|
ECOG performance status 0–1 2+ Missing |
468 (54) 133 (16) 261 (30) |
357 (48) 80 (11) 305 (41) |
343 (56) 142 (23) 124 (21) |
|
Stage at initial diagnosis 0–II III IIIA IIIB IV Missing |
103 (12) 11 (1) 86 (10) 93 (11) 551 (64) 18 (2) |
233 (31) 59 (8) 19 (3) 42 (6) 226 (30) 163 (22) |
53 (9) 36 (6) N/A N/A 222 (36) 298 (49) |
|
Modified Charlson Comorbidity Index score 0 1 2+ |
411 (48) 335 (39) 116 (14) |
540 (73) 113 (15) 89 (12) |
302 (50) 203 (33) 104 (17) |
|
Number of unique organ sites of metastases at or prior to first CPI None 1 2 ≥ 3 |
61 (7) 331 (38) 244 (28) 226 (26) |
53 (7) 251 (34) 186 (25) 252 (34) |
32 (5) 273 (45) 157 (26) 147 (24) |
|
Metastatic sites prior to first CPI Liver Bone Lung Brain Lymph nodes |
173 (20) 356 (41) 406 (47) 181 (21) 168 (20) |
194 (26) 195 (26) 423 (57) 172 (23) 218 (29) |
157 (26) 193 (32) 209 (34) 15 (3) 353 (58) |
|
PD-L1/PD-1 statusc Positive Negative Unknown/pending/equivocal//missing |
104 (12) 101 (12) 657 (76) |
22 (3) 33 (4) 687 (93) |
20 (3) 41 (7) 548 (90) |
|
First CPI line of therapy 1 L 2 L 3 L+ |
163 (19) 477 (55) 222 (26) |
653 (88) 73 (10) 16 (2) |
178 (29) 340 (56) 91 (15) |
| Number of CPI administrations/orders, median (IQR)d | 6 (4–14) | 6 (4–15) | 4 (2–9) |
| Follow-up time from CPI start date to end of follow-up (IQR), monthse | 7.6 (3.2–13.5) |
11.4 (4.7–19.8) |
5.0 (2.4–9.9) |
|
Mean CPI dose during baseline, mgf Nivolumab Pembrolizumab Atezolizumab |
227 185 1200 |
243g 172 N/A |
236 197 1199 |
|
Recorded CS useh Ever CS use Prior to baseline Baseline (bCS) |
821 (95) 747 (87) 258 (30) |
500 (67) 120 (16) 182 (25) |
527 (87) 486 (80) 116 (19) |
|
Type of bCS usei Oral only IM or IV administration only Oral + IM or IV |
(n = 258) 110 (43) 93 (36) 55 (21) |
(n = 182) 97 (53) 53 (29) 32 (18) |
(n = 116) 36 (31) 62 (53) 18 (16) |
|
Specific bCS usei,j Dexamethasone Prednisone Methylprednisolone |
342 (63) 94 (17) 65 (12) |
214 (58) 82 (22) 49 (13) |
152 (73) 34 (16) 16 (8) |
1L, first-line; 2L, second-line; 3L, third-line; ALP, alkaline phosphatase; aMel, advanced melanoma; aNSCLC, advanced non-small-cell lung cancer; aUC, advanced urothelial carcinoma; CS, corticosteroid; CPI, immune checkpoint inhibitor; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; HR, hazard ratio; IM, intramuscular; IQR, interquartile range; IV, intravenous; N/A, not available; OS, overall survival.
aSmoking status is not collected from medical records for patients with aMel. Data were missing for < 4 patients with aNSCLC and 10 patients with aUC.
bAcademic practice type was comprised of EHR system records from National Cancer Institute-Designated Cancer Centers.
cPD-L1/PD-1 status determined through abstraction of the EHR for any clinical/pathological notes or data on expression/staining intensity.
dCombination treatment of nivolumab + ipilimumab (when given at the same time and on the same date) was counted as 2 CPI administrations; 28% of aMel patients had 2 administrations.
eEnd of follow-up occurred at the earliest of a TTNT event, death, or last visit date in the Flatiron Health database.
fDefined as the mean per-person CPI dose during the baseline period. Approved monotherapy dosing (per OPDIVO, KEYTRUDA, or TECENTRIQ US package inserts during the study period): nivolumab at 240 mg every 2 weeks or 480 mg every 4 weeks; pembrolizumab at 2 mg/kg every 3 weeks or 200 mg every 3 weeks; atezolizumab at 1200 mg every 3 weeks.
gIncludes nivolumab monotherapy only (no ipilimumab + nivolumab combinations for aMel).
hDefined as any time prior to, during, or after CPI treatment. Prior to baseline was defined as > 14 days prior to CPI start date. Baseline CS use was defined as the 14-day period prior to CPI start date and up to 30 days later. During CPI included the duration of the entire line of therapy.
iProportions of patients are based on those who received bCSs.
jPatients could have received > 1 specific bCS.
Corticosteroid use
The majority of patients (67%-95%) had evidence of CS use (≥1 administration of IM or IV, or oral CS order) at any time in their patient record across tumor types. When we focused on the baseline period around CPI start date (inclusive of 14 days prior to and up to 30 days after), 19% to 30% of patients across tumor types received CSs during the baseline period (Table 1). Baseline characteristics were generally similar by bCS use (Table 2), with the exception of statistically significant differences observed in patients with bCSs who had brain metastases (all groups, P < .05) or liver metastases (aNSCLC, P< .001; aUC, P < .05) at CPI initiation, stage IV disease at diagnosis (aNSCLC, P < .05), CCI < 2 (aMel, P < .05), and ECOG performance status ≥2 at the start of CPI treatment (aUC, P < .01; Table 2). Dexamethasone was the most commonly used steroid across tumor types (58%-73%), followed by prednisone and methylprednisolone (Table 1). These three compounds accounted for >90% of the CS use across tumor types during the baseline period and during the entire CPI line of therapy. Data were missing on dose for oral CSs, which was the majority of CS use across all indications.
Table 2.
Patterns and patient characteristics related to bCS use.
| Patients, n (%) unless noted | aNSCLC (n = 862) |
aMel (n = 742) |
aUC (n = 609) |
|||
|---|---|---|---|---|---|---|
| Key characteristics by bCS use |
No bCS (n = 604) |
bCS (n = 258) |
No bCS (n = 560) |
bCS (n = 182) |
No bCS (n = 493) |
bCS (n = 116) |
| Age at first CPI, mean (SD) | 69 (9.8) | 68 (9.6) | 67 (13.0) | 66 (13.0) | 73 (8.9) | 73 (8.9) |
| Male | 332 (55) | 134 (52) | 389 (69) | 131 (72) | 365 (74) | 85 (73) |
|
Race/ethnicity Non-Hispanic White Non-Hispanic Black or African American Non-Hispanic Asian or other Hispanic or Latino Missing |
421 (70) 42 (7) 61 (10) 20 (3) 60 (10) |
183 (71) 17 (7) 24 (9) 8 (3) 26 (10) |
475 (85) 4 (< 1) 29 (5) 9 (2) 43 (8) |
154 (85) < 4 (< 1) 8 (4) < 4 (< 1) 18 (10) |
368 (75) 21 (4) 32 (7) 24 (5) 48 (10) |
79 (68) 5 (4) 14 (12) 5 (4) 13 (11) |
| Modified Charlson Comorbidity Index < 2 | 527 (87) | 219 (85) | 484 (86) | 169 (93)* | 411 (83) | 94 (81) |
| Smoking history, yes | 538 (89) | 221 (86) | N/A | N/A | 350 (71) | 86 (74) |
|
First CPI line of therapy 1 L 2 L 3 L+ |
115 (19) 336 (56) 153 (25) |
48 (19) 141 (55) 69 (27) |
490 (88) 58 (10) 12 (2) |
163 (90) 15 (8) 4 (2) |
148 (30) 272 (55) 73 (15) |
30 (26) 68 (59) 18 (15) |
| Stage IV at initial diagnosis | 367 (61) | 184 (71)* | 164 (29) | 62 (34) | 173 (35) | 49 (42) |
|
ECOG performance status 0–1 2+ Missing |
328 (54) 89 (15) 187 (31) |
140 (54) 44 (17) 74 (29) |
262 (47) 63 (11) 235 (42) |
95 (52) 17 (9) 70 (39) |
294 (60) 103 (21) 96 (20) |
49 (42)** 39 (34)** 28 (24) |
|
Metastatic sites prior to first CPI Liver Bone Lung Brain |
103 (17) 240 (40) 299 (50) 113 (19) |
70 (27)** 116 (45) 107 (41)* 68 (26)* |
151 (27) 144 (26) 312 (56) 116 (21) |
43 (24) 51 (28) 111 (61) 56 (31)** |
118 (24) 154 (31) 178 (36) 9 (2) |
39 (34)* 39 (34) 31 (27) 6 (5) |
*P < 0.05 vs No bCS; **P < 0.01 vs No bCS.
1L, first-line; 2L, second-line; 3L, third-line; ALP, alkaline phosphatase; aMel, advanced melanoma; aNSCLC, advanced non-small-cell lung cancer; aUC, advanced urothelial carcinoma; bCS, baseline corticosteroid; CPI, immune checkpoint inhibitor; ECOG, Eastern Cooperative Oncology Group; N/A, not available.
Overall survival and time to next treatment
At least half of the patients died during the study period, including 47% (347 of 742) with aMel, 61% (530 of 862) with aNSCLC, and 62% (379 of 609) with aUC. Patients who did not receive bCSs had longer median OS than those who received bCSs: aNSCLC, 10.6 vs 6.6 months (P = .00018); aMel, 21.5 vs 16.4 months (P = .095); and aUC, 7.7 vs 4.1 months (P = .0012) in unadjusted analyses. Multivariable analyses adjusted for key demographic and clinical characteristics, including prior CS use, showed worse OS in patients with aNSCLC and aUC who received bCSs than those who did not receive bCSs – HR, 1.34 (95% CI, 1.12–1.61), and 1.44 (95% CI, 1.12–1.87), respectively – but no significant difference in patients with aMel (HR, 1.24 [95% CI, 0.97–1.57]; Figure 1).
Figure 1.
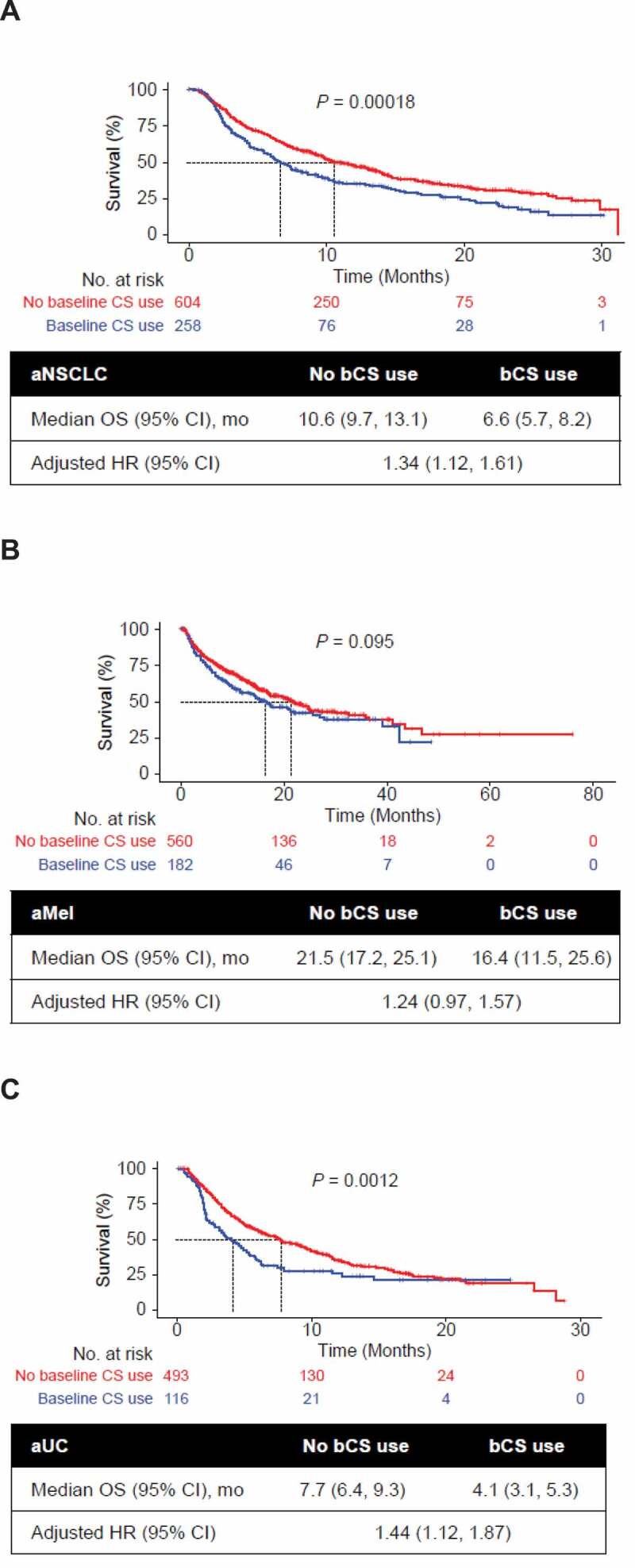
Univariate Kaplan-Meier curves of OS by bCS use and tumor type. Analyses are shown for patients with (a) aNSCLC, (b) aMel, and (c) aUC. Multivariable association of bCS use with OS was adjusted for baseline demographic and clinical characteristics including prior CS use (yes/no), age at CPI start, sex, stage at initial diagnosis (0-II, IIIA, IIIB, IV, missing), race/ethnicity (White, other, missing), Eastern Cooperative Oncology Group performance status at CPI start (< 2, 2+, missing), modified Charlson Comorbidity Index (CCI) score, treatment sequence, brain metastases, smoking status (aNSCLC, aUC), histology (aNSCLC; squamous, nonsquamous, not specified) and grade (aUC). P values were generated by log-rank test. aMel, advanced melanoma; aNSCLC, advanced non-small-cell lung cancer; aUC, advanced urothelial carcinoma; bCS, baseline corticosteroid; CPI, immune checkpoint inhibitor; HR, hazard ratio; OS, overall survival.
More than half of the patients (58%-61%) experienced a TTNT event during the study period. Across all tumor types, the median TTNT was shorter in patients who received bCSs than those who did not (3.5 vs 6.4 months in patients with aNSCLC, respectively; 3.0 vs 5.5 months with aMel; 2.1 vs 4.1 months with aUC). Multivariable analyses adjusting for key demographic and clinical characteristics, including prior CS use, showed a shorter TTNT across tumor types in patients who received bCSs than in those who did not (Figure 2).
Figure 2.
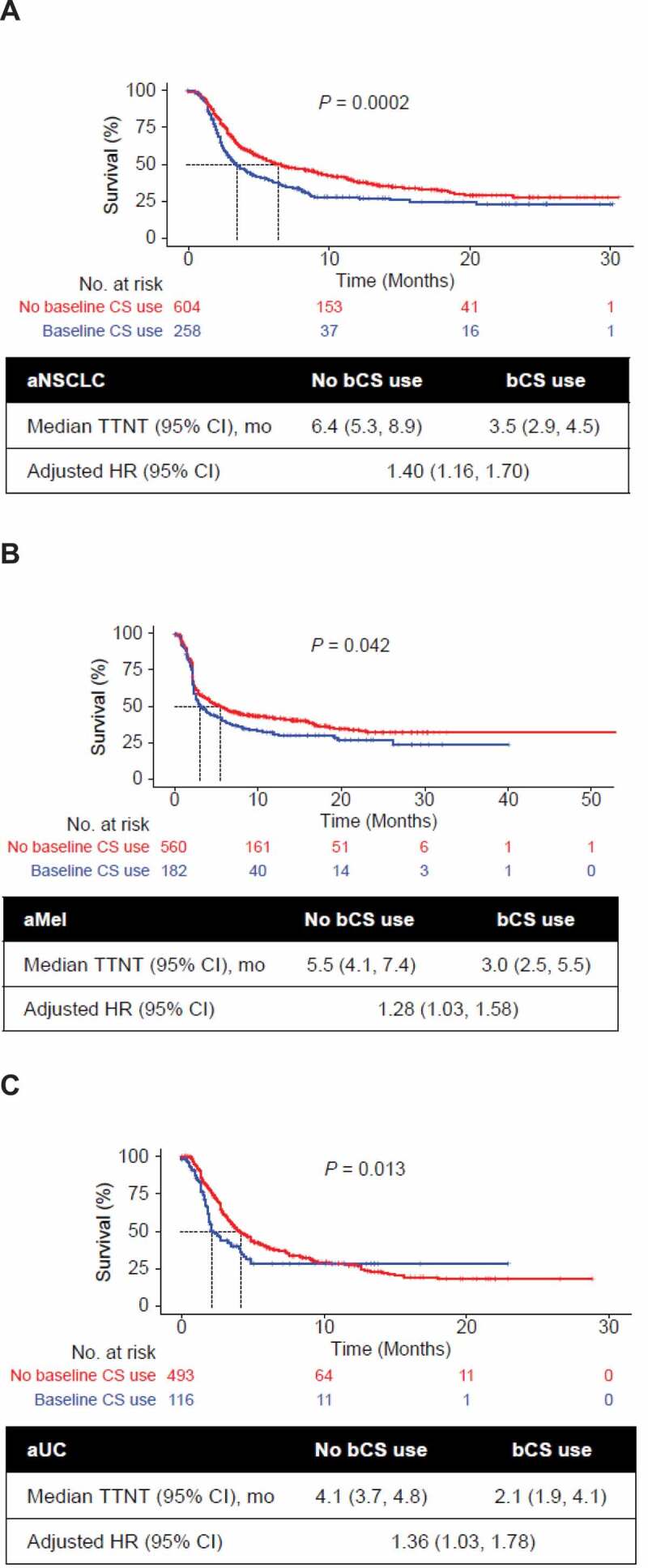
Univariate Kaplan-Meier curves of TTNT by bCS use and tumor type. Analyses are shown for patients with (a) aNSCLC, (b) aMel, and (c) aUC. The base model adjusted for baseline demographic and clinical characteristics including prior CS use (yes/no), age at CPI start, sex, stage at initial diagnosis (0-II, IIIA, IIIB, IV, missing), race/ethnicity (White, other, missing), Eastern Cooperative Oncology Group performance status at CPI start (< 2, 2+, missing), modified Charlson Comorbidity Index (CCI) score, treatment sequence, brain metastases, smoking status (aNSCLC, aUC), histology (aNSCLC; squamous, nonsquamous, not specified) and grade (aUC). P values were generated by log-rank test. aMel, advanced melanoma; aNSCLC, advanced non-small-cell lung cancer; aUC, advanced urothelial carcinoma; bCS, baseline corticosteroid; CPI, immune checkpoint inhibitor; HR, hazard ratio; TTNT, time to next treatment.
Sensitivity analyses showed that the associations of bCS use with OS and with TTNT were consistent after adjusting for important characteristics in additional models, such as presence of liver metastases, abnormal hemoglobin at baseline, abnormal alkaline phosphatase at baseline, and chemotherapy prior to CPI. Additional sensitivity analyses limited to the subset of patients who completed at least 3 CPI cycles of treatment or had their first CPI during early lines of therapy (first- and second-line only), or patients with aMel who took CIT monotherapy, yielded similar findings (Supplementary Tables S1 and S2).
Discussion
This retrospective observational study examined the use of bCS in patients receiving CPI treatment for aNSCLC, aMel, or aUC. Overall CS use was highly prevalent across tumor types, including bCS use in one-fifth to one-third of patients. Baseline CS use was associated with shorter OS and TTNT for patients with aNSCLC or aUC after adjusting for baseline characteristics, including prior CS use and other measured potential confounders. This study was focused on the baseline period around the CPI start date and did not evaluate the impact of CSs during later periods of CPI. CS use during these later CPI treatment periods may have different biological implications and different underlying reasons, such as management of immune-mediated adverse events.18,23 Our findings are consistent with recent reports of clinical outcomes associated with CPI treatment and bCS use in many of the same tumor types, including shorter OS and/or progression-free survival (PFS) in patients who received concomitant CSs with CPI20–22,27,30,38,39 – although some differences have emerged in smaller study populations or subgroups, such as those receiving CSs related versus unrelated to cancer care.24,30,38
When interpreting our findings in the context of similar published reports, between-study differences in contributing factors should be considered, such as the prevalence and definition of CS use, time window of CS use, source populations (US vs EU, academic vs community-based, single center vs oncology network), baseline conditions (autoimmune diseases), and analytic approaches to address confounding comorbidities and/or reasons for CS use. Nevertheless, six studies consistently reported significantly shorter OS and PFS with CS use during CPI treatment in final or multivariable models adjusting for baseline characteristics and conditions (Table S4).20–22,26,27,39 Spakowicz and colleagues evaluated the effect of five types of concomitant medications (antibiotics, CSs, proton pump inhibitors, histamine 2 blockers, and non-steroid anti-inflammatory drugs) on OS among 690 CPI-treated patients with advanced/metastatic melanoma (29%), NSCLC (23%), or other cancers. Multivariate HRs showed associations of OS with CS use within 28 days of the CPI start date for patients with melanoma (HR, 1.57; P = .01) or NSCLC (HR, 1.85; P < .01).22 In a study of 640 patients with NSCLC, Arbor and colleagues compared those who received ≥10 mg/day prednisone equivalent at the start of CPI treatment with those who received <10 mg and found significantly shorter OS (5.4 vs. 12.1 months; P < .001) and PFS (1.9 vs. 2.6 months; P = .001) in analyses adjusting for smoking history, performance status, and brain metastases (HR for OS, 1.66 [P < .001]; HR for ORR, 0.42 [P = .053]; HR for PFS, 1.31 [P = .03]).21 Martínez Bernal also reported a significantly worse OS (3 vs 10 months; P = .002) and more progressive disease (P = .011) in patients with NSCLC receiving ≥20 mg daily prednisone during CPI treatment.39 In a smaller study of CS use in 27 patients receiving CPIs for aNSCLC, aMel, or renal cell carcinoma and >10 mg/day prednisone equivalent, Pan27 reported poorer clinical outcomes in patients who received CSs for >2 weeks than in those with shorter courses of CSs (≤2 weeks).27 In a study of 151 patients with aNSCLC, Fuca and colleagues observed a lower median OS (4.9 vs 15.1 months; P < .001) and PFS (2.0 vs 3.9 months; P = .003) in patients who had CS exposure ≥10 mg daily for ≥1 day during the first 28 days of CPI treatment vs <10 mg per day. Corresponding changes were observed at the cellular level, including neutrophil count, neutrophil-to-lymphocyte ratio, and eosinophil count at 4 and 6 weeks after CPI initiation.20 Recent evidence suggests that dexamethasone may differentially impact immune checkpoint inhibition compared with prednisone.22,40 A sub-analysis of our study focused on patients receiving baseline dexamethasone exclusively yielded stronger associations for both endpoints across all three indications (multivariate HRs for OS in aNSCLC, 1.49 [95% CI: 1.19, 1.88]; aMel, 1.51 [1.10, 2.08]; and aUC, 1.55 [1.15, 2.09]).
A few studies have reported that the reason for CS use can be an independent predictor of outcomes or that the association of CS use with OS or PFS is restricted to those patients who receive CSs for cancer-related symptoms (Table S4).24,30,38 Riudavets and colleagues evaluated a 6-month period around CPI start date (± 3 months) in 267 patients with NSCLC and reported shorter OS (6.5 vs 16.5 months; P < .001) in patients who received bCS for conditions at baseline (rather than for treatment of immune-related adverse events).30 Ricciuti and colleagues reported shorter OS (4.9 vs 11.2 months; P < .001) and PFS (2.0 vs 3.4 months; P = .01) in patients receiving ≥10 mg/day prednisone during CPI treatment (n = 91); this difference was not significant in patients receiving CSs for reasons unrelated to cancer.38 De Giglio24 reported no negative impact on prognosis in patients with aNSCLC who received ≥10 mg prednisone-equivalent CSs within the first 8 weeks of CPI if the indication for CS use was not cancer-related symptoms.24 We were unable to adequately capture reasons for steroid use in our study, although, as done in other studies,20,21 we adjusted for history of prior CS use, CCI score, and brain metastases, which are related to baseline conditions and/or cancer-related symptoms. We also conducted sensitivity analyses further adjusting for prior chemotherapy (a proxy for cancer-related symptoms).
Defining the duration of “early” CS use may also be an important factor in interpreting our findings along with those of other recent studies. Although poorer clinical outcomes were observed in patients who received CSs for >2 weeks vs ≤2 weeks,27 studies that focused on longer time periods – such as 8 weeks24 or 3 months30 to define “early” CS use after starting CPI treatment – did not find significant associations. Svaton41 did not find significant differences for a 2-month period of CS use around CPI treatment, which the authors attributed to a small number of low-dose CS users (22 out of 224 patients).41 Fuca and colleagues also found that significant differences in PFS, OS, and peripheral blood immune cells were limited to the first 28 days of CPI treatment and did not persist when considering the entire CPI treatment period. Spakowickz et al. 22 also evaluated a 28-day CPI window for multiple concomitant medications and found the strongest effects for CS at day 0 of CPI start (Table S4).
To our knowledge, this study is the first of its size to evaluate CS use with CPI treatment across multiple indications. We analyzed a largely community-based real-world setting from a national US oncology EHR-derived database with detailed data on treatment and reliable follow-up information. Detailed evaluation of bCS use was available, and our analysis accounted for prior CS exposure along with a multitude of important potential confounders, including demographic and clinical characteristics; however, residual confounding from measured factors and unmeasured variables may still have influenced the results. We observed consistent findings of CS use with CPI monotherapy across multiple indications, suggesting these findings may be generalizable across tumor types; however, the magnitude of effect may be different from other tumors. Findings from the sensitivity analyses accounting for potentially influential factors were consistent with those of the primary analyses.
Because the data source did not provide reliable data on reasons for CS use and was limited to cancer-related encounters, we did not have access to information on non-cancer-related reasons for CS use, which may have provided further insight into potential confounding factors. Additionally, PD-L1/PD-1 status was not available in the medical records for most patients (76%-93% across tumor types), which was not unexpected considering the study period included diagnoses as early as January 2011, before such testing was required. Use of other immunosuppressive treatments was not included in the analysis, which may have impacted levels of immunosuppression and subsequent outcomes.
TTNT is commonly used as a proxy for disease progression in real-world data sources and is likely the best possible measure of progression in EHR-based research42–44 however, it should be noted that TTNT does not directly evaluate tumor response patterns, and treatment changes could have been due to reasons other than disease progression (such as toxicity or treatment preferences). In addition, tumor burden assessments in real-world settings are not subject to trial-based RECIST methodology and are conducted at the discretion of treating physicians. For long-term outcomes, although OS has been validated with high sensitivity in Flatiron Health,33 other outcome measures such as objective response were not available. Evaluations of dose and duration of CS use were not possible because complete data on CS dosing and frequency were available only for IM or IV administrations, which represented 29% to 53% of CS use across tumor types. As a result, the dose of IV/IM administration-only CSs was high and not representative of all CS use. Use of oral CSs was based on orders and did not indicate if the CS was taken by the patient; this study assumed that every ordered CS was received by the patient. Since this was a US clinical practice–based EHR database, health system factors and clinical practice patterns related to cancer care, CS use, and CPI treatment will differ in other parts of the world, potentially limiting generalizability to other settings.
CPI-treated patients with aNSCLC or aUC receiving CSs at baseline had worse OS, and patients across tumor types had shorter TTNT, than those who did not receive bCSs; this observation persisted when adjusted for available demographic and clinical characteristics, including prior CS exposure. A shorter treatment duration may limit the potential long-term benefits of CPI use. Additional studies are needed to further explore these observations, including patterns of CS use prior to CPI and prospective evaluations that may capture timing, dosing, and reasons related to CS use. These data suggest that CS use at initiation of CPIs should be carefully considered.
Supplementary Material
Acknowledgments
This study is sponsored by F. Hoffmann-La Roche Ltd. Medical writing support was provided by Jeff Frimpter, MPH, of Health Interactions and funded by F. Hoffmann-La Roche Ltd.
Disclosure of potential conflicts of interest
This study is sponsored by F. Hoffmann-La Roche Ltd. All authors disclose medical writing support funded by F. Hoffmann-La Roche Ltd.
Alexandra Drakaki reports an advisory role with AstraZeneca, BMS, and Radmetrix; research funding (to institution) from Kite Pharma; travel expenses from Eli Lilly and AstraZeneca; Stock from UroGen, Allogene, Kynan Pharma; and employment with UCLA.
Preet K Dhillon, Stephen Y Chui, Jinjoo Shim, Matthew Kent, Viraj Degaonkar, Tien Hoang, Virginia McNally, and Patricia Luhn are employees of Roche/Genentech.
Stephen Y Chui, Jinjoo Shim, Viraj Degaonkar, Tien Hoang, Virginia McNally, and Patricia Luhn own Roche stock.
Heather Wakelee reports honoraria from Novartis and AstraZeneca; compensated advisory board participation with AstraZeneca, Xcovery, Janssen, Mirati, and Daiichi Sankyo; uncompensated advisory board participation with Merck, Takeda, Genentech/Roche, and Cellworks; research funding (to institution) from ACEA Biosciences, Arrys Therapeutics, AstraZeneca/Medimmune, BMS, Celgene, Clovis Oncology, Exelixis, Genentech/Roche, Gilead, Lilly, Merck, Novartis, Pfizer, Pharmacyclics, and Xcovery; and travel funds from AstraZeneca.
Ralf Gutzmer reports honoraria from Roche Pharma, Bristol Myers Squibb, Novartis, Merck Sharp & Dohme, Almirall-Hermal, Amgen, Merck-Serono, Sun, Pierre-Fabre, Bayer, Sanofi; an advisory/consultancy role with Roche Pharma, Bristol Myers Squibb, Novartis, Merck Sharp & Dohme, Almirall-Hermal, Amgen, Pierre-Fabre, Merck-Serono, 4SC, Sun, Sanofi, and Pfizer; research funding (to institution) from Pfizer, Johnson & Johnson, Novartis, Amgen, Sanofi, and Merck-Serono; travel funds from Roche Pharma, Bristol Myers Squibb, Pierre-Fabre, Sun, and Merck-Serono.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–9. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicenter, open-label, phase 3 randomized controlled trial. Lancet. 2018;391(10122):748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 8.Galsky MD, Arranz Arija JA, Aristotelis Bamias A, Davis ID, De Santis M, Kikuchi E, Garcia-Del-Muro X, De Giorgi U, Mencinger M, Izumi K, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547–1557. doi:10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 9.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 11.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 14.Beaulieu E, Ngo D, Santos L, Yang YH, Smith M, Jorgensen C, Escriou V, Scherman D, Courties G, Apparailly F, et al. Glucocorticoid-induced leucine zipper is an endogenous anti-inflammatory mediator in arthritis. Arth Rheum. 2010;62:2651–2661. doi: 10.1002/art.27566. [DOI] [PubMed] [Google Scholar]
- 15.Davis TE, Kis-Toth K, Szanto A, Tsokos GC.. Glucocorticoids suppress T cell function by upregulating microRNA-98. Arth Rheum. 2013;65:1882–1890. doi: 10.1002/art.37966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network . Antiemesis. Version 1.2020. [Accessed 2020 Mar 31] https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
- 17.National Comprehensive Cancer Network . Management of immunotherapy-related toxicities. Version 1.2020. [Accessed 2020 Mar 31] https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- 18.Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob -J-J, Cowey CL, Lao CD, Chesney J. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35(34):3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuca G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, Ferrara R, Zilembo N, Ganzinelli M, Sica A, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4(1):e000457. doi: 10.1136/esmoopen-2018-000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 22.Spakowicz D, Hoyd R, Muniak M, Husain M, Bassett JS, Wang L, Tinoco G, Patel SH, Burkart J, Miah A, et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: causal modeling, timing, and classes of concomitant medications. BMC Cancer. 2020;20(1):383. doi: 10.1186/s12885-020-06882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Khattak A, Carlino MS, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6(4):519–527. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Giglio A, Mezquita L, Auclin E, Blanc-Durand F, El-Amarti L, Caramella C, Bernal GM, Hendriks L, Ferrara R, Naltet C, et al. Impact of early introduction of steroid on immune-checkpoint inhibitors (ICI) in patients with advanced non-small cell lung cancer treated. Ann Oncol. 2019;30(suppl 11):xi16–xi32. doi: 10.1093/annonc/mdz449. [DOI] [Google Scholar]
- 25.Leighl N, Gandhi L, Hellmann MD. Pembrolizumab for NSCLC: immune-mediated adverse events and corticosteroid use. J Thorac Oncol. 2015;10:S233. [Google Scholar]
- 26.Scott SC, Pennell NA. Early use of corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol. 2018;13:1771–1775. doi: 10.1016/j.jtho.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Pan EY, Merl MY, Lin K. The impact of corticosteroid use during anti-PD1 treatment. J Oncol Pharm Pract. September 2019;7. doi: 10.1177/1078155219872786. [DOI] [PubMed] [Google Scholar]
- 28.Mehra N, Sharp A, Lorente D, Dolling D, Sumanasuriya S, Johnson B, Dearnaley D, Parker C, de Bono J. Neutrophil to lymphocyte ratio in castration-resistant prostate cancer patients treated with daily oral corticosteroids. Clin Genitourin Cancer. 2017;15(6):678–684. doi: 10.1016/j.clgc.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol. 2017;120:86–92. doi: 10.1016/j.critrevonc.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Riudavets M, Mosquera J, Garcia Compelo R, Serra J, Anguera G, Gallardo P, Sullivan IG, Barba A, Majem M. Impact of corticosteroids and antibiotics on efficacy of immune-checkpoint inhibitors in patients with advanced non-small cell lung cancer. J Thoracic Oncol. 2019;14(10):S728. doi: 10.1016/j.jtho.2019.08.1557. [DOI] [Google Scholar]
- 31.Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43(11):1216–1227. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 32.Webb R, Singer M. Oxford handbook of critical care. New York (NY): Oxford University Press; 2005. [Google Scholar]
- 33.Curtis MD, Griffith SD, Tucker M. Development and validation of a high-quality composite real-world mortality endpoint. Health Serv Res. 2018;53(6):4460–4476. doi: 10.1111/1475-6773.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM. Validation analysis of a composite real-world mortality endpoint for US cancer patients [abstract]. In: Proceedings of the Annual Meeting of the American Association for Cancer Research 2020; 2020 Apr 27-28 and Jun 22-24. Philadelphia (PA): AACR; Cancer Res 2020; 80(16Suppl):Abstract nr 5772. [Google Scholar]
- 35.Griffith SD, Miksad RA, Calkins G, You P, Lipitz NG, Bourla AB, Williams E, George DJ, Schrag D, Khozin S, et al. Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non-small-cell lung cancer data set. JCO Clin Cancer Inform. 2019;3:1–13. doi: 10.1200/CCI.19.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J-M, Sundararajan V. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 38.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non–small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019;37(22):1927–1934. doi: 10.1200/JCO.19.00189. [DOI] [PubMed] [Google Scholar]
- 39.Martínez Bernal G, Mezquita L, Auclin E, Ferrara R, Planchard D, Remon Masip J, Lahmar J, Boucher M-E, Caramella C, Adam J, et al. Baseline corticosteroids (CS) could be associated with absence of benefit to immune checkpoint inhibitors (ICI) in advanced non-small cell lung cancer (NSCLC) patients. Ann Oncol. 2017;28(suppl 5):v460–v496. doi: 10.1093/annonc/mdx380.025. [DOI] [Google Scholar]
- 40.Okoye IS, Xu L, Walker J, Elahi S. The glucocorticoids prednisone and dexamethasone differentially modulate T cell function in response to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade. Cancer Immunol Immunother. 2020;69(8):1423–1436. doi: 10.1007/s00262-020-02555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svaton M, Zemanova M, Zemanova P, Kultan J, Fischer O, Skrickova J, Jakubikova L, cernovska M, Hrnciarik M, Jirousek M, et al. Impact of concomitant medication administered at the time of initation of nivolumab therapy on outcome in non-small cell lung cancer. Anticancer Res. 2020;40:2209–2217. doi: 10.21873/anticanres.14182. [DOI] [PubMed] [Google Scholar]
- 42.Rier HN, Levin MD, van Rosmalen J, Bos MMEM, Drooger JC, de Jong P, Portielje JEA, Elsten EMP, Ten Tije A-J, Sleijfer S, et al. First-line palliative HER2-targeted therapy in HER2-positive metastatic breast cancer is less effective after previous adjuvant trastuzumab-based therapy. Oncologist. 2017a;22(8):901–909. doi: 10.1634/theoncologist.2016-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MCJM, Levin M-D. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast. 2017b;31:9–15. doi: 10.1016/j.breast.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Liang C, Li L, Fraser CD, Ko A, Corzo D, Enger C, Patt D. The treatment patterns, efficacy, and safety of nab-paclitaxel for the treatment of metastatic breast cancer in the United States: results from health insurance claims analysis. BMC Cancer. 2015;15:1019. doi: 10.1186/s12885-015-2027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


