Abstract
Purpose
Gait impairment is a common clinical symptom of patients with Parkinson’s disease (PD). Detecting specific gait parameters’ changes in order to guide clinical intervention is at present lacking. The present study aimed to (1) quantify gait impairments in different PD subtypes and (2) explore whether the results of quantitative gait analysis are beneficial to clinical treatment.
Patients and Methods
We enrolled 86 patients with PD (48 men, and 38 women) from the Department of Geriatrics of the Affiliated Brain Hospital of Nanjing Medical University. Unified Parkinson’s Disease Rating Scale (UPDRS) and Hoehn-Yahr Scale were used to evaluate the motor symptoms of PD. All patients stopped anti-Parkinsonian medication for 24 hours (72 hours for controlled release medicine). The patients were divided into two subtypes, namely, postural instability gait difficulty (PIGD; n=56) and tremor dominant (TD; n=30) subtypes according to UPDRS. All patients completed the instrumented stand and walk test, and a set of JiBuEn gait analysis system was used in gait data collection.
Results
We observed a shorter stride length (p=0.021), a longer stride time (p=0.036), a faster cadence (p=0.036), and a more variable stride length (p=0.012) in the PIGD group compared with the TD group. In addition, compared with the TD group, we found that the toe-off angle (p=0.005) and the range of motion of ankle joint (p=0.009) decreased in the PIGD group.
Conclusion
Our study demonstrated that the gait performance of patients with PIGD is worse than those with TD from the perspective of quantitative gait analysis. We extended previous research and found the PIGD group exhibited severe gait impairments in some specific spatiotemporal and kinematic gait parameters. The different manifestations of these gait impairments may guide in choosing appropriate treatment of patients with different PD subtypes.
Keywords: Parkinson’s disease, postural instability gait difficulty, tremor, wearable sensors, gait
Introduction
Gait impairment is a common clinical symptom of patients with Parkinson’s disease (PD). Impaired gait reduces the patient’s life quality and even increases the risk of falls and death. Although there are more and more strategies to treat PD, the selection of an appropriate pharmacological or rehabilitative therapy intervention that best matches the needs of patients is a challenge for clinicians.1,2 Each medicine has a relatively special effect. For example, stride length is improved most obviously, followed by gait velocity, stride time, and cadence after treatment with levodopa.3,4 Catechol-O-Methyl transferase (COMT) inhibitors and dopamine agonists are beneficial for the improvement of gait velocity.5–7 Patients with PD on Monoamine oxidase type B inhibitors (MAOB-I) have demonstrated a lower probability of developing freezing of gait8 and have a lower postural instability gait difficulty (PIGD) scores.9 Cholinesterase inhibitors (ChE-I) can improve gait velocity, gait variability, and fall risk.10,11 Detecting specific gait parameters’ changes in order to guide pharmacological intervention are at present lacking.12 PD is often divided into two types according to motor symptoms: PIGD and tremor dominant (TD) subtypes.13 Quantitative gait analysis has become possible with the introduction of wearable devices. However, little is known about the specific gait characteristics of different PD subtypes. Accordingly, the present study aimed to (1) quantify gait impairments in different PD subtypes; and (2) explore whether the results of quantitative gait analysis are beneficial to clinical treatment. In-depth knowledge of specific gait impairments might aid in the individualized treatment of PD and enhance the life quality of patients.
Methods
Patients
We enrolled 86 patients (48 men and 38 women; mean PD duration was 5.99 ± 4.69 years, and mean Hoehn-Yahr stage was 2.55±0.92) in our study. These patients were recruited from the Department of Geriatrics of the Affiliated Brain Hospital of Nanjing Medical University between October 2018 and March 2020. All patients were diagnosed according to the Movement Disorder Society (MDS) criteria.14 In addition, these patients were free of cerebrovascular disease, spinal column diseases, and musculoskeletal disease which may affect gait performance. The study was approved by the Medical Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University. All patients signed written informed consent before the study.
PD Subtypes
All subjects stopped anti-Parkinsonian medication for 24 hours (72 hours for controlled release medicine) prior to evaluation. We used the Unified Parkinson’s Disease Rating Scale (UPDRS) and Hoehn-Yahr Scale15 to evaluate the motor symptoms of PD. The patients were divided into two subtypes, namely, the PIGD and TD groups, according to UPDRS. Those patients were selected and included in the TD group if the ratio of the mean tremor scores (eight items: 2.16 in Part 2 and 3.20 and 3.21 in Part 3 of UPDRS) to the mean PIGD scores (five items: 2.13, 2.14 and 2.15 in Part 2 and 3.29 and 3.30 in Part 3 of UPDRS) ≥1.5, or were included in the PIGD group if the ratio ≤1.13,16
Equipment and Gait Data Collection
A set of JiBuEn gait analysis system was used in data collection. Previous research has demonstrated the detailed algorithm for gait parameters and experimental design.17 The device consisted of a pair of smart shoes and five inertial micro-electro-mechanical system sensors tied to the upper and lower limbs and waist. The system collected gait data and transmitted the data to a computer. By using the latest high-order low-pass filter, zero-correction algorithm, and hexahedral calibration technique, the system can reduce accumulative errors, reduce high-frequency noise and installation errors produced by the wearable sensor devices. Finally, by the JiBuEn gait analysis system, we can collect spatiotemporal gait parameters and kinematic gait parameters quickly and precisely.
All patients were instructed to complete the instrumented stand and walk test (ISAW).18 Firstly, all patients stood for 30 seconds, walked 7 meters, then turned 180 degrees, and finally returned to their original place. Before the test, we have introduced the procedures to the patients in detail. In addition, all patients practiced the procedure twice to get more familiar with ISAW. We instructed the patients to walk in a comfortable and relaxed way during the test.
Statistical Analysis
Data were shown as mean ± SD, and the statistical significance level was set at α=0.05. Two-sample t-tests were used to compare the differences in normally distributed measurement data, and Mann–Whitney U-Test was used for measurement data that were not normally distributed. χ2 test was used for qualitative data. Variability of stride length, stride time, stance phase time, and swing phase time were calculated separately using Eq.1 and then combined using Eq.2.19 All data were analyzed using SPSS software version 25 (IBM Corp, Armonk, New York). Figures were configured using GraphPad Prism version 8.0.1.
Eq.1: 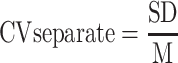
Eq.2: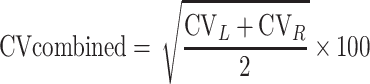
where CV means coefficient of variation. SD means standard deviation. M indicates mean value. The subscripts L and R represent the left and right sides of patients, respectively.
Results
Clinical Characteristics of Participants
We enrolled 86 patients in our study and their demographic characteristics are listed in Table 1. These patients were divided into the PIGD and TD groups. We found no statistical difference in the baseline data between the two groups.
Table 1.
Clinical Characteristics of Participants
| Whole Sample | PIGD | TD | P | |
|---|---|---|---|---|
| N | 86 | 56 | 30 | |
| Male (%) | 48 (55.81%) | 31 (55.36%) | 17 (56.67%) | 0.907 |
| Age (years) | 66.36±8.92 | 66.52±9.39 | 66.07±8.13 | 0.825 |
| Height (cm) | 164.28±8.04 | 163.36±8.07 | 166.00±7.81 | 0.147 |
| Weight (kg) | 65.77±9.56 | 65.06±9.84 | 67.10±9.04 | 0.349 |
| Shoe size | 40.06±2.09 | 40.04±1.95 | 40.10±2.37 | 0.759 |
| MMSEs | 24.43±5.64 | 24.57±5.29 | 24.17±6.32 | 0.532 |
| Duration of PD (years) | 5.99±4.69 | 6.15±4.64 | 5.68±4.83 | 0.659 |
| H-Y stage | 2.55±0.92 | 2.67±0.91 | 2.33±0.91 | 0.102 |
| UPDRS III scores | 34.76±17.46 | 32.66±14.71 | 38.67±21.41 | 0.341 |
Note: Data is shown as mean ± SD.
Abbreviations: PIGD, postural instability gait difficulty subtype; TD, tremor dominant subtype; MMSEs, Mini-Mental State Examination scores; H-Y stage, Hoehn-Yahr stage; UPDRS III, Unified Parkinson’s Disease Rating Scale part 3.
Differences in Spatiotemporal Gait Parameters
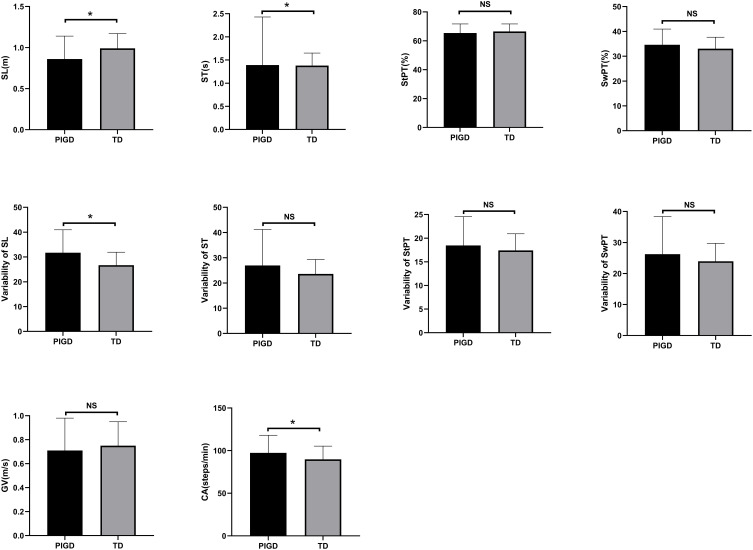
We collected the following spatiotemporal gait parameters: gait velocity, cadence, stride length, stride time, stance phase time, and swing phase time. In addition, variability of stride length, stride time, stance phase time and swing phase time were collected. We only observed a shorter stride length, a longer stride time, a faster cadence and a more variable stride length in PIGD group compared with the TD group (Figure 1).
Figure 1.
Spatiotemporal gait parameters of different PD subtypes.
Abbreviations: SL, stride length; ST, stride time; StPT, stance phase time; SwPT, swing phase time; GV, gait velocity; CA, cadence; “ns”, means no significance.
Note: *P < 0.05.
Differences in Kinematic Gait Parameters
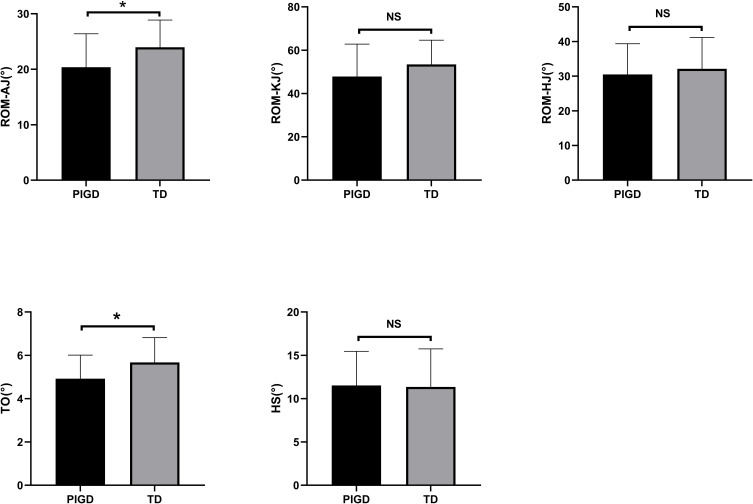
We collected the range of motion (ROM) of hip joint (HJ), knee joint (KJ), and ankle joint (AJ) in the sagittal plane. The difference of maximum and minimum angles of above three joints were defined as ROM. In addition, we measured heel strike (HS) and toe-off (TO) angles in our study. We observed remarkable difference in TO angle and ROM-AJ between the two groups. ROM-AJ and TO angles decreased in the PIGD group, whereas the ROM-KJ, ROM-HJ, and HS of the PIGD and TD groups had no difference (Figure 2).
Figure 2.
Kinematic gait parameters of different PD subtypes.
Abbreviations: ROM, range of motion; AJ, ankle joint; KJ, knee joint; HJ, hip joint; TO, toe-off angle; HS, heel strike angle; “ns”, means no significance.
Note: *P < 0.05.
Discussion
The present study aimed to (1) quantify gait impairments in different PD subtypes; and (2) explore whether the results of quantitative gait analysis are beneficial to clinical treatment. The novel and main finding of our study was that the PIGD group had more severe spatiotemporal gait parameters and distal joint motor deficits during walking than the TD group. These specific impairments may shed more light on clinicians’ decision-making, leading to individualized treatment of PD.
For spatiotemporal gait parameters, previous studies have demonstrated that patients with PD exhibited a decreased stride length, reduced gait velocity, slower turns and smaller arm swing than normal.2,4 In our study, the PIGD group exhibited a smaller stride length compared with the TD group. This finding is similar to previous reports, which demonstrated reduced stride length in PIGD group.20,21 A shorter stride length is related to a weakened forward strength22 and an impaired ability to control the center of body mass in patients.4 In addition, we extended the scope of the previous study and found that the PIGD group showed a longer stride time and a faster cadence compared with the TD group. These characteristics of the PIGD group reflected a more bradykinetic gait pattern compared with TD group. The increased stride time means that the PIGD group had more difficulty in completing the same walking task than the TD group. Patients with PD would increase their cadence to compensate for their small stride length.23 However, an increased cadence is not always beneficial for gait, particularly in people with balance difficulty. Notably, our study suggests that the PIGD group suffer a higher fall risk than the TD group. Previous studies found that stride length is improved most obviously, followed by gait velocity, stride time and cadence after taking levodopa.3,4 Compared with the OFF state, the stride length, gait velocity and cadence increased significantly by ∼22.22%, ∼17.57%, ∼7.31% in the ON state in patients with PD, respectively. Stride time decreased remarkably by∼10.14% in the ON state than that of the OFF state in patients with PD. This finding indicates that levodopa may be a priority for the PIGD group in the absence of contraindications. COMT inhibitors, in conjunction with levodopa, can improve the gait velocity of patients with PD.7 Pramipexole and apomorphine can also improve gait velocity of PD.5,6 These studies indicated that gait performance may not have a guiding value in choosing from these two kinds of drugs because we did not find a difference in gait velocity between the PIGD and TD groups. It has been well known that gait variability was increased in patients with PD. A more variable gait pattern may be associated with a higher variability of lower extremity.24 Our study supported the findings of a previous research, that is, the PIGD group exhibited a higher stride length variability than TD group.25 High variability indicates an inconstant gait performance, which increases the risk of fall.4 Levodopa can only improve the variability in patients with PD to some extent.20 ChE-I can improve gait variability and fall risk.10,11 In addition, patients with PIGD have more non-motor symptoms26,27 and more obvious cognitive impairment than the TD group.28 Above all, the administration of ChE-I may have a double benefit for the PIGD group. Methylphenidate, a rarely used drug, is used in the treatment of attention deficits in patients. Methylphenidate can also improve gait variability.29 Given the higher possibility of cognitive deficits and a higher stride length variability in the PIGD group, methylphenidate may be suitable for this group in the absence of contraindications. It has been demonstrated that a lower probability of developing freezing of gait,8 a potential modify disease progression value, and a lower PIGD scores9 in patients with PD on MAOB-I. What is different from our perceptual knowledge, the disease progression of TD group is faster than PIGD group.30 The combined results showed that both groups may benefit from MAOB-I.
A few quantitative studies investigated kinematic gait parameters. Our study extended previous research and took kinematic gait parameters into consideration. We found remarkable difference in TO angle and ROM-AJ between the two groups. The PIGD group exhibited smaller TO angle and ROM-AJ than the TD group. These results are in line with recent works that the AJ movement is decreased in PD,3 and patients with PIGD have severely impaired distal movements.21 The decreased TO and HS angles indicate a reduced foot clearance and may result in tripping.31 HS angle is decreased whereas TO is not reduced in patients with PD.3,12,32 However, in our study, the HS of both PD subtypes showed no difference. TO was remarkably decreased in the PIGD group. This finding indicated that the PIGD group had worse foot clearance ability. Interestingly, the attentional strategy of focusing on the feet while walking can be employed to normalize foot clearance ability in PD.31 Compared with the TD group, the PIGD group may benefit more from this strategy. Patients with PD exhibit an impaired ability to produce maximal muscle force compared with healthy people.24,33 Our study extended previous research and found that the PIGD group produced a smaller AJ movement than the TD group. These results may shed more light into rehabilitative therapy. A rehabilitative therapy that focuses on strengthening the distal muscles of the lower limbs may be a priority to PIGD group. Our previous research showed that increased stiffness is associated with decreased AJ movement.3 Rigid muscles showed the best response to levodopa, and levodopa can improve AJ movement in patients with PD.34,35 Our previous and current studies indicated that levodopa may be a good choice for the PIGD group.
Our study had several limitations. First, only 86 patients with PD were enrolled in our study, and this small sample size may affect the generalizability of our results. Second, PD is a heterogeneous disease with motor and non-motor symptoms. Some non-motor symptoms, such as anxiety and depression, can affect gait performance in patients with PD. However, we evaluated the patient’s cognitive level to avoid this widely been recognized factor that would affect gait. Third, this study was not a de novo group, and anti-PD drugs may influence gait. However, we minimized the influence of pharmacological intervention by stopping anti-Parkinsonian medication for 24 hours (72 hours for controlled release medicine).
Conclusion
In conclusion, our study demonstrated the idea that the gait performance of patients with PIGD subtype is worse than the TD Group from the perspective of quantitative gait analysis. One of our novel findings is that the PIGD group exhibited specific gait impairments, such as shorter stride length, longer stride time, faster cadence and more variable gait pattern compared with the TD group. In addition, the patients with PIGD had more severe impaired distal joint movements than the TD group. Another novel finding is that levodopa, ChE-I and methylphenidate may be a priority for the PIGD group from the perspective of qualitative gait analysis. Attentional strategy, which involves focusing on the feet while walking, and a kind of rehabilitative therapy, which focuses on strengthening the distal muscles of the lower limbs may improve the gait performance of the PIGD group better.
Acknowledgments
We would like to acknowledge all patients for participating in our study. In addition, we are grateful to JiBuEn gait analysis system for their timely technical help. This study was supported by 1) Special Funds of the Jiangsu Provincial Key Research and Development Projects [grant NO. BE2018610 and BE2019612], 2) Nanjing Medical Science and technique Development Foundation [grant NO. ZKX17031]. 3) Key Research and Development Project of the Ministry of Science and Technology [grant NO. 2016YFC1306600], and 4) Jiangsu Provincial Cadre Health Research Projects [grant NO. BJ16001].
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author.
Ethics Approval and Informed Consent
The study was approved by the Medical Ethics Committee of Affiliated Brain Hospital of Nanjing Medical University. All patients signed informed written consent before the study.
In addition, all mentioned procedures in our study were performed according to the declaration of Helsinki.
Consent for Publication
The authors declare that there are no conflicts of interest regarding the publication of this paper. Consent for publication was obtained from all the authors.
Disclosure
The authors declare no conflicts of interest regarding this work.
References
- 1.Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323(6):548–560. doi: 10.1001/jama.2019.22360 [DOI] [PubMed] [Google Scholar]
- 2.Nonnekes J, Ruzicka E, Nieuwboer A, Hallett M, Fasano A, Bloem BR. Compensation strategies for gait impairments in Parkinson disease: a review. JAMA Neurol. 2019;76(6):718–725. doi: 10.1001/jamaneurol.2019.0033 [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, Jiang X, Zhong M, et al. Wearable sensors measure ankle joint changes of patients with Parkinson’s disease before and after acute levodopa challenge. Parkinson’s Disease. 2020;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smulders K, Dale ML, Carlson-Kuhta P, Nutt JG, Horak FB. Pharmacological treatment in Parkinson’s disease: effects on gait. Parkinsonism Relat Disord. 2016;31:3–13. doi: 10.1016/j.parkreldis.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky MA, Park BS, Nutt JG. Effects of a dopamine agonist on the pharmacodynamics of levodopa in Parkinson disease. Arch Neurol. 2010;67(1):27–32. doi: 10.1001/archneurol.2009.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ondo W, Hunter C, Almaguer M, Gancher S, Jankovic J. Efficacy and tolerability of a novel sublingual apomorphine preparation in patients with fluctuating Parkinson’s disease. Clin Neuropharmacol. 1999;22(1):1–4. doi: 10.1097/00002826-199901000-00001 [DOI] [PubMed] [Google Scholar]
- 7.Ondo WG, Hunter C, Vuong KD, Jankovic J. The pharmacokinetic and clinical effects of tolcapone on a single dose of sublingual apomorphine in Parkinson’s disease. Parkinsonism Relat Disord. 2000;6(4):237–240. doi: 10.1016/S1353-8020(00)00019-5 [DOI] [PubMed] [Google Scholar]
- 8.Giladi N, McDermott MP, Fahn S, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56(12):1712–1721. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic J, Berkovich E, Eyal E, Tolosa E. Symptomatic efficacy of rasagiline monotherapy in early Parkinson’s disease: post-hoc analyses from the ADAGIO trial. Parkinsonism Relat Disord. 2014;20(6):640–643. doi: 10.1016/j.parkreldis.2014.02.024 [DOI] [PubMed] [Google Scholar]
- 10.Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75(14):1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson EJ, Lord SR, Brodie MA, et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, Phase 2 trial. Lancet Neurol. 2016;15(3):249–258. doi: 10.1016/S1474-4422(15)00389-0 [DOI] [PubMed] [Google Scholar]
- 12.Schlachetzki JCM, Barth J, Marxreiter F, et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS One. 2017;12(10):e0183989. doi: 10.1371/journal.pone.0183989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Movement Disorders. 2013;28(5):668–670. [DOI] [PubMed] [Google Scholar]
- 14.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591–1601. doi: 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 15.Goetz CG, Poewe W, Rascol O, et al. Movement disorder society task force report on the hoehn and yahr staging scale: status and recommendations. Movement Disorders. 2004;19(9):1020–1028. doi: 10.1002/mds.20213 [DOI] [PubMed] [Google Scholar]
- 16.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/WNL.40.10.1529 [DOI] [PubMed] [Google Scholar]
- 17.Tao S, Zhang X, Cai H, Lv Z, Hu C, Xie H. Gait based biometric personal authentication by using MEMS inertial sensors. J Ambient Intell Humaniz Comput. 2018;9(5):1705–1712. doi: 10.1007/s12652-018-0880-6 [DOI] [Google Scholar]
- 18.Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galna B, Lord S, Rochester L. Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol. Gait Posture. 2013;37(4):580–585. doi: 10.1016/j.gaitpost.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 20.Herman T, Weiss A, Brozgol M, Giladi N, Hausdorff JM. Gait and balance in Parkinson’s disease subtypes: objective measures and classification considerations. J Neurol. 2014;261(12):2401–2410. doi: 10.1007/s00415-014-7513-6 [DOI] [PubMed] [Google Scholar]
- 21.Vervoort G, Bengevoord A, Nackaerts E, Heremans E, Vandenberghe W, Nieuwboer A. Distal motor deficit contributions to postural instability and gait disorder in Parkinson’s disease. Behav Brain Res. 2015;287:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Movement Disorders. 1997;12(2):206–215. [DOI] [PubMed] [Google Scholar]
- 23.ME M. Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol, Neurosurgery, Psychiatry. 1994;57(12):1532–1534. doi: 10.1136/jnnp.57.12.1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skinner JW, Christou EA, Hass CJ. Lower extremity muscle strength and force variability in persons with Parkinson disease. JNPT. 2019;43(1):56–62. [DOI] [PubMed] [Google Scholar]
- 25.Brognara L, Palumbo P, Grimm B, Palmerini L. Assessing gait in Parkinson’s disease using wearable motion sensors: a systematic review. Diseases. 2019;7:1. doi: 10.3390/diseases7010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Ng SY, Chia NS, et al. Non-motor symptoms in early Parkinson’s disease with different motor subtypes and their associations with quality of life. European J Neurol. 2019;26(3):400–406. [DOI] [PubMed] [Google Scholar]
- 27.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Movement Disorders. 2016;31(8):1095–1102. doi: 10.1002/mds.26510 [DOI] [PubMed] [Google Scholar]
- 28.ML M, KA F, M P, et al. β-Amyloid and postural instability and gait difficulty in Parkinson’s disease at risk for dementia. Movement Disorders. 2013;28(3):296–301. doi: 10.1002/mds.25213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2006;29(1):15–17. [DOI] [PubMed] [Google Scholar]
- 30.Galna B, Lord S, Burn DJ, Rochester L. Progression of gait dysfunction in incident Parkinson’s disease: impact of medication and phenotype. Movement Disorders. 2015;30(3):359–367. doi: 10.1002/mds.26110 [DOI] [PubMed] [Google Scholar]
- 31.Ginis P, Pirani R, Basaia S, et al. Focusing on heel strike improves toe clearance in people with Parkinson’s disease: an observational pilot study. Physiotherapy. 2017;103(4):485–490. doi: 10.1016/j.physio.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 32.Shin KJ, Park J, Ha S, et al. Decreased foot height may be a subclinical shuffling gait in early stage of Parkinson’s disease: A study of three-dimensional motion analysis. Gait Posture. 2019;76:64–67. doi: 10.1016/j.gaitpost.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 33.Skinner JW, Lee HK, Roemmich RT, Amano S, Hass CJ. Execution of activities of daily living in persons with Parkinson disease. Med Sci Sports Exerc. 2015;47(9):1906–1912. [DOI] [PubMed] [Google Scholar]
- 34.Sethi K. Levodopa unresponsive symptoms in Parkinson disease. Movement Disorders. 2008;23(Suppl 3):S521533. [DOI] [PubMed] [Google Scholar]
- 35.Kurz MJ, Hou JG. Levodopa influences the regularity of the ankle joint kinematics in individuals with Parkinson’s disease. J Comput Neurosci. 2010;28(1):131–136. doi: 10.1007/s10827-009-0192-0 [DOI] [PubMed] [Google Scholar]