Abstract
Purpose
Cutaneous squamous cell carcinoma (cSCC) is the most common second basal cell carcinoma in our population. Wogonoside, the main in vivo metabolite of wogonin, possesses anti-inflammatory, anti-angiogenesis and anti-cancer activities. Nevertheless, the effectiveness of wogonoside therapy on cSCC has not been clarified.
Methods
In this study, we investigated the effects of wogonoside on cell proliferation, invasion, epithelial–mesenchymal transition (EMT) and cancer stem-like cell (CSC) properties of SCL-1 and SCC12 cell lines, and the effects on tumor formation in vivo. In vitro, cells were treated with 0, 25, 50 and 100 μM wogonoside for 48 h. In vivo, SCL-1 cells were subcutaneously injected into the right thigh of mice to form xenograft tumors. Animals were randomly divided into two groups (n=10): the control group and the 80 mg/kg wogonoside group.
Results
The results showed that wogonoside attenuated proliferation, invasion and EMT of SCL-1 and SCC12 cell lines, and enhanced the rate of apoptosis. Meanwhile, wogonoside efficiently abolished the CSC traits of cSCC; the expression of CSC markers (ALDH1, SOX-2, Oct4 and CD44) and the percentage of CD133+ cells were remarkably downregulated. In addition, we found that wogonoside repressed the activation of both PI3K/AKT and Wnt/β-catenin pathways. In vivo, wogonoside significantly inhibited tumor formation.
Conclusion
The results indicated that wogonoside could attenuate cSCC by reducing EMT, invasion and CSC properties. The efficacy of intervention may be related to inhibition of the PI3K/Akt and Wnt/β-catenin pathways. These novel findings could furnish new ideas on the potential therapeutic application of wogonoside in cSCC cancellation and cancer intervention.
Keywords: cSCC, ALDH1, Wnt/β-catenin pathway, PI3K/AKT pathway, EMT
Introduction
Cutaneous squamous cell carcinoma (cSCC) is a common non-melanoma skin cancer associated with basal cell carcinoma.1–3 Its main feature is invasiveness, which always triggers metastasis. Despite considerable advances in cSCC therapy, the incidence of cSCC has been increasing in several countries in recent years.4 At present, the main curative approach is surgical resection. Radiotherapy and chemotherapy are also typical treatments. However, most of these methods are associated with side-effects and recurrences. Therefore, the selection of appropriate and effective therapeutic drugs is of important clinical significance.
The PI3K/AKT pathway plays an important role in cancer stem cells (CSCs), including the ability to maintain colony formation and proliferation.5,6 At the same time, targeting the PI3K/AKT pathway could drastically reduce the bulk tumor burden and slow down the metabolism of CSCs.6,7 The PI3K/AKT pathway is involved in the treatment of a variety of squamous cell carcinomas.8,9 Evidence revealed that the Wnt/β-catenin pathway initiated a variety of CSC aberrations. After activation of the Wnt signal, β-catenin is separated from the disruption complex and transferred into the nucleus, where it forms a complex with the T-cell factor/lymphoid enhancer and activates the transcription of downstream genes, including Cyclin D1, c-Myc, CD44 and ALDH.10 The Wnt/β-catenin pathway has also been confirmed as a carcinogenic pathway and plays a key role in maintaining CSC activity. Research has shown that wogonoside has effects on both the PI3K/Akt and Wnt/β-catenin pathways.11,12
Scutellaria baicalensis georgi (Chinese name: Huangqin) is one of the most well-known herbs in traditional Chinese medicine (TCM), the root of which is included in the 50 fundamental herbs.13,14 Scutellaria baicalensis georgi is widely used in the traditional medical systems of China and Japan, and is generally used to treat many diseases and conditions, such as gingivitis, inflammation, pneumonia, tumors and cancer.15–17 Wogonoside is a naturally bioactive flavonoid derived from the root of Scutellaria baicalensis georgi. Wogonoside possesses a variety of biological activities, including anti-inflammatory, anti-angiogenesis and anti-cancer activities.18–20 Although wogonoside is a potential cancer treatment drug, its therapeutic effect on cSCC has not been studied, and the relevant mechanism has not been elucidated. Therefore, this study aimed to investigate the suppressive effects of wogonoside on cSCC and its mechanism of action.
Materials and Methods
Materials
Wogonoside (purity >98%) was purchased from Langze Pharmaceutical Co. Ltd (Nanjing, China) and the solvent was dimethylsulfoxide (DMSO). The chemical structure of wogonoside is shown in Figure 1A. RPMI medium, fetal bovine serum (FBS), streptomycin double antibody and trypsin were purchased from Kaixin Bioengineering Co. Ltd.
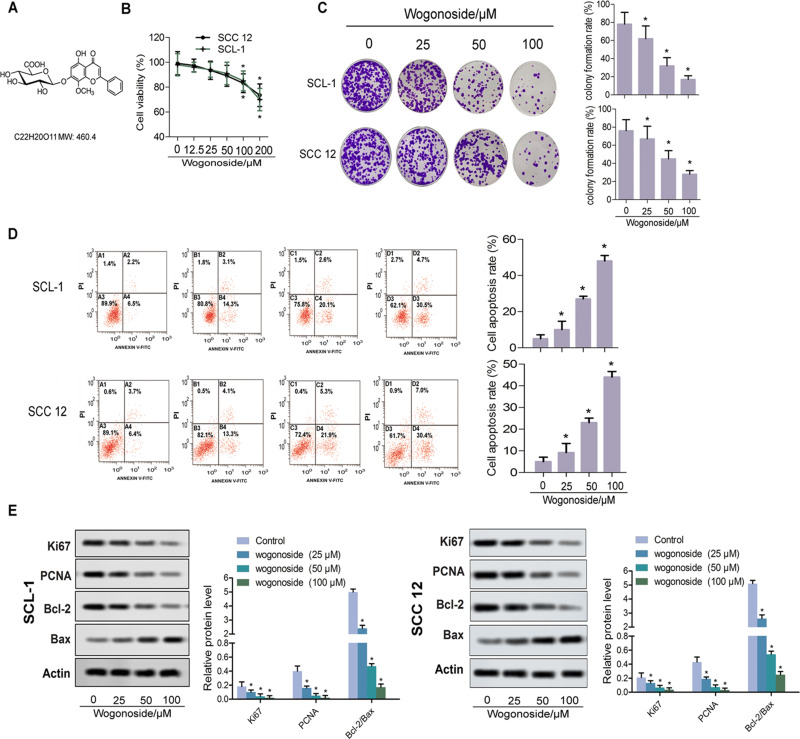
Figure 1.
Wogonoside suppressed proliferation of SCL-1 and SCC12 cells. (A) Chemical structure of wogonoside. (B) Effect of wogonoside on the viability of SCL-1 and SCC12 cells, detected by the CCK-8 assay. (C) Proliferation of SCL-1 and SCC12 cells, detected by the clonal formation assay. (D) Apoptosis rates of SCL-1 and SCC12 cells, detected by flow cytometry. (E) Western blot assay for the detection of relative protein levels of apoptotic proteins (Ki67, PCNA, Bcl-2 and Bax). *p<0.05 vs control.
Cell Culture
SCL-1 cells and SCC12 cells (Ginny Ginson Material Technology Co. Ltd) were cultured in RPMI-1640 medium with 10% serum, 0.1 mg/mL streptomycin and 100 U/mL penicillin in a cell culture chamber under 90% air and 5% CO2 at 37°C. When 90% of the cells had adhered to the wall, they could be subcultured once in 2–3 days. The logarithmic growth cells were taken for experiments.
Animal Models
Twenty BALB/c nude mice (male, 7–8 weeks old) were provided by the Animal Center of Luoyang Central Hospital affiliated to Zhengzhou University. All mice had free access to food and water under feeding conditions, at 25±3°C with 55–60% humidity under a 12-h light/dark cycle. After a week of adjustment, mice were divided into two groups (n=10): control group and 80 mg/kg wogonoside group (intragastric administration, dosing frequency once every other day).11 A week later, SCL-1 cells were subcutaneously injected into the right thigh to form xenograft tumors. Survival curves was continuously measured within 25 days after injection. Tumor weight was determined on the 25th day after injection. After experimentation, mice were killed by intraperitoneal injection of pentobarbital sodium (200 mg/kg body weight). Tumors were collected for subsequent testing in vivo.
The animal experiments in the present study were carried out according to the Guidelines for the Care and Use of NIH Experimental Animals and approved by Luoyang Central Hospital affiliated to Zhengzhou University (LYZX0037).
Cell Viability Assay and Cell Proliferation
The cytotoxicity of wogonoside on SCL-1 and SCC12 cells was examined by Cell Counting Kit-8 (CCK-8; Sigma-Aldrich, USA). Cells were seeded at a density of 5×103 cells/well into 96-well plates (Corning, New York, NY, USA). After 24 h of culture, the cells were treated with different concentrations of wogonoside (25, 50 and 100 μM) for 48 h in an incubator at 37°C with 5% CO2. Next, CCK-8 was added and the cells were incubated for 4 h under the same conditions. Each group consisted of five parallel wells. Afterwards, 10 μL/well of CCK-8 dye (5 mg/mL) was added, and then the plate was incubated at 37°C for an additional 4 h in a 5% CO2 atmosphere. Then, the absorbance at 450 nm was measured by a microplate reader (GENios; Tecan, Männedorf, Switzerland).21
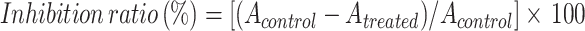 |
Colony Formation Assay
For colony formation assays, 5×103 SCl-1 cells and SCC12 cells were each seeded in triplicate to six-well plates overnight. After 10 days of culture, visible cells were washed with phosphate-buffered saline (PBS), fixed with methanol and then stained with Giemsa. Colonies with N50 cells were counted.22
Immunofluorescence Assay
To estimate the changes in vimentin level in SCL-1 cells and SCC12 cells treated with wogonoside, an immunofluorescent assay was conducted. Cells were seeded onto small glass dishes and transfected with wogonoside. Then, cells were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.5% Triton X-100 for another 30 min and blocked overnight at 37°C with 5% BSA in TBST.
After being pretreated with primary antibody vimentin (#5741, CST) at 4°C overnight, cells were incubated with Alexa Fluor® 594 secondary antibody (1:2000, cat. no. Z-25307; Thermo Fisher) and stained with 4,6-diamidino-2-phenylindole (DAPI) (1:1000, cat. no. D9564; Sigma-Aldrich) at room temperature for 3 h. Subsequently, the cells were washed with PBS and analyzed by confocal microscopy (LSM 510 Meta; Zeiss, Oberkochen, Germany). The experiment was independently repeated in triplicate.
Transwell Assay
The invasive capacity of SCl-1 cells and SCC12 cells was analyzed by a transwell assay. Matrigel was diluted with serum-free RPMI-1640 medium, then applied to the middle bottom of the upper chamber and air-dried at room temperature for later use. After incubating for 24 h with serum-free medium, cell suspension was added into the upper chambers (200 mL per chamber). The lower chambers were filled with 600 μL medium containing 10% FBS. After incubation at 37°C for 24 h, the chambers were removed and washed twice with PBS. The residual cells were cleared using cotton buds. After fixation with 95% alcohol and staining with crystal violet, cells were examined under a microscope (Leica, Germany) and the average number of invasive cells was recorded. The experiments were independently repeated in triplicate.
Microtube Formation Assay
SCL-1 and SCC12 cells were each retreated with wogonoside at different concentrations (25, 50 and 100 μM) for 48 h. Matrigel was mixed with serum-free RPMI-1640 medium precooled to 4°C in a ratio of 1:1. The mixture was laid on the bottom of a 24-well plate, 300 μL per hole, and cured in an incubator at 37°C with 5% CO2 for 30 min. The SCL-1 and SCC12 cells treated with wogonoside were inoculated into the above-mentioned gelatinized 24-well plate after adjusting the concentration to 1.2×105/mL in serum-free RPMI-1640 medium. The microtubule structure was observed every 4 h. Five visual fields were randomly taken under the microscope (100×). Microvision Saisam software was used to analyze the images, and the microtubule-like structures were counted, with five visual fields per hole. The experiments were independently repeated in triplicate.
Tumorsphere Formation Assay
The method is described in previous research literature.23 In brief, cells were inoculated into an ultra-low-attachment 24-well plate at a density of 5×103 cells/well with the addition of fresh medium every 2 days. Three concentrations of wogonoside (25, 50 and 100 μM) were used to interrupt SCL-1 and SCC12 sphere-forming cells and 0.1% DMSO was used as the control. After culturing for 7 days, the tumorspheres of each concentration were imaged with a microscope (d >50 μm).
Flow Cytometry
Following treatment with each concentration of wogonoside, the numbers of CD133+ cells in sphere-forming cells were measured, referring to the previous report.23 In brief, the detection of CD133+ cells was performed according to the manufacturer’s protocol and measured by flow cytometry analysis.
TUNEL Assay
The prepared paraffin sections were dewaxed, rehydrated and washed. Then, the sections were incubated with TUNEL reaction mixture (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions. Apoptotic cells were detected by fluorescence microscopy (Olympus, Japan).
Immunohistochemistry
The expression of vimentin, VEGF and SOX-2 in the nude mouse model was evaluated by immunohistochemistry. After dewaxing, rehydration and repair, sections were sealed with 5% normal goat serum for 1 h and co-incubated with the primary antibodies vimentin (#5741, CST), VEGF (#2479, CST) and SOX-2 (#3728, CST) overnight at 4°C. Then, sections were treated according to the manufacturer’s instructions and analyzed by microscopy.
Western Blot Analysis
Cells were collected after 48 h of pretreatment with wogonoside. After rinsing, cells were treated with radioimmunoprecipitation assay lysis buffer, dissolving the protease inhibitors. Western blotting was performed according to the previous literature.19 The primary antibodies were as follows: Ki-67, PCNA, E-cadherin, N-cadherin, fibronectin, VEGF, MMP-9, MMP-14, CD44, Bcl-2, Bax, SOX-2, ALDH1, Oct4, AKT, p-AKT, β-catenin, p-β-catenin, Actin, STAT3, p-STAT3, p65, p-p65, vimentin and CD133+. They were all obtained from Santa Cruz Biotechnology (USA). The relative protein level was analyzed by ImageJ software. The experiments were independently repeated in triplicate.
Statistical Analysis
Data are shown as mean ± SD. Multiple comparisons of parametric data were measured by one-way ANOVA, followed by Bonferroni’s multiple test between group pairs (SPSS 21.0; IBM Corp.,). p<0.05 was considered statistically significant.
Results
Wogonoside Suppressed Proliferation of SCL-1 and SCC12 Cells
The toxicity of wogonoside on SCL-1 and SCC12 cells was detected by the CCK-8 assay. Figure 1B shows that 100 μM and 200 μM wogonoside inhibited the cell viability of SCL-1 and SCC12 cells significantly. Thus, concentrations of 25, 50 and 100 μM wogonoside were used in subsequent experiments. Compared with the control group, different concentrations of wogonoside significantly suppressed the colony formation rate of SCL-1 and SCC12 cells (Figure 1C), and improved their apoptosis rate (Figure 1D). Western blot assay further confirmed the interventional role of wogonoside on SCL-1 and SCC12 cells. From Figure 1E, it can be seen that, compared with the control group, the levels of PCNA and Ki67 were significantly reduced in SCL-1 and SCC12 cells treated with wogonoside. It is worth noting that the ratio of Bcl-2/Bax was decreased after wogonoside treatment (Figure 1E). These results suggest that wogonoside suppressed the proliferation of SCL-1 and SCC12 cells, and improved their apoptosis rate.
Wogonoside Inhibited the Epithelial–Mesenchymal Transition (EMT) of SCL-1 and SCC12 Cells
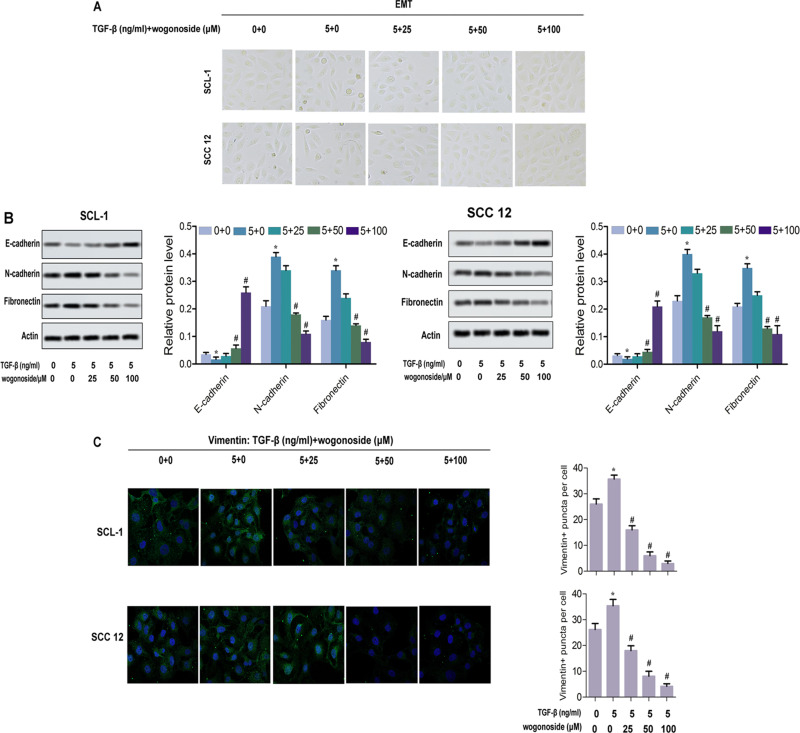
In this study, TGF-β was used to induce EMT of SCL-1 and SCC12 cells. As shown in Figure 2A, compared with the control group, high concentrations of wogonoside prevented EMT in both cell lines. Therefore, wogonoside obviously restrained the morphological epithelial and mesenchymal transformation. Then, the expression levels of EMT marker proteins were detected by Western blot. After treatment with wogonoside, the levels of N-cadherin and fibronectin were significantly downregulated in both SCL-1 and SCC12 cells compared with the control group. Meanwhile, the level of the epithelial marker protein E-cadherin was increased by wogonoside (Figure 2B). To confirm the effect of wogonoside on EMT of SCL-1 cells, the content of vimentin was detected by immunofluorescence. As illustrated in Figure 2C, wogonoside treatment obviously downregulated the number of vimentin-positive puncta in both cell types. Hence, these observations imply that wogonoside effectively inhibited the EMT of SCL-1 cells.
Figure 2.
Wogonoside inhibited the EMT of SCL-1 and SCC12 cells. (A) Typical pictures of EMT. (B) Western blot assay for the expression levels of labeled proteins (E-cadherin, N-cadherin and fibronectin) in SCL-1 and SCC12 cells. (C) Immunofluorescence detected vimentin puncta in both SCL-1 and SCC12 cells. *p<0.05 vs control, #p<0.05 vs TGF-β-treated group.
Wogonoside Attenuated the Athletic Ability of SCL-1 and SCC12 Cells
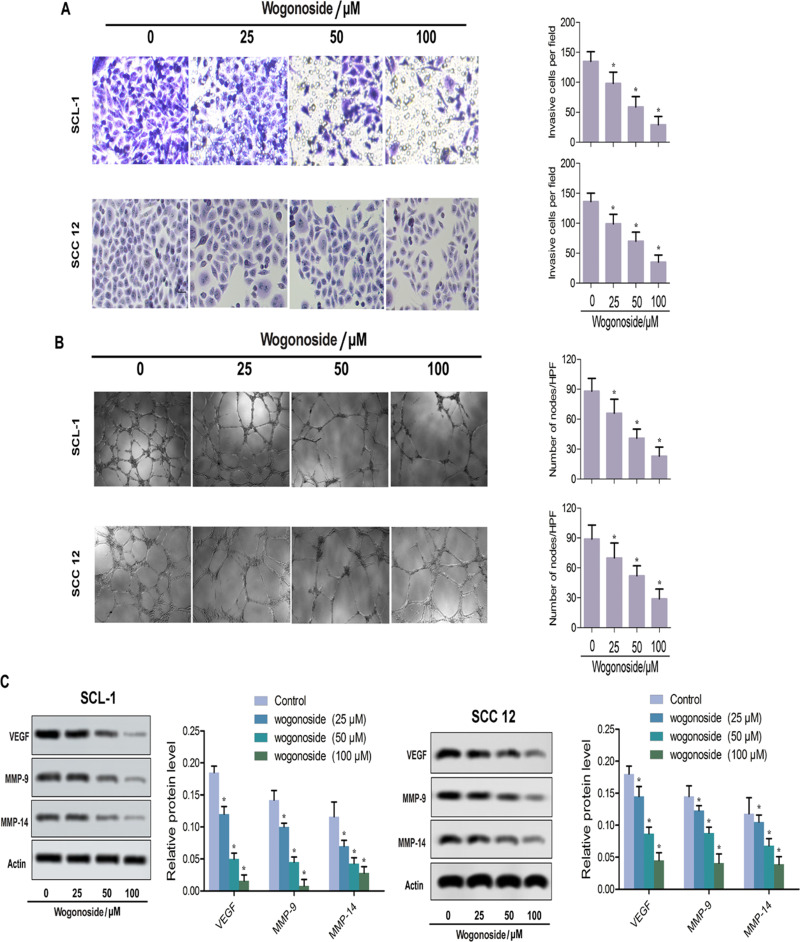
In the transwell assay, SCL-1 and SCC12 cells were treated with three concentrations of wogonoside (25, 50 and 100 μM) for 48 h. After wogonoside treatment, the invasive ability of cells was decreased (Figure 3A). In the microtubule formation assay, wogonoside suppressed the formation of microtubule-like structures (Figure 3B). With wogonoside treatment, the levels of VEGF, MMP-9 and MMP-14 were obviously downregulated in both SCL-1 and SCC12 cells (Figure 3C). These results suggest that wogonoside affects the athletic ability of SCL-1 and SCC12 cells.
Figure 3.
Wogonoside attenuated the athletic ability of SCL-1 and SCC12 cells. (A) Typical pictures of cells from the transwell assay and invasive cells per field. (B) Ultrastructural changes of microtubules in SCL-1 and SCC12 cells and number of nodes/HPF. (C) Western blot assay for the expression of marked proteins (VEGF, MMP-9 and MMP-14). *p<0.05 vs control.
Wogonoside Attenuated the Stem Cell Characteristics of SCL-1 and SCC12 Cells
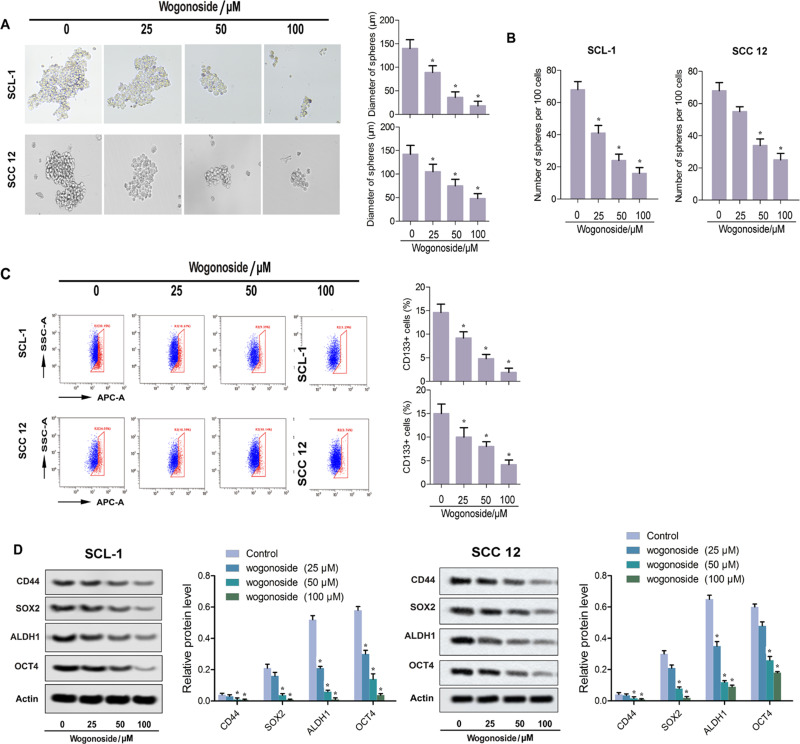
As shown in Figure 4A, stable tumorspheres of SCL-1 and SCC12 cells were formed under three concentrations of wogonoside treatment. Compared with the control group, wogonoside treatment obviously suppressed the formation of tumorspheres, while the average diameter of spheres and number of spheres per 100 cells were reduced significantly (Figure 4A and B). The expression levels of CSC markers, CD44, CD133+, SOX, ALDH1 and Oct4, were analyzed to further characterize spherical cells. The percentage of CD133+ cells was decreased dramatically after wogonoside treatment (Figure 4C). The expression of CD44, SOX, ALDH1 and Oct4 all declined obviously in SCL-1 sphere-forming cells in the wogonoside treatment groups compared with the control group. Meanwhile, the relative protein level of each marker protein was also downregulated significantly with increasing wogonoside concentrations (Figure 4D). Together, these results suggest that wogonoside attenuated the stem cell characteristics of SCL-1 and SCC12 cells.
Figure 4.
Wogonoside attenuated the stem cell characteristics of SCL-1 and SCC12 cells. (A) Representative pictures of tumorspheres and diameter of spheres in each group. (B) Number of spheres per 100 cells. (C) Flow cytometry for the determination of CD133 activities. (D) Western blot assay for the expression of stem cell marker proteins (CD44, SOX2, ALDH1 and Oct4). *p<0.05 vs control.
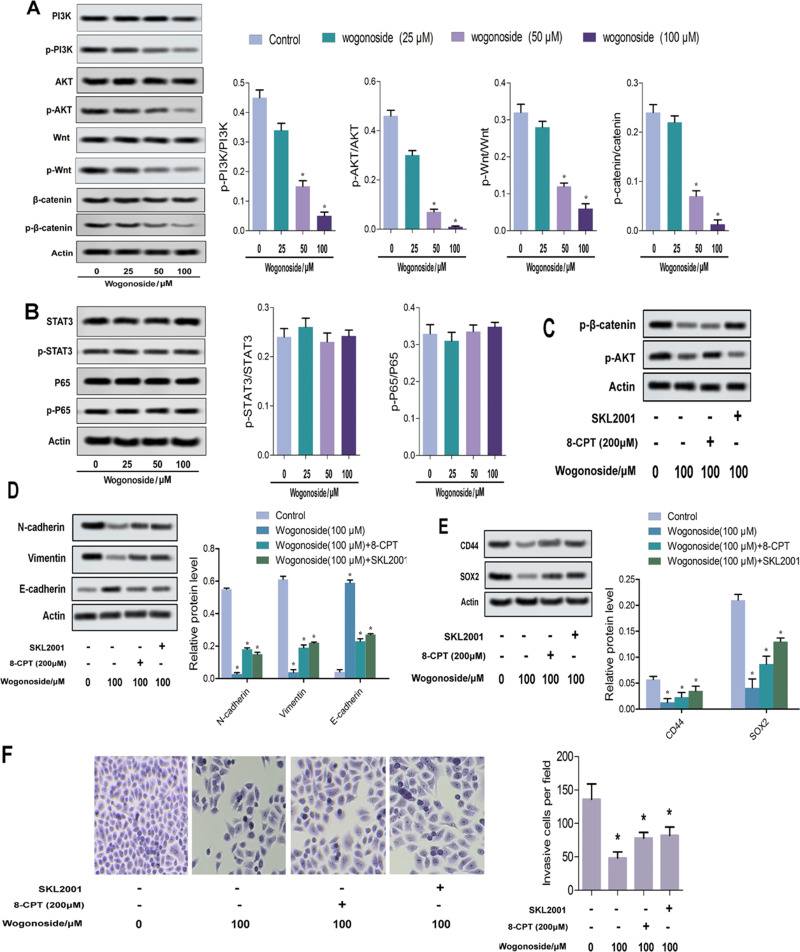
Wogonoside Suppressed the Activities of Wnt/β-Catenin and PI3K/Akt Pathways
It has been documented that wogonoside mediates several cancer processes by inhibiting the activities of several pathways, of which Wnt/β-catenin and PI3K/Akt are the two most common.12,24 In this study, we explored the effects of wogonoside on four pathways, namely Wnt/β-catenin, PI3K/Akt, STAT3 and NF-κB/p65, to determine the wogonoside inhibition pathway in cSCC. As shown in Figure 5A and B, the expression levels of p-PI3K, p-Wnt, p-β-catenin and p-AKT protein in the wogonoside group were significantly lower than those in the control group, while the expression levels of p-STAT3 and p-p65 protein did not change (Figure 5A and B). As shown in Figure 5C, wogonoside could partially counteract the effects of Akt agonist 8-cpt (200 μM) and β-catenin agonist skl2001. In order to further confirm the effect of wogonoside, we verified the effect of wogonoside on EMT, cell stem characteristics and invasion of SCL-1 cells (Figure 5D–F). The results are consistent with those in Figure 5C.
Figure 5.
Wogonoside suppressed the activities of Wnt/β-catenin and PI3K/Akt pathways. (A and B) Western blot assay detected the expression of pathway proteins. (C–F) Wogonoside partially counteracted the activation of Akt and β-catenin by agonists in vitro. *p<0.05 vs control.
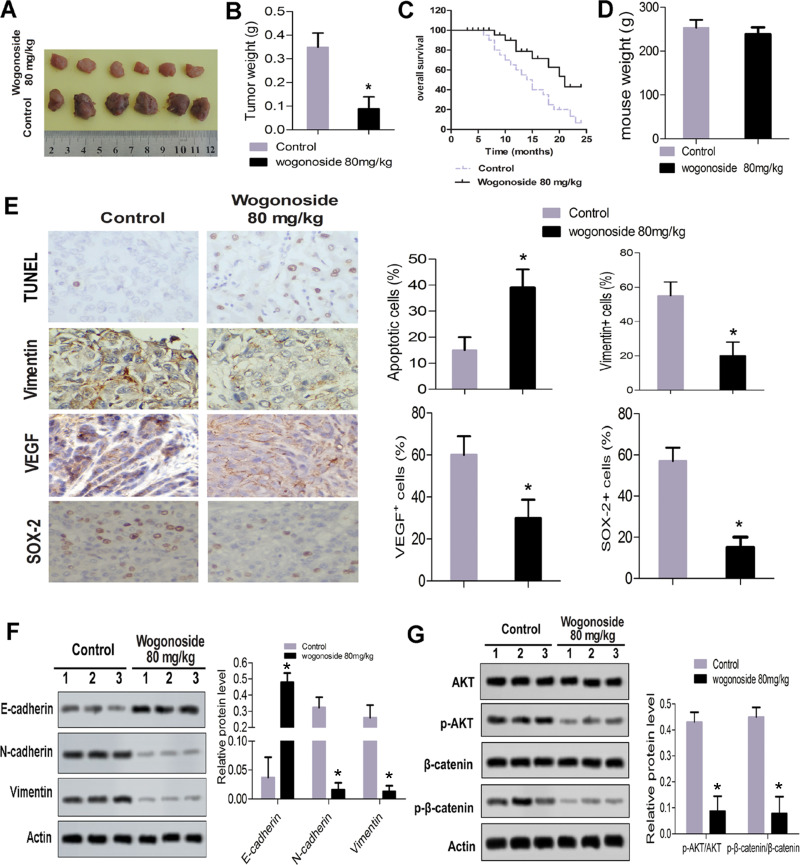
Wogonoside Inhibited Tumor Formation In Vivo
As shown in Figure 6A, the sizes of tumor tissue in the 80 mg/kg wogonoside group were significantly smaller than in the control group. Compared with the control group, the mean weight of tumor in the 80 mg/kg wogonoside group was reduced markedly (Figure 6B). As shown in Figure 6C, the Kaplan–Meier curve shifted obviously to the right. This result revealed that wogonoside treatment increased the survival rate of nude mice. As shown in Figure 6D, wogonoside treatment had no effect on the weight of mice. The results indicate that wogonoside had no toxic effect on nude mice and inhibited tumor growth in vivo. The TUNEL experiment results showed that the percentage of apoptotic cells was upregulated dramatically compared to the control group (Figure 6E). As shown in Figure 6E, the percentage of SOX-2, one of the most basic stem cell genes, was downregulated significantly in the 80 mg/kg wogonoside group compared with the control group. Similarly, compared with the control group, the percentages of VEGF and vimentin were downregulated obviously in the 80 mg/kg wogonoside group (Figure 6E). As shown in Figure 6F, the effect of wogonoside on EMT-related proteins in vivo was consistent with that in vitro. As shown in Figure 6G, the phosphorylation of p-AKT/AKT and p-β-catenin/β-catenin was reduced significantly. These results suggest that wogonoside inhibited the activities of the Wnt/β-catenin and PI3K/AKT pathways.
Figure 6.
Wogonoside inhibited tumor formation in vivo. (A) Sizes of the tumor tissue. (B) Mean weight of tumor. (C) Kaplan–Meier curve of nude mouse model. (D) Wogonoside had no toxic effect on mice. (E) Results of immunohistochemical analysis. (F) Effect of wogonoside on EMT in vivo. (G) Western blot assay for the expression of pathway proteins (AKT, p-AKT, β-catenin and p-β-catenin). *p<0.05 vs control.
Discussion
Scutellaria baicalensis georgi is a common TCM herb with a long history and a broad spectrum of cancer treatment applications. Wogonin, one of major active components of Scutellaria baicalensis georgi, has exhibited anti-cancerous and outstanding tumor-inhibitory properties.25–27 Wogonin has been confirmed to exert anti-metastatic effects in several solid tumors, such as hepatocellular cancer, melanoma and breast cancer.28–30 Wogonoside, a main in vivo metabolite of wogonin, possessed the same anti-metastatic effect on breast cancer.20 Although the anti-metastatic effect of wogonoside was confirmed, the potential mechanism of wogonoside has not been clearly elucidated. Meanwhile, whether wogonoside possessed anti-metastatic effects on cSCC was not reported. In the present study, we determined the inhibitory effects of wogonoside on cSCC and revealed its possible mechanism.
The ability of clonal proliferation is a typical feature of tumor cells. It has been reported that wogonin can effectively inhibit the proliferation of tumor cells, which is consistent with our experimental results.31 The results indicated that wogonoside inhibited cell proliferation by decreasing the expression levels of Ki67 and PCNA in SCL-1 and SCC12 cells. The ratio of Bcl-2/Bax is one of the key indicators for characterizing apoptosis. When the Bcl-2/Bax ratio decreases, it suggests the occurrence of apoptosis.32 Li et al found that wogonoside caused apoptosis of Bel-7402, a cell line separated from hepatocellular carcinoma, by regulating Bax/Bcl-2.33 Therefore, it is speculated that the suppression of SCL-1 and SCC12 cell proliferation may be related to the apoptosis induced by the wogonoside treatment.
EMT is an indispensable part of embryonic development and organogenesis. EMT is currently considered to be one of the key and initial links in the invasion and metastasis of tumor cells.22,34,35 EMT is generally recognized as a key factor in the dissociation of cancer cells from primary tumors and subsequent infiltration into blood vessels.36 During the process of EMT, cells lose their epithelial traits, including cell adhesion and polarity, and obtain a mesenchymal morphology and the ability to migrate. Biochemically, cells switch off the expression of epithelial markers such as the adherens junction protein E-cadherin, and turn on mesenchymal markers including vimentin and fibronectin.37,38 Wei et al found that wogonoside could inhibit the occurrence of EMT.39 Similarity, the present study showed that wogonoside downregulated the expression of fibronectin, N-cadherin and vimentin, and upregulated the expression of E-cadherin, both in cells and in a mouse model. The results indicated that wogonoside suppressed the EMT process of cSCC both in vitro and in vivo.
Tumor metastasis is the leading cause of cancer-related death, in which infiltration plays a critical role. During the infiltration, tumor cells first lose their intercellular junctions, then degrade, remodel and adhere to the surrounding extracellular matrix (ECM), and finally migrate to distant places via the ECM.40 VEGF is a highly specific pro-vascular endothelial growth factor that promotes increased vascular permeability, ECM degeneration, vascular endothelial cell migration, proliferation and angiogenesis. It has been reported that wogonin inhibited the autocrine secretion of VEGF in endothelial cells, and suppressed the secretion of VEGF and restrained angiogenesis in tumor cells.41 We found that wogonoside was able to suppress invasion and microtubule formation in SCL-1 and SCC12 cells. Simultaneously, abnormal expression of MMP-9, MMP-14 and VEGF in SCL-1 and SCC12 cells led to enhanced invasion and weakened ability to form microtubules.
CSCs are considered as rare tumor cells with the ability to continuously self-renew and differentiate.23 In our study, wogonoside inhibited the formation of tumorspheres and the percentage of CD133+ dramatically. On the other hand, wogonoside decreased the expression of CD44, SOX2, ALDH1 and Oct4. Collectively, wogonoside repressed the CSC-like properties of cSCC.
Numerous studies have shown that wogonoside exerts different anti-tumor effects by regulating different pathways. Han et al found that wogonoside inhibited the growth of human colon cancer cells via the PI3K/AKT/mTOR/p70S6K signaling pathway and induced mitochondria-mediated autophagy-associated apoptosis.12 Huang et al reported that wogonoside suppressed angiogenesis in breast cancer by inhibiting the Wnt/β-catenin pathway.11 Xiao et al found that wogonoside had a growth-inhibiting effect on T-acute lymphoblastic leukemia through the STAT3 pathway.42 Sun et al reported that wogonoside activates NF-κB by inhibiting the PI3K/Akt pathway, thereby preventing the development of colitis-associated colorectal cancer and the progression of colon cancer in an inflammation-related microenvironment.24 On the other hand, pathways related to cSCC have also been investigated by researchers. Ci et al found that downregulation of kynureninase inhibited proliferation of cSCC and restrained the PI3K/AKT pathway.43 Li et al reported that microRNA-374 affected the proliferation, migration, invasion and apoptosis of cSCC cells by targeting Gadd45a through the p53 signaling pathway.44 However, the mechanism of wogonoside on cSCC has not been clarified. We found that phosphorylation of AKT and β-catenin was significantly reduced by wogonoside treatment, while it had little effect on STAT3 and p65. In addition, wogonoside can partially counteract the activation of Akt and β-catenin by agonists in vitro. The PI3K/AKT signaling pathway is regulated by multiple factors and steps. It has been reported that phosphorylation of Ser473 of AKT activates various downstream factors and further regulates various biological activities of cells.9 These data suggest that wogonoside can reduce the phosphorylation of AKT and β-catenin in SCL-1 and SCC12 cells, presumably by delaying the activation of the PI3K/AKT and Wnt/β-catenin signaling pathways to delay the process of cSCC. Experiments in vivo illustrated that 80 mg/kg wogonoside suppressed tumor growth and shifted the overall survival line to the right. Compared to the control, 80 mg/kg wogonoside obviously enhanced the percentage of apoptotic cells and decreased the percentage of vimentin, VEGF and SOX-2. We speculate that these outcomes are related to regulation of the PI3K/Akt and Wnt/β-catenin pathways by wogonoside.
Conclusion
Wogonoside could inhibit the proliferative capacity, EMT process, invasion and microtubule-forming abilities of SCL-1 cells; moreover, wogonoside could inhibit the stem cell characteristics of SCL-1 cells in vitro. Wogonoside also inhibited tumor formation in a nude mouse model in vivo. The main mechanism may involve inhibition of the PI3K/AKT and Wnt/β-catenin signaling pathways. Therefore, wogonoside may be a promising cSCC therapeutic.
Author Contributions
Xiuyong Wang, Yuan Chang, Ming Gao and Fan Zhang made a significant contribution to the work reported in the conception, study design, execution, acquisition of data, analysis and interpretation. Xiuyong Wang and Ming Gao wrote the manuscript. Xiuyong Wang and Yuan Chang have critically reviewed the article. All authors have read and approved the final manuscript. All authors have agreed on the journal to which the article will be submitted. All authors agree to take responsibility and be accountable for the contents of the article.
Abbreviations
cSCC, cutaneous squamous cell carcinoma; CSC, cancer stem cell; TCM, traditional Chinese medicine; DMSO, dimethylsulfoxide; PBS, phosphate-buffered saline; DAPI, 4,6-diamidino-2-phenylindole; EMT, epithelial–mesenchymal transition; ECM, extracellular matrix.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sadek H, Azli N, Wendling JL, et al. Treatment of advanced squamous cell carcinoma of the skin with cisplatin, 5-fluorouracil, and bleomycin. Cancer. 1990;66(8):1692–1696. doi: [DOI] [PubMed] [Google Scholar]
- 2.Jagdeo J, Weinstock MA, Piepkorn M, Bingham SF. Reliability of the histopathologic diagnosis of keratinocyte carcinomas. J Am Acad Dermatol. 2007;57(2):279–284. doi: 10.1016/j.jaad.2007.03.021 [DOI] [PubMed] [Google Scholar]
- 3.Filipowicz E, Adegboyega P, Sanchez RL, Gatalica Z. Expression of CD95 (Fas) in sun-exposed human skin and cutaneous carcinomas. Cancer. 2002;94(3):814–819. doi: 10.1002/cncr.10277 [DOI] [PubMed] [Google Scholar]
- 4.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187 [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Wulfkuhle J, Zhang H, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104(41):16158–16163. doi: 10.1073/pnas.0702596104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marhold M, Tomasich E, El-Gazzar A, et al. HIF1alpha regulates mTOR signaling and viability of prostate cancer stem cells. Mol Cancer Res. 2015;13(3):556–564. doi: 10.1158/1541-7786.MCR-14-0153-T [DOI] [PubMed] [Google Scholar]
- 7.Kolev VN, Wright QG, Vidal CM, et al. PI3K/mTOR dual inhibitor VS-5584 preferentially targets cancer stem cells. Cancer Res. 2015;75(2):446–455. doi: 10.1158/0008-5472.CAN-14-1223 [DOI] [PubMed] [Google Scholar]
- 8.Xi R, Pan S, Chen X, et al. HPV16 E6-E7 induces cancer stem-like cells phenotypes in esophageal squamous cell carcinoma through the activation of PI3K/Akt signaling pathway in vitro and in vivo. Oncotarget. 2016;7(35):57050–57065. doi: 10.18632/oncotarget.10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan S, Sun Y, Sui D, et al. Lobaplatin promotes radiosensitivity, induces apoptosis, attenuates cancer stemness and inhibits proliferation through PI3K/AKT pathway in esophageal squamous cell carcinoma. Biomed Pharmacother. 2018;102:567–574. doi: 10.1016/j.biopha.2018.03.109 [DOI] [PubMed] [Google Scholar]
- 10.Kim YM, Kahn M. The role of the Wnt signaling pathway in cancer stem cells: prospects for drug development. Res Rep Biochem. 2014;4:1–12. doi: 10.2147/RRBC.S53823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Zhao K, Hu Y, et al. Wogonoside inhibits angiogenesis in breast cancer via suppressing Wnt/β-catenin pathway. Mol Carcinog. 2016;55(11):1598–1612. doi: 10.1002/mc.22412 [DOI] [PubMed] [Google Scholar]
- 12.Han C, Xing G, Zhang M, et al. Wogonoside inhibits cell growth and induces mitochondrial-mediated autophagy-related apoptosis in human colon cancer cells through the PI3K/AKT/mTOR/p70S6K signaling pathway. Oncol Lett. 2018;15(4):4463–4470. doi: 10.3892/ol.2018.7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Lee YJ, Kim HY, et al. Beneficial effects of scutellaria baicalensis on penile erection in streptozotocin-induced diabetic rats. Am J Chin Med. 2016;44(2):305–320. doi: 10.1142/S0192415X1650018X [DOI] [PubMed] [Google Scholar]
- 14.Chen JJ, Huang CC, Chang HY, et al. Scutellaria baicalensis ameliorates acute lung injury by suppressing inflammation in vitro and in vivo. Am J Chin Med. 2017;45(1):137–157. doi: 10.1142/S0192415X17500100 [DOI] [PubMed] [Google Scholar]
- 15.Arweiler NB, Pergola G, Kuenz J, Hellwig E, Sculean A, Auschill TM. Clinical and antibacterial effect of an anti-inflammatory toothpaste formulation with Scutellaria baicalensis extract on experimental gingivitis. Clin Oral Investig. 2011;15(6):909–913. doi: 10.1007/s00784-010-0471-1 [DOI] [PubMed] [Google Scholar]
- 16.Kim EH, Shim B, Kang S, et al. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J Ethnopharmacol. 2009;126(2):320–331. doi: 10.1016/j.jep.2009.08.027 [DOI] [PubMed] [Google Scholar]
- 17.Cheng CS, Chen J, Tan HY, Wang N, Chen Z, Feng Y. Scutellaria baicalensis and cancer treatment: recent progress and perspectives in biomedical and clinical studies. Am J Chin Med. 2018;46(1):25–54. doi: 10.1142/S0192415X18500027 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Lu N, Ling Y, et al. Wogonoside inhibits lipopolysaccharide-induced angiogenesis in vitro and in vivo via toll-like receptor 4 signal transduction. Toxicology. 2009;259(12):10–17. doi: 10.1016/j.tox.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 19.Lim BO. Effects of wogonin, wogonoside, and 3,5,7,2ʹ,6ʹ-pentahydroxyflavone on chemical mediator production in peritoneal exduate cells and immunoglobulin E of rat mesenteric lymph node lymphocytes. J Ethnopharmacol. 2003;84(1):23–29. doi: 10.1016/S0378-8741(02)00257-X [DOI] [PubMed] [Google Scholar]
- 20.Yao Y, Zhao K, Yu Z, et al. Wogonoside inhibits invasion and migration through suppressing TRAF2/4 expression in breast cancer. J Exp Clin Cancer Res. 2017;36(1):103. doi: 10.1186/s13046-017-0574-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Wang H, Li W, et al. Pazopanib, a novel multi-kinase inhibitor, shows potent antitumor activity in colon cancer through PUMA-mediated apoptosis. Oncotarget. 2017;8(2):3289–3303. doi: 10.18632/oncotarget.13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Song Y, Ling Z, et al. R-spondin 2-LGR4 system regulates growth, migration and invasion, epithelial-mesenchymal transition and stem-like properties of tongue squamous cell carcinoma via Wnt/beta-catenin signaling. E Bio Medicine. 2019;44:275–288. doi: 10.1016/j.ebiom.2019.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Zhu JY, Yang X, Chen Y, et al. Curcumin suppresses lung cancer stem cells via inhibiting Wnt/beta-catenin and sonic hedgehog pathways. Phytother Res. 2017;31(4):680–688. doi: 10.1002/ptr.5791 [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Zhao Y, Wang X, et al. Wogonoside prevents colitis-associated colorectal carcinogenesis and colon cancer progression in inflammation-related microenvironment via inhibiting NF-kappaB activation through PI3K/Akt pathway. Oncotarget. 2016;7(23):34300–34315. doi: 10.18632/oncotarget.8815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev. 2009;35(1):57–68. doi: 10.1016/j.ctrv.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Huynh DL, Sharma N, Kumar Singh A, et al. Anti-tumor activity of wogonin, an extract from Scutellaria baicalensis, through regulating different signaling pathways. Chin J Nat Med. 2017;15(1):15–40. doi: 10.1016/S1875-5364(17)30005-5 [DOI] [PubMed] [Google Scholar]
- 27.Tai MC, Tsang SY, Chang LYF, Xue H. Therapeutic potential of wogonin: a naturally occurring flavonoid. CNS Drug Rev. 2006;11(2):141–150. doi: 10.1111/j.1527-3458.2005.tb00266.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Tian S, Liu M, Jian L, Zhao L. Wogonin inhibits the proliferation and invasion, and induces the apoptosis of HepG2 and Bel7402 HCC cells through NFkappaB/Bcl-2, EGFR and EGFR downstream ERK/AKT signaling. Int J Mol Med. 2016;38(4):1250–1256. doi: 10.3892/ijmm.2016.2700 [DOI] [PubMed] [Google Scholar]
- 29.Zhao K, Wei L, Hui H, et al. Wogonin suppresses melanoma cell B16-F10 invasion and migration by inhibiting Ras-medicated pathways. PLoS One. 2014;9(9):e106458. doi: 10.1371/journal.pone.0106458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen P, Lu N, Ling Y, et al. Inhibitory effects of wogonin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Toxicology. 2011;282(3):122–128. doi: 10.1016/j.tox.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 31.Abd El-Hafeez AA, Khalifa HO, Mahdy EAM, et al. Anticancer effect of nor-wogonin (5, 7, 8-trihydroxyflavone) on human triple-negative breast cancer cells via downregulation of TAK1, NF-kappaB, and STAT3. Pharmacological Rep. 2019;71(2):289–298. doi: 10.1016/j.pharep.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Um HD. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: a review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget. 2016;7(5):5193–5203. doi: 10.18632/oncotarget.6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Tu M, Cheng C, et al. Wogonoside induces apoptosis in Bel-7402, a hepatocellular carcinoma cell line, by regulating Bax/Bcl-2. Oncol Lett. 2015;10(3):1831–1835. doi: 10.3892/ol.2015.3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Wever O, Pauwels P, De Craene B, et al. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130(3):481–494. doi: 10.1007/s00418-008-0464-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das V, Bhattacharya S, Chikkaputtaiah C, Hazra S, Pal M. The basics of epithelial-mesenchymal transition (EMT): A study from a structure, dynamics, and functional perspective. J Cell Physiol. 2019;234:14535–14555. doi: 10.1002/jcp.28160 [DOI] [PubMed] [Google Scholar]
- 36.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi: 10.1126/science.1228522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay ED. Interaction of embryonic surface and cytoskeleton with extracellular matrix. Am J Anat. 1982;165(1):1–12. doi: 10.1002/aja.1001650102 [DOI] [PubMed] [Google Scholar]
- 38.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15(2):117–134. doi: 10.1007/s10911-010-9178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei C, Jing J, Zhang Y, Fang L. Wogonoside inhibits prostate cancer cell growth and metastasis via regulating Wnt/β-Catenin pathway and epithelial-mesenchymal transition. Pharmacology. 2019;104(56):312–319. doi: 10.1159/000502400 [DOI] [PubMed] [Google Scholar]
- 40.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3(5):362–374. doi: 10.1038/nrc1075 [DOI] [PubMed] [Google Scholar]
- 41.Song X, Zhou Y, Zhou M, et al. Wogonin influences vascular permeability via Wnt/beta-catenin pathway. Mol Carcinog. 2015;54(7):501–512. doi: 10.1002/mc.22093 [DOI] [PubMed] [Google Scholar]
- 42.Xiao R, Gan M, Jiang T. Wogonoside exerts growth-suppressive effects against T acute lymphoblastic leukemia through the STAT3 pathway. Hum Exp Toxicol. 2017;36(11):1169–1176. doi: 10.1177/0960327116679716 [DOI] [PubMed] [Google Scholar]
- 43.Ci C, Wu C, Lyu D, et al. Downregulation of kynureninase restrains cutaneous squamous cell carcinoma proliferation and represses the PI3K/AKT pathway. Clin Exp Dermatol. 2019. [DOI] [PubMed] [Google Scholar]
- 44.Li XJ, Li ZF, Wang JJ, Han Z, Liu Z, Liu BG. Effects of microRNA-374 on proliferation, migration, invasion, and apoptosis of human SCC cells by targeting Gadd45a through P53 signaling pathway. Biosci Rep. 2017;37:4. doi: 10.1042/BSR20170710 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]