ABSTRACT
The small hydrophobic (SH) glycoprotein of human respiratory syncytial virus (RSV) is a transmembrane protein that is poorly accessible by antibodies on the virion but has an ectodomain (SHe) that is accessible and expressed on infected cells. The SHe from RSV strain A has been formulated in DPX, a unique delivery platform containing an adjuvant, and is being evaluated as an RSV vaccine candidate. The proposed mechanism of protection is the immune-mediated clearance of infected cells rather than neutralization of the virion. Our phase I clinical trial data clearly showed that vaccination resulted in robust antibody responses, but it was unclear if these immune responses have any correlation to immune responses to natural infection with RSV. Therefore, we embarked on this study to examine these immune responses in older adults with confirmed RSV infection. We compared vaccine-induced (DPX-RSV(A)) immune responses from participants in a Phase 1 clinical trial to paired acute and convalescent titers from older adults with symptomatic laboratory-confirmed RSV infection. Serum samples were tested for anti-SHe IgG titers and the isotypes determined. T cell responses were evaluated by IFN-γ ELISPOT. Anti-SHe titers were detected in 8 of 42 (19%) in the acute phase and 16 of 42 (38%) of convalescent serum samples. IgG1, IgG3, and IgA were the prevalent isotypes generated by both vaccination and infection. Antigen-specific T cell responses were detected in 9 of 16 (56%) of vaccinated participants. Depletion of CD4+ but not CD8+ T cells abrogated the IFN-γ ELISPOT response supporting the involvement of CD4+ T cells in the immune response to vaccination. The data showed that an immune response like that induced by DPX-RSV(A) could be seen in a subset of participants with confirmed RSV infection. These findings show that older adults with clinically significant infection as well as vaccinated adults generate a humoral response to SHe. The induction of both SHe-specific antibody and cellular responses support further clinical development of the DPX-RSV(A) vaccine.
KEYWORDS: Respiratory syncytial virus, vaccine, T cell, B cell, immunotherapy, antibody, natural infection, immunization, aged, adult
Introduction
Respiratory syncytial virus (RSV) is the leading cause of early childhood bronchiolitis and pneumonia worldwide and results in significant hospitalizations in infants, the elderly, and immunocompromised individuals.1–4 There is an increasing recognition of the unmet medical need for an effective RSV vaccine for older persons.1 To this end, several groups have predominantly focused their vaccine development efforts toward targeting the transmembrane fusion (F) protein or glycoprotein (G) of the two RSV subtypes5-7 and more than 10 are in development for this age group.8 The majority of these vaccine candidates were designed to potentially function via antibody-mediated virus neutralization.9 To date, no prophylactic RSV vaccine has been approved for clinical use.
Novel approaches to induce non-neutralizing antibodies targeting the infected cells rather than the virion itself are being explored for vaccine development against other intracellular pathogens. For example, targeting the ectodomain of the matrix protein 2 (M2e) of the influenza A virus has shown effective protection against influenza A virus infection in pre-clinical models.10 HIV vaccine candidates that induce non-neutralizing antibodies acting through antibody-dependent cellular cytotoxicity (ADCC) have also been reported.11
The small hydrophobic glycoprotein (SH) of RSV is a type II transmembrane protein that forms pentameric transmembrane pores in infected cells.12 It functions as a viral ion channel13 and might impact viral fusion.14 RSV mutants lacking the SH protein are attenuated in vivo but not in vitro, indicating that the SH protein is most likely involved in pathogenesis and interferes with the host immune response.15,16 In this context the SH protein is known to inhibit TNF-α signaling and to either enable or inhibit inflammasome activation.17–19 Interestingly, SH protein is poorly accessible to targeted antibodies whereas it is highly expressed on the surface of infected cells,20 making it a potentially unique non-neutralizing RSV vaccine candidate. Antibodies toward the ectodomain of SH (SHe), induced by a SHe peptide fused to a carrier, have been shown to control RSV infection in mice and cotton rats.20 These pre-clinical studies of the candidate SHe-based vaccine demonstrated that the suppression of viral replication depends on the activation of Fcγ receptors, in particular, FcγRI and FcγRIII, as well as the involvement of alveolar macrophages. This immune response suggests that the likely mechanism of action of viral suppression is ADCC or antibody-dependent cellular phagocytosis (ADCP).20 While there are limited data on the anti-SHe human response, it has been proposed that natural infection may not induce a strong immune response to SHe in mice and rats due to its cellular location.20,21 An advantage of targeting SHe is that it provides an alternative avenue for immune protection and may be complementary to natural immunity.
DPXTM is a lipid-in-oil delivery platform that facilitates antigen delivery to regional lymph nodes and has been demonstrated to induce robust T cell and B cell responses in pre-clinical and clinical studies for both cancer and infectious disease, respectively.22–25 We previously demonstrated in mice that a novel DPX formulation incorporating the SHe peptide of group A RSV (DPX-RSV(A)) elicited more robust immune responses compared to an alum-adjuvanted vaccine.26 Furthermore, a Phase 1 clinical trial in 50–64 year old healthy adults demonstrated DPX-RSV(A) to be safe and well-tolerated, and induced robust antigen-specific serum IgG responses in the DPX-RSV(A) 25-μg (high dose) group that were sustained for more than a year.27 Binding of clinical trial participant anti-SHe IgG antibodies to SH-expressing mammalian cells was observed, supporting the proposed infected cell-based mechanism of action in response to DPX-RSV(A). The objective of this study is to further characterize the immune response to DPX-RSV(A) of this adult population in order to compare with the responses of adults with symptomatic laboratory-confirmed RSV infection.
Materials and methods
Human samples
Healthy participants (50–64 years) were randomly allocated to a two-dose schedule (study day (D) 0, 56) of low or high doses (10 µg or 25 µg) of DPXTM-RSV(A) (n = 8 low dose, n = 8 high dose) RSV(A)-Alum (n = 8 low dose, n = 8 high dose) on D0 (placebo on D56), or placebo on D0 and D56. Serum was collected on D0, D7, D28, D56, D63, D84, and D236; in the high-dose arm D421 was also collected. PBMCs were collected on D0, D56, and D84. The study (Clinical Trials Registration NCT02472548) was undertaken in compliance with Good Clinical Practice (GCP) guidelines, the Declaration of Helsinki, and national regulatory requirements and was approved by the local Institutional Review Board (IRB).
Confirmed RSV-infected participants
Serum samples from RSV-confirmed participants (ages range 58–91) were collected by the University of Rochester at the time of illness (acute) and 4 to 6 weeks later (convalescent). RSV infection was confirmed by viral culture or Reverse Transcription Polymerase Chain Reaction (RT-PCR).
ELISA
Anti-SHeA antibodies from serum samples were detected by indirect ELISA as described.27 Briefly, 96-well ELISA plates were coated with SHe strain A antigen and twofold dilutions of serum were added at a starting dilution of 1:100. Bound antibodies were detected by anti-human IgG horseradish peroxidase detection antibody. Positive response defined as OD reading twice the assay background.
Flow cytometry
PBMCS from healthy participants (50–64 years) enrolled in Phase 1 clinical trial NCT0247254827 were characterized for various B cell and T cell subsets. Within the B cell population, we analyzed plasma cells (CD20−CD27+CD138+CD126+CD38+), exhausted memory B cells (CD19+CD21lowCD27−), IgM memory B cells (CD19+CD27+IgMhi), activated memory B cells (CD19+CD21lowCD27+), isotype-switched memory B cells (CD19+CD27+IgG+), nBregs (CD10−CD5hi), plasmablast (CD19+CD21−CD27+), activated B cells (CD19+HLA-DR+CD38+), naïve B cells (CD19+CD21+CD27−) resting memory B cells (CD19+CD21+CD27+). Within the T cell population, we analyzed CD4+ T cells, whether there was proliferation as determined by Ki67 expression, whether they are Th1 or Th2 polarized cells via expression levels of CXCR3, Tbet, TNF, and IFN-γ for Th1, and CCR4, GATA3, and IL-4 for Th2, and whether the T cells were naïve, effector, or memory cells via expression of CD45RA and CCR7. B and T cells were evaluated by flow cytometry using the following antibodies; CD19-Alexa Fluor 700 (Clone HIB19), CD10-BV605 (Clone HI10a), CD20-BV786 (Clone 2H7), CD27-BV510 (Clone L128), CD38-BV650 (Clone HIT2), CD138-PE-CF594 (Clone MI15), CD21-APC (Clone B-ly4), IgM-BB515 (Clone G20-127), IgG-PE (Clone G18-145), CD5-APC-Cy7 (Clone UCHT2), HLA-DR-PerCP-Cy5.5 (Clone G46-6), CD126-BV421 (Clone M5), CD4-PerCP-Cy5.5 (Clone SK3), T-Bet-PE-CF594 (Clone O4-46), TNF-PE (Clone MAb11), Ki-67-AF488 (Clone B56), CD45RA-APC-H7 (Clone HI100), CD3-AF700/CD3-PeCy7 (Clone UCHT1), IFN-γ-APC (B27), IL-4-BV786 (Clone MP4-25D2), CCR7-BV650 (Clone 3D12), CD194 (CCR4)-BV605 (Clone 1G1), CD183-BV510 (Clone 1 C6/CXCR3), and GATA3-BV421 (Clone L50-823) obtained from BD Biosciences or BioLegend. Data were acquired on the BD FACS Celesta (BD Biosciences) and was analyzed by FlowJo Software (v10)
Antibody isotyping
Anti-SHe specific antibody isotyping was performed by flow cytometry analysis. For each sample, 107 polystyrene beads (Bangs Laboratory) were coated with 100 ug SHe dimer.20 Human serum was incubated with beads coated with SHe-antigen for 2 h, then a cocktail of fluorescent-labeled antibodies toward human IgM, IgD, IgG1, IgG2, IgG3, IgG4, IgA, IgE were used including: Mouse anti-human IgG1 Fc-PE (Clone HP6001) (Southern Biotech), Mouse anti-human IgG2-FITC (Clone HP-6014) (Sigma), Mouse anti-human IgG3-Hinge PE (Clone HP6050) (Southern Biotech), Mouse anti-human IgG4-pFc’AF647 (Clone HP6023) (Southern Biotech), Mouse anti-human IgA-FITC (Clone M2A) (EMD Millipore), Mouse anti-human IgE BV650 (Clone G7-26) (BD Bioscience), Mouse anti-human IgD BV605 (Clone 1A6-2) (BD Bioscience), and Mouse anti-human IgM BV421 (Clone G20-127) (BD Bioscience). Bound antibodies were detected by flow cytometry on a BD FACS Celesta (BD Biosciences) and UltraComp eBeads (Invitrogen) were used for compensation control. Data were analyzed by FlowJo Software (v10).
IFN- γ ELISPOT assay
IFN-γ ELISPOT was performed on SHe peptide stimulated PBMCs28 using the Human IFN-γ ELISPOT Kit (CTL). Briefly, freshly thawed PBMCs were incubated at 300,000 cells per well with CTL-Test medium and were stimulated or not with SHe peptide (50 μg/ml) or Cell Stimulation Cocktail (Invitrogen) (positive control) for 22 ± 2 h at 37ºC and 5% CO2. After overnight culture, plates were developed to detect spot forming units (SFU). For CD4+ or CD8+ T cell depletion ELISPOTs, selected cell types were depleted prior to ELISPOT using a magnetic bead positive selection kit (StemCell Technologies).
Results
Isotype profile of DPX-RSV(A) induced SHe-specific antibodies
We first characterized the isotype and subclass profile of antibodies to DPX-RSV(A) vaccination using serum samples of the Phase 1 clinical trial (NCT02472548), in which two vaccine doses were used (Step 1, 10 µg, n = 8 or Step 2, 25 µg, n = 8). The majority (11/16) of the DPX-RSV(A) responsive participants demonstrated an increase in the IgG1, IgG3, or IgA anti-SHe subclasses. More specifically, of the 11 DPX-RSV(A) vaccinated participants in which antibodies were detected posttreatment 10 (91%) generated IgG1, 6 (55%) generated IgG3, 4 (36%) generated IgA, while only 2 (18%) generated IgG2 isotypes. The increase in IgG1 and/or IgG3 isotypes demonstrated a trend in a time-dependent manner and correlated with the previous ELISA results (representative examples in Figure 1A,B; Table 1). In contrast, the serum samples from the DPX-RSV(A) non-responsive participants as well as the majority of the participants in the RSV(A)-Alum and placebo arms did not demonstrate a similar increase in any anti-SHe antibody isotypes (representative example, Figure 1C; Table 1). Of the RSV-Alum group examined (n = 9 out of 16), 2 out of 9 (22%) generated IgG1 and 1 out of 9 (11%) generated IgE isotypes but no IgG3 or any other of the other isotypes examined (IgG2, IgG4, IgA, IgD, IgM) (Table 1).
Figure 1.
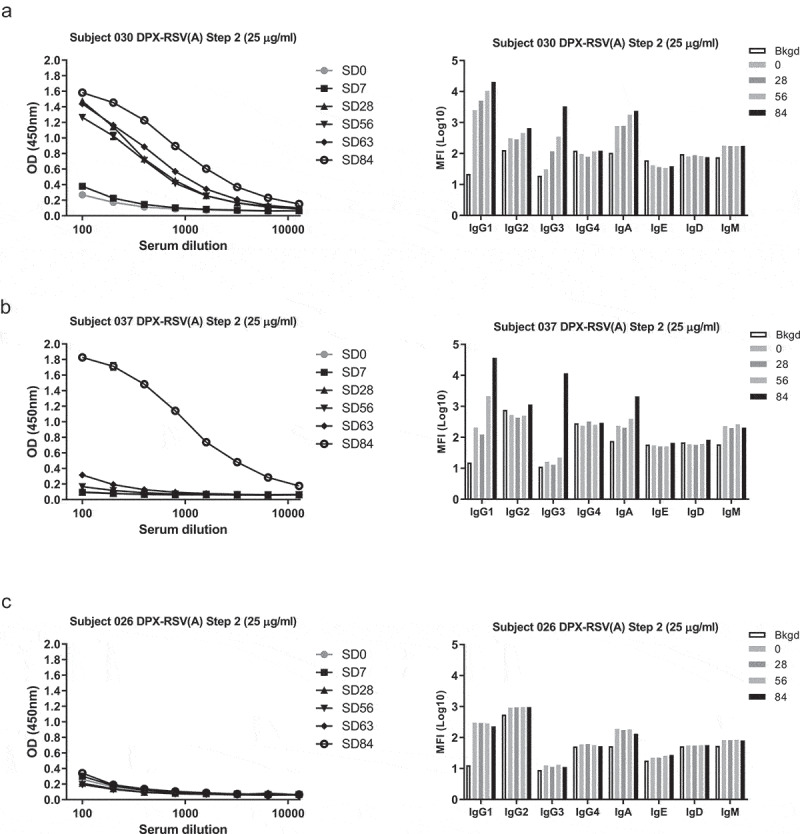
Characterization of anti-SHe(A) response induced by DPX-RSV(A). Subjects in clinical trial NCT02472548 received two doses of low dose (10 μg SHe(A) antigen, n = 8) or high dose (25 μg SHe(A) antigen, n = 8) in DPX-RSV(A). Anti-SHe(A) responses toward SHe(A) were detected in serum by ELISA (line graphs). Anti-SHe(A) isotyping was performed by flow-cytometry. Subject 030 (A), Subject 037 (B), and Subject 026 (C), are representative subjects from DPX-RSV(A) high dose cohort. Bar graphs represent mean florescent intensity (MFI) of anti-human IgG1, IgG2, IgG3, IgG4, IgA, IgD, IgE and IgM antibodies bound to SHe(A) labeled beads for the background (Bkgd), D0 (0), D28 (28), D56 (56), and D84 (84).
Table 1.
Relative change in SHe(A)-specific antibody isotypes in all Cl1204 trial DPX-RSV(A) (n = 16) and representative Alum-RSV(A) (n = 9 of 16) or Placebo (n = 5 of 8) treated subjects from D0 to D84 with corresponding ELISA and ELISPOT results.
| Isotypea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Treatment Group | IgG1 | IgG2 | IgG3 | IgG4 | IgA | IgE | IgD | IgM | ELISAb | ELISPOTc |
| 1 | DPX1-Step 1 | ++ | * | ||||||||
| 5 | DPX2-Step 1 | ||||||||||
| 7 | DPX3-Step 1 | ++ | ++ | +++ | +++ | ||||||
| 11 | DPX4-Step 1 | + | + | + | |||||||
| 14 | DPX5-Step 1 | + | ++ | + | |||||||
| 15 | DPX6-Step 1 | ||||||||||
| 18 | DPX7-Step 1 | + | |||||||||
| 19 | DPX8-Step 1 | + | + | * | |||||||
| 24 | DPX1-Step 2 | ++ | + | ||||||||
| 26 | DPX2-Step 2 | ||||||||||
| 30 | DPX3-Step 2 | + | + | +++ | + | ++ | +++ | ||||
| 31 | DPX4-Step 2 | ++ | ++ | + | * | ||||||
| 32 | DPX5-Step 2 | ++ | ++ | ++ | * | ||||||
| 35 | DPX6-Step 2 | + | +++ | ||||||||
| 37 | DPX7-Step 2 | +++ | + | +++ | ++ | +++ | +++ | ||||
| 41 | DPX8-Step 2 | + | + | * | |||||||
| 4 | Alum1-Step1 | ||||||||||
| 9 | Alum3-Step1 | ||||||||||
| 13 | Alum5-Step1 | * | |||||||||
| 16 | Alum6-Step1 | + | |||||||||
| 20 | Alum8-Step1 | ++ | |||||||||
| 25 | Alum3-Step2 | +++ | |||||||||
| 34 | Alum5-Step2 | ||||||||||
| 38 | Alum7-Step2 | ||||||||||
| 40 | Alum8-Step2 | ||||||||||
| 2 | Placebo1-Step1 | ||||||||||
| 12 | Placebo3-Step1 | + | |||||||||
| 21 | Placebo4-Step1 | ||||||||||
| 28 | Placebo2-Step2 | ||||||||||
| 39 | Placebo3-Step2 | ||||||||||
†Isotyping legend: Relative change D0-D84: >2-fold but <10-fold (+), ≥ 10-fold (++), ≥ 100-fold (+++). Preexisting response: >10-fold higher than background at D0 (gray shading).
††ELISA legend: Relative change D0-D84 in OD (450 nm): <0.4 (*), >0.4 (+), >0.8 (++), >1.6 (+++). Preexisting response: >0.2 OD (450 nm) at SD0 (gray shading).
†††ELISPOT legend: Relative change D0-D84 in SFU per 300,000 cells; >5 to <40 (*), >40 (+), >80 (++), >160 (+++). Preexisting response: >20 SFU per 300,000 cells at D0 (gray shading).
Cellular immune response to DPX-RSV(A) vaccination includes T cell involvement
To evaluate the immune-phenotype associated with the DPX-RSV(A) vaccine immunization, longitudinally collected PBMC samples (D0, D56, D84) from participants vaccinated with DPX-RSV(A) (Step 1, 10 µg, n = 8 or Step 2, 25 µg, n = 8), RSV(A)-Alum (Step 1, 10 µg, n = 8 or Step 2, 25 µg, n = 8); or placebo (Step 1, n = 4; Step 2, n = 4) were evaluated by flow cytometry. Comprehensive analyses of B cell phenotypes including the plasma cells, exhausted memory cells, activated memory B cells, natural B regulatory cells, IgM+ memory B cells, IgG+ memory B cells, naïve B cells, plasmablasts, resting memory B cells and activated B cells revealed that none of these B cell sub-populations were altered between different treatment groups or over time within a specific vaccine group (data not shown).
Similarly, within the T cell population, the presence of CD4+ and CD8+ phenotypes, their respective proliferation status (Ki67 expression) as well as the Th1/Th2 subpopulations were assessed along with the naïve, effector or memory cell phenotypes. While no significant difference was observed in the total CD4+ cells (data not shown), participants immunized with RSV(A)-Alum (Step 2, 25 µg dose) demonstrated a significant increase in the proliferating CD4+ T cells (CD4+-Ki67+) by D56 and D84 compared to respective D0 values (Figure 2A). Both RSV(A)-Alum (Step 2, 25 µg dose) participants and DPX-RSV(A) (Step 2, 25 µg dose) participants had a significant increase in CD4+IFN-γ+ T cells (Figure 2B) suggesting that the vaccination with the SHe antigen may elicit an active cellular immune response.
Figure 2.
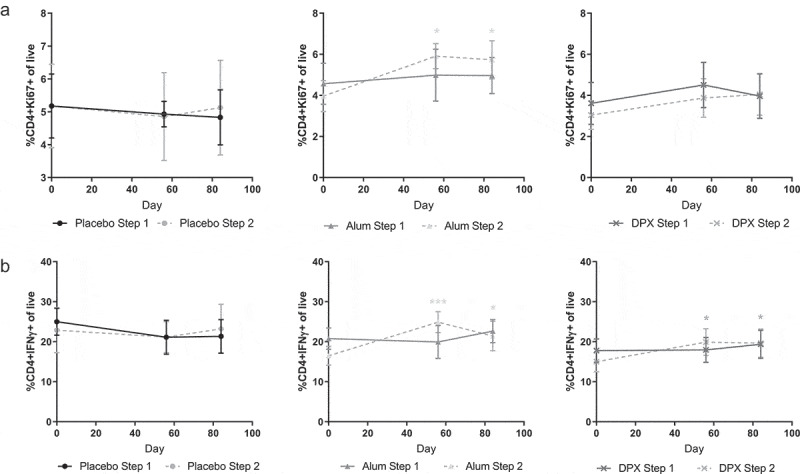
Percentage of key CD4+ T cell populations in PBMCs. Percentage Ki67+ CD4+ T cells (A), and IFN-γ+ CD4+ T cells (B) of total live PBMCs, of Cl1204 subjects on D0, D56, and D84 postvaccination. Live cells were gated based on forward scatter and side scatter. Data shown as the average of eight subjects per Alum or DPX treatment group, four subjects in placebo groups, ± SEM. *p < .05, ***p < .001, two-way ANOVA with Tukey’s posttest. Light gray * indicates significance for Alum Step 2 samples compared to D0. Dark gray* indicates significance for DPX-RSV(A) Step 2 samples compared to D0.
DPX-RSV(A) vaccination induces a CD4+ T cell response
To further characterize the functional cellular response to SHe vaccination, the longitudinally collected PBMC samples (D0, D56, D84) from participants immunized with DPX-RSV(A) (Step 1, 10 µg, n = 8 or Step 2, 25 µg, n = 8), RSV(A)-Alum (Step 1, 10 µg, n = 8 or Step 2, 25 µg, n = 8), or placebo (Step 1, n = 4; Step 2, n = 4) were assayed for IFN-γ secretion using an ELISPOT assay. Of note, only DPX-RSV(A) vaccinated participants demonstrated a trend toward positive IFN-γ response (Figure 3A). The DPX-RSV(A) high-dose (Step 2, 25 µg dose) subgroup response was found to be significantly higher than the other study arms with a mean of 377 SFU per 106 PBMC on D84 (p = .002). Moreover, the IFN-γ ELISPOT responses correlate (p = .0124) with DPX-RSV(A) participants demonstrating high antibody titers by ELISA (Table 1).
Figure 3.
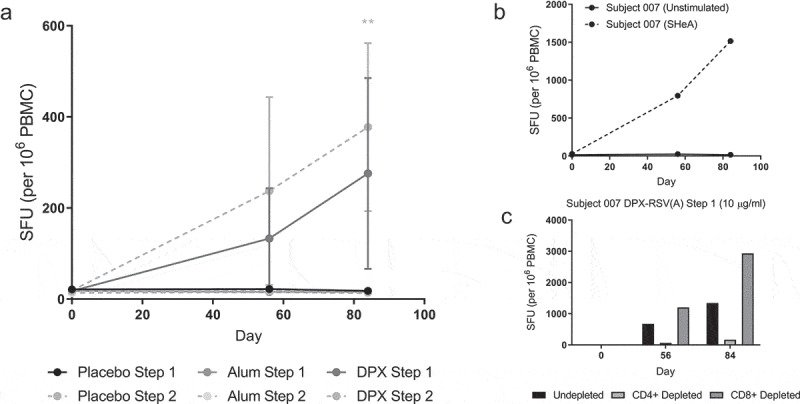
DPX-RSV(A) induces SHe(A) specific CD4 + T cells. Subjects treated with DPX-RSV(A) (Step 1, n = 8; Step 2, n = 8), RSV(A)-Alum (Step 1, n = 8; Step 2, n = 8), or placebo (Step 1, n = 4; Step 2, n = 4). IFN-γ ELISPOT response performed using PBMC samples (A). Representative IFN-ɣ ELISPOT (B). Representative example of IFN-ɣ ELISPOT performed using PBMCs whole or CD8+ or CD4+ depleted (C). Responses to SHe(A) stimulation shown, background responses were >51 SFU per 10^6 PBMC. Statistics by 2way ANOVA and Tukey’s multiple comparisons test.
To further understand the respective contribution of CD4+ and CD8+ T cells in the IFN-γ response to SHe(A) stimulation, we depleted either CD4+ or CD8+ T cells in PBMCs of DPX-RSV(A) vaccinated participants and assessed IFN-γ ELISPOT response (representative example, Figure 3B-C). The IFN-γ ELISPOT response was abrogated upon depletion of CD4+ T cells but not that of CD8+ T cells suggesting that the IFN-γ response upon DPX-RSV(A) vaccination is primarily driven by CD4+ T cell (Figure 3C). We also observed positive IL-4 ELISPOT response in two representatives DPX-RSV(A) vaccinated participants that generated an IFN-γ response (data not shown).
Antibody responses to SHe can be detected during natural RSV infection in older adults
With data to support the generation of anti-SHe antibodies in response to DPX-RSV(A) vaccination, we were interested to determine whether anti-SHe antibodies are also generated during the course of natural infection in the older adult population (aged 58–91). For this, paired acute and convalescent serum samples from older adults with symptomatic confirmed RSV infection were obtained from the University of Rochester and screened for anti-SHe IgG titers. ELISA analysis indicated the presence of anti-SHe IgG in only 9 out of 42 (21%) acute serum samples (Table 2). Detection of anti-SHe in convalescent serum did not correlate with clinical variables27 or RSV strain type but was more likely to occur in subjects with detectable anti-SHe in acute samples (p = .13). Sixteen out of 42 (38%) of the convalescent serum samples from RSV-infected adults tested positive for the anti-SHe titers, nine of which (56%) were also positive in the corresponding acute sample (Table 2). None of the samples were positive in acute but negative in convalescent. Stratifying this analyses on the basis of the infecting RSV strain (A vs B), we further observed that only strain A infections were characterized by a detectable rise (≥2x) in the anti-SHe titers above the corresponding acute sample (Table 2). Five out of the fifteen (33%) confirmed RSV A infections resulted in an anti-SHe response, as detected by ELISA. In comparison, all subjects had detectable F and G antibody at baseline with most demonstrating robust increases to both antigens with infection (Table 2).
Table 2.
Anti-SHe(A), anti-F, and anti-G titers detected in subjects with confirmed RSV infection at acute and convalescent stages from the University of Rochester and their corresponding strain.
| Anti-SHe(A) Titres |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Convalescent |
Anti-F Titres |
Anti-G Titres |
|||||||||||||
| Subject ID | RSV Strain Type | Detected in Acute | Detected in Conv | 2-Fold rise over Background | 4-Fold rise over Background | 2x Above Respective Acute | F Acute | F Conv | F Rise | GA Acute | GA Conv | GA Rise | GB Acute | GB Conv | GB Rise |
| S1 | A | + | + | + | + | + | + | + | 2 | + | + | 2.5 | + | + | 1.5 |
| S2 | A | + | + | + | + | + | + | + | 8 | + | + | 5.5 | + | + | 5.5 |
| S6 | A | + | + | + | + | - | + | + | 4 | + | + | 4.5 | + | + | 3 |
| S9 | A | - | + | + | + | + | + | + | 7 | + | + | 5.5 | + | + | 5 |
| S10 | A | - | + | + | + | + | + | + | 4 | + | + | 4 | + | + | 4 |
| S14 | A | - | + | + | - | + | + | + | 5 | + | + | 6.5 | + | + | 6 |
| S17 | A | - | - | - | - | - | + | + | 4 | + | + | 4.5 | + | + | 2 |
| S18 | A | - | - | - | - | - | + | + | 6 | + | + | 4 | + | + | 3.5 |
| S19 | A | - | - | - | - | - | + | + | 8 | + | + | 7 | + | + | 7 |
| S23 | A | - | - | - | - | - | + | + | 6 | + | + | 4.5 | + | + | 3.5 |
| S26 | A | - | - | - | - | - | + | + | 5 | + | + | 6 | + | + | 5.5 |
| S28 | A | - | - | - | - | - | + | + | 5 | + | + | 5 | + | + | 6.4 |
| S36 | A | - | - | - | - | - | + | + | 3 | + | + | 1 | + | + | 1.5 |
| S39 | A | - | - | - | - | - | + | + | 5 | + | + | 3.5 | + | + | 2.5 |
| S41 | A | - | - | - | - | - | + | + | 6 | + | + | 3.5 | + | + | 4 |
| S5 | B | + | + | + | + | - | + | + | 3 | + | + | 2 | + | + | 3 |
| S8 | B | + | + | + | - | - | + | + | 3 | + | + | 0.68 | + | + | 3 |
| S21 | B | - | - | - | - | - | + | + | 4 | + | + | 1.5 | + | + | 2.5 |
| S22 | B | - | - | - | - | - | + | + | 8 | + | + | 4.5 | + | + | 4 |
| S24 | B | - | - | - | - | - | + | + | 4 | + | + | 3.5 | + | + | 4.5 |
| S25 | B | - | - | - | - | - | + | + | 6 | + | + | 3.5 | + | + | 5.06 |
| S27 | B | - | - | - | - | - | + | + | 4 | + | + | 3 | + | + | 3.5 |
| S29 | B | - | - | - | - | - | + | + | 0 | + | + | 1.5 | + | + | 2.5 |
| S30 | B | - | - | - | - | - | + | + | 2 | + | + | 4 | + | + | 3.5 |
| S31 | B | - | - | - | - | - | + | + | 3 | + | + | 3 | + | + | 4 |
| S34 | B | - | - | - | - | - | + | + | 4 | + | + | 2 | + | + | 2.5 |
| S37 | B | - | - | - | - | - | + | + | 3 | + | + | 2 | + | + | 3 |
| S40 | B | - | - | - | - | - | + | + | 2 | + | + | 3 | + | + | 2 |
| S3 | NA | + | + | + | + | + | + | + | 2 | + | + | 1.5 | + | + | 3 |
| S4 | NA | + | + | + | + | + | |||||||||
| S7 | NA | + | + | + | - | - | + | + | 3 | + | + | 0 | + | + | 0 |
| S11 | NA | + | + | + | + | - | + | + | 1 | + | + | 2 | + | + | 1 |
| S12 | NA | - | + | + | + | + | + | + | 5 | + | + | 5.68 | + | + | 6 |
| S13 | NA | - | + | + | + | + | + | + | 2 | + | + | 3 | + | + | 3 |
| S15 | NA | - | + | + | - | + | + | + | 3 | + | + | 2 | + | + | 3 |
| S16 | NA | - | + | + | - | - | + | + | 4 | + | + | 3.5 | + | + | 2 |
| S20 | NA | - | - | - | - | - | + | + | 2 | + | + | 2 | + | + | 2 |
| S32 | NA | - | - | - | - | - | + | + | 2 | + | + | 1.5 | + | + | 2 |
| S33 | NA | - | - | - | - | - | + | + | 3 | + | + | 0.5 | + | + | 3 |
| S35 | NA | - | - | - | - | - | + | + | 2 | + | + | 1 | + | + | 2 |
| S38 | NA | - | - | - | - | - | + | + | 1 | + | + | 2.5 | + | + | 1 |
| S42 | NA | - | - | - | - | - | + | + | 1 | + | + | 0 | + | + | -1 |
RSV strain confirmed by PCR and anti-SHe(A), anti-F, and anti-G titers determined by ELISA. NA – RSV Strain unknown. Anti-SHe(A) positive response was defined as an optical density (OD) reading twice the background at 1:100 dilution. Anti- SHe(A) titers detected in the acute and convalescent phase of RSV-infected subjects with 2 and 4-fold rise in titers above background in convalescent phase and 2 times above the respective acute phase. GA and GB indicate anti-G responses that are specific to RSV type A and B, respectively.
Isotype characterization of serum samples from these naturally infected adult participants revealed that similar to DPX-RSV(A) vaccination, RSV infection also primarily generates IgG1, IgG3, and IgA isotypes which further corresponds to the anti- SHe ELISA titers (Figure 4A-B). Analysis of the University of Rochester samples (Table 3) also shows that about half of the patients have anti-SHe antibodies in the acute sample of either one or a combination of these three isotypes (IgG1, IgG3, IgA) and that the infection led to an increase from acute to convalescent in IgG1 (34%), IgG3 (25%), or IgA (16%). Moreover, we observed that the ELISA response was associated with either an increase in, or acute infection levels of, IgG1 (Table 3).
Figure 4.
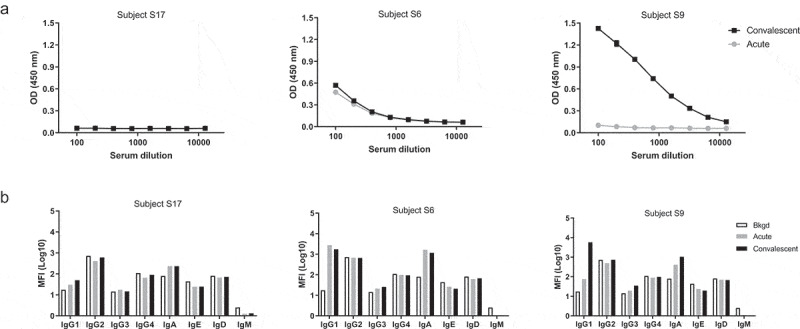
Representative ELISA data of anti-SHe detection in confirmed RSV(A) infection patient serum and anti-SHeA antibody isotyping. Serum was assayed for SHe antibodies by ELISA in doubling dilutions from 1:100 (A). Positive response defined as OD reading twice the assay background at 1:100 dilution. Isotyping of anti-SHe(A) antibodies (B). Acute serum sample (light gray), convalescent serum sample (black).
Table 3.
Relative change in SHeA-specific serum antibody isotypes of natural infection subjects from acute to convalescent with corresponding ELISA results and confirmation by PCR of RSV infection type.
| Isotypea |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject ID | RSV Strain Type | IgG1 | IgG2 | IgG3 | IgG4 | IgA | IgE | IgD | IgM | ELISAb |
| S6 | A | |||||||||
| S9 | A | ++ | + | +++ | ||||||
| S17 | A | |||||||||
| S18 | A | |||||||||
| S19 | A | |||||||||
| S28 | A | + | ||||||||
| S36 | A | + | + | + | ||||||
| S39 | A | |||||||||
| S41 | A | |||||||||
| S5 | B | + | ||||||||
| S8 | B | |||||||||
| S21 | B | |||||||||
| S29 | B | + | ||||||||
| S30 | B | + | + | |||||||
| S31 | B | + | + | |||||||
| S34 | B | |||||||||
| S37 | B | ++ | + | |||||||
| S40 | B | |||||||||
| S3 | NA | + | + | + | + | |||||
| S4 | NA | ++ | + | ++ | ||||||
| S7 | NA | |||||||||
| S11 | NA | ++ | + | + | ||||||
| S12 | NA | ++ | + | + | ||||||
| S13 | NA | + | ++ | |||||||
| S15 | NA | + | + | + | ||||||
| S16 | NA | + | ||||||||
| S20 | NA | |||||||||
| S32 | NA | |||||||||
| S33 | NA | + | ||||||||
| S35 | NA | + | + | |||||||
| S38 | NA | |||||||||
| S42 | NA | |||||||||
aIsotyping legend: Relative change from acute to convalescent: >2-fold but <10-fold (+), ≥10-fold (++), ≥100-fold (+++). Pre
existing response: >10-fold higher than the background in acute (gray shading).
bELISA legend: Relative change from acute to convalescent by OD (450 nm): <0.4 (+), >0.4 (++), >0.8 (+++). Preexisting response: >0.2 OD (450 nm) in acute (gray shading).
Discussion
Enhanced understanding of the role of the SH protein in RSV infection and the mechanism of DPX-RSV(A) vaccination is important to validate the utility of incorporating the SHe(A) peptides in the DPX formulation. Our previous clinical work highlighted the safety and efficacy of DPX-RSV(A) compared to both placebo and an alum-based vaccine. It also demonstrated that vaccination with DPX-RSV(A) leads to a robust and highly durable anti-SHe immune response in the majority of participants.27 The trial also established, in vitro, a significant increase in binding of anti-SHe IgG antibodies to SH-expressing human cells exposed to sera of DPX-RSV(A)-vaccinated participants.27 In this work, we performed a detailed characterization of the DPX-RSV(A) elicited an immune response in the context of severe disease in the elderly population to further understand its mechanism of action and potential clinical utility as a novel vaccine candidate against RSV in this population.
Antibodies of various isotypes that bind to the same target can elicit different immune responses. Varied vaccine formulations differ in their ability to generate a repertoire of isotypes for specific antigenic targets. Here we further characterized the immune responses of participants immunized with DPX-RSV(A) vaccine and demonstrate that the robust response consists of IgG1, IgG3, and IgA isotypes (Table 1). In contrast, the comparator RSV(A)-alum formulation mainly induced an IgG1 response, and in a very small proportion of participants (n = 2/9 participants evaluated). Thus, these data show that DPX-RSV(A) vaccination elicits a wider variety of anti-SHe isotypes, including IgG3 which is considered a robust mediator of effector T cell functions and ADCP or ADCC against invading pathogens.29,30 As expected, we observed a time-dependent increase in these isotypes which correlates with an increase in total anti-SHe antibody titers.
Induction of the IgA isotype has been a challenge and goal in vaccine development as IgA plays a role in mucosal immunity and is the first line of defense against pathogens entering by this route.31 The fact that the DPX-RSV(A) vaccine immunization is also associated with an increase in anti-SHe IgA isotypes is therefore noteworthy since the upper respiratory tract is the initial site of RSV infection. The generation of high-affinity IgA isotypes is known to be mediated by a T cell-dependent pathway.31 These findings highlight the unique mechanism of action of the DPX-RSV(A) approach and pave the way for future functional and mechanistic studies in this direction.
Interestingly, we observed that the DPX-RSV(A) vaccination also induced CD4+ T cell-dependent IFN-γ responses that tended to be correlated with a strong increase in IgG3 (Table 1). These preliminary findings support the premise that the DPX-RSV(A) vaccine induces the Th1 antigen-specific CD4+ T cell response which sustains the development and maintenance of memory B cell population. The fact that we also observed positive IL-4 ELISPOT response in two representatives DPX-RSV(A) treated participants, further supports the stimulation of B cells by the DPX-RSV(A) vaccination platform. IL-4 is associated with Th2 polarized CD4 + T cells involved in humoral immunity. The ability for this platform to generate both a Th1 as well as a Th2 response may prove to be an advantage over traditional alum-based vaccines. Typically, alum-based vaccines elicit a Th2 response.32 Of note, the production of IL-4 in response to natural RSV infection or vaccination has been implicated in the reduction of RSV-specific cytotoxic T-cells in vivo.33
RSV is known to modulate the host immune system to evade the mechanisms for the development of memory response making persons susceptible to reinfections throughout life.34 Natural RSV infection is also known to involve the production of virus-neutralizing antibodies and T cell-specific host immunity.35 Vaccine candidates mimicking and amplifying this natural immune response might hold promising clinical utility. Most adults have high levels of RSV-neutralizing antibodies due to multiple RSV infections. Yet a prior assessment of five standardized sera samples from humans failed to demonstrate anti-SHe antibodies.20 However, a potential limitation of this analysis may be the fact that these samples were not collected at the time of infection but were randomly collected samples that had anti-F and -G antibodies, indicating prior RSV infection. Testing pre- and post-infection human serum may, therefore, provide more accurate information about the acute increase in SHe-specific antibodies generated, yet does not explore the potential duration of these responses. Here we report that nine out of 42 (21%) of the participants of the University of Rochester cohort had anti-SHe IgG as detected by ELISA. Five out of 15 (33%) of participants with confirmed RSV Strain A infection generated anti-SHe IgG at the convalescent stage that is at least twofold above the corresponding acute samples. Notably, none of the Strain B RSV infected subjects in the Rochester cohort had a response to SHe by ELISA.
Similar antibody responses have been observed for the ectodomain of the influenza A M2 protein, which is highly reminiscent to the RSV SH protein including bearing a very small ectodomain. The low levels of serum antibodies directed against the M2 ectodomain (M2e) that can be detected in human sera have illustrated that the anti-M2e antibody responses upon natural infections are weak and/or transient.36,37 Interestingly, the frequency and level of anti-M2e serum antibodies is very low in children and young adults but increases with age.37 In adults aged >40 years up to 50% had detectable preexisting anti-M2e serum antibodies. This observation indicates that to acquire detectable levels of serum anti-M2e antibodies reoccurring influenza infections are required. As RSV reinfects throughout life it would be interesting to test if the level and frequency of preexisting anti-SHe antibodies increases with age. It was demonstrated that natural infection with 2009 pandemic H1N1 influenza A virus elicited anti-M2e antibody responses in 47% of patients. Analogous to our SHe results, these responses were clearly more robust in patients with preexisting anti-M2e antibodies.37 This observation suggests that vaccine-induced antibody responses to these small and poorly immunogenic viral proteins could potentially be boosted by infection. In contrast to natural influenza infections vaccines based on M2e (M2e-VLP + QS21 and M2e-flagelin) were able to elicit anti-M2e antibody responses in almost all (95-97%) volunteers.38,39 However, in contrast to DPX- RSV(A) vaccination those antibody responses clearly dropped over time.
Isotype analysis of the immune response during measles virus infection showed that compared to the convalescent samples, the acute samples were characterized by an initial surge of IgG1 response followed by IgG2, IgG3, and IgG4.40 Interestingly, the percentage of IgG2 and IgG3 dropped during the memory phase, meanwhile IgG1 and IgG4 remained detectable for years after infection.40 Our data from similar isotype characterization of paired serum samples from RSV-infected subjects demonstrate that the natural RSV infection generated an isotype repertoire of anti-SHe antibodies very similar to that induced by the DPX-RSV(A) vaccination with IgG1, IgG3, and IgA being the primary isotypes identified (Figure 4A-B, Table 3). While we could not differentiate the preexisting levels from infection-induced changes, this analysis supports our primary finding in the University of Rochester cohort. In conclusion, we find that natural RSV infection leads to the production of similar isotypes as those generated by DPXTM-RSV(A) with a clear trend toward IgG1, IgG3, and IgA. Although baseline SHe antibody levels and responses to natural infection were less frequent than those to F and G RSV antigens (Table 2), it is worth noting that natural immunity is both transient and incomplete.
The proposed mechanism of action of DPX-RSV(A) is that: 1, DPX facilitates active uptake of antigens delivered in DPX at the site of injection; 2, antigen that travels to the regional lymph node where CD4+ T cells are activated to support and maintain B cells; and 3, induces a robust SHe immune response (Figure 5A). We speculate that SHe-specific antibodies limit infection by clearance of RSV-infected airway epithelial cells. Specifically, antibodies access the RSV-infected airway epithelia and bind to SH on the infected cell surface. This binding stimulates phagocytosis by alveolar macrophages, thereby limiting viral infection in the lung (Figure 5B). Supportively, in addition to strong antibody titers, we have observed that antibodies generated by this platform can bind to mammalian cells expressing SH, mimicking the context of natural binding conditions.27
Figure 5.
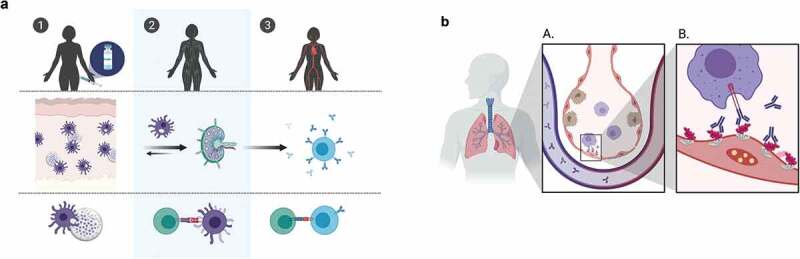
DPX-RSV(A) induces strong systemic antibody responses. (A) DPX platform facilitates active uptake of DPX components from the site of injection (1), traveling to regional lymph nodes where CD4 + T cells are activated (2) and provide T cell help to B cells, inducing robust SHeA-specific immune responses (3). (B) SHeA-specific antibodies limit infection by phagocytosis of RSV-infected airway epithelial cells. Antibodies access the RSV-infected airway epithelia, binding to SH on the infected cell surface. This stimulates phagocytosis by alveolar macrophages, limiting viral infection in the lung.
While future studies must confirm these preliminary findings in large randomized cohorts, our data demonstrates that a unique DPX-RSV(A) formulation targeting the SH ectodomain of RSV Strain A produces a robust immune response characterized by an increase in anti-SHe IgG1, IgG3, and IgA antibodies as well as a CD4+ T cell-dependent IFN-γ response. When compared to natural acute and convalescent samples, the immune response to DPX-RSV(A) is at least equivalent to the response induced by natural RSV infection. This suggests that the vaccine can generate or enhance preexisting humoral immunity to the SHe antigen, to a similar extent as natural infection. One limitation of this study was that only acute and convalescent serum samples were available from this natural infection cohort. Therefore, our analysis was limited to contemporaneous infection assessment and we could not examine sustained response, nor could we examine T cell response. Interestingly, a panel of human RSV reference serum from the National Institutes of Health (NIH) was previously screened20 and while the immune response to the F and G protein was widespread, the response to SHe was low suggesting that there is a limited natural sustained response to SHe. This contrasts with what we observed with DPX-RSV(A) vaccination. As such, opportunity exists to augment the natural immunity by prophylactic vaccination with DPX-RSV(A). Importantly, this study did not examine the protective effects of the vaccine. Future studies should investigate whether preexisting immunity to SHe can limit re-infection and if so, whether prophylactic treatment with DPX-RSV(A) would be effective. In conclusion, DPX-RSV(A) induced a humoral immune response that mimics that of the natural RSV infection and may prove to be a robust platform for eliciting durable protection.
Acknowledgments
Thank you to all involved in collecting this data at IMV Inc., VIB, CCfV, and the University of Rochester. Thank you to the participants, patients, and families involved in our clinical trial.
Funding Statement
IMV Inc. is a publicly traded company. B.S. was a postdoctoral assistant at the Department for Biomedical Molecular Biology at Ghent University and was also supported by FWO-EOS project Vir-EOS.
Disclosure of potential conflicts of interest
H.T., V.K., Y.B., G.W., and M.S. are employees and/or stock shareholders of IMV Inc. VIB and UGent hold patent rights on SHe-based vaccines and treatment options for RSV (patent application WO2012/065997 [24.05.2012]). B.S. and X.S. are named as inventors on this patent, which is licensed to IMV Inc.
References
- 1.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE.. Respiratory syncytial virus infection in elderly and high risk adults. Med Mal Infect. 2005;352(17):1749–59. doi: 10.1016/j.medmal.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Khushalani NI, Bakri FG, Wentling D, Brown K, Mohr A, Anderson B, Keesler C, Ball D, Bernstein ZP, Berstein SH, et al. Respiratory syncytial virus infection in the late bone marrow transplant period: report of three cases and review. Bone Marrow Transplant. 2001;27(10):1071–73. doi: 10.1038/sj.bmt.1703046. [DOI] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ.. Bronchiolitis-associated hospitalizations among US children, 1980-1996. J Am Med Assoc. 1999;282(15):1440–46. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–58. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Cyr SL, Burt DS, Anderson R. Murine host responses to respiratory syncytial virus (RSV) following intranasal administration of a Protollin-adjuvanted, epitope-enhanced recombinant G protein vaccine. J Clin Virol. 2009;44(4):287–91. doi: 10.1016/j.jcv.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Hsu KHL, Lubeck MD, Bhat BM, Bhat RA, Kostek B, Selling BH, Mizutani S, Davis AR, Hung PP. Efficacy of adenovirus-vectored respiratory syncytial virus vaccines in a new ferret model. Vaccine. 1994;12(7):607–12. doi: 10.1016/0264-410X(94)90264-X. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus : infection, detection, and new options for. Am Soc Microbiol. 2017;30(1):277–319. doi: 10.1128/CMR.00010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Program for Appropriate Technology in Health (PATH) . RSV vaccine and mAb snapshot. Communication; 2019. August 28 [accessed 2020 January30]. https://path.azureedge.net/media/documents/RSV-snapshot-2019_08_28_High_Resolution_PDF.pdf.
- 9.Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239(1):149–66. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, Van Rooijen N, Verbeek S, Fiers W, Saelens X. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: fc receptors and alveolar macrophages mediate protection. J Immunol. 2011;186(2):1022–31. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 11.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen XI, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86(21):11521–32. doi: 10.1128/jvi.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan SW, Tan E, Lin X, Yu D, Wang J, Tan GMY, Vararattanavech A, Yeo CY, Soon CH, Soong TW, et al. The small hydrophobic protein of the human respiratory syncytial virus forms pentameric ion channels. J Biol Chem. 2012;287(29):24671–89. doi: 10.1074/jbc.M111.332791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez M, Garcia-Barreno B, Melero JA, Carrsasco L, Guinea R. Membrane permeability changes induced in Escherichia coli by the SH protein of human respiratory syncytial virus. Virology. 1997;235(2):342–51. doi: 10.1006/viro.1997.8696. [DOI] [PubMed] [Google Scholar]
- 14.Heminway BR, Yu Y, Tanaka Y, Perrne KG, Gustafson E, Bernstein JM, Galinski MS. Analysis of respiratory syncytial Virus F, G, and SH proteins in cell fusion. Virology. 1994;200(2):801–05. doi: 10.1006/viro.1994.1245. [DOI] [PubMed] [Google Scholar]
- 15.Bukreyev A, Whitehead SS, Murphy BR, Collins PL. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71(12):8973–82. doi: 10.1128/JVI.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitehead SS, Burkreyev A, Teng MN, Firestone CY, St.Claire M, Elkins WR, Collin PL, Murohy BR. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73(4):3438–42. doi: 10.1128/JVI.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuentes S, Tran KC, Luthra P, Teng MN, He B. Function of the respiratory syncytial virus small hydrophobic protein. J Virol. 2007;81(15):8361–66. doi: 10.1128/jvi.02717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell RF, McDonald JU, Ivanova M, Zhong Z, Bukreyev A, Tregoning JS. Partial attenuation of respiratory syncytial virus with a deletion of a small hydrophobic gene is associated with elevated Interleukin-1β responses. J Virol. 2015;89(17):8974–81. doi: 10.1128/jvi.01070-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triantafilou Kar S, Vakasis E, Kotecha S, Triantafilou M. Human respiratory syncytial virus viroporin SH: A viral recognition pathway used by the host to signal inflammasome activation. Thorax. 2013;68(1):66–75. doi: 10.1136/thoraxjnl-2012-202182. [DOI] [PubMed] [Google Scholar]
- 20.Schepens B, Sedeyn K, Vande Ginste LV, De Baets S, Schotsaert M, Roose K, Houspie L, Van Ranst M, Gilbert B, van Rooijen N, et al. Protection and mechanism of action of a novel human respiratory syncytial virus vaccine candidate based on the extracellular domain of small hydrophobic protein. EMBO Mol Med. 2014;6(11):1436–54. doi: 10.15252/emmm.201404005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rixon HWML, Brown G, Aitken J, McDonald T, Graham S, Sugrue RJ. The small hydrophobic (SH) protein accumulates within lipid-raft structures of the Golgi complex during respiratory syncytial virus infection. J Gen Virol. 2004;85(5):1153–65. doi: 10.1099/vir.0.19769-0. [DOI] [PubMed] [Google Scholar]
- 22.Berinstein NL, Karkada M, Oza AM, Odunsi K, Villella J, Nemunaitis JJ, Morse MA, Pejovic T, Bentley J, Buyse M, et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology. 2015;4(8):37–41. doi: 10.1080/2162402X.2015.1026529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weir GM, Hrytsenko O, Quinton T, Berinstein NL, Stanford MM, Mansour M. Anti-PD-1 increases the clonality and activity of tumor infiltrating antigen specific T cells induced by a potent immune therapy consisting of vaccine and metronomic cyclophosphamide. J Immunother Cancer. 2016;4(1):1–13. doi: 10.1186/s40425-016-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berinstein NL, Karkada M, Morse MA, Nemunaitis JJ, Chatta G, Kaufman H, Odunsi K, Nigam R, Sammatur L, MacDonald L, et al. First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian, breast and prostate cancer patients. J Transl Med. 2012;10(1):1. doi: 10.1186/1479-5876-10-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir GM, Hrytsenko O, Stanford MM, Berinstein NL, Karkada M, Liwski R, Mansour M. Metronomic cyclophosphamide enhances HPV16E7 peptide vaccine induced antigen-specific and cytotoxic T-cell mediated antitumor immune response. Oncoimmunology. 2014;3(8):37–41. doi: 10.4161/21624011.2014.953407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald LD, MacKay A, Kaliaperumal V, Weir G, Penwell A, Rajagopalan R, Langley JM, Halperin S, Mansour M, Stanford MM. Type III hypersensitivity reactions to a B cell epitope antigen are abrogated using a depot forming vaccine platform. Hum Vaccines Immunother. 2017;14(1):59–66. doi: 10.1080/21645515.2017.1375637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langley JM, MacDonald LD, Weir GM, MacKinnon-Cameron D, Ye L, McNeil S, Schepens B, Saelens X, Stanford MM, Halperin SA. A respiratory syncytial virus vaccine based on the small hydrophobic protein ectodomain presented with a novel lipid-based formulation is highly immunogenic and safe in adults: A first-in-humans study. J Infect Dis. 2018;218(3):378–87. doi: 10.1093/infdis/jiy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patton K, Aslam S, Lin J, Yu L, Lambert S, Dawes G, Esser MT, Woo J, Janetzki S, Cherukuri A. Enzyme-linked immunospot assay for detection of human respiratory syncytial virus F protein-specific gamma interferon-producing T cells. Clin Vaccine Immunol. 2014;21(5):628–35. doi: 10.1128/CVI.00736-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damelang T, Rogerson SJ, Kent SJ, Chung AW. Role of IgG3 in infectious disease. Trends Immunol. 2019;40(3):197–211. doi: 10.1016/j.it.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Jounai N, Yoshioka M, Tozuka M, Inoue K, Oka T, Miyaji K, Ishida K, Kawai N, Ikematsu H, Kawakami C, et al. Age-specific profiles of antibody responses against respiratory syncytial virus infection. EBioMedicine. 2017;16:124–35. doi: 10.1016/j.ebiom.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyaka PN. Inducing mucosal IgA: a challenge for vaccine adjuvants and delivery systems. J Immunol. 2017;199(1):9–16. doi: 10.4049/jimmunol.1601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garlapati S. Do we know the Th1/Th2/Th17 determinants of vaccine response? Expert Rev Vaccines. 2012;11(11):1307–10. doi: 10.1586/erv.12.111. [DOI] [PubMed] [Google Scholar]
- 33.Aung S, Tang Y-W, Graham BS. Interleukin-4 diminishes CD8+ respiratory syncytial virus-specific cytotoxic T-Lymphocyte activity in vivo. J Virol. 1999;73(11):8944–49. doi: 10.1128/jvi.73.11.8944-8949.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canedo-Marroquín G, Acevedo-Acevedo O, Rey-Jurado E, Saavedra JM, Lay MK, Bueno SM, Riedel CA, Kalergis AM. Modulation of host immunity by human respiratory syncytial virus virulence factors: A synergic inhibition of both innate and adaptive immunity. Front Cell Infect Microbiol. 2017;7(AUG):1–10. doi: 10.3389/fcimb.2017.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev. 1999;12(2):298–309. doi: 10.1128/cmr.12.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng JQ, Zhang M, Mozdzanowska K, Zharikova D, Hoff H, Wunner W, Couch RB, Gerhard W. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virol J. 2006;3:1–13. doi: 10.1186/1743-422X-3-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong W, Reed C, Blair PJ, Katz JM, Hancock K. Influenza Serology Working Group. Serum antibody response to matrix protein 2 following natural infection with 2009 pandemic influenza A(H1N1) virus in humans. J Infect Dis. 2014;209(7):986–94. doi: 10.1093/infdis/jit811. [DOI] [PubMed] [Google Scholar]
- 38.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, Kavita U, Stanberry L, Shaw A. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29(32):5145–52. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 39.Schepens B, De Vlieger D, Saelens X. Vaccine options for influenza: thinking small. Curr Opin Immunol. 2018;53:22–29. doi: 10.1016/j.coi.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Isa MB, Martínez L, Giordano M, Zapata M, Passeggi C, De Wolff MC, Nates S. Measles virus-specific immunoglobulin G isotype immune response in early and late infections. J Clin Microbiol. 2001;39(1):170–74. doi: 10.1128/JCM.39.1.170-174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Program for Appropriate Technology in Health (PATH) . RSV vaccine and mAb snapshot. Communication; 2019. August 28 [accessed 2020 January30]. https://path.azureedge.net/media/documents/RSV-snapshot-2019_08_28_High_Resolution_PDF.pdf.


