Abstract
Background:
Acute kidney injury (AKI) is independently associated with increased morbidity and mortality. Quality improvement has been identified as an important goal in the care of patients with AKI. Different settings can be targeted to improve AKI care, broadly classified these include the inpatient and outpatient environments. In this paper, we will emphasize quality indicators associated with the management and secondary prevention of AKI in hospitalized patients to limit the severity, duration, and complications.
Methods:
During the 22nd Acute Disease Quality Initiative (ADQI) consensus conference, a multidisciplinary group of experts discussed the evidence and used a modified Delphi process to achieve consensus on recommendations for AKI-related quality indicators (QIs) and care processes to improve patient outcomes. The management and secondary prevention of AKI in hospitalized patients were discussed, and recommendations were summarized.
Results:
The first step in optimizing the quality of AKI management is the determination of baseline performance. Data regarding each institution’s/center’s performance can provide a reference point from which to benchmark quality efforts. Quality program initiatives should prioritize achievable goals likely to have the highest impact according to the setting and context. Key AKI quality metrics should include improvement in timely recognition, appropriate diagnostic workup, and implementation of known interventions that limit progression and severity, facilitating recovery, and mitigating AKI-associated complications. We propose the Recognition-Action-Results framework to plan, measure, and report the progress toward improving AKI management quality.
Conclusions:
These recommendations identified and outlined an approach to define and evaluate the quality of AKI management in hospitalized patients.
Keywords: Acute kidney injury, Quality improvement initiative, Outcomes
1. Introduction
The short- and long-term consequences of acute kidney injury (AKI) are well-recognized [1–3]. The number of AKI episodes, their severity, and duration are critical determinants of adverse outcomes, including de novo or progressive chronic kidney disease (CKD), progression to end-stage kidney disease (ESKD), cardiovascular disease, readmissions, increased cost of care, and mortality. In 2009, an audit of the quality of care in the United Kingdom provided to hospitalized patients who died with AKI indicated that improving management and care quality warranted urgent attention [4]. Clinical adjudicators determined that <50% of the care provided to AKI patients was considered appropriate, and 29% of patients received inadequate clinical management. The primary deficiencies in management comprised simple process interventions, such as the absence of kidney function assessment over time (e.g., with routine serum creatinine evaluation), lack of urine output monitoring, and failure to avoid potential nephrotoxins or adjust doses of drugs eliminated by the kidney.
To address these inconsistencies in care quality, the Kidney Disease Improving Global Outcomes (KDIGO) and the National Institute for Health and Care Excellence (NICE) groups developed initial guidelines aimed at improving early detection and interventions for AKI and reducing practice variation in management. These recommendations are supported by recent evidence that suggests that early AKI detection and prevention may attenuate AKI severity and complications [5–7]. A systematic application of relatively simple quality improvement measures has favorably altered short- and long-term outcomes of AKI [8–11]. Yet, while these interventions contributed to improve AKI care quality and better outcomes [12–14], evidence indicates that such interventions are not systematically applied [7,15]. One possible reason for the poor compliance with prevention and management bundles is a lack of clarity in the optimal approach to measuring baseline and improvements in AKI care quality. The Scottish Patient Safety Programme (SPSP) found low baseline compliance of 45-60% with the AKI care bundle. After feedback from stakeholders and adapting the delivery of the bundle information, the compliance improved only slightly. They found that other factors affecting the implementation include the ‘bundle fatigue’ and that maintenance of the motivation to comply with the bundles to be the main issue that most likely needs to be tackled differently in various institutions and settings. Thus, it is of utmost importance that the implementation of quality improvement projects is designed in a manner to accept and update feedback from all stakeholders continually. Appendix A provides an example for a quality improvement project for hospitalized patients who have developed AKI.
To address the need to improve care quality in AKI, the 22nd Acute Disease Quality Initiative (ADQI) conference was convened. A critical recommendation of the consensus panel was that improvement in AKI care quality requires prioritization and implementation of care process enhancements using deliberate and measurable methods. The main questions that are addressed in this article are:
Question 1 -What are the key quality indicators for improving diagnosis and evaluation of AKI episodes in the hospital?
Question 2 - What are the key quality indicators for limiting the duration and severity of AKI and enhancing the chances of AKI resolution?
Question 3 - What are the key quality indicators for reducing the complications related to AKI?
2. Methods
The 22nd ADQI Consensus Conference was held over 2-days in San Diego, CA, in October 2018, and included an interdisciplinary group of clinicians and researchers from North and South America, Asia, and Europe. Relevant disciplines were well represented, including adult and pediatric nephrology, critical care, advanced practice provider, pharmacy, epidemiology, and health services research. This consensus meeting followed the established ADQI process, as previously described [16,17]. The broad objective of ADQI is to provide expert-based statements and interpretation of current knowledge for use by clinicians according to professional judgment and to identify evidence gaps to establish research priorities.
The secondary prevention of AKI workgroup sought to develop consensus statements to improve the management of AKI for hospitalized patients. The consensus-building process informed by an objective review of articles identified through a PubMed search by workgroup members was applied. The results of this consensus conference are not based on a formal systematic review process. A modified Delphi method was used to reach consensus, with evidence where possible, with the ultimate goal of addressing the key questions and articulating a research agenda to address existing knowledge gaps. A summary of the consensus conference has been previously published [16]. Within this article, we provide more detailed recommendations regarding the quality of AKI management and secondary prevention within the hospital and present case studies to be used as a framework for the implementation of AKI quality improvement projects.
3. Results
Question 1
What are the key considerations for developing quality programs that evaluate contributors to an episode of AKI?
3.1. Consensus Statement 1
We propose that for each patient diagnosed with AKI during hospitalization, the goal is recovery to baseline kidney function in the shortest period of time with a minimum number of complications. This is best achieved by timely and accurate diagnosis and management of AKI, and prevention of complications. These goals might be achieved by using a Recognition-Action-Results framework.
Rationale.
The main goal in the management of established AKI is to achieve rapid and complete recovery of kidney function to baseline levels. Early and accurate AKI detection and identification of its reversible causes along with the implementation of patient- and context-specific appropriate secondary prevention could potentially reduce the severity and duration of disease and associated complications (Fig. 1) [18]. AKI is a heterogeneous syndrome. Hence, quality improvement initiatives should consider different contextual factors, including the clinical setting (e.g., community- versus hospital-acquired, medical versus post-surgical, critical care versus non-critical care), underlying causes, the trajectory and severity at the time of AKI identification, and the goals and resource availabilities of the healthcare system [19]. Failure to identify reversible AKI episodes can result in missed opportunities to deter progression, complications, and prevent its recurrence [7,10]. In order to achieve these goals, we suggest that clinicians apply the Recognition-Action-Results (RAR) framework to identified areas for improvement (Table 1). The domains that QI projects should focus on include diagnosis and workup, prevention of progression and facilitating recovery, and finally avoiding complications that are known to be directly or indirectly related to AKI.
Fig. 1.
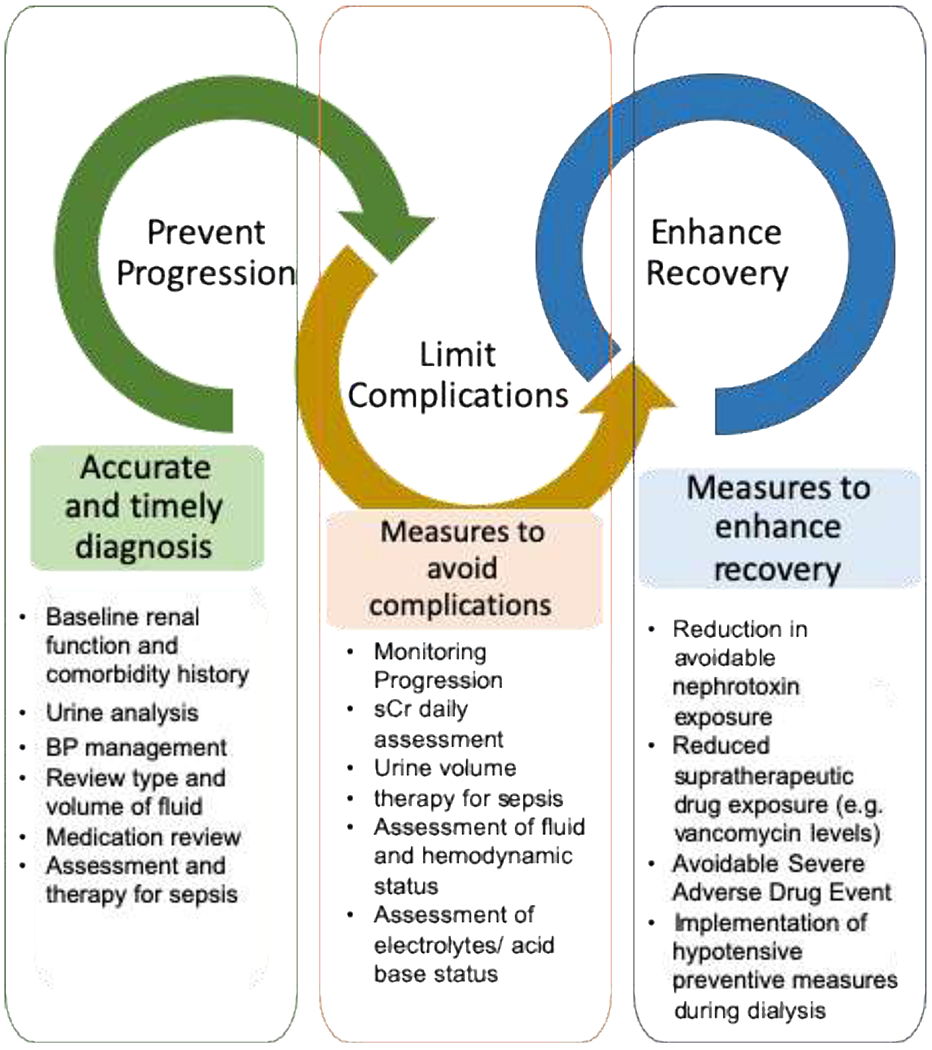
Timing and measures to early recognize and treat complications.
The key steps for managing established AKI includes preventing progression, limiting complications, function. Identification of risk and facilitating the recovery of kidney factors for AKI, early detection, and adequate monitoring can potentially reduce the severity and duration of disease and associated complications.
Table 1.
Key indicators of assessments following episodes of AKI.
| Recognition Diagnosis and evaluation | Action Monitoring | Therapy | Documentation | Results Metrics |
|---|---|---|---|---|
| Baseline renal function and comorbidity history Previous AKI history Physical examination Urine analysis Protein/ Albumin excretion |
• sCr • Urine volume |
• Assessment and therapy for sepsis • BP management • Review type and volume of fluid • Medication review |
• Medication review and reconciliation • Progression of kidney function • Frequency of sCr assessment |
• Proportion of patients with AKI in different settings • Proportion of patients with appropriate sCr assessment |
| AKI severity | • Progression (sCr daily assessment) • Urine output changes • Volume and hemodynamic assessment • Adverse Drug Events |
• Volume infused • Type of fluids used • Diuretic usage • Need for RRT |
• Severity, peak serum creatinine • Oliguria incidence • Duration of AKI • Need for RRT • RRT indication • Days in RRT |
• Proportion of patients with documentation of AKI severity • Proportion of AKI recovery by severity • Proportion of patient with AKI-D • Days in RRT |
| Minimum work up / context-specific to determining etiology Hemodynamic variables Radiology and serology tests Other context-specific tests |
• Complications: hyperkalemia/ metabolic acidosis /symptoms or complications of uremia (for example, pericarditis or encephalopathy)/ fluid overload/ pulmonary edema. | • Appropriate treatment of AKI complications based on previous guidelines | • Work up for complications determining etiology • Documentation of appropriate monitoring and treatment |
• Proportion of patients with complications • Appropriate management of complications |
Like any other quality improvement project identifying the quality indicators that could be used and benchmarked with best practices is essential. These indicators are related to the structure, process, and outcomes. For example, if timely diagnosis of AKI is the primary goal of the QI project, quality indicators could include 1) structure indicators, i.e., capacity of the laboratory to measure kidney function or injury biomarkers, time to serum creatinine result from ordering by the clinician, percentage of patients with accurate urine output documentation, access to electronic health records where information about AKI could be documented, 2) process indicators, i.e., using RAR format for development of process indicators. AKI quality indicators for Recognition could include the proportion of AKI patients who have measured baseline and current serum creatinine, and hourly urine output monitoring and documentation, time to clinical notification of AKI from the time patients reach the AKI definition criteria, and finally proportion of AKI patients who have documentation of AKI in their medical records. The ratio of patients with appropriate action taken, such as a urinalysis within 24 hours of the diagnosis of AKI (unless anuric), or the proportion with documentation of medication profile assessment to manage nephrotoxin-exposures is the next set of process indicators. The result could include the proportion of AKI patients that continue to receive nephrotoxic drugs after AKI identification. Outcome indicators could include identifying the proportion of AKI patients who receive patient- and context-specific secondary preventive interventions or the proportion of patients with moderate or severe AKI stages. These examples of quality indicators could also be applied to appropriate workup and effective secondary prevention among AKI patients, and minimization of AKI-related complications.
As achieving the goals of this consensus statement requires a great effort and includes complex sets of interventions by multiple teams and individuals, project prioritization is of critical importance to maximize impact with the invested effort. A stepwise approach should be developed to focus on achievable goals and incremental progress towards meeting these quality measures.
3.2. Consensus Statement 2
Quality improvement surrounding the diagnostic evaluation of AKI should attempt to maximize the proportion of patients who undergo a context-appropriate and timely evaluation while avoiding unnecessary testing.
Following the diagnosis of AKI, a timely evaluation of the etiology should be completed. This should include the context and timeline of the events, medical and surgical history, physical examination findings, and laboratory assessments (Table 1). These data should be targeted to facilitate timely development of a differential diagnosis list.
Identification of AKI etiology may require additional tests such as evaluation of urine sediment, detection of proteinuria (as with urine albumin/creatinine ratio), imaging studies including ultrasonography examination of the kidney and effective blood volume status, use of other functional or injury biomarkers, serological assessment, or even kidney biopsy. In some situations, further patient workup will be necessary to determine the cause of AKI, such as tumor lysis syndrome, glomerulonephritis, and thrombotic microangiopathy. In these cases, the completeness and timeliness of a context-appropriate evaluation and execution of cause-specific interventions should be considered as quality indicators (e.g., timely and specific treatment initiation).
Given the heterogeneity of AKI, current evidence does not support that all patients with AKI require the same intensity of evaluation. Most hospitalized patients will have multiple exposures and are at higher risk of AKI. Kidney function should be assessed in patients with comorbidities such as diabetes mellitus, heart failure, liver disease, those presenting hypotension, hypovolemia, infections or using nephrotoxic drugs. Clinical practice guidelines and expert opinion generally favor a stepwise approach based on the environment and underlying illness severity, trajectory, or persistence of AKI [13,20–25]. The focus of specific quality programs should vary with regard to these contextual considerations (examples are provided using the RAR framework in Table 2A). In patients with undifferentiated AKI, quality indicators could include the proportion of patients with a urinalysis performed within 24 hours of the diagnosis. In those with community-acquired AKI, where hypovolemia is a common etiology, the proportion of patients with documentation of a volume assessment could be a quality indicator. In other scenarios where a specific diagnosis may predominate, tests should be tailored appropriately. As an example, a quality program measuring the frequency of kidney ultrasound evaluation in patients with AKI immediately after cardiac surgery, a setting where other diagnoses predominate and bladder catheterizations are customary, may have a lower yield than after gynecologic surgery when ureteral injuries can be a primary cause of AKI and can be identified by ultrasound. Lastly, in more complex populations, such as oncology patients who are receiving chemotherapy and develop AKI, quality programs might choose to target the proportion of patients completing timely screening and prevention of tumor lysis syndrome.
Table 2A.
Example Quality Improvement Initiatives for Diagnostic Evaluation of AKI
| Recognition | Action | Results |
|---|---|---|
| High readmission rates for persistent AKI after cardiac angiography were not properly screened for AKI. | • Assessment of risk for AKI pre angiography and increasing follow-up serum creatinine assessment post-angiography in those at high risk to improve initial detection and determine reversibility | • Documentation of risk assessment for AKI pre angiography • Improved proportion of patients with AKI that have a follow-up serum creatinine post angio • Reduced rehospitalizations for severe/complications of AKI |
| High incidence of progressive AKI following gynecologic surgery | • Assessment of urine volume following surgery, or screening for nephrotoxin use (e.g., NSAIDs) • Protocol for imaging with the detection of decreased urine volume or elevation of serum creatinine • Improved imaging frequency |
Documentation of serial assessment of urine volume after surgery • Improved frequency of evaluation for ureteral injury • Decreased AKI frequency? /reduce nephrotoxin exposure? |
| General Hospital Population with persistent AKI (any stage) | • Determining populations and setting with increased AKI frequency • Assessment of risk factors for AKI in the specific population • Protocol for urinalysis, follow-up serum creatinine, nephrotoxin evaluation, ultrasound in patients with risk factors |
• Documentation decreasing rates of AKI frequency in specific populations and settings • Increased detection of potential nephrotoxin exposures • Reduced severity/duration of AKI |
Context-appropriate diagnostic evaluations should not only be referent to the patient population of interest but also the resources of the environment. The availability of diagnostic tools for AKI evaluation will vary among the healthcare systems. In most settings, the implementation of the basic workup requires no additional infrastructure (e.g., assessment of chronic risk factors and acute exposures). More complex invasive procedures (e.g., kidney biopsy), technology (e.g., computer-based clinical decision support), or resources (e.g., dedicated allied health staff) may not always be available. Obviously, as with any quality improvement project, the counterbalancing point of enhanced diagnostic evaluation needs to be monitored, reported, and managed. These may be straightforward, as with the cost and resource utilization of enhanced diagnostic testing, or less obvious as with project fatigue that could jeopardize responsiveness to many different practice improvement efforts.
Ultimately, we suggest an approach to quality initiatives surrounding diagnostic evaluation that considers the clinical context and the likelihood that the test will increase the identification of actionable causes in a timely manner but is also sensitive to the healthcare system characteristics and availability of resources.
Question 2
What are the key considerations for developing quality improvement programs focused on limiting the duration and severity of AKI?
Consensus Statement 3
Quality improvement programs should include the implementation and reporting of the proportion of patients that receive timely and diagnosis-appropriate interventions. Adherence with the locally agreed upon preventive interventions should be audited and shared with clinicians periodically.
3.3. Rationale
Once diagnostic evaluations of AKI are concluded, reducing the severity and duration of AKI rests on timely and effective management of the underlying causes, and decreased exposure to other potentially modifiable risk factors. As an example, if the cause for AKI in a group of patients is identified as acute tubulointerstitial nephritis associated with a beta-lactam antibiotic, a quality program could measure the time to withdrawal of that agent, the proportion of patients with documentation of the adverse effect in the electronic health record, and institution of an appropriate alternative medication during this episode and in the future (Table 2B).
Table 2B.
Example Quality Initiations to Avoid the Progression and Duration of AKI
| Recognition | Action | Results |
|---|---|---|
| High prevalence of continued nephrotoxin-exposure after AKI | • Improved alerting (manual/electronic) of potential nephrotoxin exposures • Development of a “Nephrotoxin Stewardship” Program • Implementation of automated recommendations for therapeutic drug monitoring |
• Reduction of avoidable nephrotoxin exposure among patient with AKI • Reduced supratherapeutic drug exposure (e.g. vancomycin levels) in patients with AKI • Reduction in AKI Severity/Duration |
| High incidence of intradialytic hypotension (IDH) | • Education of providers regarding possible strategies to prevent IDH • Development of protocols for frequent BP assessments and rapid correction of hypotensive episodes during iHD • Incorporate in the dialysis prescription possible interventions to prevent IDH |
• Reduced frequency of IDH • Reduce the duration of IDH • Increase proportion of dialysis that achieve UP goals without hypotensive episodes • Reduction of AKI duration |
In other cases, the cause of AKI may be unclear, multifactorial, or a direct consequence of the underlying systemic condition (e.g., shock, sepsis). Thus, quality programs may focus on instituting management and secondary prevention strategies that are broadly applicable as with the KDIGO bundle. [26] These include optimization of hemodynamics, volume status, hyperglycemia management, nephrotoxin stewardship, and in some cases, specialist (e.g., nephrologist) consultation.[27–29] The role of bundled interventions in patients at risk for or with AKI has been recognized in recent studies [28–31]. These bundles have prevented AKI and reduced its severity in a few small studies. [10,29,31,32] In one such study, 276 patients undergoing cardiac surgery with elevated kidney biomarkers indicative of early injury were randomly assigned to routine care or implementation of the AKI KDIGO prevention bundle. Postoperative AKI was significantly lower in the protocolized prevention bundle group (55% vs. 72%; absolute risk reduction of 17% and number needed to treat of 5.8; P = 0.004). This was since reproduced in a cohort of general surgery patients [28]. While promising, more research is required to determine the effectiveness of each element of the bundled care intervention, the role for electronic alert systems and clinical decision support, and the potential of injury biomarkers to provide predictive or prognostic enrichment of cohorts and select patients at risk for worse outcomes or those most likely to benefit from such interventions.
Independent of AKI etiology, identification, and reduction of nephrotoxin exposure is always indicated and could be a focused element of quality programs. Failure to address nephrotoxin use accounts for up to 28% of potential adverse drug events in patients with established AKI [33]. The concept of nephrotoxin stewardship encompasses coordinated interventions designed to improve the appropriate use of nephrotoxic medications, analogous to stewardship efforts for other classes of medications, including antimicrobials and opiate analgesics. [34] The Nephrotoxic Injury Negated by Just-in-time Action (NINJA) program is one such example of a carefully implemented and successful nephrotoxin stewardship plan. The NINJA program engaged a multidisciplinary care team, including dedicated pharmacists, to review cases of hospitalized non-critically ill children with a high nephrotoxin burden (3 or more potentially nephrotoxic agents). Through the promotion of frequent serum creatinine monitoring in these patients, this nephrotoxin stewardship effort resulted in a sustained reduction in exposure to potentially toxic medications and a 64% reduction in AKI rate [35].
Not all nephrotoxins can be avoided. For example, antimicrobials with nephrotoxic potential may be unavoidable in patients with serious infections where effective therapeutic options are limited (e.g., resistant pathogens). Also, in cases where making a rapid and accurate diagnosis necessitates the use of contrast media, the benefit may outweigh the risk. Quality care, which includes nephrotoxin stewardship, prioritizes the measurement, evaluation, and appropriateness of nephrotoxin exposures rather than systematic discontinuation of all nephrotoxins, which would be wholly infeasible and likely harmful. In cases where nephrotoxin use is essential as with the above examples, attentiveness to cumulative nephrotoxin burden (the total amount of nephrotoxins the patient is exposed to), may reveal an opportunity to omit or change non-essential nephrotoxins. In addition, some nephrotoxin exposures are amenable to risk-reduction strategies that could be implemented (e.g., volume expansion with isotonic crystalloid prior to iodinated contrast exposure; once-daily aminoglycoside dosing). In all patients with nephrotoxin exposure, quality indicators could measure the nephrotoxin burden, frequency of kidney function monitoring, access to and appropriate utilization of drug level testing in high-risk patients, implementation of risk reduction strategies (e.g., volume expansion), and appropriateness of alternative medications or dose adjustment. Quality programs could use these indicators to determine where investment of additional resources (e.g., dedicated pharmacist) is most needed and suitable.
Outside of broad nephrotoxin stewardship, specific agents and drug classes have been targeted for quality programs given their breadth of use and potential risks in hospitalized patients. Nonsteroidal anti-inflammatory drugs (NSAIDs) contribute to both direct and indirect nephrotoxicity. Direct nephrotoxicity of NSAIDs most commonly presents as acute interstitial nephritis, but can also include minimal change disease, glomerulopathies, and papillary necrosis. [36] Indirect nephrotoxicity may result from altered intraglomerular hemodynamics. This impact on glomerular perfusion is of particular importance in contexts where intravascular volume or hemodynamics may be already significantly deranged (e.g., cardiovascular surgery). Similarly, in patients with AKI from fluid or hemodynamic alterations, the continuation of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers may warrant justification and documentation. [26] Regarding iodinated radiocontrast, although recent studies suggest that the association with AKI may not be as strong as previously described [37,38], quality indicators could measure the risk/benefit evaluation of utilization as well as the institution of prophylactic measures. Collectively attentiveness to these stewardship efforts should limit the incidence, severity, and adverse outcomes of AKI [25,28–30,39].
In addition to AKI primary and secondary prevention bundles and nephrotoxin stewardship programs, another factor that may represent quality care is the involvement of a Nephrology specialist to guide AKI management in high-risk patients [40]. In a prospective controlled non-randomized interventional study, early renal service involvement, defined as a one-time nephrology consultation within 18 hours of the onset of hospital-acquired AKI was associated with a lower peak SCr level (i.e., lower AKI severity), but larger studies are needed to determine if progression of AKI can be influenced by early nephrologist involvement [41]. The early involvement of a nephrologist may present an advantage in terms of proper volume assessment, recognition of systemic diseases that affect the kidney, adjustment of medication regimens to prevent further hemodynamic or toxic injury to the kidney, and prompt initiation of renoprotective strategies. While nephrologists may provide benefits in the care and management of patients with AKI, this resource is not infinite or universally available. A quality indicator could measure the proportion of patients in a high-risk subset who had a timely nephrology consultation, for example, those with stage II and III AKI.
Question 3
What Are the Key Considerations for Developing Quality Improvement Programs Focused on Reducing the Complications of AKI?
3.4. Consensus Statement 4
Quality indicators for prevention of avoidable AKI-related complications include monitoring and reporting the context-specific adverse events (to patient advocates, clinicians, administrators, and regulatory bodies), and implementation of risk reduction strategies. Quality indicators for the prevention of AKI complications include the proportion of patients with AKI who receive appropriate post-AKI care and the percentage of patients who develop AKI-related complications.
Rationale.
Uremia-related complications of AKI and risks associated with the initiation of kidney replacement therapy (e.g., bloodstream infection, electrolyte abnormalities, hypotension) or other AKI-related management options (e.g., diuresis, withholding nephrotoxins) should be monitored and reported. We propose that quality programs focus on determining the incidence of complications to appropriately guide effort allocation toward the monitoring of complications (i.e., recognition of hyperkalemia), the response (e.g., action to treat it), and whether risk reduction strategies are in place (e.g., low potassium diet, no potassium maintenance in fluids).
Patients with AKI are at risk for developing several complications and worse patient outcomes. [42] AKI directly affects immune dysfunction, inflammatory response, organ crosstalk, and organ failure. [43,44]. Complications related to AKI can include direct consequences of diminished clearance of endogenous or exogenous substances (e.g., hyperkalemia, renally eliminated nephrotoxic drugs), therapies used to manage AKI (e.g., catheter-related bloodstream infections for patients on renal replacement therapy), or complications of concurrent interventions for other acute or chronic conditions (e.g., accumulation of renally-eliminated medications, progression of cancer due to limited chemotherapy treatment options in those with kidney dysfunction, etc.) (Table 3).
Table 3.
Examples of monitoring, management, and documentation of most common AKI complications.
| Recognition | Action Monitoring | Risk reduction | Management | Results Result |
|---|---|---|---|---|
| Hyperkalemia (K>6) | • Serial Monitoring (Daily while in AKI) | • Discontinuation of potentiating therapies (K replacement, K-sparing diuretics, K affecting drugs) | • Time to intervention, effectiveness, follow-up | • Frequency of severe hyperkalemia, dialysis, death from hyperkalemia |
| Avoidable Severe Adverse Drug Event (e.g. renal-eliminated opiates) | • Detection of no-go meds, complication monitoring | • Stop ‘no-go’ meds (e.g., morphine, meperidine) • Use of alternative analgesics, dose adjustment (challenging), augmented monitoring for ADEs |
• Time to intervention, effectiveness, follow-up | • Avoidable ADE (e.g., respiratory depression, sedation, death), • Improved Therapeutic Drug Monitoring |
| Volume Overload > 10% of admission body weight | • strict I/O, daily weights | • Daily Assessment, voidance of ‘maintenance IV fluid’ | • Time to intervene | • Measure % volume overload in EHR flowsheet |
Quality programs to reduce complications of AKI should begin with determining the local incidence of AKI-related complications to guide selecting projects with a lower effort to impact ratio. Using the RAR framework, initial quality efforts could focus on monitoring of the incidence of complications (e.g., recognition of hyperkalemia), proportion of patients who receive adequate response (e.g., action taken to manage and treat hyperkalemia), or whether proactive risk reduction strategies are in place (e.g., avoiding unnecessary potassium supplementation or withholding angiotensin-converting enzyme inhibitors). Following the recognition of complication and implementation of actions to manage them, monitoring the results of the interventions is critically important (e.g., proportion of patients with AKI who have life-threatening hyperkalemia). Broadly this quality improvement project focuses on decreasing the proportion of patients who developed hyperkalemia.
Early detection and management of life-threatening fluid and electrolyte abnormalities related to AKI are important interventions considered in many AKI care bundles. Fluid overload is the most frequent complication of AKI and has been associated with increased rates of AKI non-recovery and mortality [45, 46]. Although the preferred tool to measure intravascular volume in ICU patients has been debated, what is not controversial is the need to monitor cumulative fluid balance and daily weights in critically ill patients to avoid the adverse outcomes associated with fluid overload. The proportion of patients who receive appropriate fluid and electrolyte management could be considered as a quality indicator. Process metrics could include implementation of daily weight documentation, frequency of unnecessary fluid administration, type of fluid selected (balanced crystalloid versus chloride –rich solutions among non-hypochloremic patients), or percentage of fluid overload in relation to patient admission bodyweight could be considered.
AKI complications may also result from acute conditions that are not primarily kidney-related (e.g., critical illnesses, cancer treatment, pain management). For example, in the presence of dynamically changing kidney function, the pharmacokinetics and pharmacodynamics of renally eliminated drugs can be unpredictable. It would be infeasible and inappropriate to establish quality programs which avoid all kidney eliminated drugs in patients with developing or recovering AKI, but quality indicators could measure the use of appropriate alternatives, where suitable, adequacy of monitoring, and dose adjustment (i.e., dose decreases in worsening AKI, and dose increases in renal recovery). For example, morphine is an opiate analgesic that can accumulate during kidney dysfunction resulting in prolonged therapeutic or toxic effects. In patients with AKI, quality indicators could focus on measuring the percentage of patients where morphine was appropriately exchanged for an alternative opiate, frequency of respiratory monitoring during therapy (i.e., signs and symptoms of opiate overdose), dose/interval adjustment in patients with changing kidney function, and finally the proportion of AKI patients who needed transfer to ICU or higher level of care due to respiratory failure secondary to the opiate accumulation.
4. Summary
Quality indicators of AKI management can be structured in the Recognition-Action-Results framework. This framework can be applied in the context of auditing AKI progression and complications. Goals for quality programs should be specific to the population and clinical setting. Engagement of dedicated multidisciplinary personnel is an essential component for the development of quality improvement programs aiming to enhance AKI management.
Supplementary Material
Acknowledgments
The ADQI consensus meeting received unrestricted grants from Baxter International Inc., La Jolla Pharmaceutical Company, Astute Medical Inc., MediBeacon Inc., AM-Pharma B.V., and AbbVie Inc. Corporate sponsors were allowed to attend all meeting sessions as observers but were not allowed to participate in the consensus process. Corporate sponsors had no input into the preparation of final recommendations or this manuscript.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2020.04.056.
References
- [1].Chua HR, et al. Extended mortality and chronic kidney disease after septic acute kidney injury. J Intensive Care Med 2018. 885066618764617. [DOI] [PubMed] [Google Scholar]
- [2].Fiorentino M, et al. Acute Kidney Injury to Chronic Kidney Disease Transition. Contrib Nephrol 2018;193:45–54. [DOI] [PubMed] [Google Scholar]
- [3].Forni LG, et al. Renal recovery after acute kidney injury. Intensive Care Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Death NCEiPOa. Acute Kidney Injury: Adding Insult to Injury. Improving the quality of Healthcare; 2009. [Google Scholar]
- [5].James MT, et al. Improving prevention, early recognition and management of acute kidney injury after major surgery: results of a planning meeting with multidisciplinary stakeholders. Can J Kidney Health Dis 2014;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tsui A, et al. Improving recognition and management of acute kidney injury. Acute Med 2014;13(3): 108–12. [PubMed] [Google Scholar]
- [7].Kolhe NV, et al. Impact of compliance with a care bundle on acute kidney injury outcomes: a prospective observational study. PLoS One 2015;10(7):e0132279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ozrazgat-Baslanti T, et al. Acute and chronic kidney disease and cardiovascular mortality after major surgery. Ann Surg 2016;264(6):987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Korenkevych D, et al. The Pattern of Longitudinal Change in Serum Creatinine and 90-Day Mortality After Major Surgery. Ann Surg 2016;263(6): 1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chandrasekar T, et al. A whole system approach to improving mortality associated with acute kidney injury. QJM 2017;110(10):657–66. [DOI] [PubMed] [Google Scholar]
- [11].Bhagwanani A, Carpenter R, Yusuf A. Improving the management of Acute Kidney Injury in a District General Hospital: Introduction of the DONUT bundle. BMJ Qual Improv Rep 2014;2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khwaja A KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin Pract 2012;120(4):179–84. [DOI] [PubMed] [Google Scholar]
- [13].Ftouh S, Lewington A. i.a.w.T.R.C.o. Acute Kidney Injury Guideline Development Group convened by the National Clinical Guidelines Centre and commissioned by the National Institute for Health and Care Excellence, Prevention, detection and management of acute kidney injury: concise guideline. Clin Med (Lond) 2014;14(1):61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Logan R, et al. Care bundles for acute kidney injury: a balanced accounting of the impact of implementation in an acute medical unit. BMJ Open Qual 2018;7(4):e000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aitken E, et al. Acute kidney injury: outcomes and quality of care. QJM 2013;106(4):323–32. [DOI] [PubMed] [Google Scholar]
- [16].Kashani K, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol 2019;14(6):941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kellum JA, et al. The first international consensus conference on continuous renal replacement therapy. Kidney Int 2002;62(5): 1855–63. [DOI] [PubMed] [Google Scholar]
- [18].Sykes L, et al. Reducing acute kidney injury incidence and progression in a large teaching hospital. BMJ Open Qual 2018;7(4):e000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kanagasundaram S. The NICE acute kidney injury guideline: questions still unanswered. Br J Hosp Med (Lond) 2013;74(12):664–5. [DOI] [PubMed] [Google Scholar]
- [20].Group KAW. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012;2(Supplement):1–138. [Google Scholar]
- [21].Hobson C, Ruchi R, Bihorac A. Perioperative acute kidney injury: risk factors and predictive strategies. Crit Care Clin 2017;33(2):379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kashani K, et al. Acute kidney injury risk assessment: differences and similarities between resource-limited and resource-rich countries. Kidney Int Rep 2017; 2(4):519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moore PK, Hsu RK, Liu KD. Management of acute kidney injury: core curriculum 2018. Am J Kidney Dis 2018;72(1): 136–48. [DOI] [PubMed] [Google Scholar]
- [24].Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care 2016;20(1):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hobson C, et al. Epidemiology, outcomes, and management of acute kidney injury in the vascular surgery patient. J Vase Surg 2018;68(3):916–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kidney Disease Improving Global Outcomes (KDIGO). Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Sppl 2012;2(1):1–138. [Google Scholar]
- [27].Selby NM, et al. Design and Rationale of ‘Tackling Acute Kidney Injury’, a Multicentre Quality Improvement Study. Nephron 2016;134(3):200–4. [DOI] [PubMed] [Google Scholar]
- [28].Gocze I, et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann Surg 2018; 267 (6): 1013–20. [DOI] [PubMed] [Google Scholar]
- [29].Meersch M, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017;43(11): 1551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schanz M, et al. Urinary [TIMP-2MIGFBP7]-guided randomized controlled intervention trial to prevent acute kidney injury in the emergency department. Nephrol Dial Transplant 2018. [DOI] [PubMed] [Google Scholar]
- [31].Kolhe NV, et al. A simple care bundle for use in acute kidney injury: a propensity score-matched cohort study. Nephrol Dial Transplant 2016;31(11):1846–54. [DOI] [PubMed] [Google Scholar]
- [32].Ebah L, et al. A Multifaceted Quality Improvement Programme to Improve Acute Kidney Injury Care and Outcomes in a Large Teaching Hospital. BMJ Qual Improv Rep 2017;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cox ZL, et al. Adverse drug events during AKI and its recovery. Clin J Am Soc Nephrol 2013;8(7):1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Barreto EF, et al. beta-Lactams: the competing priorities of nephrotoxicity, neurotoxicity, and stewardship. Ann Pharmacother 2018;52(11): 1167–8. [DOI] [PubMed] [Google Scholar]
- [35].Goldstein SL, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 2016;90(1):212–21. [DOI] [PubMed] [Google Scholar]
- [36].Bakhriansyah M, et al. Risk of nephrotic syndrome for non-steroidal anti-in-flammatory drug users. Clin J Am Soc Nephrol 2019;14(9): 1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McDonald JS, et al. Post-contrast acute kidney injury in intensive care unit patients: a propensity score-adjusted study. Intensive Care Med 2017;43(6):774–84. [DOI] [PubMed] [Google Scholar]
- [38].Kashani K, Levin A, Schetz M. Contrast-associated acute kidney injury is a myth: we are not sure. Intensive Care Med 2018;44(1): 110–4. [DOI] [PubMed] [Google Scholar]
- [39].Barreto EF, et al. Innovative use of novel biomarkers to improve the safety of renally eliminated and nephrotoxic medications. Pharmacotherapy 2018;38(8):794–803. [DOI] [PubMed] [Google Scholar]
- [40].Soares DM, et al. Delayed Nephrology Consultation and High Mortality on Acute Kidney Injury: a meta-Analysis. Blood Purif 2017;43(l-3):57–67. [DOI] [PubMed] [Google Scholar]
- [41].Balasubramanian G, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am J Kidney Dis 2011;57(2):228–34. [DOI] [PubMed] [Google Scholar]
- [42].Faubel S, Shah PB. Immediate consequences of acute kidney injury: the impact of traditional and nontraditional complications on mortality in acute kidney injury. Adv Chronic Kidney Dis 2016;23(3): 179–85. [DOI] [PubMed] [Google Scholar]
- [43].Liborio AB, et al. AKI complications in critically ill patients: association with mortality rates and RRT. Clin J Am Soc Nephrol 2015;10(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jaber S, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, ran-domised controlled, phase 3 trial. Lancet 2018;392(10141):31–40. [DOI] [PubMed] [Google Scholar]
- [45].Bagshaw SM, et al. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care 2008; 12(4):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol 2016;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


