Abstract
Understanding immune responses to severe acute respiratory syndrome coronavirus 2 is crucial to understanding disease pathogenesis and the usefulness of bridge therapies, such as hyperimmune globulin and convalescent human plasma, and to developing vaccines, antivirals, and monoclonal antibodies. A mere 11 months ago, the canvas we call COVID-19 was blank. Scientists around the world have worked collaboratively to fill in this blank canvas. In this Review, we discuss what is currently known about human humoral and cellular immune responses to severe acute respiratory syndrome coronavirus 2 and relate this knowledge to the COVID-19 vaccines currently in phase 3 clinical trials.
Introduction
In the past 18 years, three novel coronaviruses have crossed the species barrier to infect humans and cause human-to-human transmission. In addition, four seasonal human coronaviruses (ie, 229E, NL63, OC43, and HKU1) have been identified as causing up to a third of community-acquired upper respiratory tract infections. Coronaviruses compose a family within the Nidovirales order and replicate by use of a nested set of mRNAs. Although most human coronaviruses have been betacoronaviruses, two of the seasonal viruses (ie, 229E and NL63) are alphacoronaviruses, which shows that both viral subgroups are important human pathogens.
In December, 2019, a novel coronavirus causing severe acute respiratory syndrome (SARS) emerged in Wuhan, China. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has since caused a pandemic involving virtually every country. As of Oct 5, 2020, our knowledge of SARS-CoV-2 has only had months to accumulate (figure 1 ). Although researchers understand more about immunity to other human coronaviruses than about immunity to SARS-CoV-2, that knowledge is also sparse. For reasons that are poorly understood, immunity to seasonal human coronaviruses tends to be short in duration, lasting from 80 days to a few years. Reinfections have been documented with three of the four seasonal human coronaviruses (ie, 229E, NL63, and OC43). Reinfection, after documented infection, has been shown in patients with SARS-CoV-2.1 Whether such reinfection represents non-durable protective immunity, different strains of the same virus, or both, is unclear.2
Figure 1.
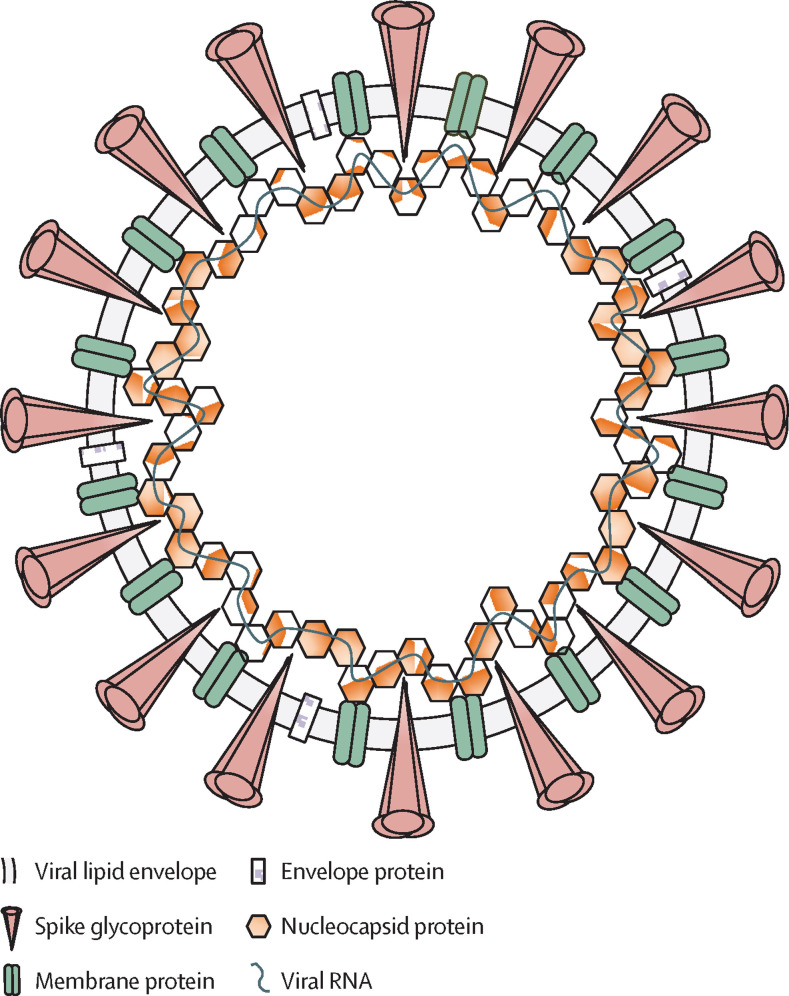
The structure of the SARS-CoV-2 virion
SARS-CoV-2 is a spherical, enveloped virus, with three structural proteins present in the lipid bilayer: the spike glycoprotein, the membrane protein, and the envelope protein. The nucleocapsid protein is associated with the membrane protein and is complexed with the viral RNA genome. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
In patients infected with either severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) or Middle East respiratory syndrome coronavirus, detection of humoral markers of immunity were measurable for 2–3 years, but these markers were absent when patients were re-tested 5–6 years later.3, 4 Understanding the mechanisms for short-duration immunity after a live viral infection is important because these processes might have considerable implications for the protection and durability of immunity induced by vaccines.
As the number of patients infected with SARS-CoV-2 continues to rise, identifying, evaluating, and understanding the immune response to SARS-CoV-2 infection becomes even more essential (figure 2 ). There is little knowledge of post-infection immunity to SARS-CoV-2, and the biological and genetic factors responsible for the broad spectrum of disease severity remain unclear. Data suggest that uncoordinated or partially neutralising antibodies, and responses from CD4+ and CD8+ T cells, might be associated with COVID-19 severity, with age being a risk factor.5 Information concerning the durability of immunity to SARS-CoV-2 infection, and the targets of B-cell and T-cell responses, can assist in the continued development of succeeding generations of new vaccines and therapeutics. We therefore review current knowledge relevant to humoral and cellular immunity to SARS-CoV-2 in humans and its application to vaccine development.
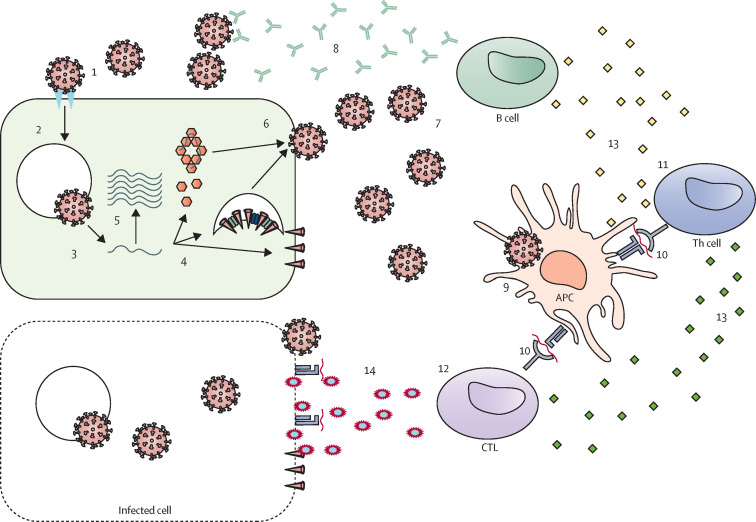
Figure 2.
SARS-CoV-2 infection and the development of immunity
The illustration depicts the major steps in the viral lifecycle and in the development of immune responses. (1) Attachment of the SARS-CoV-2 virion to the cell surface via interactions with the ACE2 cellular receptor. (2) Entry into the cell. Viral proteins can be recognised by pattern recognition receptors (eg, TLR3, TLR4, and TLR7), leading to the release of danger-associated molecular patterns, the inflammatory response, and the activation of innate anti-viral pathways. (3) Membrane fusion and release of RNA into the cell. (4) RNA translation to produce viral proteins. (5) RNA genome is copied and attached to the nucleocapsid protein. (6) Assembly of daughter SARS-CoV-2 virions. (7) Recognition of the spike glycoprotein and nucleocapsid protein (structural proteins) by the B-cell receptor. (8) B cell produces spike glycoprotein-binding antibodies and neutralising antibodies targeting the RBD region of the spike glycoprotein. (9) Viral uptake by APCs. (10) Presentation of antigens, including epitopes from structural and non-structural proteins, to T cells. (11) Activation of Th cells. (12) Activation of CTLs. (13) Th cells produce cytokines (mainly IFNγ, IL-2, and TNFα). (14) CTL recognition and killing of infected cells. ACE2=angiotensin-converting enzyme 2. APC=antigen-presenting cell. CTL=cytotoxic T lymphocyte. RBD=receptor-binding domain. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. Th=T-helper. TLR=toll-like receptor. TNF=tumour necrosis factor.
Humoral immunity to SARS-CoV-2
Humoral immune responses to SARS-CoV-2 are mediated by antibodies that are directed to viral surface glycoproteins, mainly the spike glycoprotein and the nucleocapsid protein (figure 3 ). Such antibodies neutralise viral infection of human cells and tissues expressing angiotensin-converting enzyme 2 (ACE2).
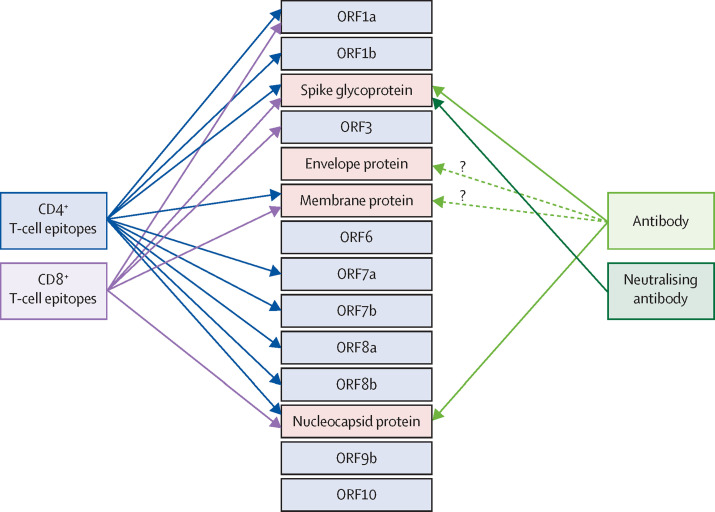
Figure 3.
SARS-CoV-2 proteins targeted by adaptive immune responses
The four structural proteins are shown in the red boxes. Non-structural proteins and accessory factors are shown in the blue boxes. Arrows link antibodies to the viral proteins they target and identify viral proteins shown to contain epitopes targeted by CD4+ T cells or CD8+ T cells. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
The 180 kDa spike glycoprotein contains two subunits (ie, N-terminal S1 and C-terminal S2) and is considered an important antigenic determinant capable of inducing a protective immune response.6 The S1 subunit holds a receptor-binding domain (RBD; residues 331–524), which mediates viral binding to functional ACE2 receptors on susceptible cells and is the main target for SARS-CoV-2 neutralising antibodies.7, 8 The major role of neutralising antibodies is antigen binding and interaction with cells bearing Fc γ-receptors to modulate subsequent immune responses. Considerable IgG responses against SARS-CoV-2 proteins (eg, nucleocapsid protein, S1, ORF9b, nsp5, and others; figure 3) have been detected in convalescent serum samples from patients who have recovered from COVID-19 by use of SARS-CoV-2 proteome microarray technology.9
Functional neutralising antibodies specific to SARS-CoV-2 that are produced following infection, vaccination, or both (anti-spike glycoprotein and anti-RBD) are considered important for viral neutralisation and viral clearance, and are quantified by use of in vitro neutralisation assays. For these reasons, antibody titres might be good biomarkers for the protective efficacy of antibodies and successful humoral immune responses after SARS-CoV-2 exposure.10 Indeed, a strong correlation (r range 0·87–0·94) between neutralising antibody responses against the spike glycoprotein, the nucleocapsid protein, and RBD proteins detected by the plaque reduction neutralisation test and those detected by ELISAs has been reported in patients with PCR-confirmed COVID-19.10 IgG, IgM, and IgA responses to the SARS-CoV-2 cysteine-like protease have also been reported in patients with COVID-19, and these responses correlate well with antibody titres to the nucleocapsid protein.11 High-quality studies that examine the duration of protection by functional neutralising antibodies and the potential for reinfection are needed in large cohorts of patients with COVID-19 to further understand and characterise SARS-CoV-2-specific immunity.
Most patients with COVID-19 or those who are convalescent have virus-specific IgM, IgA, and IgG responses in the days after infection, suggesting that antibodies mediate protective immunity to SARS-CoV-2.12, 13 The overall kinetics of the antibody response against SARS-CoV-2 are analogous to those for SARS-CoV-1, which are characterised by robust seroconversion (IgM and IgG) 7–14 days following symptom onset and antibody concentrations persisting for weeks to months after infection and viral clearance.14 A longitudinal study assessing the kinetics of spike glycoprotein-specific antibodies in patients with COVID-19 found that IgA antibodies were produced early (in the first week) and peaked in concentration at 20–22 days, whereas IgM antibodies reached high titres at 10–12 days that subsequently waned 18 days after the onset of symptoms.13 A seroprevalence study that examined IgG responses to spike glycoprotein in 40 patients with COVID-19 after symptom onset reported that IgG titres increased during the first 3 weeks and began to decrease by 8 weeks.15 In individuals with mild COVID-19, a rapid decline of RBD-specific IgG titres within 2–4 months has been observed in several studies, suggesting that SARS-CoV-2-induced humoral immunity might not be long-lasting in individuals with mild disease.16, 17 Similar results have been reported with antibody responses specific to SARS-CoV-2 nucleocapsid protein.18
A prospective study of 67 patients with COVID-19 who had high titres (peak titre >2 fold the cutoff value for a positive result) of nucleocapsid-specific IgM (up to 1:800) and IgG (up to 1:60) after symptom onset found that antibody titres were significantly higher in patients with severe disease than they were in patients without severe disease and were associated with clinical outcomes.19 Furthermore, patients with COVID-19 and low IgG titres (ie, peak titre 1–2 fold the cutoff value for a positive result) had a higher rate of viral clearance than did patients with COVID-19 and high IgG titres (ie, strong antibody responders), again suggesting that strong antibody responses might be associated with more severe disease, and low antibody responses might be associated with higher rates of viral clearance.19 However, a comprehensive study of adaptive immunity to SARS-CoV-2, which also examined the association with disease severity, showed that the concentration of neutralising antibody was not correlated with COVID-19 severity, indicating that cellular immune responses are also important for resolving SARS-CoV-2 infection.5 A report on the immunological assessment of patients with symptomatic and asymptomatic SARS-CoV-2 acute infections found that IgG titres were much higher in symptomatic individuals than they were in asymptomatic individuals (median signal-to-cutoff ratio 20·5 [IQR 5·8–38·2] vs 3·4 [1·6–10·7]; p=0·005) in the 3–4 weeks after SARS-CoV-2 exposure.20 In the convalescent phase (ie, 8 weeks after discharge from hospital), IgG titres in symptomatic individuals remained significantly higher than those in asymptomatic individuals (p=0·002). Notably, IgG titres declined during the convalescent phase in 30 (97%) of the 31 symptomatic individuals and in 28 (93%) of the 30 asymptomatic individuals, with four (13%) symptomatic individuals and 12 (40%) asymptomatic individuals becoming IgG seronegative within 2–3 months following infection.20 Other COVID-19 studies have reported similar results indicating that antibody responses after SARS-CoV-2 infection have a short duration (eg, 3–4 months).16, 21 A study from Iceland reported that 1107 (91·1%) of 1215 individuals who tested positive for SARS-CoV-2 by PCR remained seropositive 4 months following diagnosis, with no reduction in antibody titres.22 Additional longitudinal studies are necessary to further understand the dynamics of SARS-CoV-2-induced antibodies in populations and the role that these antibodies have in the risk and severity of COVID-19. Such knowledge is relevant to diagnosing patients with COVID-19 early and for examining the incidence of infections (clinical and subclinical) in different populations.
It is important to understand whether, and how, other factors (eg, age, race, ethnicity, sex, body-mass index, and smoking status) might influence serological and immune responses during SARS-CoV-2 infection. In a cohort of 20 patients with COVID-19, S1-specific IgG responses were significantly higher in older women (>40 years) than in younger women (<40 years) and men, implying that older women might develop antibody responses more effectively than do other groups.9 In 149 convalescent individuals, however, men had significantly higher anti-RBD and anti-spike glycoprotein neutralising IgG titres than did women.23 Sex-specific antibody responses against SARS-CoV-2 have also been found in the Icelandic population,22 and a small study has shown sex-specific differences in innate, antibody, and T-cell responses to SARS-CoV-2 infection.24 With regards to age, older (60–85 years) and middle-aged (40–59 years) patients had significantly higher titres of SARS-CoV-2 neutralising antibodies than did younger patients (15–39 years).25 Two studies have identified inborn genetic mutations that disrupt type I interferon responses26 and the genetically driven production of autoantibodies blocking type I interferon function27 as risk factors for severe COVID-19. These findings suggest that this innate immune response has an important role in protection against SARS-CoV-2 infection and provide a potential explanation for the wide variety of clinical disease phenotypes.
The effective mitigation of SARS-CoV-2 transmission is estimated to begin once herd immunity reaches, and is sustained at, 70%.28 There is no pre-existing immunity to SARS-CoV-2 in the population, except through cross-reactivity (shared viral antigens or epitopes) with other coronaviruses. Whether pre-existing immunity to common human seasonal coronaviruses might offer some degree of cross-protection is unknown. Clearly, an effective and safe vaccine for COVID-19 would be an ideal way to achieve herd immunity.28 Understanding the kinetics and durability of, and the extent of protection from, vaccine-induced antibody responses will be crucial. The immunological correlates of protection against SARS-CoV-2 are unknown, and the roles of specific antibodies (and T cells) in the elimination of infection have not yet been definitively identified in humans. Data from studies of rhesus macaques infected with SARS-CoV-2 have shown the protective role of neutralising antibodies against viral challenge; however, this role has not been established in humans.29 At this stage, the clinical development and evaluation of vaccines against SARS-CoV-2 would be considerably facilitated by the identification of a correlate of vaccine-induced protection.
Cellular immunity to SARS-CoV-2
Initial reports on cellular immunity to SARS-CoV-2 have consisted of case reports with small numbers of patients,30, 31, 32 which have indicated that the proportion of CD38+, HLA-DR+ T cells (both CD4+ and CD8+) increases during the first 7–10 days of COVID-19 symptoms and begins to return to baseline around day 20. SARS-CoV-2-specific T cells express perforin 1 and granzymes upon in vitro restimulation with viral antigens. In some reports but not others, the increase in the proportion of SARS-CoV-2-specific T cells seemed to correlate with disease severity;33, 34 this finding represents an important unanswered question that could affect vaccine development. Serious illness has also been linked to a greater reduction in peripheral CD4+ and CD8+ T cell counts compared with non-serious illness, suggesting a link between disease severity and the size of the cellular immune response; however, larger studies are necessary to further support a correlation.
Braun and colleagues35 evaluated T-cell responses to peptides derived from SARS-CoV-2 spike glycoprotein using the expression of activation markers (4-1BB ligand receptor and CD40-L) to identify epitope-specific CD4+ T cells. HLA-DR+ and CD38+ activated T cells specific to the spike glycoprotein were detectable in 15 (83%) of 18 patients with COVID-19.30, 31, 32 Notably, Braun and colleagues35 identified T cells reactive to spike glycoprotein in 24 (35%) of 68 healthy participants who had not tested positive for COVID-19. The role of these pre-existing SARS-CoV-2-reactive cells in COVID-19 is unknown, but Braun and colleagues35 speculated that the presence and absence of these cells might contribute to the different clinical manifestations of COVID-19.
Grifoni and colleagues33 used HLA prediction algorithms and peptide megapools to identify SARS-CoV-2-specific T cells in ten patients with COVID-19 and 11 healthy, unexposed control participants. Virus-specific CD4+ T-cell responses were detected in seven (70%) patients with COVID-19 and virus-specific CD8+ T-cell responses were detected in all ten patients with COVID-19, further indicating that most individuals can develop T-cell responses to SARS-CoV-2. Recognition of SARS-CoV-2 antigens by pre-existing and cross-reactive T cells created during previous infection with human coronaviruses might also contribute to the frequent presence of T cells reactive to SARS-CoV-2 in patients with COVID-19. The CD4+ T-cell response predominantly consisted of T-helper-1 (Th1) cells, characterised by high concentrations of IFNγ secretion and a propensity for the structural spike glycoprotein, the membrane protein, and the nucleocapsid protein (in that order), although non-structural proteins (ie, nsp3, nsp4, and ORF8) were also targeted. CD8+ T-cell responses specific to SARS-CoV-2 produced IFNγ and tumour necrosis factor (TNF)α, also reflective of a response skewed towards Th1 cells. The immunodominance pattern differed from the Th cell response, but the pattern also showed a preference for structural proteins over non-structural proteins (in order of preference: spike glycoprotein, membrane protein, nsp6, nucleocapsid, ORF8, and ORF3a; figure 3). Unexposed donors also had CD4+ T cells (six [60%] of ten) and CD8+ T cells (four [36%] of 11) reactive to SARS-CoV-2 peptides, suggesting that T-cell cross-reactivity might be common. Peng and colleagues36 evaluated T-cell responses in 42 patients who had recovered from COVID-19 and 19 unexposed controls using an overlapping peptide pool strategy covering each viral protein, except for ORF1. Peng and colleagues36 also noted that both the CD4+ T-cell and the CD8+ T-cell responses were mainly skewed towards Th1 cells, with the production of IFNγ, IL-2, and TNFα, and found that the spike glycoprotein was immunodominant. In both studies, the strength and breadth of the immune response was increased in patients with severe disease compared with patients with mild disease, and there was considerable inter-individual variability in the response; however, a few peptides were more commonly targeted than were others. A more in-depth evaluation of T-cell responses in 203 patients with COVID-19 found that virus-specific T cells displayed an activated, cytotoxic phenotype during acute infection, whereas virus-specific T cells evaluated during the convalescent phase had a memory phenotype and were polyfunctional, with both CD4+ T cells and CD8+ T cells expressing IFNγ, IL-2, and TNFα.37 Notably, T-cell responses were detectable in individuals recovering from mild COVID-19 who did not have detectable antibody responses to SARS-CoV-2.37
In the first report of a SARS-CoV-2 vaccine (an adenovirus serotype-5-vectored vaccine expressing the spike glycoprotein) in humans, T-cell responses in the 108 vaccine recipients were measured by an IFNγ enzyme-linked immunospot and intracellular cytokine staining after stimulation with overlapping spike glycoprotein peptides.38 T-cell responses from CD4+ T cells and CD8+ T cells were detectable 14 and 28 days after vaccination. The responding T cells also produced IL-2, TNFα, or both. CD4+ T cells were more likely to be polyfunctional than were CD8+ T cells. Pre-vaccination T-cell responses to SARS-CoV-2 spike glycoprotein were minimal or non-existent in all patients, suggesting that this population did not have cross-reactive T-cell immunity. The extent of cross-reactivity between T-cell responses to SARS-CoV-1 and SARS-CoV-2 remains to be seen.
Responses from T follicular helper (Tfh) cells are crucial to the development of robust humoral immunity through the formation of germinal centres and provision of co-stimulation (eg, CD40–CD40-L interactions) and cytokines (eg, IL-21) to B cells.39 A post-mortem study of individuals who died of COVID-19 found an absence of germinal centres and an absence of BCL-6+ Tfh cells,40 suggesting that inadequate activation of the Tfh response is one possible mechanism for the shortfall in durable antibody responses to SARS-CoV-2. However, a single-cell RNA sequencing study of the CD4+ T-cell response to SARS-CoV-2 found an increased proportion of Tfh cells in patients with severe disease compared with patients with mild disease.41 The authors reported that cell clusters enriched in SARS-CoV-2-specific CD4+ T cells expressed canonical Tfh genes (eg, CXCL13, IL21, and BTLA), indicating that SARS-CoV-2 infection does lead to the production of Tfh cells. Risk factors for severe COVID-19 are also associated with increased numbers of T-helper-17 (Th17) cells,42 and there is evidence that Th17 cells accumulating in the lungs can contribute to the excessive inflammation seen in COVID-19.43, 44
Several early reports have found a statistically significant reduction in T-cell counts in patients with COVID-19,45, 46 with additional studies reporting functional exhaustion of the remaining T cells.47, 48, 49 However, the aforementioned studies that examined cellular immune responses specific to SARS-CoV-2 did not report similar findings, although CD4+ T-cell responses are clearly more robust than are CD8+ T-cell responses. Possibly, differences in study timing (eg, during acute illness vs during the convalescent phase), varying definitions of mild and severe disease, and other factors contributed to the conflicting results.
A report by Laing and colleagues50 sought to identify an immune signature in patients with COVID-19 that could be used to guide clinical care and treatment. In addition to the development of humoral and cell-mediated immune responses specific to SARS-CoV-2, the authors found several additional characteristics that could distinguish between patients with COVID-19 and patients who had recovered from COVID-19 and non-exposed patient controls. These characteristics in patients with COVID-19 included the upregulation of IL-6, IL-8, IL-10, and C-X-C motif chemokine 10; rapidly cycling T cells expressing exhaustion markers (PD-1 and HAVcr-2); depletion of both αβT cells and γδT cells; decreases in natural effector and CD5+ B cells; increased neutrophil numbers; and a shift in the frequency of CD11+ versus CD11− myeloid dendritic cells. Further examination of these alterations might provide insight into disease presentation. Targeted therapies to reverse or minimise these changes (eg, suppression of inflammatory cytokine production) could also provide clinical benefit.
Overall, the current data show that both CD4+ T-cell and CD8+ T-cell responses occur in most patients infected by SARS-CoV-2 within 1–2 weeks after symptom onset and produce mainly Th1 cytokines. The frequency of CD4+ T cells targeted to the spike glycoprotein correlates with neutralising antibody titres,33 suggesting that the T-cell response might also vary among individuals with different disease severities. Two small studies37, 51 have also suggested that some individuals exposed to SARS-CoV-2 develop specific T-cell memory responses in the absence of specific antibodies, indicating that cellular immunity might be induced by SARS-CoV-2 in the absence of humoral immune responses. The contribution of cellular immunity to protection against COVID-19 is not currently clear; however, a balanced immune response consisting of high titres of neutralising antibodies and Th1-biased T cells will probably be optimal. The role of CD8+ T-cell responses in protection against COVID-19 is also unclear, with some evidence suggesting that CD8+ T-cell responses are stronger in patients with mild disease than in patients with severe disease.36, 37 Additional research into the cellular immune response to SARS-CoV-2 and COVID-19 vaccines will be necessary to test this hypothesis. Some, but not all, of the phase 1/2 trials of COVID-19 vaccines examined cellular immunity; therefore, this hypothesis cannot be fully answered.
Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2 that elicit protective immune responses are crucial to the prevention and mitigation of the morbidity and mortality caused by SARS-CoV-2 infection. Current understanding suggests that a balanced humoral and Th1-directed cellular immune response might be important for protection from COVID-19 and the avoidance of vaccine-enhanced disease.52 Various candidate vaccines are being developed and tested, including nucleic acid vaccines, inactivated virus vaccines, live attenuated vaccines, protein or peptide subunit vaccines, and viral-vectored vaccines (table ). Each approach has advantages and disadvantages, which have been reviewed elsewhere.53, 54, 55 The front runner candidates are all administered by the intramuscular route; therefore, focus is on evaluating immune responses in the blood rather than those in the mucosal surfaces. The role of mucosal immunity should not be discounted, and several intranasal vaccine formulations are under investigation.56, 57, 58
Table.
COVID-19 vaccine clinical trials
| Vaccine type | Location | Trial number | |
|---|---|---|---|
| Phase 1 trials only | |||
| Inovio | DNA (INO-4800) | USA | NCT04336410 |
| Genexine | DNA (GX-19) | South Korea | NCT04445389 |
| Academy of Military Sciences; Suzhou Abogen Biosciences; Walvax Biotechnology | mRNA (ARCoV) | China | .. |
| ReiThera; Lazzaro Spallanzani National Institute for Infectious Diseases | Gorilla adenovirus vector (GRAd-CoV2) | Italy | NCT04528641 |
| Clover Pharmaceuticals; Dynavax Technologies | Protein (SCB-2019) | .. | NCT04405908 |
| Vaxine | Protein | Australia | NCT04453852 |
| Medicago; GSK; Dynavax Technologies | Virus-like particle | USA | NCT04450004 |
| University of Queensland; CSL | Proteins | Australia | NCT04495933 |
| Kentucky Bioprocessing | Plant | USA | NCT04473690 |
| Medigen; Dynavax Technologies | Protein (MVC-COV1901) | Taiwan | NCT04487210 |
| Adimmune | Protein (AdimrSC-2f) | Taiwan | NCT04522089 |
| West China Hospital of Sichuan University | Protein | China | NCT04470609 |
| Sanofi; GSK | Protein | .. | NCT04537208 |
| Merck; Pasteur Institute | Measles vector | France | NCT04497298 |
| Research Institute for Biological Safety Problems | Inactivated virus (QazCovid) | Kazakhstan | NCT04530357 |
| Themis; Merck; University of Pittsburgh Center for Vaccine Research | Vesicular stomatitis virus-vectored (COVID-19–101) | Belgium; France | NCT04497298 |
| Symvivo | Oral (bacTRL-Spike) | USA; Canada | NCT04334980 |
| Phase 1 and phase 2 trials | |||
| Imperial College London; Morningside Ventures | Self-amplifying RNA | UK | .. |
| AnGes; Osaka University; Takara Bio | DNA (AG0302-COVID19) | Japan | NCT0452708; NCT04463472 |
| Arcturus; Duke-NUS Medical School | mRNA (LUNAR-COV19) | Singapore | NCT04480957 |
| Johnson & Johnson; Beth Israel Deaconess Medical Center | Adenovirus serotype 26 vector (Ad26.COV2-S) | USA | NCT04436276 |
| Novavax | Nanoparticle (NVX-CoV2373) | USA; South Africa | NCT04533399 |
| Finlay Vaccine Institute | Protein (Soberana 1) | Cuba | .. |
| Vector Institute | Peptide (EpiVacCorona) | Russia | NCT04527575 |
| Bharat Biotech; Indian Council of Medical Research; National Institute of Virology | Inactivated virus (Covaxin) | India | NCT04471519 |
| Anhui Zhifei Longcom Biopharmaceutical; Institute of Microbiology of the Chinese Academy of Sciences | Protein | China | .. |
| Zydus Cadila | DNA (ZyCoV-D) | India | .. |
| Curevac | mRNA (CVnCoV) | Germany, Belgium | NCT04449276, NCT04515147 |
| Phase 3 trials | |||
| AstraZeneca; University of Oxford (30 000 participants) | Chimpanzee adenovirus (ChAdOx1/AXD1222) | UK; India; Brazil, South Africa; USA | NCT04516746 |
| Moderna; National Institutes of Health (30 000 participants) | RNA (mRNA-1273) | USA | NCT04470427 |
| Pfizer; BioNTech (44 000 participants) | RNA (BNT162b1 and BNT162b2) | USA | NCT04368728 |
| The Janssen Pharmaceutical Companies of Johnson & Johnson (60 000 participants) | Adenovirus serotype 26 vector (Ad26.COV2.S) | USA; Argentina; Brazil; Chile; Columbia; Mexico; Peru; Philippines; South Africa; Ukraine | NCT04505722 |
| The Gamaleya National Research Centre for Epidemiology and Microbiology; Academy of Military Medical Sciences (40 000 participants) | Adenovirus serotype 5 vector and adenovirus serotype 26 vector (Sputnik V) | Russia | NCT04530396 |
| CanSino Biologics; Academy of Military Medical Sciences (40 000 participants) | Adenovirus serotype 5 vector (Ad5CoV) | China; Pakistan | NCT04526990 |
| Sinovac Biotech (9000 participants) | Inactivated virus (CoronaVac) | Brazil; Indonesia | .. |
| Sinopharm; Wuhan Institute of Biological Products (21 000 participants) | Inactivated virus | The United Arab Emirates; Bahrain; Peru; Morocco; Argentina; Jordan | .. |
| Sinopharm; Beijing Institute of Biological Products (5000 participants) | Inactivated virus (BBIBP-CorV) | The United Arab Emirates | .. |
By August, 2020, multiple phase 3 clinical vaccine trials, each involving tens of thousands of participants, had commenced in various geographical locations (eg, the USA, the UK, the United Arab Emirates, Morocco, Argentina, Peru, Brazil, Indonesia, Russia, China, and South Africa). Interim results from these trials are expected to be available at the end of 2020 and will provide a first indication of the efficacy and safety of COVID-19 vaccines. Notably, some phase 3 trials are designed and statistically powered around the primary outcome of preventing severe COVID-19. This design could be problematic in terms of sufficient numbers of participants. In the USA, the Food and Drug Administration has issued guidance stating that a COVID-19 vaccine would have to protect at least 50% of vaccinated people to be considered efficacious.59 In addition, establishing safety will be limited in statistical power in most trials, particularly for uncommon adverse events. Notably, few trials include people younger than 18 years and are likely to enrol sufficiently large numbers of people older than 55 years (particularly those in congregate living situations), and all trials currently exclude women who are pregnant. Many mutations of SARS-CoV-2 have been identified;60 therefore, vaccine development could be obstructed if the virus later evades immunity to the spike glycoprotein used to construct the vaccine—the so-called Achilles heel of COVID-19 vaccines.61 We now review the vaccine candidates currently in phase 3 trials.
AstraZeneca
Oxford University (Oxford, UK) and AstraZeneca have developed a chimpanzee adenovirus-vectored investigational vaccine (ChAdOx1/AZD1222) encoding the spike glycoprotein of SARS-CoV-2.62 The vaccine showed both immunogenicity and protective efficacy in non-human primates given a prime-boost vaccination schedule (2·5 × 1010 viral particles in each dose, with the second dose given 28 days after the first).62 A phase 1/2 trial with 543 individuals receiving the AZD1222 vaccine tested a prime (5·0 × 1010 viral particles) and a prime-boost (2·5 × 1010 or 5·0 × 1010 viral particles) schedule.63 The study showed the induction of humoral responses, characterised by anti-spike glycoprotein IgG and neutralising antibodies, and IFNγ T-cell responses in most recipients after the first dose of vaccine and an additional increase in humoral immune outcomes after the second dose of vaccine. Humoral immune outcomes in vaccine recipients were similar to those observed in convalescent plasma from patients who had recovered from COVID-19. Adverse events (eg, pain and tenderness at the injection site, chills, fatigue, fever, headache, malaise, muscle aches, and nausea) were mostly mild and largely occurred within 4–5 days of vaccination. The trial protocol was amended to include the prophylactic use of paracetamol, which reduced local and systemic reactions to the vaccine. The phase 1/2 trial was briefly paused after a participant developed neurological symptoms, which were later linked to multiple sclerosis. A large phase 3 trial of the AZD1222 vaccine involving 30 000 adults (20 000 vaccine recipients and 10 000 controls) began in August, 2020, in multiple worldwide locations (table). The phase 3 trial was paused after a vaccine recipient developed symptoms consistent with transverse myelitis. Although the UK trial resumed shortly after the pause, as of Oct 5, 2020, the US trial has not yet resumed. This vaccine requires refrigeration, which could be problematic for use in low-income countries.
Moderna
Moderna and the National Institutes of Health have jointly developed an mRNA-based vaccine (mRNA-1273) consisting of a sequence-optimised mRNA encoding the spike glycoprotein encapsulated in lipid nanoparticles.64 Studies in non-human primates have shown the vaccine's immunogenicity and protective efficacy after two doses (10 μg or 100 μg) given 4 weeks apart.64 In a phase 1, dose-escalation trial, this vaccine induced both spike glycoprotein binding and virus-neutralising antibody responses in recipients aged 18–55 years.65 These humoral immune responses were similar to those observed in convalescent plasma from patients who had recovered from COVID-19. Vaccine recipients also developed cellular responses, mainly biased towards CD4+ Th1 cells. CD8+ T-cell responses were marginal, except for those in recipients of two vaccinations with the higher dose (100 μg). No important safety concerns were noted with this vaccine, with mild local and systemic side-effects including pain at the injection site, chills, fatigue, myalgia, and fever occurring within a few days of vaccination. A phase 3 clinical trial of mRNA-1273 started in August, 2020, in the USA (table). This trial will include adults 18 years and older, with 20 000 vaccine recipients and 10 000 controls. One potential issue for vaccine deployment is that a storage temperature of −20°C is required.
Pfizer and BioNtech
Pfizer and BioNtech have also developed a series of mRNA-based COVID-19 vaccines. Early results from phase 1/2 trials66 testing two vaccines (BNT162b1 and BNT162b2) in 45 participants indicate that BNT162b1, a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine, elicited RBD-binding IgG and neutralising antibodies, with mostly mild side-effects (eg, injection site pain, fatigue, headache, chills, muscle pain, and joint pain). The participants, aged 18–55 years, were randomly assigned to receive two intramuscular doses, separated by 21 days, of either 10 μg, 30 μg, or 100 μg of BNT162b1 (given as 0·5 mL doses and stored at −80°C). A second dose of 100 μg of the vaccine was not administered due to increased reactogenicity. At day 21 after the first vaccine dose, geometric mean titres of RBD-specific IgG were measurable, ranging from 534 U/mL to 1778 U/mL, and were similar to, or more than, those observed in a human convalescent serum panel. By 2 weeks after the second dose, geometric mean titres of neutralising antibody were 1·9 times higher after the 10 μg vaccine dose and 4·6 times higher after the 30 μg vaccine dose than the geometric mean titres of neutralising antibody of the convalescent panel, suggesting the presence of antibody affinity maturation.66 Safety, and cellular and humoral immune responses, 2 weeks after the second dose of vaccine were assessed in this trial. BNT162b1 and BNT162b2 elicited similar dose-dependent SARS-CoV-2 geometric mean titres of neutralising antibody in both younger (18–55 years) and older (65–85 years) participants; however, BNT162b2-vaccinated individuals had higher CD4+ and CD8+ T-cell responses against the spike glycoprotein and RBD than did the BNT162b1-vaccinated participants. Because BNT162b2 produced a higher breadth of T-cell responses and had a favourable safety profile, BNT162b2 was the candidate vaccine selected for evaluation in phase 3 trials. BNT162b2 requires storage at −80°C, a fact that could pose logistical problems. A phase 3 trial of approximately 44 000 individuals (aged 18–85 years) is now taking place in the USA (table).
Johnson & Johnson
The Janssen Pharmaceutical Companies of Johnson & Johnson have initiated a randomised, double-blind, placebo-controlled, phase 3 trial (in 60 000 participants aged 18 years and older) of their replication-defective Ad26.COV2.S vaccine, which expresses full-length spike glycoprotein.67 Results have shown that a single immunisation with this adenovirus serotype 26-vectored vaccine (1·0 × 1011 viral particles by the intramuscular route without adjuvant) induces strong neutralising antibody responses and provides protection against SARS-CoV-2 challenge in rhesus macaques aged 6–12 years.68 This candidate vaccine, which requires storage at 2–8°C, is now being tested in a phase 1/2 trial involving 1045 participants (aged 18–55 years and ≥65 years) in the USA and Belgium. The company has not yet publicly released details of the vaccine's safety profile and efficacy. The phase 3 trial of this vaccine started on Sept 23, 2020 (table).
Gamaleya
The Gamaleya National Research Centre for Epidemiology and Microbiology (Russian Federation) have published the results of two phase 1/2 clinical trials of their COVID-19 vaccine consisting of recombinant adenovirus serotype 26 (rAd26) vector and recombinant adenovirus serotype 5 (rAd5) vector, both carrying the gene for the SARS-CoV-2 spike glycoprotein (rAd26-S and rAd5-S).69 These candidate vaccines (1·0 × 1011 viral particles per vaccine dose) were tested in 76 healthy individuals aged 18–60 years (38 participants in each study). In each study, patients were given either a single dose of the rAd5-S vaccine in phase 1, a single dose of the rAd26-S vaccine in phase 1, or both rAD5-S and rAd26-S in phase 2. The first study examined frozen vaccine formulations (0·5 mL per dose; stored at −18°C), and the second study examined lyophilised formulations (1·0 mL per dose; stored at 2–8°C). Local and systemic reactions were mild, and 100% of recipients in both studies seroconverted, with RBD ELISA titres and neutralising antibody titres equal to or more than titres observed in convalescent plasma from patients who had recovered from COVID-19. CD4+ and CD8+ Th cell immune responses were detected in all volunteers and peaked at day 28 after vaccination. The Institute of Biology at the Academy of Military Medical Sciences announced the approval (in small population groups) of their adenovirus-vectored vaccine (Sputnik V; formerly known as Gam-COVID-Vac) on Aug 12, 2020, before the phase 3 clinical studies had started. A phase 3 safety and efficacy trial will recruit 40 000 participants from different age and risk groups. Concerns have been raised about the vaccine's safety and efficacy, given that the vaccine has not yet been tested in a phase 3 clinical trial.70 Because of these concerns, verifying the integrity of data (including safety and efficacy data) generated in these clinical trials of COVID-19 vaccines is important.70
CanSino Biologics
China-based CanSino Biologics have developed a recombinant adenovirus serotype 5-vectored COVID-19 vaccine that expresses the SARS-CoV-2 full-length spike glycoprotein from the Wuhan-Hu-1 virus strain.38 This candidate vaccine was tested in a phase 1 clinical trial38 of 108 healthy adults aged 18–60 years. Participants received a single vaccination consisting of either 5·0 × 1010 viral particles, 1·0 × 1011 viral particles, or 1·5 × 1011 viral particles. Neutralising antibody titres increased by at least four times from baseline in 11 (31%) of 36 participants in the middle dose group at day 14 and in 18 (50%) at day 28, and in 15 (42%) of 36 participants in the high-dose group at day 14 and in 27 (75%) at day 28. Adverse reactions included pain, redness, and swelling at the injection site, fever, headache, fatigue, and muscle or joint pain, most of which were mild, transient, and occurred within 1 week of vaccination. CanSino Biologics and the Institute of Biology at the Academy of Military Medical Sciences announced the approval of their adenovirus serotype 5-vectored vaccine on June 25, 2020, before the start of phase 3 testing.71 The phase 3 trial includes 40 000 participants aged 18 years and older and is underway in Pakistan and China. Information on storage conditions has not yet been released for this vaccine, but storage conditions are likely to be similar to those of other vaccines based on adenovirus vectors and might involve either refrigeration or storage at −20°C.
Sinovac Biotech
This vaccine (CoronaVac) is a chemically inactivated, whole-virus preparation administered in a two-dose regimen (at day 0 and day 28) and was granted an emergency use authorisation by Chinese authorities in July, 2020, before the initiation of phase 3 studies. This authorisation reportedly resulted in nearly 90% of company employees being immunised with the vaccine.71 Phase 1/2 clinical trials, which enrolled healthy volunteers aged 18–59 years, have been completed (eg, NCT04352608). The phase 1 trial included 143 participants. In the phase 2 trial, 600 participants were randomly assigned to receive, in two intramuscular injections, either 3 μg per 0·5 mL or 6 μg per 0·5 mL of the trial vaccine, or placebo, either on day 0 and day 14, or on day 0 and day 28.73 No serious adverse events were reported. The vaccine elicited anti-RBD antibodies, as measured by ELISA, and neutralising antibodies 14 days after the second dose of vaccine in 92·4% of individuals receiving the vaccine at 0 and 14 days, and in 97·4% of those receiving the vaccine at 0 and 28 days. Importantly, neutralising antibody responses were significantly higher in younger adults (aged 18–39 years) than in older adults (aged 40–59 years), and stronger responses were noted in participants given the second dose on day 28 than in those given the second dose on day 14. No data have been published in regard to measures of cellular immune responses to this vaccine. A phase 3 trial has been launched in Brazil and Indonesia, with the trial in Brazil aiming to enrol 9000 health-care personnel.
Sinopharm
Sinopharm have developed and are testing two inactivated whole-virus, alum-adjuvanted vaccines. The first vaccine candidate (New Crown COVID-19) was developed by the Wuhan Institute of Biological Products. Both phase 1 and 2 study data have been published.74 The phase 1 trial (n=96) examined a three-dose series, and the phase 2 trial (n=224) studied a 5 μg dose across two study groups: vaccination on day 0 and day 14 (n=84) versus alum only (n=28), or vaccination on day 0 and day 21 (n=84) versus alum only (n=28). This trial included adults aged 18–59 years. The phase 2 trial did not include any comparator, such as convalescent human plasma, but did measure neutralising antibody titres, which were generally similar in concentration to those produced by other COVID-19 vaccines and were higher in the group vaccinated on days 0 and 21 (geometric mean titre 247, 95% CI 176–345) than in the group vaccinated on days 0 and 14 (121, 95–154). In addition, lymphocyte cell subsets and cytokines were measured by flow cytometry and did not show changes across study groups, suggesting that cellular immune responses might not have been generated. The safety profile of this vaccine was excellent, with local and systemic reactions generally on par with those in the alum-only group of the study. A phase 1/2 clinical trial of individuals 6 years and older has also been initiated. A phase 3 clinical trial began in July, 2020, and plans to enrol 21 000 participants in the United Arab Emirates, Bahrain, Peru, Morocco, Argentina, and Jordan. In late August, 2020, Sinopharm researchers revealed that they had already begun to administer the vaccine to health-care personnel and groups at high risk of becoming infected.
The second vaccine candidate being tested by Sinopharm was developed by the Beijing Institute of Biological Products. A phase 3 trial (n=5000) is taking place in the United Arab Emirates (table). The United Arab Emirates has granted emergency use of the vaccine in health-care providers. Sinopharm have reportedly administered these experimental vaccines to hundreds of thousands of people under an emergency use condition approved by the Chinese Government.72
Enhancement of disease
Antibody response is an important component of protective immunity during SARS-CoV-2 infection.75, 76 In antibody-dependent enhancement, heterotypic (non-neutralising) antibodies might have the potential to facilitate viral entry into cells through interactions with Fc receptors or complement. Even in the absence of active viral replication in immune cells, this process might lead to the activation of macrophages, monocytes, and B cells, and IL-6, TNFα, and IL-10 production.77 Cases of antibody-dependent enhancement induced by vaccines have been reported after the use of formalin-inactivated vaccines against respiratory syncytial virus and measles, and after the use of a vaccine against dengue virus.78, 79, 80 Concerns have therefore been raised regarding the potential for antibody-dependent enhancement in individuals who are infected with SARS-CoV-2 after vaccination with a COVID-19 candidate vaccine.38 The potential risk of antibody-dependent enhancement mediated by Fc receptors could be increased with mutations in the SARS-CoV-2 spike glycoprotein, which could weaken the primary host antibody response. Monocyte, macrophage, and B-cell infection might occur in numerous tissues as a result of subsequently unstable virus-antibody complexes, leading to extensive apoptosis of immune cells and the production of inflammatory cytokines.52 There was evidence of vaccine-enhanced disease after SARS-CoV-1 vaccine administration in subsequently challenged animal models.81 Yang and colleagues82 hypothesised that the molecular mechanism of enhancement might involve the interaction of antibodies with conformational epitopes in the ACE2-binding domain. A study of antibody-dependent infection of human macrophages by SARS-CoV-1 has shown the role of anti-spike glycoprotein IgG in the infection of immune cells and that antibody-dependent enhancement is triggered by downstream signalling pathways of FcγRII receptors.83, 84, 85 Although previous SARS-CoV-2 exposure could have a role in antibody-dependent enhancement, candidate SARS-CoV-2 vaccines administered to small animals and non-human primates produced antibody-mediated protection with no signs of acute lung injury and immunopathology, as was seen after SARS-CoV-1 vaccination in animal models.75, 86 Antibody-dependent enhancement poses a theoretical obstacle to vaccine development and is being carefully evaluated.52, 87 The extent to which pre-existing antibodies to SARS-CoV-2 (and potentially to SARS-CoV-1) might contribute to antibody-dependent enhancement and disease severity remains in question;81 however, no evidence of antibody-dependent enhancement has been found in animal models or in humans in phase 3 clinical trials.
Conclusions
Much remains to be learned regarding coronavirus immunity in general and SARS-CoV-2 immunity in particular, including the protective immunity induced by vaccines and the maintenance of immunity against this virus. Furthermore, multiple vaccine types will probably be needed across different populations (eg, immune-immature infants, children, pregnant women, immunocompromised individuals, and immunosenescent individuals aged ≥65 years). In addition to the adaptive immune response, there are some data suggesting that trained innate immunity might also have a role in protection against COVID-19.88, 89 Multiple clinical trials (eg, NCT04327206, NCT04328441, NCT04414267, and NCT04417335) are examining whether unrelated vaccines, such as the measles, mumps, and rubella vaccine and the Bacillus Calmette–Guérin vaccine, can elicit trained innate immunity and confer protection against COVID-19. It is crucial that research focuses on understanding the genetic drivers of infection and vaccine-induced humoral and cellular immunity to SARS-CoV-2, defining detailed targets of humoral and cellular immune responses at the epitope level, characterising the B-cell receptor and T-cell receptor repertoire elicited by infection or vaccination, and establishing the long-term durability, and maintenance, of protective immunity after infection or vaccination. A safe regulatory pathway leading to licensing must also be defined for use of these vaccines in children, pregnant women, immunocompromised people, and nursing home residents. Some have called for further shortening of the vaccine development process through the use of controlled human challenge models.90 As of Oct 5, 2020, no such studies have occurred, but the UK is considering initiating such trials in early 2021.
Search strategy and selection criteria
We searched PubMed for articles published in English from database inception to Sept 24, 2020, using the search terms “SARS-CoV-2”, “COVID-19”, “MERS-CoV”, “SARS-CoV-1”, “coronavirus”, “vaccines”, “pandemic”, “spike protein”, and “neutralizing antibody” in various combinations. Publications specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) did not exist before the COVID-19 outbreak that began in late 2019; however, when appropriate, results from studies of seasonal coronaviruses, SARS-CoV-1, and the Middle East respiratory syndrome coronavirus were considered for inclusion in this Review.
Contributors
All authors contributed equally to the drafting and review of the manuscript. RBK designed the figures.
Declaration of interests
GAP is the chair of a safety evaluation committee for novel investigational vaccine trials being done by Merck Research Laboratories, offers consultative advice on vaccine development to Merck & Co, Medicago, GSK, Sanofi Pasteur, Emergent Biosolutions, Dynavax, Genentech, Eli Lilly and Company, Janssen Global Services, Kentucky Bioprocessing, and Genevant Sciences, holds patents related to vaccinia, influenza, and measles peptide vaccines, and has received grant funding from ICW Healthcare Ventures for preclinical studies to develop a peptide-based COVID-19 vaccine. IGO holds patents related to vaccinia, influenza, and measles peptide vaccines and has received grant funding from ICW Healthcare Ventures for preclinical studies to develop a peptide-based COVID-19 vaccine. RBK holds patents related to vaccinia, influenza, and measles peptide vaccines, has received grant funding from ICW Healthcare Ventures for preclinical studies to develop a peptide-based COVID-19 vaccine, and has received funding from Merck Research Laboratories to study waning immunity to the mumps vaccine.
References
- 1.To KK-W, Hung IF-N, Ip JD. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1275. published online Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavanaugh D. Coronaviruses and toroviruses. In: Zuckerman AJ, Banatvala JE, Pattinson JR, Griffiths P, Schoub B, editors. Principles and practice of clinical virology. 5th edn. John Wiley & Sons; London, UK: 2004. pp. 379–397. [Google Scholar]
- 3.Wu LP, Wang NC, Chang YH. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13:1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne DC, Iblan I, Rha B. Persistence of antibodies against Middle East respiratory syndrome coronavirus. Emerg Infect Dis. 2016;22:1824–1826. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moderbacher CR, Ramirez SI, Dan JM. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020 doi: 10.1016/j.cell.2020.09.038. published online Sept 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ou X, Liu Y, Lei X. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11 doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong SK, Li W, Moore MJ, Choe H, Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai W, He L, Zhang X. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H-w, Li Y, Zhang H-n. Global profiling of SARS-CoV-2 specific IgG/IgM responses of convalescents using a proteome microarray. medRxiv. 2020 doi: 10.1101/2020.03.20.20039495. published online March 27. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okba NMA, Müller MA, Li W. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Fleta P, Alfranca A, González-Álvaro I. SARS-Cov-2 cysteine-like protease (Mpro) is immunogenic and can be detected in serum and saliva of COVID-19-seropositive individuals. medRxiv. 2020 doi: 10.1101/2020.07.16.20155853. published online July 18. (preprint) [DOI] [PubMed] [Google Scholar]
- 12.Ni L, Ye F, Cheng ML. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971. doi: 10.1016/j.immuni.2020.04.023. 77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padoan A, Sciacovelli L, Basso D. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin Chim Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou B, Li T-D, Zheng S-F. Serology characteristics of SARS-CoV-2 infection since exposure and post symptom onset. Eur Respir J. 2020 doi: 10.1183/13993003.00763-2020. published online May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams ER, Ainsworth M, Anand R. Antibody testing for COVID-19: a report from the National COVID Scientific Advisory Panel. medRxiv. 2020 doi: 10.1101/2020.04.15.20066407. published online July 7. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibarrondo FJ, Fulcher JA, Goodman-Meza D. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Röltgen K, Wirz OF, Stevens BA. SARS-CoV-2 antibody responses correlate with resolution of RNAemia but are short-lived in patients with mild illness. medRxiv. 2020 doi: 10.1101/2020.08.15.20175794. published online Aug 17. (preprint) [DOI] [Google Scholar]
- 18.Liu T, Wu S, Tao H. Prevalence of IgG antibodies to SARS-CoV-2 in Wuhan—implications for the ability to produce long-lasting protective antibodies against SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.06.13.20130252. published online June 16. (preprint) [DOI] [Google Scholar]
- 19.Tan W, Lu Y, Zhang J. Viral kinetics and antibody responses in patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.24.20042382. published online March 26. (preprint) [DOI] [Google Scholar]
- 20.Long QX, Tang XJ, Shi QL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 21.Seow J, Graham C, Merrick B. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.07.09.20148429. published online July 11. (preprint) [DOI] [Google Scholar]
- 22.Gudbjartsson DF, Norddahl GL, Melsted P. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020 doi: 10.1056/NEJMoa2026116. published online Sept 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbiani DF, Gaebler C, Muecksch F. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi T, Ellingson MK, Wong P. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020 doi: 10.1038/s41586-020-2700-3. published online Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin JM, Bai P, He W. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Bastard P, Liu Z. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020 doi: 10.1126/science.abd4570. published online Sept 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastard P, Rosen LB, Zhang Q. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020 doi: 10.1126/science.abd4585. published online Sept 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontanet A, Cauchemez S. COVID-19 herd immunity: where are we? Nat Rev Immunol. 2020;20:583–584. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng W, Bao L, Liu J. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369:818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thevarajan I, Nguyen THO, Koutsakos M. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuri-Cervantes L, Pampena MB, Meng W. Immunologic perturbations in severe COVID-19/SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.05.18.101717. published online May 18. (preprint) [DOI] [Google Scholar]
- 33.Grifoni A, Weiskopf D, Ramirez SI. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489. doi: 10.1016/j.cell.2020.05.015. 501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Bert N, Tan AT, Kunasegaran K. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 35.Braun J, Loyal L, Frentsch M. SARS-CoV-2 reactive T cells in healthy donors and patients with COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2598-9. published online July 29. [DOI] [PubMed] [Google Scholar]
- 36.Peng Y, Mentzer AJ, Liu G. Broad and strong memory CD4 (+) and CD8 (+) T cells induced by SARS-CoV-2 in UK convalescent COVID-19 patients. bioRxiv. 2020 doi: 10.1101/2020.06.05.134551. published online June 8. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekine T, Perez-Potti A, Rivera-Ballesteros O. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. bioRxiv. 2020 doi: 10.1101/2020.06.29.174888. published online June 29. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu F-C, Li Y-H, Guan X-H. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 40.Kaneko N, Kuo H-H, Boucau J. The loss of Bcl-6 expressing T follicular helper cells and the absence of Germinal Centers in COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.08.025. published online Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meckiff BJ, Ramirez-Suastegui C, Fajardo V. Single-cell transcriptomic analysis of SARS-CoV-2 reactive CD4 (+) T cells. SSRN. 2020 doi: 10.2139/ssrn.3641939. published online July 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orlov M, Wander PL, Morrell ED, Mikacenic C, Wurfel MM. A case for targeting Th17 cells and IL-17A in SARS-CoV-2 infections. J Immunol. 2020;205:892–898. doi: 10.4049/jimmunol.2000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotez PJ, Bottazzi ME, Corry DB. The potential role of Th17 immune responses in coronavirus immunopathology and vaccine-induced immune enhancement. Microbes Infect. 2020;22:165–167. doi: 10.1016/j.micinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Li S, Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G, Wu D, Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diao B, Wang C, Tan Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Biasi S, Meschiari M, Gibellini L. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng M, Gao Y, Wang G. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laing AG, Lorenc A, Del Molino Del Barrio I. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020 doi: 10.1038/s41591-020-1038-6. published online Aug 17. [DOI] [PubMed] [Google Scholar]
- 51.Gallais F, Velay A, Wendling M-J. Intrafamilial exposure to SARS-CoV-2 induces cellular immune response without seroconversion. medRxiv. 2020 doi: 10.1101/2020.06.21.20132449. published online June 22. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 53.Poland GA, Ovsyannikova IG, Crooke SN, Kennedy RB. SARS-CoV-2 vaccine development: current status. Mayo Clin Proc. 2020 doi: 10.1016/j.mayocp.2020.07.021. published online Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaur SP, Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassan AO, Kafai NM, Dmitriev IP. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020 doi: 10.1016/j.cell.2020.08.026. published online Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bloomberg News China starts testing COVID-19 nasal spray vaccine. Sept 11, 2020. https://www.bloomberg.com/news/articles/2020-09-11/china-starts-testing-covid-19-nasal-spray-vaccine-in-world-first
- 58.MediciNova MediciNova announces that its intranasal COVID-19 vaccine successfully induced systemic IgG and mucosal IgA neutralizing antibodies against SARS-CoV-2 in mice using BC-PIV vector technology. Sept 23, 2020. https://finance.yahoo.com/news/medicinova-announces-intranasal-covid-19-103000673.html
- 59.US Food and Drug Administration Coronavirus (COVID-19) update: FDA takes action to help facilitate timely development of safe, effective COVID-19 vaccines. June 30, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-action-help-facilitate-timely-development-safe-effective-covid
- 60.Long SW, Olsen RJ, Christensen PA. Molecular architecture of early dissemination and massive second wave of the SARS-CoV-2 virus in a major metropolitan area. medRxiv. 2020 doi: 10.1101/2020.09.22.20199125. published online Sept 23. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poland GA. Tortoises, hares, and vaccines: a cautionary note for SARS-CoV-2 vaccine development. Vaccine. 2020;38:4219–4220. doi: 10.1016/j.vaccine.2020.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Doremalen N, Lambe T, Spencer A. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. published online May 13. (preprint) [DOI] [PubMed] [Google Scholar]
- 63.Folegatti PM, Ewer KJ, Aley PK. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corbett KS, Flynn B, Foulds KE. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020 doi: 10.1056/NEJMoa2024671. published online July 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jackson LA, Anderson EJ, Rouphael NG. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. published online July 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mulligan MJ, Lyke KE, Kitchin N. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020 doi: 10.1038/s41586-020-2639-4. published online Aug 12. [DOI] [PubMed] [Google Scholar]
- 67.Loftus P. For COVID-19 vaccine, J&J plans 60,000-subject pivotal trial. Aug 20, 2020. https://www.wsj.com/articles/for-covid-19-vaccine-j-j-plans-60-000-subject-pivotal-trial-11597936496
- 68.Mercado NB, Zahn R, Wegmann F. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020 doi: 10.1038/s41586-020-2607-z. published online July 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Logunov DY, Dolzhikova IV, Zubkova OV. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bucci E. Note of concern. Sept 17, 2020. https://cattiviscienziati.com/2020/09/07/note-of-concern/
- 71.Wee S-L, Simões M. In coronavirus vaccine race, China strays from the official paths. July 16, 2020. https://www.nytimes.com/2020/07/16/business/china-vaccine-coronavirus.html
- 72.Deng C. China injects hundreds of thousands with experimental COVID-19 vaccines. Sept 12, 2020. https://www.wsj.com/articles/china-injects-hundreds-of-thousands-with-experimental-covid-19-vaccines-11599834029
- 73.Zhang Y-J, Zeng G, Pan H-X. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. medRxiv. 2020 doi: 10.1101/2020.07.31.20161216. published online August 10. (preprint) [DOI] [Google Scholar]
- 74.Xia S, Duan K, Zhang Y. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arvin AM, Fink K, Schmid MA. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- 76.Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22:72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev. 2015;268:340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruckwardt TJ, Morabito KM, Graham BS. Immunological lessons from respiratory syncytial virus vaccine development. Immunity. 2019;51:429–442. doi: 10.1016/j.immuni.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Iankov ID, Pandey M, Harvey M, Griesmann GE, Federspiel MJ, Russell SJ. Immunoglobulin g antibody-mediated enhancement of measles virus infection can bypass the protective antiviral immune response. J Virol. 2006;80:8530–8540. doi: 10.1128/JVI.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katzelnick LC, Gresh L, Halloran ME. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lambert PH, Ambrosino DM, Andersen SR. Consensus summary report for CEPI/BC March 12-13, 2020 meeting: assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine. 2020;38:4783–4791. doi: 10.1016/j.vaccine.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang ZY, Werner HC, Kong WP. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc Natl Acad Sci USA. 2005;102:797–801. doi: 10.1073/pnas.0409065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yip MS, Leung NH, Cheung CY. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol J. 2014;11:82. doi: 10.1186/1743-422X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaume M, Yip MS, Cheung CY. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J Virol. 2011;85:10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu L, Wei Q, Lin Q. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chandrashekar A, Liu J, Martinot AJ. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smatti MK, Al Thani AA, Yassine HM. Viral-induced enhanced disease illness. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peignier A, Parker D. Trained immunity and host-pathogen interactions. Cell Microbiol. 2020 doi: 10.1111/cmi.13261. published online Sept 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xing Z, Afkhami S, Bavananthasivam J. Innate immune memory of tissue-resident macrophages and trained innate immunity: re-vamping vaccine concept and strategies. J Leukoc Biol. 2020;108:825–834. doi: 10.1002/JLB.4MR0220-446R. [DOI] [PubMed] [Google Scholar]
- 90.WHO Key criteria for the ethical acceptability of COVID-19 human challenge studies. May 6, 2020. https://apps.who.int/iris/bitstream/handle/10665/331976/WHO-2019-nCoV-Ethics_criteria-2020.1-eng.pdf?ua=1 [DOI] [PMC free article] [PubMed]