Abstract
In addition to direct anti-viral activity, NK cells regulate viral pathogenesis by virtue of their cytolytic attack on activated CD4 and CD8 T cells. To gain insight into which differentiated T cell subsets are preferred NK targets, transgenic T cells were differentiated in vitro into Th0, Th1, Th2, Th17, Treg, Tc1, and Tc2 effector cells and then tested for lysis by enriched populations of lymphocytic choriomeningitis virus (LCMV)-induced activated NK cells. There was a distinct hierarchy of cytotoxicity in vitro and in vivo, with Treg, Th17, and Th2 cells being more sensitive and Th0 and Th1 cells more resistant. Some distinctions between in vitro vs in vivo generated T cells were explainable by type 1 interferon induction of class 1 histocompatibility antigens on the effector T cell subsets. NK receptor (NKR)-deficient mice and anti-NKR antibody studies identified no one essential NKR for killing, though there could be redundancies.
1. Introduction
It should come as no surprise that NK cells can regulate adaptive immunity by killing T cells, as transformed T cell lines were among the first targets described for NK cells (Herberman et al., 1975; Kiessling et al., 1975), and immature thymocytes were the first documented non-transformed natural targets (Hansson et al., 1979a). These immature thymocytes express low levels of class 1 major histocompatibility complex (MHC) proteins. Viral infections induce type 1 interferon (IFN) and up-regulate MHC antigens in thymocytes and thereby protect them from NK cell-mediated lysis(Bukowski and Welsh, 1986; Hansson et al., 1980). Naïve resting mature T cells express high levels of class 1 MHC antigens and lack sufficient adhesion molecules to be sensitive to NK cells. However, activated T cells can become susceptible to lysis and seem to be particularly sensitive to elimination by NK cells in vivo if they lack receptors for type 1 IFN, such that they cannot escape lysis by responding to IFN (Crouse et al., 2014). When activated, effector T cells may express certain NK cell receptor (NKR) ligands, such as those for NKG2D and DNAM-1, that may make them susceptible to lysis (Cerboni et al., 2007; Nielsen et al., 2012), and infection of cultured T cells with viruses such as HIV can enhance or alter their sensitivity to NK cells under certain conditions (Cohen et al., 1999; Ward et al., 2007).
Despite this well-documented phenomenon that NK cells can attack T cells, it is only recently that the full impact of NK cells on regulating adaptive anti-viral immunity and immunopathology has been realized. Using the lymphocytic choriomeningitis virus (LCMV) infection of mice model, it has been shown that NK cells can regulate the magnitude of the T cell response and thereby alter viral clearance, persistence, and immune pathology, with profound impacts on morbidity and mortality (Cook and Whitmire, 2013; Lang et al., 2012; Waggoner et al., 2011). The primary targets for the activated NK cells appear to be activated CD4 T cells, though activated CD8 T cells can also be lysed. Further, there are profound secondary downstream effects on CD8 T cells and germinal center (GC) B cells that occur as a consequence of NK cell killing of CD4 T cells. Several aspects of adaptive immunity can be impacted (Rydyznski et al., 2015). T follicular helper (Tfh) cells help GC B cells, and the numbers of both are elevated in virus-infected mice when NK cells are depleted. In vivo cytotoxicity assays suggest that day 3 NK cells can directly kill otherwise undefined, activated CD4 T cells early in infection. It is not clear whether the NK cell-dependent changes in Tfh cell numbers detected as early as day 5 after infection are a consequence of direct NK cell killing of Tfh cells or of their precursors. In contrast, regulatory T cell (Treg) numbers seem relatively unaffected by NK cells early during LCMV infection (Che et al., 2015).
In the mouse, the preferential in vivo lysis of CD4 rather than CD8 T cells by NK cells has been linked to the higher expression of CD48 on the CD8 vs. CD4 T cells activated in vivo (Waggoner et al., 2010). CD48 interacts with the molecule 2B4 (CD244) on NK cells, driving a negative signal that protects high CD48-expressing CD8 T cell targets from lysis. In 2B4-deficient mice, there is elevated but equivalent killing of activated CD4 and CD8 T cells by activated NK cells, as demonstrated by in vivo cytotoxicity assays, while in wild type mice lysis of low CD48-expressing CD4 T cells is favored (Waggoner et al., 2011).
Here we sought greater insight into the subsets of activated T cells directly lysed by NK cells and in the nature and possible function of NKR in this process. We questioned whether an in vitro cytotoxicity assay could be developed to compare the relative sensitivities of the diverse types of differentiated effector T cells. We thus examined NK cell-mediated cytotoxicity against naïve TcR transgenic T cells differentiated in vitro into distinct, well-defined effector T cell subsets. We show here that these in vitro generated T cells are highly sensitive to lysis by purified in vivo -activated NK cells, and that there are distinct hierarchies of sensitivity, suggesting that NK cells may exert complex regulatory roles, following viral infection.
2. Materials and Methods
2.1. Virus-infection of mice
Six to 8-week old male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) or mutant mice on the C57BL/6 background were inoculated intraperitoneally with 5 ×104 plaque-forming units (PFU) of LCMV, strain Armstrong. These mice were harvested at day 3 post-infection as a source of in vivo-activated NK cells. Other strains of mice used were NKG2D KO (Zafirova et al., 2009), NCR1 (NKp46) KO (Gazit et al., 2006), DNAM-1 KO (Gilfillan et al., 2008), DNAM-1 x NKG2D double KO mice crossed by Dr. Steven Hatfield, UMMS, and dap10/dap12 double KO mice kindly provided by Dr. Toshiyuki Takai, Tohoku University, and Dr. Lewis Lanier, University of California, San Francisco (Marshall et al., 2017). FoxP3-GFP knock in mice were a gift from Dr. Vijay Kuchroo (Harvard) and bred in our facility (Bettelli et al., 2006). Perforin KO and type 1 IFNRα KO mice were purchased from Jackson Labs, Bar Harbor, ME.
For NK cell depletion experiments, mice were inoculated with a dose (25 µg) of mAb to NK 1.1 (PK136) or an isotype control mouse mAb the day before infection. OT-I and OT-II T cell receptor transgenic mice were used to generate CD8 and CD4 T cell effector populations, respectively. Both transgenic T cells recognize H-2b-restricted peptide epitopes of chicken egg ovalbumin. All mice were bred or maintained at the University of Massachusetts Medical School vivarium, and the work conducted was approved by the Animal Care and Use Committee of the University of Massachusetts Medical School.
2.2. Naïve transgenic T cell isolation and effector generation
To harvest naïve T cells, the spleen and peripheral lymph nodes were isolated from unmanipulated OT-I and OT-II transgenic T cell receptor donor mice, and a single cell suspension was obtained by gently pressing the organs through a wire mesh with the plunger of a 3 mL syringe. The single cell suspensions were further purified by passage over nylon wool (Polysciences, Warrington, PA) and Percoll gradient centrifugation (GE Healthcare, Wauwatosa, WI) to enrich for small, resting T cells. Positive MACS selection (Miltenyi Biotec, Auburn, CA) was used to isolate CD4 or CD8 T cell subsets using CD8 or CD4 beads, respectively. It was important to purify naïve T cells without contamination from memory T cells, as the memory cells would already have been polarized and differentiated, and that would introduce variability in the effector cultures. The resulting MACS-purified T cells were greater than 95% pure and expressed a prototypical naïve phenotype (CD62Lhigh, CD44low), as in previous studies (Hamada et al., 2009; McKinstry et al., 2007; McKinstry et al., 2009).
For all cultures, naïve CD4 and CD8 T cells were plated at 2 × 105 cells/mL together with irradiated antigen-presenting cells (APC) at 2 × 105/mL, such that the total initial number of cells per mL was 4 × 105, in RPMI 1640 media supplemented with 2 mM Glutamine (Corning, Corning, NY), 100 IU penicillin and 100 ug/mL streptomycin (ThermoFisher, Waltham, MA), 10 mM Hepes buffer (ThermoFisher), 50 uM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), and 7.5% fetal bovine serum (Hyclone, GE Healthcare). The APC used were T cell-depleted splenocytes obtained from naïve C57BL/6 mice following removal of CD90.2+ cells by MACS separation, and these were cultured with 25 ug/mL of both LPS and Dextran Sulfate (both from Sigma-Aldrich), for two days prior to use in T cell cultures. The APC were irradiated with 3000 Rads just prior to use in culture.
All cultures received 5 uM of the relevant OVA peptide (SIINFEKL for OT-1 CD8 cells and ISQAVHAAHAEINEAG for OT-II CD4 cells) (New England Peptide Gardner, MA) and 11 ng/mL of recombinant mouse IL-2 (Peprotech, Rocky Hill, NJ). On the second day of culture, all cultures were fed with an equal amount of complete RPMI media, as was originally plated, that now contained 11 ng/mL IL-2 without additional peptide or subset-polarizing signals. The cultures were harvested on Day 4 and centrifuge-washed thoroughly, at which time point the only cells that were present were highly viable activated effector T cells.
The following cytokines were included at the initiation of both CD4 and CD8 effector T cell cultures to differentiate them into distinct cell types: for Th0 conditions, only IL-2 at 11 ng/mL; for Th1/Tc1 conditions, anti-IL-4 (clone 11B11) at 15 ug/mL, IL-12 at 2 ng/mL; for Th2/Tc2 conditions, anti-IFNγ (clone XMG1.2) at 15 ug/mL, IL-4 at 15 ng/mL; for Th17 conditions, anti-IL-4 and anti-IFNγ, both at 15 ug/mL, IL-6 at 20 ng/mL, IL-23 at 25 ng/mL, IL-21 at 50 ng/mL, TGF-β at 0.5 ng/mL, IL-1 at 10 ng/mL, TNF at 10 ng/mL; for Treg conditions, anti-IL-4 and anti-IFNγ, both at 15 ug/mL, and TGF-β at 5 ng/mL. All blocking antibodies were purchased from BioXcell (West Lebanon, NH). All other reagents were purchased from Peprotech.
2.3. Flow Cytometry
Flow cytometry analyses were performed using BD instruments (FACS LSRII, FACS Canto II or FACS Scan), and analyses were performed using FlowJo analysis software (FlowJo LLC, Ashland, OR). For surface staining of differentiated T cell subsets and primary mouse leukocytes, cells were isolated, and 2 million cells were plated. Antibodies were incubated with the cells for 20 minutes at 4 C before the cells were washed twice with a buffer comprised of Phosphate buffered saline and serum. Fixation was done using BD Cytofix™ for 5 minutes at 4 C. Cells were washed once and analyzed. All surface antibodies used were titrated against 2 million naïve cells to ensure optimal staining conditions.
Intracellular cytokine staining was performed as previously described (Roman et al., 2002). Briefly, CD4 T cells were stimulated with Phorbol myristate acetate and ionomycin (both from Sigma-Aldrich) for 4 hours, with brefeldin A (Sigma-Aldrich) added after 2 h. Cells were then surface stained and fixed for 20 min in 4% paraformaldehyde. After washing, cells were permeabilized by 10 min incubation in 0.1% saponin buffer (PBS plus 1% FBS, 0.1% NaN3 and 0.1% saponin; Sigma-Aldrich) and stained for cytokine by the addition of anti-IFNγ, TNF, IL-4, and IL-17, fluorescently labeled Ab for 20 minutes. Treg cells were detected by surface staining for CD25 followed by nuclear staining for FoxP3 as per manufacturer’s instructions using a FoxP3 staining kit (ThermoFisher). All antibodies were obtained from ThermoFisher or BD Biosciences (San Jose, CA).
2.4. Cytotoxicity assays.
For cytotoxicity assays in vitro, a standard 51 Chromium release assay was used on 96 microwell plates, with 104 target cells/well and titrated amounts of LCMV-induced day 3 spleen leukocyte effector cells, either unfractionated or partially purified for NK cells on MAC columns. NK cells were purified using Miltenyi Biotec NK Cell Isolation kits. Briefly,107 spleen leukocytes were treated with NK Cell Biotin-antibody for 15 minutes at 4C, washed and then incubated with Anti-Biotin Microbeads for 15 minutes at 4C. Magnetic separation was achieved by passing the labeled cells over a Miltenyi LS column in a magnetic field. The flow through containing unlabeled cells was collected, representing the enriched NK cells. Assays were run at various E:T ratios for 4 hours at 37 C. For the cytotoxicity assays in vivo, the T cell cultures were labeled with Cell Trace Violet at concentrations ranging from 2.5 uM to 0.01 uM and Cell Trace DDOA Far Red at 0.5uM for 15 min at 37 C. Cells were mixed at a 1:1 ratio and transferred intravenously into day 3 LCMV-infected C57BL/6 mice, depleted or not of NK cells the day before infection. Mice were sacrificed about 4 hours after injection, and spleen leukocytes were isolated and analyzed by flow cytometry for the presence of the labeled cells. Selective loss was calculated based on the ratio of the in vitro-generated population compared to the population of intravenously injected labeled control C57BL/6 spleen leukocytes in the NK replete vs. NK cell-depleted mice.
2.5. Stastical analyses.
Most cytotoxicity data are presented as the average of three or four replica wells using effector cells that are NK cells prepared from pools of mice. Because of the high precision in the cytotoxicity assay and the presentation of data at different E:T ratios, error bars are usually not presented. However, standard deviation error bars are presented in Figures where only single E:T rations are presented or when cold target or antibody-based competitors were added to the assays. Significance was established in these and other figures by a Student’s t-test. A one-way Wilcoxon analysis was used to evaluate comprensive cytotoxicity data from a large group of experiments.
3. Results
3.1. Development of assay to measure NK cell-mediated killing of T cell subsets in vitro.
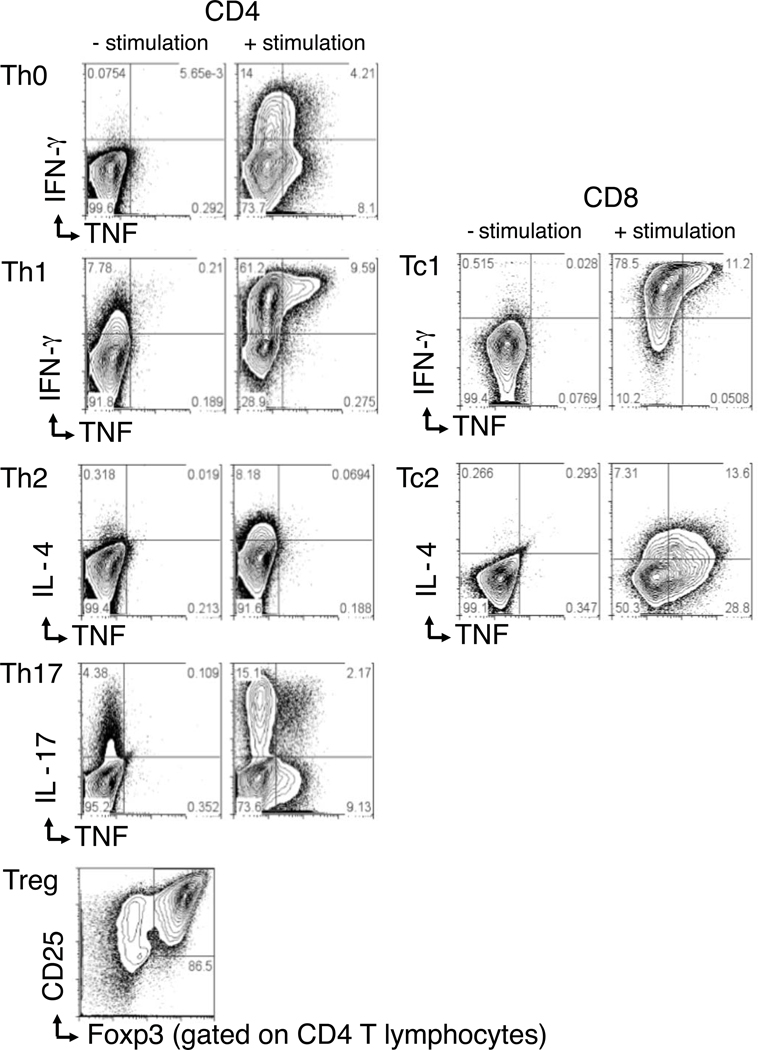
OT-I (CD8) and OT-II (CD4) transgenic T cells were stimulated with their cognate peptide ligands in vitro for 4 days, using mixtures of cytokines and anti-cytokine mAb that result in polarized T effector subsets, as described in Materials and Methods. The resulting effector cells produced cytokines and transcription factors characteristic of Tc1, Tc2, Th0, Th1, Th2, Th17, and Treg cells, although it should be noted that these cells did not differentiate in the same environment as that occurring in a viral infection in vivo. Figure 1 shows the essential properties of differentiated effector T cells used in these studies. The figure panels show representative intracellular cytokine staining (ICCS) by the different effector populations after 4 days of culture. Except for the Treg cells, all cultures were restimulated before flow cytometry analysis of ICCS. The Th0 culture made virtually no IFNγ or TNF before stimulation and only low levels after stimulation. The Th1 and Tc1 cultures produced low levels of IFNγ before stimulation, but high levels of IFNγ and TNF after stimulation. The Th2 and Tc2 cultures produced IL-4 only after stimulation. The Th17 culture produced some TNF and high levels of Th17 after stimulation. The Treg cultures constitutively expressed high levels of the alpha chain of the IL-2 receptor (CD25) and of FoxP3, the transcription factor characteristic of regulatory T cells. Thus, the different subsets exhibited the expected polarized cytokine production potential.
Figure 1. Differentiated T cell cultures.
OT-I (CD8-Tc1 and Tc2) and OT-II (CD4-Th0, Th1, Th2, Th17, and Treg) short term (4 day) transgenic T cell cultures were set up in the presence of cognate peptide and differentiation agents, as described in the Materials and Methods. Cells were stained either directly (-stimulation) or after re-stimulation with PMA and Ionomycin (+ stimulation) and analyzed by flow cytometry.
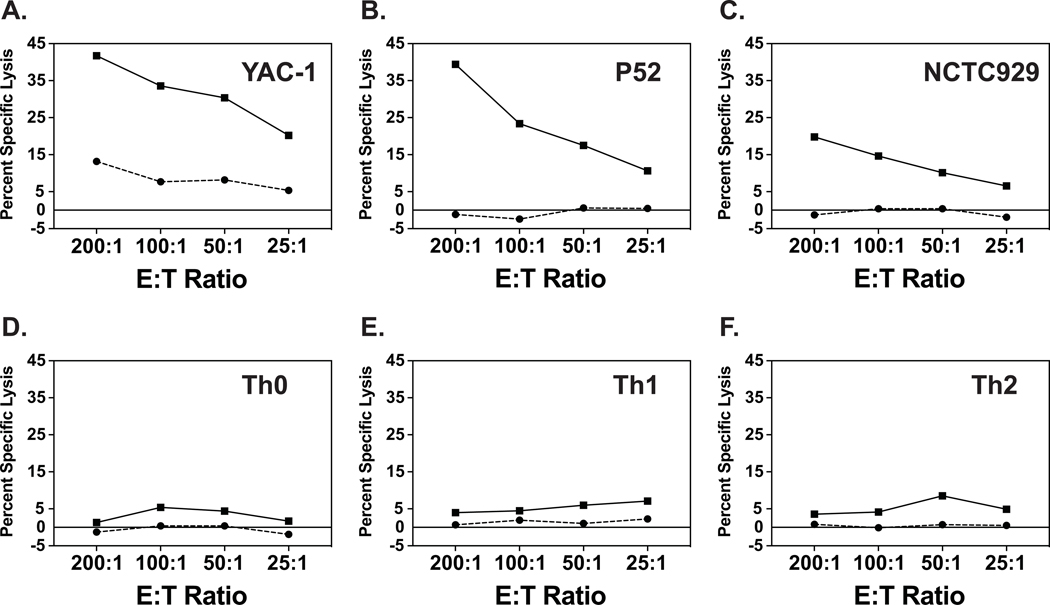
We tested Th0, Th1 and Th2 effector cell targets generated from naïve C57BL/6 mouse transgenic CD4 T cells to determine their sensitivities to NK cell killing in vitro, using 51Cr-release cytotoxicity assays with spleen leukocytes from day 3 LCMV-infected C57BL/6 mice as a source of NK cells, as described in Materials and Methods. These well-studied effector splenocyte populations are a source of highly activated NK cells and have been shown to lyse a wide variety of syngeneic, allogeneic, and xenogeneic targets in vitro at effector-to-target (E:T) ratios of 25:1 in 4–6-hour cytotoxicity assays (Welsh, 1978; Welsh and Zinkernagel, 1977). Figure 2A shows the dynamics of lysis of the highly NK-sensitive YAC-1 (H2a) cells, a target expressing low levels of class 1 MHC molecules by virtue of its production of IL-10 (Petersson et al., 1998). This panel shows that the YAC-1 cells were sensitive to spleen leukocytes from uninfected mice (bottom line) and very sensitive to day 3 spleen leukocyte effector populations (top line). Many studies have established that this target cell killing is due to CD3-, NK1.1+ NK cells. Figure 2 B, C show the day 3 leukocyte lysis of the usually less sensitive syngeneic P-52 (H2b) and allogeneic L-929 (H2k) continuous cell line targets. These targets were lysed by the day 3 activated leukocytes from LCMV-infected mice but not by leukocytes from uninfected mice. This pattern of sensitivity has been reported with many target cell types (Welsh et al., 1981).
Figure 2. Lysis of target cells by unfractionated spleen leukocytes.
Plots show 4-hour 51Cr-release cytotoxicity assays against YAC-1, P52, and NCTC929 (L-929) continuous cell lines and against cultured Th0, Th1, and Th2 differentiated effector T cell targets. Solid lines represent lysis by day 3 LCMV-induced C57BL/6 mouse spleen leukocytes, and dashed lines represent lysis by spleen leukocytes from uninfected mice.
In contrast to the killing of the continuous cell lines, none of the tested differentiated activated effector T cell subsets were lysed at levels exceeding 5% by the activated day 3 leukocytes from spleens of LCMV-infected mice at E:T ratios of up to 200 to 1 (Fig. 2D,E,F).Thus, the differentiated T cells seemed to resist killing by LCMV-induced leukocytes containing activated NK cell populations. A closer look at the kinetics of this low level of killing of the T cell subsets revealed in some cases slightly higher killing at lower rather than at higher E:T ratios. This led us to consider that there may be inhibitors within the day 3 spleen leukocyte population that interfered with the killing of T cells. Presumably, these contaminating cell types would have similarities to the labeled differentiated T cell targets.
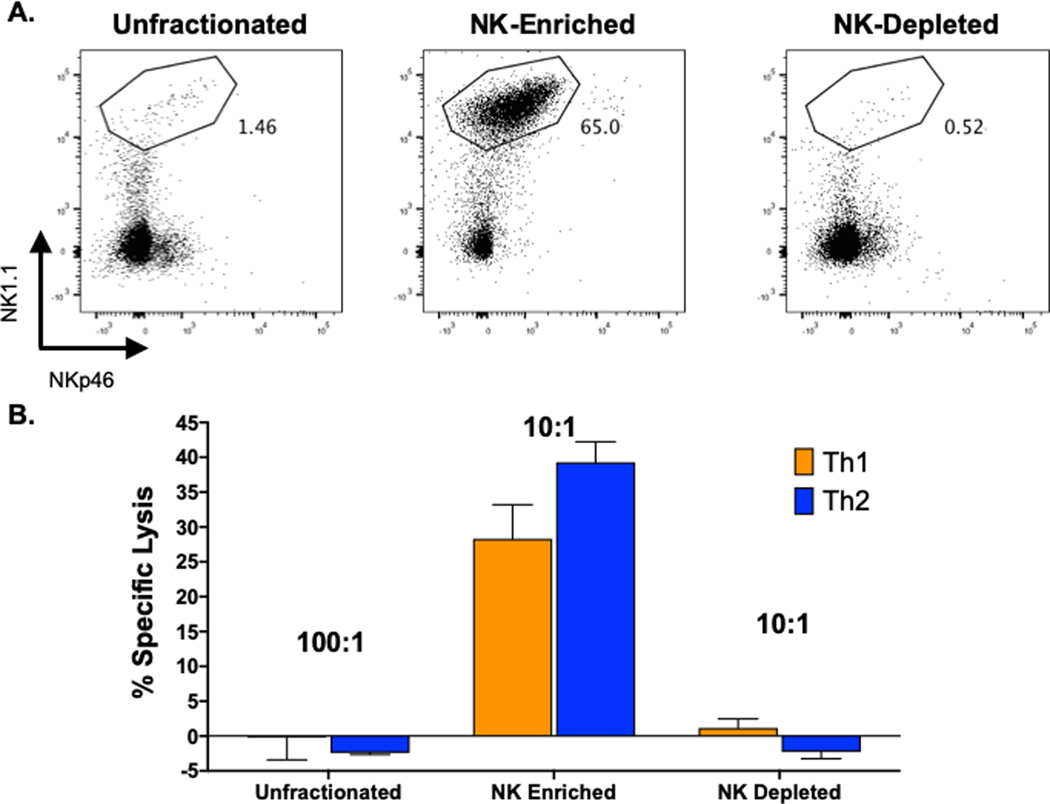
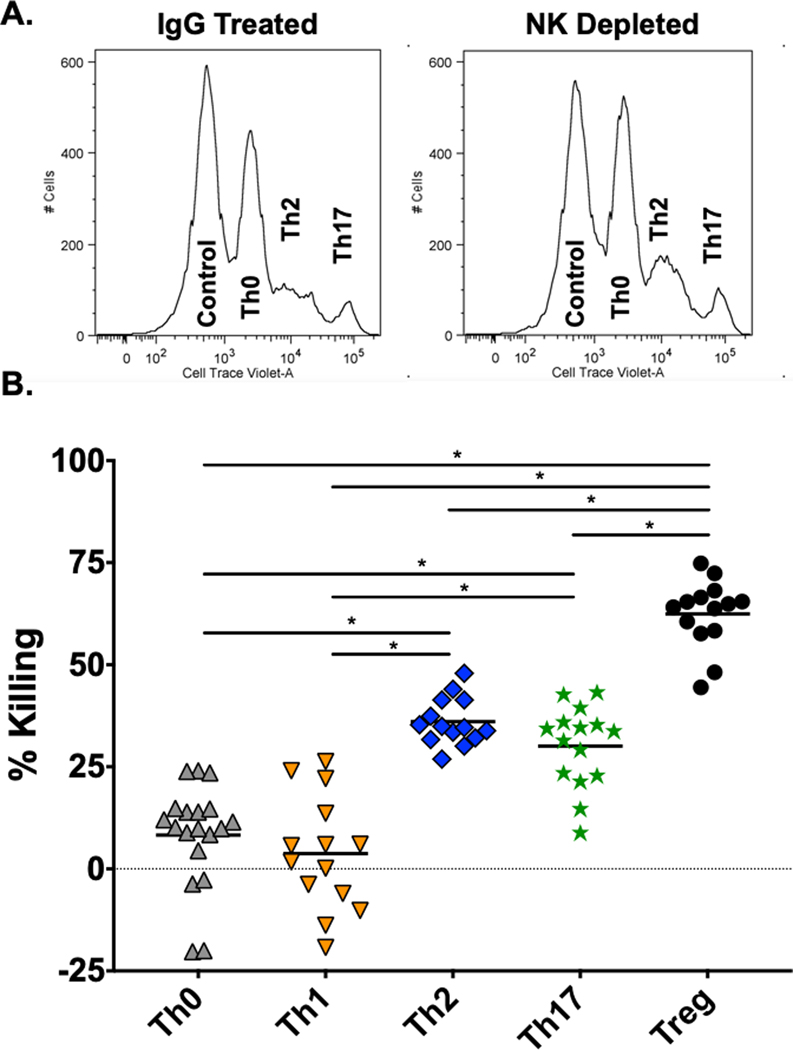
To avoid problems with inhibitory pathways mediated by unfractionated spleen leukocytes, NK cells were partially purified by passage through commercial magnetic-activated cell sorting (MACS) columns that enrich for NK cells by adhering other cell types to column-bound antibodies that do not bind to NK cells (see Materials and Methods). This enriched the NK1.1+ cell population to levels of about 50 to 85% purity, depending on the experiment, and most co-stained with mAb to NKp46 (Fig. 3a). These enriched NK cell populations, in contrast to unfractionated spleen leukocytes or the NK-depleted fraction, very efficiently lysed the effector T cells (Fig 3b). The NK-depleted fraction exhibited very little killing, confirming that the cytotoxicity seen in these assays was indeed due to NK cells.
Figure 3. MACS column-enrichment of NK cells and lysis of differentiated T cell cultures.
NK cells were partially purified from spleen leukocytes from day 3 LCMV-infected C57BL/6 mice and tested in 4-hour 51Cr-release cytotoxicity assays against Th1 and Th2 cultures. A. Upper panels are low cytometry plots against NK1.1 (y-axis) and NKp46 (x-axis). Boxes in upper panels represent %NK1.1+ cells. B. Unfractionated cytotoxicity data are plotted at E:T = 100:1, whereas enriched and depleted samples are plotted at E:T =10:1.
3.2. Hierarchies of NK cell killing of differentiated effector T cell subsets
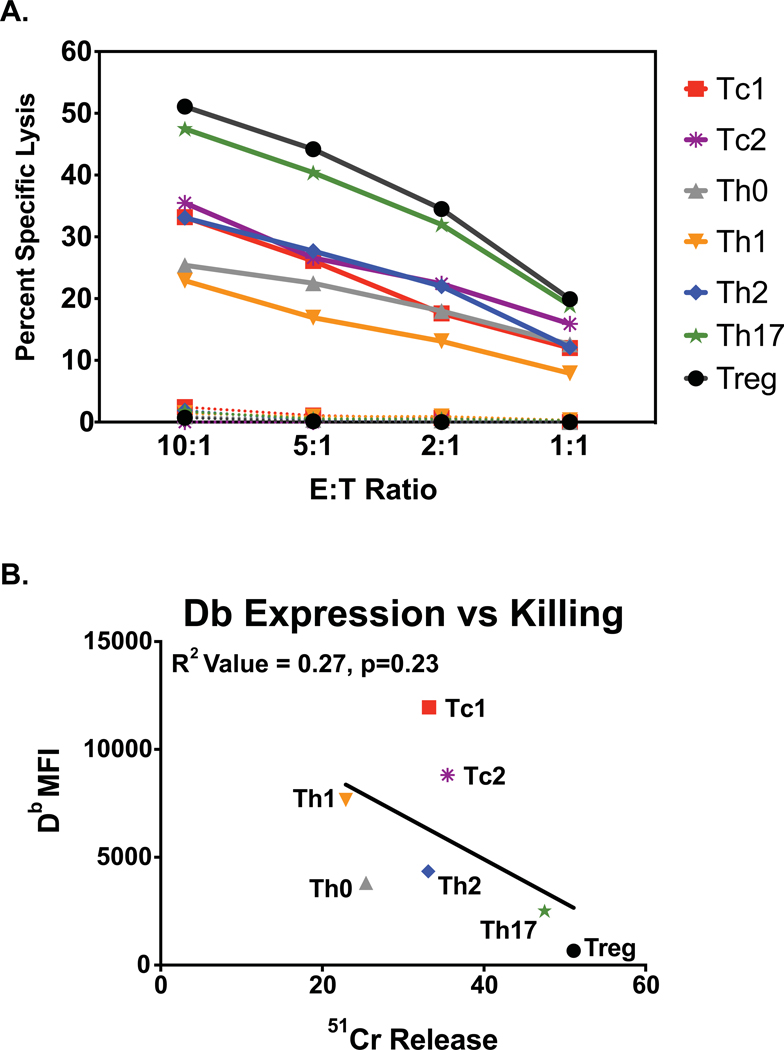
We asked whether there were differences in the sensitivities of the various types of differentiated effector T cells to the partially purified NK cells. Figure 4A shows an experiment where all effector T cell subsets we studied were examined at the same time. NK cells killed all targets at E:T ratios as low as 5 or 2.5 to 1, with good linear kinetics of killing (Fig 4). This figure shows a hierarchy of sensitivity of Treg>Th17>Th2~Tc2~Tc1>Th0~Th1. This is one of two experiments where all T cell types were tested together, and although there was some variability, the hierarchies were similar in both those and in smaller experiments with fewer targets tested. The hierarchy in which Treg and Th17 cells are most sensitive and Th0 and Th1 are least sensitive, with Th2, Tc1, and Tc2 in the middle, was reproducible in many experiments (Table 1 top) and statistically significant (Table 1 bottom).
Figure 4. Hierarchy of killing of differentiated T cell cultures.
A. In a single experiment all studied effector T cell subset cultures were tested for susceptibility to killing by NK cell-enriched populations of LCMV day 3 spleen leukocytes (solid lines) or by the NK cell-depleted population (dotted lines). Data are from a 4-hour 51Cr-release cytotoxicity assay. B. Correlation of cell surface expression of class 1 Db antigens, as analyzed by flow cytometry, with cytotoxicity (E:T=5:).
Table 1. Lysis of Differentiated T cell Effectors by LCMV-induced Activated NK Cells.
Top. This table represents a compilation of all of the cytotoxicity experiments done with enriched populations of day 3 LCMV-induced C57BL/6 mouse spleen leukocyte NK cells against effector T cell subsets in 4 hr cytotoxicity assays at E:T of 5:1. Values represent percent lysis observed. There was an additional experiment that was technically flawed and therefore not shown. Bottom. This is a one-way Wilcoxon analysis comparing the hierarchical data from all of the tabulated experiments, giving the p value of the cytotoxicity difference between each tested pair of targets. It shows that the Treg and Th17 cells are killed significantly more than the Th0 and Th1 cells, and that the Th2 cells are killed significantly more than the Th1 cells.
| Experiment | Th0 | Th1 | Tc1 | Th2 | Tc2 | Th17 | Treg |
|---|---|---|---|---|---|---|---|
| 1 | 17 | 30 | |||||
| 2 | 17 | 23 | 29 | ||||
| 3 | 22 | 17 | 26 | 28 | 27 | 40 | 44 |
| 4 | 20 | 31 | |||||
| 5 | 38 | 48 | |||||
| 6 | 13 | 41 | |||||
| 7 | 11 | 23 | 20 | 28 | 23 | ||
| 8 | 17 | 27 | 29 | 26 | 35 | ||
| 9 | 8.0 | 16 | 23 | 29 | |||
| 10 | 10 | 14 | 20 | 28 | |||
| 11 | 10 | 10 | 23 | 24 | |||
| 12 | 16 | 29 | 25 | ||||
| 13 | 35 | 55 | 31 | ||||
| 14 | 12 | 35 | 30 | ||||
| 15 | 18 | 15 | 31 | ||||
| 16 | 12 | 27 | 27 | ||||
| 17 | 10 | 27 | 25 | 44 | 34 | 33 | 30 |
| 18 | 22 | 30 | 37 | 36 | |||
| 19 | 23 | 34 | 34 | 42 | |||
| 20 | 17 | 27 | 40 | ||||
| 21 | 15 | 22 | 24 | ||||
| 22 | 6.4 | 23 | 14 | 35 | |||
| 23 | 12 | 31 | 33 | 26 |
| Th0 | Th1 | Tc1 | Th2 | Tc2 | Th17 | Treg | |
| Th0 | NA | NA | NA | NA | NA | NA | NA |
| Th1 | 0.00164 | NA | NA | NA | NA | NA | NA |
| Tc1 | 0.00142 | 0.13919 | NA | NA | NA | NA | NA |
| Th2 | 3.97E-06 | 0.01622 | 0.36275 | NA | NA | NA | NA |
| Tc2 | 0.01374 | 0.11461 | 0.25122 | 0.41116 | NA | NA | NA |
| Th17 | 2.02E-05 | 0.00451 | 0.13059 | 0.16217 | 0.45288 | NA | NA |
| Treg | 5.58E-07 | 0.00149 | 0.17805 | 0.1916 | 0.44402 | 0.62247 | NA |
Given the functional importance of NK cells killing of CD4 T cells in vivo, most of our focus was on the killing of CD4 T cell subsets. Table 1 shows cytotoxicity at E:T=5:1 in the various experiments. Of 16 experiments in which Tregs were tested, the Tregs were the most sensitive in 9 of them. In 9 experiments where Th17 targets were tested, the Th17 cells were most sensitive in 4. In 8 experiments where both Treg and Th17 cells were tested, along with other targets, either the Treg or the Th17 cells were the most sensitive in 7. In 17 experiments where Th0 cells were tested, the Th0 cells were least sensitive in 14. In 20 experiments in which Th1 cells were tested, the Th1 cells were least sensitive in 7. The Tc1, Tc2, and Th2 cells seemed of intermediate sensitivity. However, in 14 of 15 experiments where Th2 targets and either or both Th0 and Th1 targets were used, Th2 targets were most sensitive. Notably, Th2 cells were more sensitive than Th1 cells at p= .016. Variations among experiments could be due to variations in cell cultures, in the state of the mice, or in the level of purity of NK cells, but Fig. 4A reflects the usual pattern seen.
3.3. Parameters of NK cell-dependent lysis of transgenic T cell cultures.
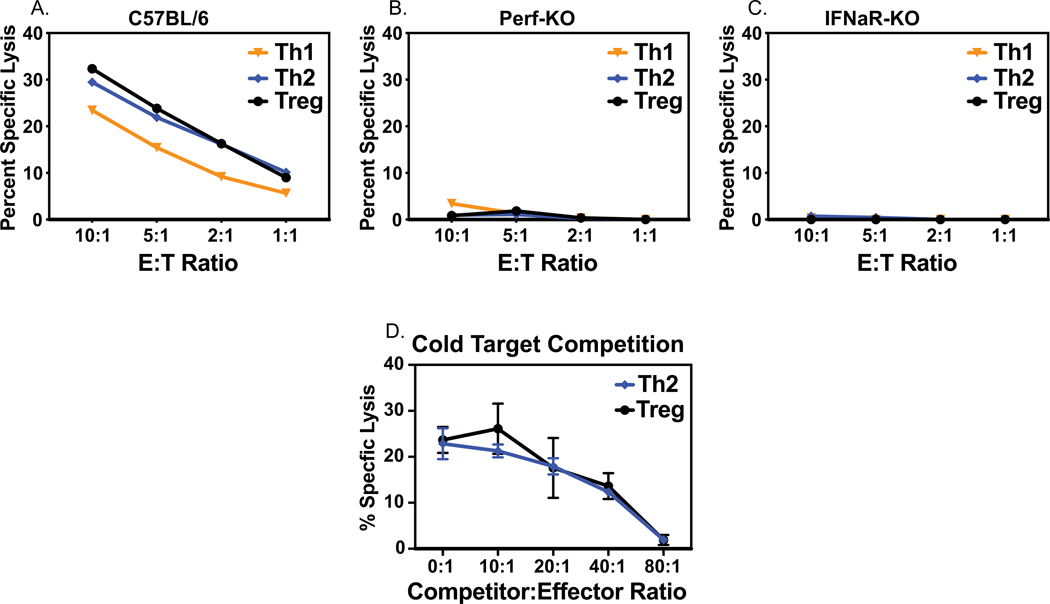
The lysis of T cell targets in vivo has been shown to be mediated by type 1 IFN-activated NK cells functioning through a perforin-dependent mechanism (Waggoner et al., 2011). We tested if representative T cell effector subsets were lysed in vitro by a similar mechanism. Figure 5A, B shows that Th1, Th2, and Treg cultures were lysed by enriched NK cells from LCMV day 3 C57BL/6 wild type mice but not by enriched NK cells from LCMV day 3 perforin KO mice. Further, Fig. 5C shows little if any lysis by enriched NK cells from LCMV day 3 type 1 IFN receptor KO mice in which the absence of the IFN receptor prevents NK activation. The requirement of NK cell enrichment to demonstrate lysis of the T cell cultures (Fig. 3) suggested that non-NK cells in spleen leukocyte populations might be competing with the killing of these targets. To test this hypothesis, cells from the NK cell-depleted day 3 spleen leukocyte fraction from C57BL/6 mice were tested as competitors and added at different competitor to target ratios to Treg and Th2 cytotoxicity assays. In both cases spleen leukocytes in the NK cell-depleted fractions competed well against the killing, with linear kinetics (Fig. 5D). Competition was also seen against the tested Th0 and Th1 targets in these assays, but only the Treg and Th2 cell data are plotted here because of the higher initial levels of cytotoxicity. Collectively, the data are consistent with the conclusion that linear killing patterns are caused by NK1.1+ NKp46+ cells working in a type 1 IFN-dependent and perforin-dependent manner, and that this killing was inhibited by NK cell-depleted spleen leukocyte competitors, presumably acting as cold targets, in a linear manner.
Figure 5. Mechanisms of NK cell killing of T cell targets.
Lysis of T cell targets by NK cell-enriched populations of LCMV-induced day 3 leukocytes from C57BL/6 (panel A), perforin KO (panel B), or type 1 IFN R KO (panel C) mice. Panel D: Cold target competition against killing of Th2 and Treg cultures by NK cell-depleted LCMV day 3 leukocyte fractions.
3.4. In vivo cytotoxicity of cultured T cells reflects the hierarchy of cytotoxicity in vitro.
An important issue was whether the degree of killing in the in vitro cytotoxicity assay against the cultured T cells would reflect their susceptibility to NK cells in vivo. To evaluate this, T cell effector subset cultures were labeled with Cell Trace Violet and Cell Trace DDOA Far Red and transferred intravenously into day 3 LCMV-infected mice, depleted or not of NK cells, in an in vivo cytotoxicity assay, as described in the Materials and Methods. Here, the recovery of these marked donor cells from the spleens of LCMV-infected recipient mice was compared to that in LCMV-infected mice depleted of NK cells. Figure 6 represents a compilation of data from individual mice from four experiments and shows that the Treg cells were most sensitive, Th2 and Th17 cells next most sensitive, and the Th0 and Th1 cells least sensitive to in vivo cytotoxicity, similar to their sensitivity hierarchies in vitro (Fig. 4; Table 1). This assay was not amenable to studying the in vivo killing of the Tc1 cells, as these CD8 cells failed to rapidly home to the spleen after transfer in vivo (data not shown).
Figure 6. In vivo cytotoxicity assay against differentiated T cell cultures.
This depicts in vivo cytotoxicity assays against cultured differentiated CD4 T cells by displaying the cytotoxic values in individual mice from 2–3 experiments per T cell subset. Details of this assay are in the Materials and Methods section. A. Representative histograms showing the survival of cell populations in NK cell replete (control IgG-treated) vs. anti-NK1.1-depleted mice. The T cell subsets are identified by the concentration of the cell trace violet dye. B. Panel showing that the cytotoxicity against Treg, Th17, and Th2 cells was significantly greater than that against the Th0 and Th1 cells, as determined by Students t test (p<0.0001).
3.5. Similarities and discrepancies between in vitro cultures and in vivo infection.
Having established that in vitro-differentiated T cell effectors can be lysed by purified populations of activated NK cells and cleared with a similar NK cell-dependent hierarchy in vivo, we questioned whether the patterns of lysis reflected those which occurred when the T cells were differentiated during infection in vivo. It is well-established that the sensitivity of target cells to NK cell killing can be regulated by target cell expression of class 1 MHC antigens, which inhibit killing by engaging inhibitory NKR. Figure 4B shows that in the comprehensive experiment revealing the hierarchy of killing of the cultured T cell targets, the Th17 and Treg cells expressed lower levels of MHC Db than other cell types, consistent with regulation of sensitivity to NK cell-mediated lyisis by class I expression level. Figure 4B also shows a broader trend that cells expressing the least class 1 MHC were more sensitive to lysis than those that expressed higher levels, but this was not statistically significant in this or other experiments. However, the low expression of class 1 MHC on Treg and Th17 cells was very reproducible. For instance, in four experiments examining Treg and two or three other targets, expressions of both class 1 Db and Kb were lowest on Treg cells in every case, much like that shown in Fig. 4B. Further, in four experiments including Treg, Th17, and other targets, Db expression on Th17 was second lowest, much like that shown in Fig. 4B.
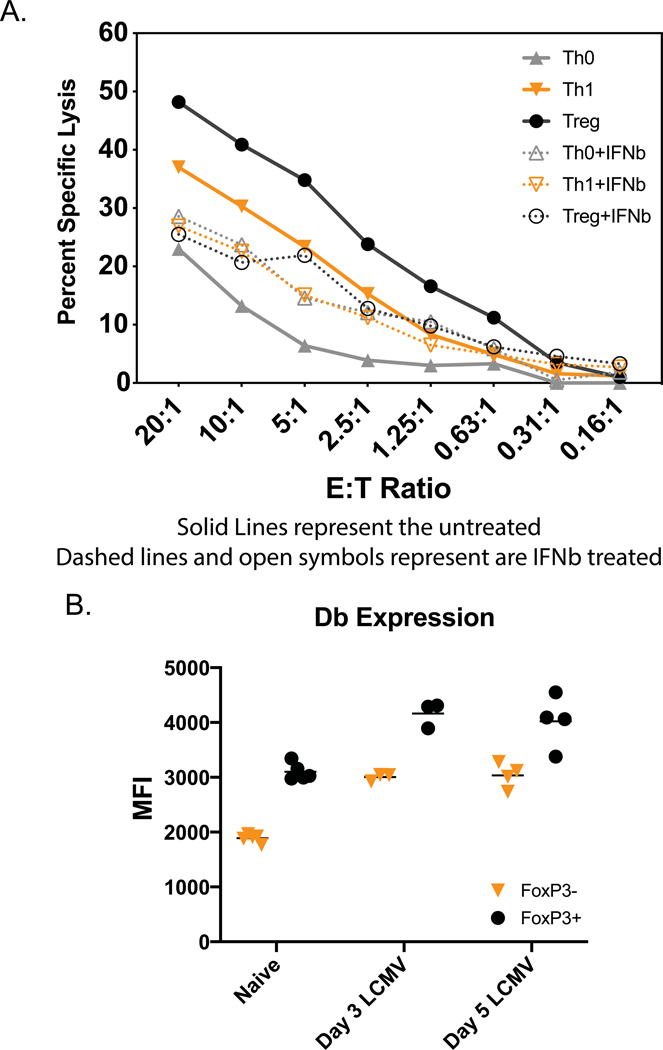
Although T cell effectors generated in vitro display phenotypes and cytokine profiles consistent with those characteristics of prototypical T cell subsets (Fig. 1), it is likely that when those cells differentiate in response to viral infection in vivo, the milieu will be quite different because of pathogen-induced inflammation. Thus, although there may be many similarities between in vitro- and in vivo-generated effector T cell subsets, there also may be differences. Given that type 1 IFN induced during the LCMV infection modulates class 1 MHC expression and the sensitivity of targets to NK cells in vivo (Bukowski and Welsh, 1986), we tested whether exposure of the T cell effector subsets to type I IFN would alter their sensitivity to killing. Th0, Th1 and Treg CD4 effector subsets were therefore exposed to 500 or 1000 units/ml of IFNβ for 24 hr prior to use as targets in cytotoxicity assays. Figure 7A displays representative data showing that exposure to IFNβ rendered Treg and Th1 cells resistant to activated NK cell-mediated killing. Tc1 cells in this and two other experiments had a similar pattern of enhanced protection (data not shown for figure clarity). However, in three experiments IFNβ rendered Th0 cells more sensitive rather than more resistant to NK cells, and we do not know the explanation for that finding. In the experiment shown in Fig. 7 IFNβ up-regulated expression of class 1 Db on most cell types, but the Th0 cells were inconsistent- mean fluorescent intensity change for Db on Th0, 6526 to 6142; Th1, 7542 to 9202; Tc1, 4458 to 5947; Treg, 1838 to 5456. There were some differences in the outcomes of IFNβ exposure. Treg cells were remarkedly protected by IFNβ, which greatly up-regulated their class 1 MHC. Th1 and Th2 cells were also protected, along with significant Db up-regulation. IFNβ consistently increased rather than decreased the sensitivity of Th0 cells, but its effect on Th0 cell Db expression varied between experiments.
Figure 7. Modulation of differentiated T cell effectors. A. IFN-induced protection of differentiated T cells in vitro.
A. Differentiated transgenic T cells were treated (or not) with 500 units/ml of IFNβ for 24 hrs and tested as targets of NK cell-enriched LCMV-induced day 3 spleen leukocytes in 4-hr 51Cr-release cytotoxicity assays. This experiment examined the Th0, Th1, Treg, and Tc1 cells, but only the first three are plotted, for better resolution of the graph. B. Expression of Class 1 Db on Treg cells in vivo. Fox-P3-GFP mice were inoculated with LCMV, and on days 0, 3, and 5 post-infection their CD4-gated spleen lymphocytes were examined for co-expression of the FoxP3-GFP transgene and for staining with mAb to class 1 Db.
The strong killing of the Treg cultures seen in Figs. 4, 6 and 7 seemed at odds with a report of Treg number stability during LCMV infection (Che et al., 2015). However, we found by using FoxP3-GFP transgenic mice to mark Treg cells in vivo, that FoxP3+ CD4 T cells expressed more Db (Fig. 7B) and Kb (not shown) than FoxP3- CD4 T cells, and that the levels of class 1 antigens were substantially increased after viral infection on day 3 and 5. Thus, the in vitro-generated Treg cells differed from those generated in vivo, in that the in vivo generated cells were relatively higher in class 1 expression compared to other CD4 T cells, whereas the in vitro generated Treg cells were relatively lower in class 1 expression compared to other T cells. In conclusion, the in vitro cultured T cell subsets, while having some similarities to T cell subsets generated in vivo, also had differences that reflected the virus-induced environment in vivo.
3.6. NKR requirement for killing of in vitro generated effector T cell subsets.
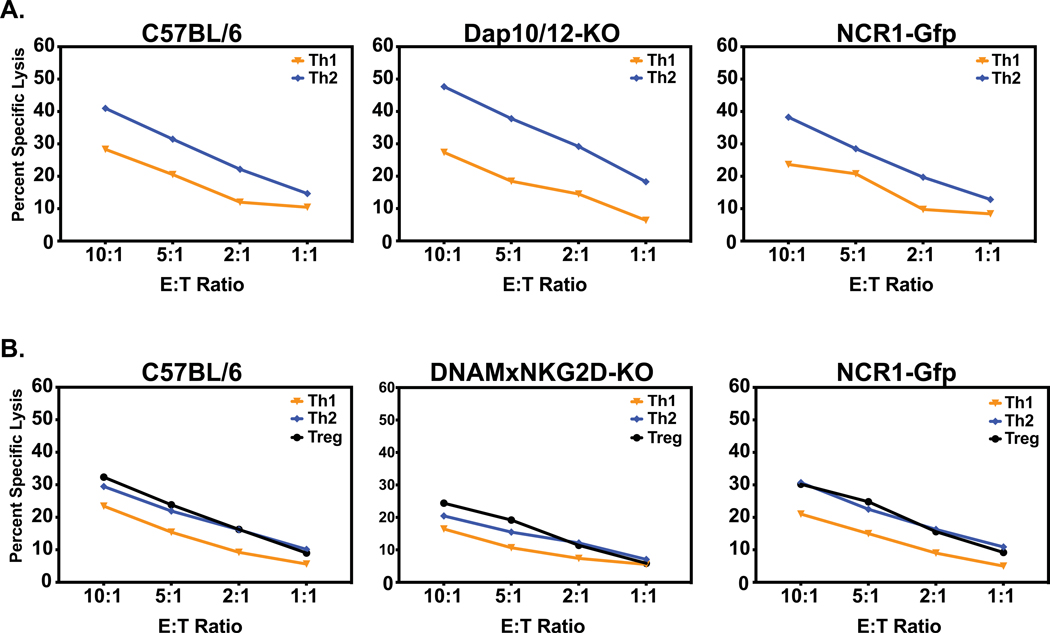
A wide variety of NKR have been identified, but it is rare that a single NKR is exclusively responsible for a biological phenotype. Exceptions to this found in our lab are the control of murine cytomegalovirus infection through the NKR Ly49H (Daniels et al., 2001) and the control of polyomavirus-induced tumors by NKG2D (Mishra et al., 2010). We therefore tested activated NK cells enriched from the spleens of day 3 LCMV-infected mice bearing mutations in various NKR on their ability to lyse the differentiated effector T cell targets. Figures 8A and B show two experiments indicating that NK cells from mice deficient in dap10/dap12, in NKp46 due to disruption of the ncr gene, and in DNAM-1 and NKG2D as the result of a double KO cross made in our lab, all kill various differentiated effector T cell cultures at wild-type mouse levels, and trending with the typical target cell hierarchies. Many activating NKR (NKG2D, Ly49D, and Ly49H) attach to dap adaptor molecules, which contain kinase regions that enable the activation signals to occur.
Figure 8. Lysis of differentiated T cell effectors by NK cell-enriched spleen leukocytes from day 3 LCMV-infected NKR mutant mice.
Panel A. Shows normal levels of lysis of T cell cultures by enriched NK cells from Day 3 LCMV-infected C57BL/6 control, dap 10/12 KO, and ncr mutant (NKp46-deficient) mice. Panel B. Shows killing by NK cell-enriched leukocytes from Day 3 LCMV-infected C57BL/6 control, DNAM1/NKG2D KO, and ncr mutant (NKp46-deficient) mice.
3.7. Blockade of killing in vitro with anti-NKR antibodies.
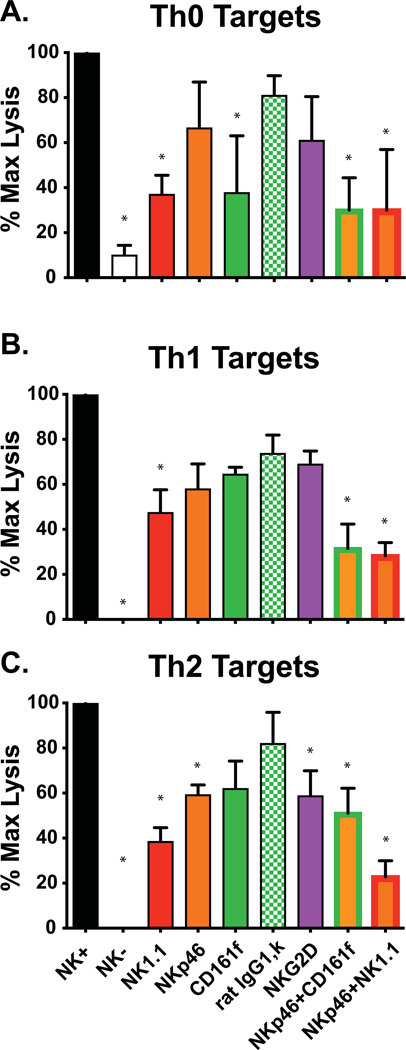
To further analyze NKR involvement in this killing, we tested the ability of mAb against activating NKR and other cell surface molecules to block NK cell-mediated cytotoxicity against differentiated effector T cell targets. These included mAb to B cell antigens CD19, IgM, and IgD (as controls) and to activating NKR CD161a (NKR-P1A) (BD Biosciences), CD161f (NKR-P1F) (Biolegend), NK1.1 (CD161b,c;NKR-P1B/C-PK136 (BD Biosciences), NKp46, NKG2D, Fcγ, and Dx5. The NKR-P1 receptors studied here are activating receptors of the killer cell lectin receptor family and bind c-type lectin-related molecules. mAb to B cell antigens (CD19, IgM, IgD) inhibited killing by <10% (data not shown). In no case was killing dramatically inhibited by any single anti-NKR antibody, though many inhibited by over 20% when compared to control antibodies. Figures 9A, B, C are representative of 5 blocking experiments, in this case showing killing against Th0, Th1, and Th2 targets by NK cell-enriched (NK+) or depleted (NK-) day 3 leukocytes, and showing the reduced killing caused by inclusion of different mAb and mAb combinations as blocking agents. The least blocking in this experiment was by a rat IgG k isotype control mAb with irrelevant specificity.
Figure 9. Blockade of NK cell-mediated cytotoxicity against differentiated T cell cultures.
Panels A, B, and C represent cytotoxicity assays against Th0, Th1, and Th2 cultures by NK cell-enriched day 3 LCMV-induced spleen leukocyte populations in 4-hr 51Cr-release cytotoxicity assays, at E:T ratio = 5:1. These were added to all samples except those in the second column, which received NK-cell depleted leukocytes from day 3 LCMV-infected mice.. Except for the first two columns, additional agents were added to block the killing. These include mAb to NKR or combinations of anti-NKR mAb. The green hatched bar represents killing in the presence of a rat IgG1, k control mAb with no specificity to NK cells. *p< .05; **, p<.00.5
Thus, the most effective reduction was achieved with combinations of mAb to NKR. Some variations were noted among these tested targets, but in general combinations of NK1.1, NKp46, CD161a and CD161f had additive effects in regards to inhibiting killing. At this time, we can only conclude that no single tested NKR is required for killing in vitro, but there may be several NKR that can participate in killing and act somewhat collaboratively or redundantly.
4. Discussion
With the exception of a subpopulation of thymocyes (Hansson et al., 1979b), freshly isolated mouse T cells have been notoriously resistant to NK cell-mediated lysis when used as targets for in vitro cytotoxicity assays, and very little has been known about differences in the NK sensitivity among differentiated T cell effector subsets. Given the importance of NK cell-mediated lysis of activated T cells in the pathogenesis of viral infections (Lang et al., 2012; Rydyznski et al., 2015; Waggoner et al., 2011; Waggoner et al., 2010), we questioned whether we could develop an in vitro cytotoxicity assay against differentiated T cell subsets and whether this would be useful for determining mechanisms of killing and in understanding NK cell-T cell interactions. The strategy we used was first to exanine the cytolytic capacities of LCMV-induced NK cell populations. The published in vivo cytotoxicity studies had shown that NK cells induced by LCMV, poly I:C, Pichinde virus, and mouse hepatitis virus, and all could lyse activated CD4 T cells in vivo, but those induced by LCMV were particularly potent in that regard. Second, we generated distinct CD4 and CD8 T cell effector subsets by differentiating OVA-specific transgenic CD4 and CD8 T cells. This was in the presence of their cognate peptide ligands and appropriate cytokines or anti-cytokine antibodies. The OVA-specific transgenic T cells were used rather than LCMV-specific transgenic T cells because the OVA systems were already set up in our lab, and this avoided issues of the T cells attacking LCMV antigens in the effector assays. Indeed, good levels of cytotoxicity were generated against the T cell subsets tested, and the cytotoxicity was mediated by activated NK cells in a typical type 1 IFN- and perforin-dependent manner with relatively linear cytotoxicity curves. This pattern of killing was consistent with a requirement for direct cell-cell interactions rather than through production of soluble factors such as lymphotoxin or TNF. What was unusual, however, was that NK cells from LCMV-induced day 3 spleen leukocytes needed to be enriched to mediate this killing. LCMV-induced NK cells have been studied since the original discovery of NK cell activation, and unenriched LCMV-induced spleen leukocytes have been shown to lyse nearly every cell type, including syngeneic, allogeneic, and xenogeneic cell lines and cells with both high and low expression of MHC antigens(Kiessling and Welsh, 1980; Welsh et al., 1981; Welsh and Zinkernagel, 1977). Further, they have been shown to lyse primary macrophages, dendritic cells, and thymocyte cultures. Figure 2 shows, however, very little lysis against the effector T cell subset cultures except after activated NK cell enrichment. This suggests that other factors in the day 3 leukocyte population may be interfering with the killing of the T cells, and, indeed, the incorporation of NK cell-depleted leukocytes into the cytotoxicity assays impaired the killing by the activated NK cells (Fig. 5). It is not clear whether any cold target competition is a factor for NK cell killing of CD4 T cells in vivo or how the NK cells and CD4 targets are topologically distributed in vivo. Further. It is not clear whether only a small subset of NK cells may be killers, though we have been unably to select such a population based on NKR expression. Nonetheless, the experiments confirm that the enriched NK cells killed all CD4 and CD8 T cell subsets, clearly establishing the sensitivity of T effector subsets to NK cell cytotoxicity. There was a hierarchy of sensitivity of the effector T cell subset cultures to killing (Fig. 4; Table 1). Treg, Th17, and Th2 cells were reproducibly most sensitive to killing, and Th0 and Th1 cells were most resistant. This finding was consistent between in vitro and in vivo cytotoxicity assays.
How faithful is this observed hierarchy to what we know that occurs during the progression of a virus infection in vivo? Actually, very little is known about the exact nature of the T cells killed in vivo, other than that the in vivo cytotoxicity assay shows activated T cells are killed early in infection when NK cells are at their peak of cytotoxicity (we have, however, reported that activated CD4 T cells taken from later stages of infection can also be killed in such assays.). At that early time point activated CD4 T cells are killed better than activated CD8 T cells, and as the infection progresses the total numbers and functional impact of both CD4 and CD8 cells are compromised by the action of NK cells. There are elevated numbers of Tfh cells later in infection when NK cells are depleted early in infection (Rydyznski et al., 2015), but the Tfh cells, which take time to differentiate in vivo, are not necessarily direct NK cell targets, and we unfortunately did not have an in vitro system available to study them. One point of interest is that the helper T cell response to LCMV is skewed toward Th1 cells (Varga and Welsh, 2000), and it is difficult to find many Th2 cells during acute LCMV infection in C57BL/6 mice. Perhaps some of this pattern could be due to preferential killing of Th2 cells by activated NK cells, a statistically significant finding in our studies. Treg cells, which were very sensitive to killing when differentiated in culture, are easy to quantify at early stages of infection, but their numbers are similar early during LCMV infection, whether or not NK cells are depleted (Che et al., 2015). This would indicate that NK cells differentially control Treg vs. Tfh cell numbers. Because of their high sensitivity to NK cells in vitro, we would have liked to observe the effects of NK cell depletion on Th17 cells in vivo but had difficulty detecting them in sufficient numbers in vivo to do the analysis.
Given the high sensitivity of differentiated Treg cultures to NK cells in vitro and in vivo, one questions how reflective the properties of these in vitro differentiated targets are of the Tregs generated in vivo. One factor might be the expression of class 1 MHC molecules, which tend to protect targets from killing by NK cells (Hansson et al., 1980; Trinchieri and Santoli, 1978; Welsh et al., 1981). Our data show that in uninfected mice Treg CD4 cells express more class 1 Db than non-Treg CD4 T cells and that by day 3 post-infection the expression of Db is even higher (Fig. 7B). We therefore tested if IFNβ would up-regulate class 1 expression on in vitro-generated Treg cells, and indeed it did dramatically and simultaneously protected them against killing by NK cells (Fig. 7A).
A second anomaly is that at early stages of infection in vivo, activated CD4 cells are reported to be more sensitive than activated CD8 cells to in vivo depletion of NK cells. Figure 4 shows that the in vitro sensitivity of in vitro-generated CD8 T cell effectors (Tc1 and Tc2) to lysis fell between the highly sensitive Th17, and Treg CD4 cells and the more resistant Th0 and Th1 CD4 cells. However, we know little about the exact nature of the activated CD4 T cells that are the targets of NK cells very early in infection in vivo. CD8 T cells activated in vivo express more CD48 than do activated CD4 T cells. CD48 binds to the negatively signaling 2B4 (CD244) surface molecule on mouse NK cells. Mice lacking 2B4 have a dramatically altered LCMV pathogenesis, and their NK cells kill activated CD4 and CD8 T cells very well and equally (Waggoner et al., 2010). Nevertheless, our extensive analyses of the cultured T cells revealed high variability in CD48 expression among experiments and T cell subsets (data not shown). Thus, we could not apply a meaningful analysis of the roles of CD48 and 2B4 in the susceptibility of the cultured T cells. In one experiment using all 7 effector T cell subsets, LCMV activated NK cells from 2B4 KO mice killed T cell subsets with a similar hierarchy to that mediated by wild type mouse NK cells (data not shown).
We hoped that studying the in vitro generated T cell effector subsets would shed light onto which NKR are critical for the NK cell mediated lysis of T cell subsets. However, it is rare that a single NKR is predominantly responsible for any in vivo phenotype. Exceptions to this include the role for Ly49H in the pathogenesis of murine cytomegalovirus infection and the role for NKG2D in the control of polyomavirus induced tumors (Daniels et al., 2001; Mishra et al., 2010). In vitro killing of the prototypic NK-sensitive YAC-1 target cells is partially but not completely blocked in our hands by mAb to NKG2D (Mishra et al., 2010), but mAb to NKG2D was poor at blocking the killing of the cultured T cell effector subsets (Fig. 9). Prior work had shown that mice deficient in the inhibitory receptor 2B4 had markedly altered pathogenesis in response to LCMV infection (Waggoner et al., 2010). However, in our present study mice deficient in various activating NKR had no altered cytotoxicity phenotype in vivo. Our preliminary studies indicate that mice deficient in NKp46, in a combination of DNAM1 and NKG2D, and in the combined adaptor molecules dap 10 and dap 12 had elevated Tfh levels after NK cell depletion, much the same as wild type C57BL/6 mice, implying that the NK cells were regulating the CD4 T cell response in these mutant mice (data not shown). In addition, LCMV-induced NK cell-enriched day 3 leukocytes from all of these mutant mice lysed the tested effector T cell subsets with hierarchies similar to that mediated by wild type mouse NK cells (Fig. 8). The dap10 and dap12 double KO mice were limited in number but were tested by flow cytometry to ensure they lacked expression of NKG2D and Ly49H, NKR that require adaptor molecules for cell surface expression. These mice were particularly interesting because of their inability to mediate NK cell killing by a variety of dap-dependent molecules. The DNAM-1/NKG2D KO and ncr (NKp46) KO mice were also confirmed to not express the relevant molecules. These results do not mean that these NKR cannot participate in killing. Rather they mean that these NKR are not required for killing. The killing could be mediated by an untested receptor, or this could simply be a highly redundant system, where any of several NKR can mediate this process. Further, no single mAb quantitatively blocked killing of the T cell cultures, but many caused some inhibition of killing compared to control antibodies, and combinations of anti-NKR mAb had some additive effects. Our interpretation of these complex experiments is that none of the tested single NKR is required for killing of the targets, but various NKR and combinations of NKR may be involved.
In conclusion, activated NK cells can kill a wide variety of effector T cell subsets generated in vitro from transgenic T cells. These effector T cell subsets may be useful for examining which NKR are necessary for killing. However, variations in the subset expression of CD48 and phenotypic differences that may occur as a consequence of virus infection-induced cytokines like IFN would indicate that caution should be taken before using them as reliable indicators of T cell susceptibility in vivo.
Acknowledgements
This study was supported by U.S. Public Health Service research grants U19 AI109858 and R01 AI118820. The views expressed are those of the authors and do not necessarily reflect the views of the National Institutes of Health. We thank Drs. Liisa Selin (UMMS) and Dario Ghersi (U. Nebraska) for help with the statistics.
References
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK, 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238. [DOI] [PubMed] [Google Scholar]
- Bukowski JF, Welsh RM, 1986. Enhanced susceptibility to cytotoxic T lymphocytes of target cells isolated from virus-infected or interferon-treated mice. J Virol 59, 735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A, 2007. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood 110, 606–615. [DOI] [PubMed] [Google Scholar]
- Che JW, Kraft AR, Selin LK, Welsh RM, 2015. Regulatory T cells resist virus infection-induced apoptosis. J Virol 89, 2112–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D, 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10, 661–671. [DOI] [PubMed] [Google Scholar]
- Cook KD, Whitmire JK, 2013. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol 190, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, Kalinke U, Vivier E, Jonjic S, Oxenius A, 2014. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity 40, 961–973. [DOI] [PubMed] [Google Scholar]
- Daniels KA, Devora G, Lai WC, O’Donnell CL, Bennett M, Welsh RM, 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med 194, 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O, 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol 7, 517–523. [DOI] [PubMed] [Google Scholar]
- Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, Colonna M, 2008. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med 205, 2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW, 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 182, 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Karre K, Kiessling R, Roder J, Andersson B, Hayry P, 1979a. Natural NK-cell targets in the mouse thymus: characteristics of the sensitive cell population. J Immunol 123, 765–771. [PubMed] [Google Scholar]
- Hansson M, Kiessling R, Andersson B, Karre K, Roder J, 1979b. NK cell-sensitive T-cell subpopulation in thymus: inverse correlation to host NK activity. Nature 278, 174–176. [DOI] [PubMed] [Google Scholar]
- Hansson M, Kiessling R, Andersson B, Welsh RM, 1980. Effect of interferon and interferon inducers on the NK sensitivity of normal mouse thymocytes. J Immunol 125, 2225–2231. [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, Holden HT, Lavrin DH, 1975. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer 16, 230–239. [DOI] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Wigzell H, 1975. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 5, 112–117. [DOI] [PubMed] [Google Scholar]
- Kiessling RW, Welsh RM Jr., 1980. Killing of normal cells by activated mouse natural killer cells: evidence for two patterns of genetic regulation of lysis. Int J Cancer 25, 611–615. [DOI] [PubMed] [Google Scholar]
- Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS, 2012. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A 109, 1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Vong AM, Devarajan P, Brauner MD, Kuang Y, Nayar R, Schutten EA, Castonguay CH, Berg LJ, Nutt SL, Swain SL, 2017. NKG2C/E Marks the Unique Cytotoxic CD4 T Cell Subset, ThCTL, Generated by Influenza Infection. J Immunol 198, 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL, 2007. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med 204, 2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL, 2009. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol 182, 7353–7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Chen AT, Welsh RM, Szomolanyi-Tsuda E, 2010. NK cells and gammadelta T cells mediate resistance to polyomavirus-induced tumors. PLoS Pathog 6, e1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N, Odum N, Urso B, Lanier LL, Spee P, 2012. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS One 7, e31959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson M, Charo J, Salazar-Onfray F, Noffz G, Mohaupt M, Qin Z, Klein G, Blankenstein T, Kiessling R, 1998. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J Immunol 161, 2099–2105. [PubMed] [Google Scholar]
- Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL, 2002. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med 196, 957–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydyznski C, Daniels KA, Karmele EP, Brooks TR, Mahl SE, Moran MT, Li C, Sutiwisesak R, Welsh RM, Waggoner SN, 2015. Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells. Nat Commun 6, 6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Santoli D, 1978. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med 147, 1314–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga SM, Welsh RM, 2000. High frequency of virus-specific interleukin-2-producing CD4(+) T cells and Th1 dominance during lymphocytic choriomeningitis virus infection. J Virol 74, 4429–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LK, Welsh RM, 2011. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481, 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Taniguchi RT, Mathew PA, Kumar V, Welsh RM, 2010. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J Clin Invest 120, 1925–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E, 2007. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood 110, 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM Jr., 1978. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med 148, 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM Jr., Zinkernagel RM, 1977. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature 268, 646–648. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Karre K, Hansson M, Kunkel LA, Kiessling RW, 1981. Interferon-mediated protection of normal and tumor target cells against lysis by mouse natural killer cells. J Immunol 126, 219–225. [PubMed] [Google Scholar]
- Zafirova B, Mandaric S, Antulov R, Krmpotic A, Jonsson H, Yokoyama WM, Jonjic S, Polic B, 2009. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity 31, 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]