Abstract
Transcatheter leadless pacemaker dislodgment is a rare and potentially fatal complication of leadless device implantation. We present the first case of multidetector computed tomography images of leadless pacemaker migration and embolization in the pulmonary middle lobe artery. The patient was managed by percutaneous retrieval of the dislodged device and re-implantation in the appropriate position.
Keywords: Arrhythmia surgery, Device, Pulmonary embolism, Pacemaker, Computed tomography
Case report
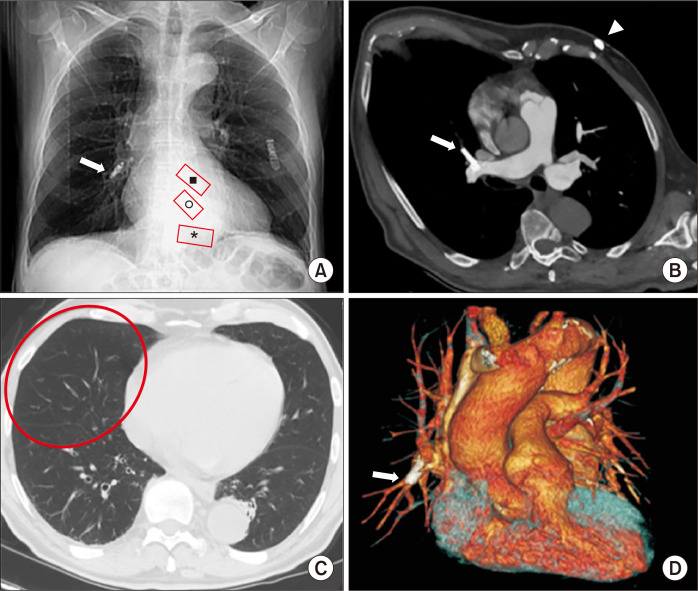
A 64-year-old man in whom a pacemaker was indicated due to bradyarrhythmia and atrial fibrillation presented to Monaldi Hospital for implantation of a transcatheter leadless pacemaker (TPS). During the device implantation procedure, unacceptable electrical measurements and fluoroscopic findings indicated possible TPS dislodgement; the procedure was immediately terminated and an emergency computed tomography (CT) scan was requested. Multi-detector CT (MDCT) angiography was quickly performed and showed that the TPS had migrated and was embolized in the pulmonary middle lobe artery. The tributary lung territory was hyperlucent due to reduced blood flow and there was no perforation or pericardial effusion on the MDCT findings (Fig. 1). Successful percutaneous retrieval of the dislodged device was readily performed; the device was snared via the proximal retrieval feature of the device and was correctly reimplanted in the next hours (Supplementary Videos 1A–G).
Fig. 1.
MDCT after TPS implantation in a 64-year-old man with bradyarrhythmia and atrial fibrillation. (A) CT scout-view shows TPS dislodgement at the right hilum (white arrow); red boxes indicate the expected location of the device in the frontal radiograph at the right ventricular apex (*), mid-septal (o), and outflow tract (■). (B) Oblique axial MIP reconstruction shows TPS embolization in the middle lobe pulmonary artery (white arrow); in the subcutaneous fat of left ventral chest wall, there is also an ECG loop recorder (arrowhead). (C) Axial MDCT (parenchymal window) shows the tributary lung territory hyperlucency due to reduced blood flow (red oval). (D) Volume-rendering coronal reconstruction clearly confirms TPS embolization in the middle lobe pulmonary artery (arrow). MDCT, multidetector computed tomography; TPS, transcatheter leadless pacemarker; CT, computed tomography; MIP, maximum intensity projection; ECG, electrocardiography.
No ethical committee approval was required for this case report, because it did not involve studies with human or animal subjects. It was not possible to obtain the patient’s consent for publication, because the patient could not be traced. All images in this case report are anonymized; therefore, the confidentiality of personal data is guaranteed.
Discussion
TPS therapy has recently been introduced into clinical practice to overcome the short- and long-term complications of traditional transvenous pacemakers (including pneumothorax, pocket hematoma/infection, lead dislodgement/fractures/infections, endocarditis, and pacemaker syndrome); it shows great promise and its applications are expanding, even though current real-world clinical experience remains limited [1,2]. In patients who require solely single-chamber ventricular pacing, the TPS is implanted directly into the right ventricle (RV) via the femoral vein and affixed near the apex or at the midpoint of the RV septum where the operator attains acceptable electrical measurements, eliminating the need for either a lead or subcutaneous pocket. There are currently 2 commercially available devices: Micra (Medtronic, Dublin, Ireland) and Nanostim (Abbott, Abbott Park, IL, USA) leadless pacemakers. The main differences between these 2 systems relate to the fixation mechanisms and the size of the delivery system used for the implant. Both devices weigh about 2 g. The Abbott device (Nanostim) is longer and thinner (length, 42 mm; diameter, 5.99 mm; introducer, 18F), and anchors to the myocardium with a helical screw-in active fixation electrode. The Medtronic device (Micra) is shorter and thicker (length, 25.9 mm; diameter, 6.7 mm; introducer, 23F), and relies on passive fixation with multiple tiny nitinol tines. The appropriate final position of the TPS is confirmed on the basis of acceptable electrical measurements (capture threshold ≤1.0 V at 0.24–0.4 ms, R-wave >6 mV, impedance >500 Ω); usually, a post-procedural chest radiograph confirms the expected device location in the region of the mid-distal RV septum or apex.
In the LEADLESS II trial total cohort of 526 patients, the rate of device-related serious adverse events was 6.5%, including cardiac perforation in 1.5% of the patients, device dislodgement in 1.1%, and device retrieval due to elevated pacing thresholds in 0.8% [2]. A worldwide post-approval registry of the Micra device reported a 1-year major complication rate of 2.7% (95% confidence interval, 2.0%–3.7%) and 1 case (0.06%) of device embolization during implant [3]. As with other implanted devices, there is a learning curve for TPS implantation. A rigorous training program and operator experience may help reduce serious adverse device events at implantation, defined as any undesirable effect related to the device or implant procedure that results in death, life-threatening illness, prolongation of hospitalization, persistent/significant disability, or incapacity [4]. The device cylinder of both currently available systems is anchored to a ventricular wall that is constantly subjected to cycles of energetic contraction and relaxation, which entails the possibility of pulmonary anterograde embolization or retrograde caval embolization. Although infrequent, these complications appear to be specific to the release systems of these new pacemakers and must be kept in mind.
Supplemental Materials
Supplementary materials can be found via https://doi.org/10.5090/kjtcs.19.185. Supplementary Videos 1A–G. Fluoroscopic videos showing the initial Micra retrieval process. The device was hooked and removed from the peripheral pulmonary circulation.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Magnusson P, Pergolizzi JV, Jr, LeQuang JA. Leadless pacemakers. In: Min M, editor. Cardiac pacing and monitoring: new methods, modern devices [Internet] IntechOpen; London: 2019. [cited 2019 Nov 2]. Available from: https://doi.org/10.5772/intechopen.83546. [DOI] [Google Scholar]
- 2.El Amrani A, Campos B, Alonso-Martin C, et al. Performance of the Micra cardiac pacemaker in nonagenarians. Rev Esp Cardiol (Engl Ed) 2020;73:307–12. doi: 10.1016/j.recesp.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–35. doi: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 4.El-Chami MF, Al-Samadi F, Clementy N, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018;15:1800–7. doi: 10.1016/j.hrthm.2018.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.