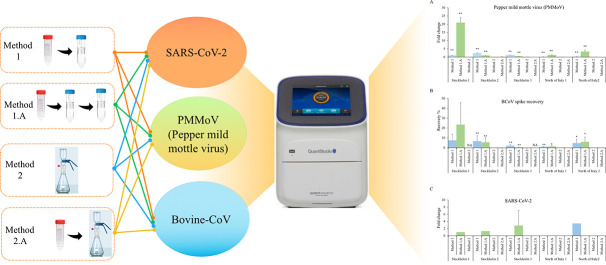
Abstract
Wastewater-based epidemiology offers a cost-effective alternative to testing large populations for SARS-CoV-2 virus, and may potentially be used as an early warning system for SARS-CoV-2 pandemic spread. However, viruses are highly diluted in wastewater, and a validated method for their concentration and further processing, and suitable reference viruses, are the main needs to be established for reliable SARS-CoV-2 municipal wastewater detection. For this purpose, we collected wastewater from two European cities during the Covid-19 pandemic and evaluated the sensitivity of RT-qPCR detection of viral RNA after four concentration methods (two variants of ultrafiltration-based method and two adsorption and extraction-based methods). Further, we evaluated one external (bovine corona virus) and one internal (pepper mild mottle virus) reference virus. We found a consistently higher recovery of spiked virus using the modified ultrafiltration-based method. This method also had a significantly higher efficiency (p-value <0.01) for wastewater SARS-CoV-2 detection. The ultracentrifugation method was the only method that detected SARS-CoV-2 in the wastewater of both cities. The pepper mild mottle virus was found to function as a potentially suitable internal reference standard.
Keywords: SARS-CoV-2, Bovine corona virus, Pepper mild mottle virus, Virus concentration method, Municipal wastewater, RT-qPCR
Graphical abstract
1. Introduction
The first cases of the current global pandemic of severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) infections were reported in December 2019, in China (WHO, 2020). Survival of other coronaviruses in water and wastewater has been previously confirmed (Gundy et al., 2009), making wastewater based epidemiology (WBE) a possible tool in developing an early warning or surveillance system for infections or rises of the SARS-CoV-2 virus. WBE has been previously used as a successful approach to grasp the severity and prevalence of pathogenic outbreaks in Sweden (Hellmér et al., 2014) and Israel (Kopel et al., 2014). Therefore, several studies have focused on identification of SARS-CoV-2 in wastewater during the current pandemic (La Rosa et al., 2020b; Medema et al., 2020; Randazzo et al., 2020).
One of the hurdles is the recovery methods, which are primarily developed for nonenveloped viruses. However, the novel SARS-CoV-2 belongs to the coronaviridae family (Gorbalenya et al., 2020) of enveloped viruses with single-stranded RNA. Different functional groups on the outer layer of enveloped and nonenveloped viruses impact wastewater recovery methods (Ye et al., 2016). Ahmed et al., 2020a, Ahmed et al., 2020b have recently compared the efficiency of seven different concentrations methods for the recovery of SARS-CoV-2. Nevertheless; recovery methods of the enveloped viruses, their efficiencies, and internal and external surrogates (reference viruses) require further research (La Rosa et al., 2020a) as the existing information indicate different recovery efficiencies for each method (Ahmed et al., 2020b).
In the current study, the sensitivity of four virus concentration methods were assessed in the perspective of external and internal reference viruses for wastewater samples from two countries: Sweden and Italy. The regions of Stockholm and North of Italy were chosen, as they represented regions with high case numbers of SARS-CoV-2 infection. The four different virus concentration techniques examined in this study were; 1) ultrafiltration 1.A) modified ultrafiltration 2) adsorption-vacuum filtration and 2.A) centrifugation combined with adsorption-vacuum filtration. Bovine coronavirus (BCoV), from betacoronavirus genus, the same genus as SARS-CoV-2 is endemic in cattle. This single-stranded positive-sense enveloped RNA was used as an external reference virus. Pepper mild mottle virus (PMMoV), a non-enveloped single-stranded RNA virus, was assessed as internal reference virus. PMMoV, from the tobamovirus family, is an indicator of fecal contamination in wastewater as it is found abundantly in various aquatic environments (Kitajima et al., 2018). Following recovery of virus, RNA isolation and one-step reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) were conducted to determine the efficiency of each virus concentration technique.
2. Materials and methods
2.1. Sampling and sample preparation
Untreated municipal wastewaters were sampled from three different regions in Stockholm, Sweden, and one region from the North of Italy in May and June 2020. The Stockholm samples were kept at +4 °C, and the experiments were performed within 24 h. The North of Italy samples were kept at −20 °C and delivered to the KTH Lab (Sweden) on dry ice. Twenty μl of BCoV (stock prepared in human colorectal tumor cell line HRT-18G, ATCC CRL-11663 (Christensen and Myrmel, 2018)) were spiked to 50 ml of all wastewater samples as an external reference.
2.2. Concentration methods
In this study, four approaches were tested. Method 1- Ultrafiltration: wastewater samples were centrifuged at 4600 ×g for 30 min at 4 °C in order to remove large and coarse particles, and the supernatant (approx. 40–50 ml) was filtered through 10 kDa cut off centrifugal ultrafilters (Sartorius) at 1500 ×g for 15 min (Megastar 1.6R benchtop centrifuge) (Medema et al., 2020). Method 1.A- Double Ultrafiltration (Method 1 modified): The obtained concentrate from Method 1 was centrifuged a second time (10 kDa cut off Sartorius centrifugal ultrafilters, 1500 ×g, 15 min, 4 °C). The obtained concentrate of method 1 and 1.A varied between 3 and 5 ml and 0.5 to 1.5 ml, respectively. Method 2- Adsorption-Extraction: MgCl2 (final concentration 25 mM) was added to the wastewater samples, followed by filtration through 0.45-μm pore size electronegative membranes (Supor 450, plain) (Ahmed et al., 2020b). Method 2.A- Centrifugation combined with adsorption-extraction (Method 2 modified): Wastewater samples were centrifuged at 4600 ×g for 30 min at 4 °C in order to remove the large and coarse particles before addition of MgCl2 (final concentration 25 mM). The obtained concentrate was then passed through 0.45-μm pore size electronegative membranes (Supor 450, plain). All concentrated samples were stored at −80 °C until further analysis.
2.3. RNA extraction
RNA from Method 1 and 1.A processed municipal wastewater was extracted by adding three volumes of Trizol LS reagent for liquid samples (Thermofisher Scientific) to one volume of concentrated wastewater. For each ml of Trizol-wastewater mixture 0.2 ml of chloroform (Sigma-Aldrich) was added, and the aquous phase purified by miRNeasy Mini Kit (Qiagen, Chatsworth, CA). RNA from filter papers obtained from Method 2 and 2.A were isolated using RNeasy PowerMicrobiome Kit (Qiagen, Chatsworth, CA). The RNA was eluated in 50 μl, and stored at −80 °C.
2.4. Reverse transcriptase quantitative polymerase chain reaction (RT-qPCR)
Primers targeting the nucleocapsid (N) gene were used to detect the SARS-COV-2 gene. The specificity of N-gene primer set against human corona viruses and other viruses (respiratory) has been previously reported by Medema et al. (2020). For the internal municipal wastewater virus reference, primers targeting PMMoV were used, and for the external (spike) reference virus, primers targeting BCoV were used. All primers are listed in Table 1 . Preliminary experiments of waste water samples showed that inhibition of the RT-qPCR reaction was reduced by addition of Bovine Serum Albumin (BSA) to the reaction mixture. Therefore, in all reaction 2 μl of 4 mg/ml BSA (Sigma-Aldrich) was added. For each reaction either 8 μl (N gene detection) or 2 μl (PMMoV and BCoV detection) of RNA template was used. This corresponds to 8 ml initial wastewater volume per SARS-CoV-2 RT-qPCR reaction, and 2 ml for PMMoV detection. Since the same initial volume of wastewater sample were used for all samples and methods (for the same virus), the results are directly comparable. SYBR Green chemistry was used to detect the expression of genes, and RNA from inactivated cultured human SARS-CoV-2 (gift from the Public Health Agency of Sweden) and BCoV were used as positive controls. Negative controls were included in each qPCR run. The reaction was performed according to the manufacturer's recommendations using iTaq universal SYBR Green one-step kit (Bio-Rad) and a final reaction volume of 20 μl (SARS-CoV-2) or 10 μl (PMMoV, BCoV). Thermal cycling (50 °C 10 min, 95 °C 30 s, followed by 40 cycles of 95 °C 10 s, 60 °C for 30 s) on a CFX96 Touch System (Bio-Rad) machine were performed. Melting curve detection (65 °C to 95 °C with increment of 0.5 °C for 5 s) were analyzed for all included genes and compared to positive controls, to ensure specific amplification. Reactions were considered positive if the cycle threshold (Ct) was below 40 cycles with a single melting peak at correct temperature.
Table 1.
Primer sets and targeted genes.
| Targeted gene | Primer sets | References |
|---|---|---|
| Nucleocapsid (N)gene | FW: 5′-GGGAGCCTTGAATACACCAAAA-3′ RV: 5′-TGTAGCACGATTGCAGCATTG-3′ |
(Medema et al., 2020) |
| PMMoV | FW: 5′-GAGTGGTTTGACCTTAACGTTTGA-3′ RV: 5′-TTGTCGGTTGCAATGCAAGT-3′ |
(Ahmed et al., 2020a, Ahmed et al., 2020b) |
| BCoV | FW: 5′-TGGTGTCTATATTCATTTCTGCTG-3′ RV: 5′-GGCCACTGCCTAGGATACA-3′ |
(Christensen and Myrmel, 2018) |
2.5. RT-qPCR amplification efficiency, limit of detection and inhibition
RNA was extracted from 200 μl of cultured SARS-CoV-2 at 6 × 105 plaque-forming unit (PFU)/ml, and 80 μl of BCoV at 4.5 × 105 50% tissue-culture-infective dose (TCID50)/ml. Ten-fold serial dilutions of the RNAs were prepared and RT-qPCR performed as described above. Standard curves were generated from the log-linear regression of Ct values of replicates, and the amplification efficiencies for SARS-CoV-2 and BCoV were calculated (Nolan et al., 2013). The lowest number of diluted standards detected in duplicate assays was considered limit of detection (LOD) for the RT-qPCR assay. The presence of qPCR inhibitor in the concentrated municipal wastewater RNA sample was subsequently assessed using the PMMoV qPCR assay. RNA templates were added in series of 1 μl, 2 μl and 4 μl. The qPCR reaction was set up as described (Section 2.4). The expression of PMMoV gene was analyzed alongside non-template controls and corresponding amplification efficacy calculated (Nolan et al., 2013) and compared to that of RNA from cultured samples.
2.6. Statistical analysis
Average Ct value and standard deviation (SD) was calculated for each sampling points. Student's t-test was used for comparison, and p-value <0.05 was considered statistically significant.
3. Results
3.1. Detection of SARS-CoV-2
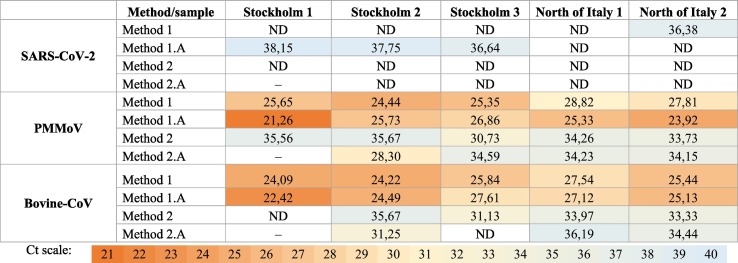
First, we investigated if SARS-CoV-2 were present in the collected samples. Four out of five samples tested positive for SARS-CoV-2 with N-gene primers (Table 2 ) using Method 1 or 1.A for wastewater virus concentration. The Ct values for positive samples were between 35 and 38 (Table 2). The N gene was detected in both replicates of the Stockholm 3 sample, however, for the three remaining positive samples (Stockholm 1, Stockholm 2 and North of Italy 2) it was only detected in one of two technical replicates. This suggests border limit detection in the assay, possibly due to low occurrence of SARS-CoV-2 in the municipal wastewater during the sample collection week or indicative of varying presence of inhibitors. To be noted, in quantitative gene expression analysis, SYBR Green and TaqMan are the two commonly used methods. The one used here, SYBR Green, is cheaper and does not require additional probes (Valasek and Repa, 2005). These are benefits during the pandemic, as there was less shortage of these reagents. This method can be less accurate, but by including melting curve analysis and positive controls, accuracy can be ensured.
Table 2.
SARS-CoV-2 detection in municipal wastewater (nd: no detection).
| Sample name | SARS-CoV-2 (N-gene) | Cq | Cq |
|---|---|---|---|
| Stockholm 1 | Positive (single result) | 38.15 | nd |
| Stockholm 2 | Positive (single result) | 37.75 | nd |
| Stockholm 3 | Positive (double result) | 37.58 | 35.71 |
| North of Italy 1 | Negative | nd | nd |
| North of Italy 2 | Positive (single result) | 36.38 | nd |
3.2. Evaluation of the concentration methods
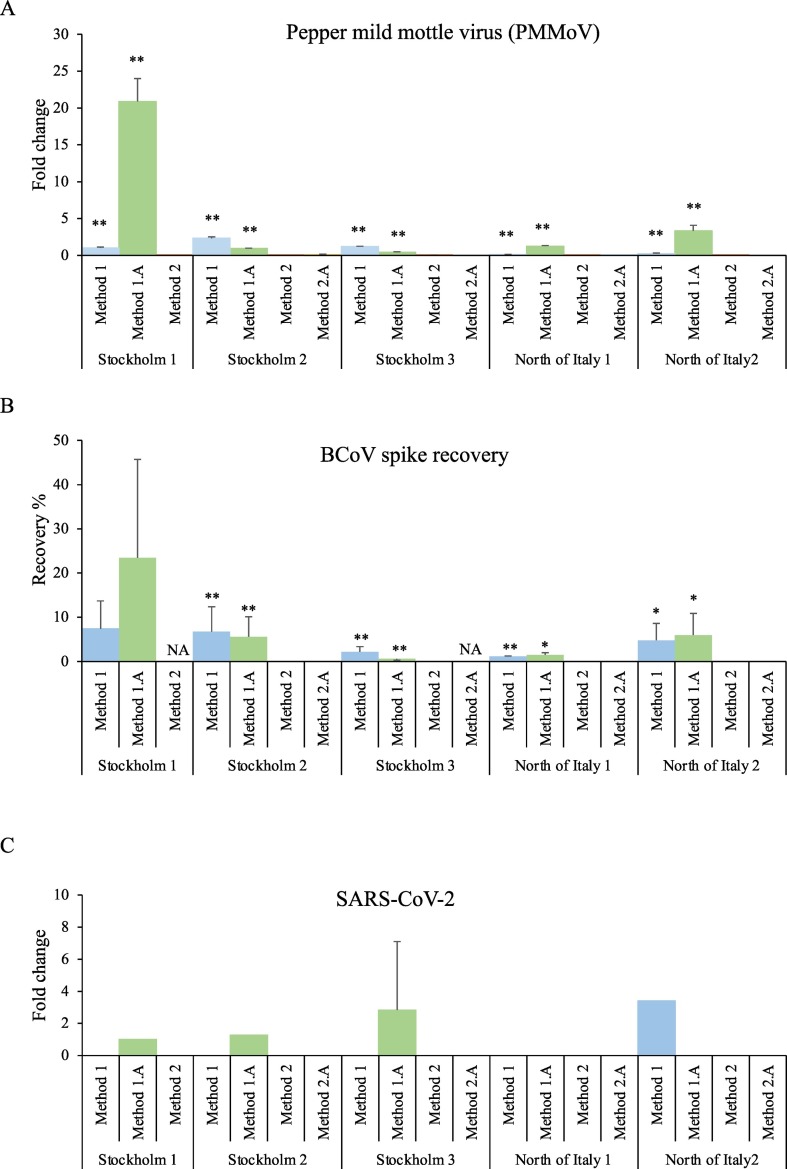
Next, we compared the detection sensitivity taken the different concentration methods into account. We evaluated three viruses, PMMoV, the spiked BCoV, and SARS-CoV-2. As presented in Table 3 and Fig. 1 , the detection of virus was highly dependent on the concentration methods used. PMMoV, a well-known potential viral indicator in municipal wastewater (Rosario et al., 2009), was readily detected in all samples and all replicates using Method 1 or 1.A (Ct 20–29, Table 3, Fig. 1A). Method 2 and 2.A detected significantly lower levels of PMMoV in all samples (Fig. 1A). The average Ct values for PMMoV detection were 24.6 ± 2 by Method 1.A; 26.4 ± 2 by Method 1, 32.8 ± 3 by Method 2.A and 34.0 ± 2 by Method 2, as determined by five sampling points. Thus, Method 1.A was the most sensitive method for PMMoV detection. The external reference virus spiked into the municipal wastewater (BCoV) was positive in all replicates using Method 1 and 1.A (Ct 22–27, Table 3). Again, the adsorption and extraction methods 2 (33.5 ± 2) and 2.A (34.0 ± 2) presented lower detection efficiencies. The recovery rate of the spiked virus was further calculated, by comparing the RT-qPCR detection of RNA extracted from equivalent amount of spiked virus. The recovery rate was substantially higher for Method 1 and 1.A, compared to method 2 and 2.A (Fig. 1B). Of note is that the recovery was low (less than 10% in most samples) also by Method 1 and 1.A. The p values of the comparison of each method were calculated and the results showed that method 1 and 1.A have significantly higher efficiency (p-value <0.01) than Method 2 and 2.A for all wastewater viruses. SARS-CoV-2 (N gene) was detected by Method 1 in one sample (Ct: 36.4) and by Method 1.A in three samples (average Ct: 37.5 ± 0.8), whereas detection could not be obtained by Method 2 or 2.A (Fig. 1C). Thus, the centrifugal ultrafilter methods, Method 1 and 1.A, enabled higher recovery rate and virus detection ability.
Table 3.
Mean amplification cycles of targeted genes for four different methods (Method 1: Ultrafiltration; Method 2- Double Ultrafiltration (Method 1 was modified); Method 3- Adsorption-Extraction; Method 4- Centrifugation combined with adsorption-extraction (Method 3 was modified). White boxes show not tested samples and ND: not detected.
Fig. 1.
RT-qPCR detection of SARS-CoV2, PMMoV, and BCoV in wastewater sample concentrated by different methods. Method 1 or 1.A performed significantly better than method 2 and 2.A for all samples. Fold change values were generated for RT-qPCR wastewater samples concentrated by four different methods from Stockholm and North of Italy using the 2−ΔCt method, and normalized to input (RNA extracted from corresponding 8 ml wastewater) with Stockholm 1 sample and Method 1.A set at value 1. A. SARS-CoV-2 N gene B. nPMMoV C. Recovery rate of spiked BCoV. Error bars: SD. Student's t-test were used to calculate statistical significance, and is indicated for comparison between Method 1 and 2, and 1A and 2A. (*p < 0.05, **p > 0.01, ***p < 0.001).
3.3. Evaluation of RT-qPCR inhibitors
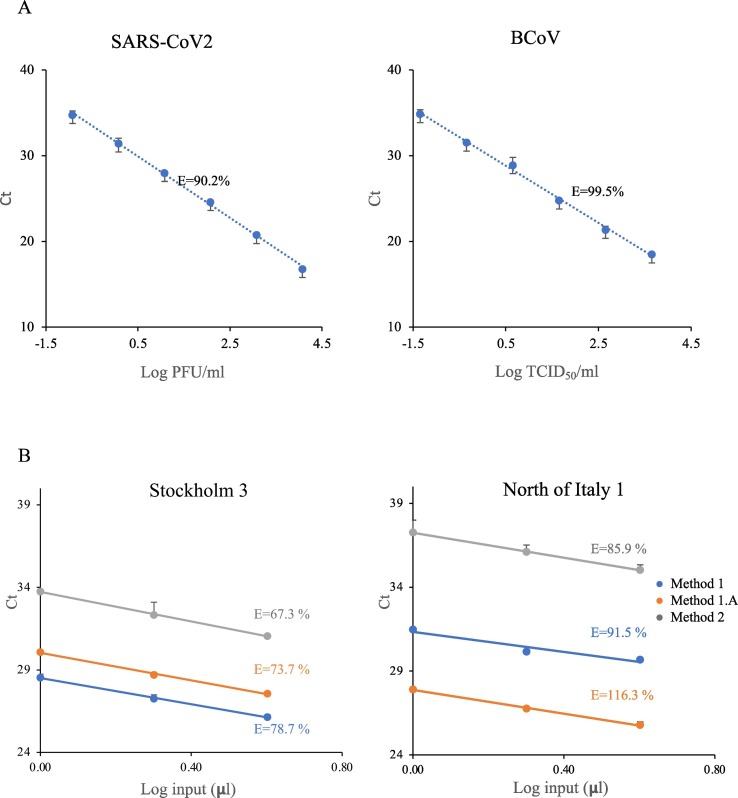
As we concluded that Method 1 and 1.A were more sensitive in regards to detection of all three viruses, but still did not detect SARS-CoV-2 in all replicates, we were interested in whether inhibitors affected the detection. We had already added BSA to counter inhibition, but in order to investigate remaining inhibition we calculated qPCR amplification efficiencies for the SARS-CoV-2 and BCoV virus primers used in this set up. We first calculated amplification efficiency using RNA purified from cultured viruses. With a theoretical optimal doubling of DNA molecules for each replication cycle, the amplification efficiency should be 100%. Desired amplification efficiencies range from 90% to 110%. Our calculations showed that amplification of pure SARS-CoV-2 and BCoV virus RNA (not from waste water) resulted in the desired range of 90% and 99.6%, respectively (Fig. 2A). Further, the limit of detection (LOD) for SARS-CoV2 was found to be 0.12 PFU/reaction and for BCoV 0.045 TCID50/reaction. It has to be noted that PFU represent the number of infectious virus particle capable of lysing the host cell and TCID50 represent the dose that infects 50% of the cells (Dulbecco and Vogt, 1954; Khatib et al., 1980), and they do not directly indicate virus copy number. Next, we calculated amplification efficiencies for PMMoV in two municipal wastewater RNA samples (Stockholm 3 and North of Italy 1) following three virus concentration methods (Method 1, 1.A and 2). This yielded amplification efficiencies of 67% to 116% (Fig. 2). An efficiency below 90% is indicative of non-optimal conditions, such as presence of inhibitors. Method 1 (single ultracentrifugation) or Method 1.A (double centrifugation) generated samples with the highest qPCR efficiencies, whereas the filter paper concentration (Method 2) had the lowest efficiency for both the Stockholm (67.3%) and Italian (85.9%) samples. We conclude that inhibitors appear to impact the amplification using the adsorption - extraction method, which likely contributes to the markedly higher Ct values and resulting lower sensitivity.
Fig. 2.
RT-qPCR efficiency in wastewater sample concentrated by different methods.
A. RT-qPCR standard curves for cultured SARS-CoV-2 and BCoV RNA. B. Standard curves for RT-qPCR efficiency determination of PMMoV in Stockholm 3 and North of Italy 1 wastewater samples, concentrated by three different methods (as indicated). E refers to the qPCR efficiency. Samples generated by Method 1 and Method 1.A had the most efficient amplification, indicative of little qPCR inhibition. Error bar: SD.
3.4. Internal or external calibrators
BCoV, which belongs to the Coronavirus genus in the Coronaviridae family, order Nidovirales, has similar genetic and serological properties as well as host range with other mammalian coronaviruses (Valarcher and Hägglund, 2010). Therefore, BCoV is classified in the same group of other mammalian coronaviruses, such as rat coronavirus, human enteric coronavirus, and human coronaviruses (Valarcher and Hägglund, 2010). In the current study, BCoV was selected as surrogate to calculate the recovery rate during sample processing and filtration, based on the similar properties. By adding a known amount of BCoV before filtration, we could estimate loss during filtration, RNA extraction, and RT-qPCR analysis. Furthermore, PMMoV is an indicator for fecal contamination in the water sources owing to its global distribution and its presence in various water sources in greater abundance than human pathogenic viruses, without substantial seasonal fluctuations (Kitajima et al., 2018). PMMoV has been used for the detection of pathogenic enteric viruses because of increased concentrations of PMMoV tend to be correlated with increased fecal contamination (Kitajima et al., 2018). In the current study, PMMoV was selected for normalization of external factors such as flow rate of the wastewater, which is changed based on wet and dry season/periods. Such fluctuation would affect the concentration of both PMMoV and SARS-CoV-2 in the samples.
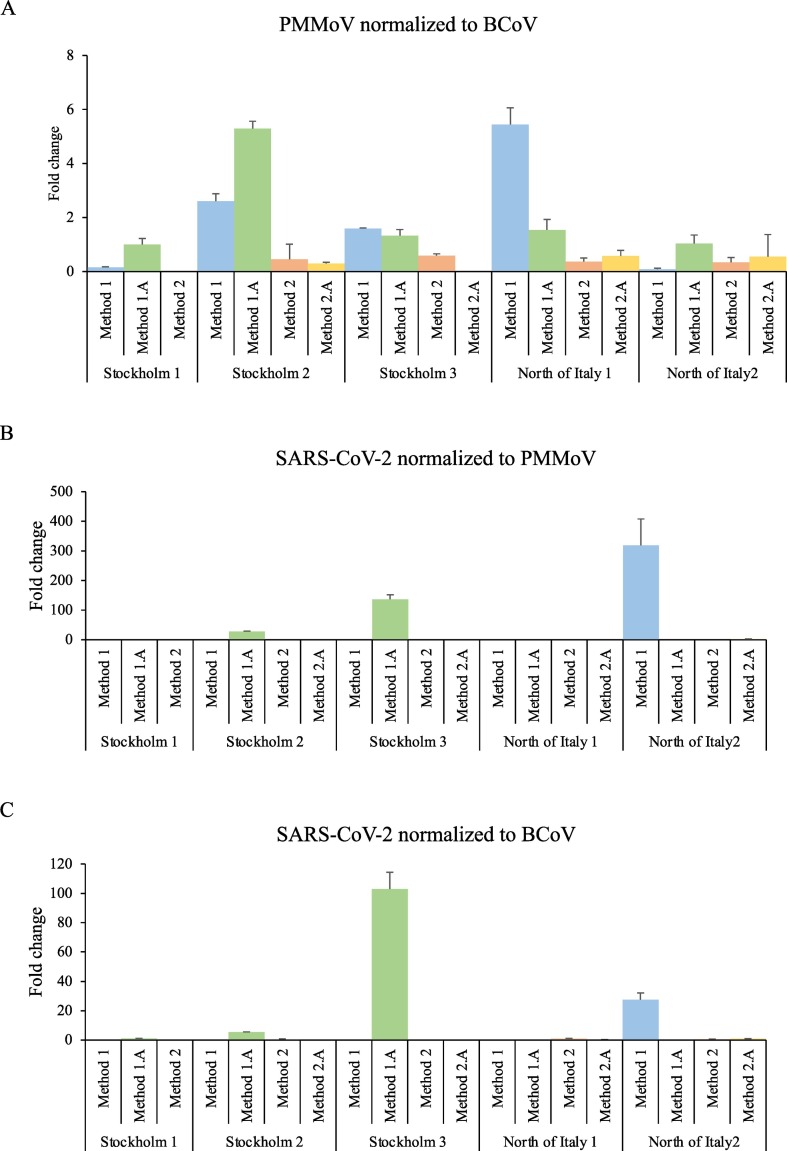
After concluding that the methods exhibited varying recovery rates for the spiked BCoV, and that they varied significantly in their detection of PMMoV and SARS-CoV-2 detection, we explored whether the internal or external reference viruses were suitable as calibrators in order to compare virus levels between samples. First, we plotted the level of PMMoV detection in relation to BCoV recovery (Fig. 3A). This showed that normalizing to the spike recovery rate can adjust the comparison to some extent, but not completely. A perfect callibration would result in equal levels of PMMoV in relation to the spike, but this was not achieved. Next, we normalized SARS-CoV-2 detection to PMMoV (Fig. 3B) or BCoV recovery rate (Fig. 3C). Both normalizations indicate that Stockholm 3 had higher levels of SARS-CoV-2 than Stockholm 1 and 2. However, the two normalization strategies rendered different relative values for the Italian sample (higher level in North of Italy 2 than the Stockholm 3 in relation to PMMoV, but lower when normalizing to spike recovery only).
Fig. 3.
Internal and external references for comparing virus levels between methods and samples. A. PMMoV detection normalized to input and recovery rate of spiked BCoV. B. SARS-CoV-2 N gene detection normalized to input and internal PMMoV reference. C. SARS-CoV-2 N gene detection normalized to input and recovery rate of spiked BCoV. Student's t-test were used to calculate statistical significance (*p < 0.05, **p > 0.01, ***p < 0.001).
3.5. Concluding remarks and recommendations
Since March 2020, there are many ongoing studies relating to the detection of SARS-CoV-2 in municipal wastewater (Ahmed et al., 2020a; Daughton, 2020; Nghiem et al., 2020). However, there is no standardized method. The most important need in wastewater-based epidemiology is to develop or improve and evaluate a standardized and sensitive method for the detection (Kitajima et al., 2020).
Ahmed et al., 2020a, Ahmed et al., 2020b compared seven concentration methods by using murine hepatitis virus as external reference (surrogate) and found that the best recovery was obtained from MgCl2 adsorption and vacuum filtration (Method 2 in the current study), in wastewater samples from Australia. This approach is cheaper and easier. However municipal wastewater characteristics might change across different countries, regions, etc. (Pons et al., 2004). The methods may also perform differently depending on type of virus (Lu et al., 2020).
In our study, municipal wastewater samples and SARS-CoV-2 from two different countries (Sweden and Italy) were tested using four different approaches in order to find out the best applicable and sensitive virus concentration method for these conditions. We also tested two reference viruses: 1) PMMoV which naturally exist in municipal wastewater, and 2) spiked animal pathogen BCoV which belongs to same genus as SARS-CoV-2. In the light of our findings:
-
1-
Ultrafiltration and modified ultrafiltration (Method 1 and 1.A) had higher recovery efficiencies than adsorption and extraction methods (Method 2 and 2.A). The latter two appeared to accumulate more PCR inhibitors. Thus, in opposition to the results obtained by Ahmed et al., 2020a, Ahmed et al., 2020b, the current study showed a better performance of the ultracentrifuge-based methods in terms of recovery efficiency and capacity for viral detection.
-
2-
Double ultrafiltration (Method 1.A) provides a reduced volume of water as starting material for the RNA isolation step, making RNA extraction less laborious and time consuming. Furthermore, detection of both SARS-CoV-2 and PMMoV were generally better with this method.
-
3-
Internal reference virus (PMMoV) was found to be a sensitive and representative standard, and adding external surrogate (BCoV) did not add meaningful additional information. We conclude that PMMoV internal standard is sufficient to inform of relative recovery and to normalize between samples.
CRediT authorship contribution statement
Mohammed Hakim Jafferali: Investigation, Methodology, Data curation, Writing - original draft, Visualization. Kasra Khatami: Investigation, Data curation, Writing - original draft. Merve Atasoy: Conceptualization, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization. Madeleine Birgersson: Investigation, Data curation, Writing - original draft. Cecilia Williams: Conceptualization, Methodology, Writing - review & editing, Supervision. Zeynep Cetecioglu: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This project is supported by Knut och Alice Wallenberg Stiftelsen (KAW 2020.0182), the Swedish Research Council (2017-01658, 2018-06169), WaterCenter@KTH, and KTH Life Science platform. The authors would like to thank the Science for Life Laboratories Environmental Virus Profile Platform, the Käppala Association, Stockholm Vatten och Avfall, the Public Health Agency of Sweden, Associate Prof. Mette Myrmel (Norwegian University of Life Sciences), and Prof Francesca Malpei (Politecnico di Milano). The authors would like to thank to Aashlesha Chekkale Vivekanand and Prachi Nandy from KTH for their support in the laboratory.
Editor: Damia Barcelo
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E., Myrmel M. Coagulant residues’ influence on virus enumeration as shown in a study on virus removal using aluminium, zirconium and chitosan. J. Water Health. 2018;16:600–613. doi: 10.2166/wh.2018.028. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Vogt M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J. Exp. Med. 1954 doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10–14. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014 doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib R., Chason J.L., Silberberg B.K., Lerner A.M. Age-dependent pathogenicity of group B coxsackieviruses in Swiss-Webster mice: infectivity for myocardium and pancreas. J. Infect. Dis. 1980 doi: 10.1093/infdis/141.3.394. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Sassi H.P., Torrey J.R. Pepper mild mottle virus as a water quality indicator. npj Clean Water. 2018 doi: 10.1038/s41545-018-0019-5. [DOI] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopel E., Kaliner E., Grotto I. Lessons from a public health emergency - importation of wild poliovirus to Israel. N. Engl. J. Med. 2014 doi: 10.1056/NEJMp1406250. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Huang Z., Luo J., Zhang X., Sha S. Primary concentration – the critical step in implementing the wastewater based epidemiology for the COVID-19 pandemic: a mini-review. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.141245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud. Chem. Environ. Eng. 2020;1(100006) doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T., Huggett J., Sanchez E. Good practice guide for the application of quantitative PCR (qPCR) Natl. Meas. Syst. 2013;50 [Google Scholar]
- Pons M.N., Spanjers H., Baetens D., Nowak O., Gillot S., Nouwen J., Schuttinga N. Wastewater characteristics in Europe - a survey. Eur. Water Manag. Online. 2004:1–10. [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario K., Nilsson C., Lim Y.W., Ruan Y., Breitbart M. Metagenomic analysis of viruses in reclaimed water. Environ. Microbiol. 2009;11:2806–2820. doi: 10.1111/j.1462-2920.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Valarcher J., Hägglund S. In: Infectious and Parasitic Diseases of Livestock. Lefevre P.C., Blancou J., Chermette R., editors. 2010. Bovine coronavirus. [Google Scholar]
- Valasek M.A., Repa J.J. The power of real-time PCR. Am. J. Physiol. - Adv. Physiol. Educ. 2005;29:151–159. doi: 10.1152/advan.00019.2005. [DOI] [PubMed] [Google Scholar]
- WHO Novel coronavirus (2019-nCoV) situation report - 1. WHO Bull. 2020:1–7. [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]