Abstract
Introduction
Ischemic stroke can occur due to disruption of blood and oxygen supply to brain tissue. White blood cells and platelets play an important role in the pathogenesis of ischemic stroke. Several studies have concluded that the lower the platelet count and the higher the number of white blood cells in ischemic stroke patients will result in a more severe stroke and had worsen prognosis. Platelet and white blood cells counts can be converted into Platelet-to-White Blood Cell Ratio (PWR) which is a comparison between the number of platelets and white blood cells, so the higher PWR will provide better clinical outcomes. Here, we examined correlation between PWR and clinical outcome in acute ischemic stroke using NIHSS tools.
Method
This research method was a retrospective analytic from 503 medical records of ischemic stroke patients from January 2015 to December 2017. Ischemic stroke divided into 2 groups: cardioembolic stroke and atherothrombotic stroke based on medical records. We calculated PWR and National Institute of Health Stroke Scale (NIHSS) for assessing clinical outcome. Statistical significance calculated with Spearman rank test, ANOVA, and multiple logistic regression.
Results
A total of 391 research subjects consisting of 213 females (54.5%) and 178 males (45.5%). The mean age of 57.14 years, and 82% subjects had hypertension as risk factor. Mean PWR of atherothrombotic stroke subjects were higher than cardioembolic stroke (33.02 vs 26.73) but had lower mean of NIHSS (5.81 vs 10.31) and had strong negative significant correlation between PWR and NIHSS (r = -0.9603; p < 0.001). From logistic regression, we found that PWR and platelet was statistically significance correlate with NIHSS (p < 0.05). The coefficient if PWR is the highest (absolute value) among other independent variables.It shows that PWR has positive effect on clinical outcome using NIHSS tools in acute ischemic stroke patients.
Conclusion
Cardioembolic stroke had higher PWR compared with atherothrombotic stroke. PWR had a strong correlation with NIHSS. The higher PWR will provide higher NIHSS and PWR has positive effect on clinical outcome using NIHSS tools in acute ischemic stroke patients.
Keywords: Neurology, Nervous system, Laboratory medicine, Clinical research, Ischemic stroke, NIHSS, Platelets, PWR, White blood cells
Neurology; Nervous system; Laboratory medicine; Clinical research; Ischemic stroke; NIHSS; Platelets; PWR; White blood cells.
1. Introduction
According to the World Health Organization (WHO), stroke caused around 15 million deaths in the world in 2015 [1]. Indonesia is a developing country with a fairly high stroke prevalence. According to the 2013 Riskesdas data, the prevalence of stroke in Indonesia is 12.1 per mile. The prevalence is up from 8.3 cases per mile in 2007 [2]. The most common stroke prevalence in Indonesia is ischemic stroke, which is 87%. In addition to the high prevalence of stroke, it is the highest cause of long-term disability [3]. Data taken from 1990 and 2013 show that globally, stroke has caused 51,429,440 disability-adjusted life years (DALY) that occur in the 20–64 age category. This figure shows a 24% increase compared to the previous calculation [4].
White blood cells and platelets play an important role in the pathogenesis of stroke infarction [5].
Platelets play an important role in the process of thrombus formation after activating in certain conditions, including atherosclerosis, inflammation, and hemoreological changes. Activated platelets will aggregate endothelial cell damage and participate in the development of atherosclerotic lesions. It can make disruption of intracranial blood flow because of arterial thrombosis or therothrombotic plaque rupture [5]. Several studies have concluded that the lower platelet count is present in the examination of ischemic stroke patients can lead to more severe strokes and had worsen prognosis. White blood cells also play a role in the pathophysiology of ischemic stroke. After ischemic occurs, infarction core will release various inflammatory mediators, such as proteases and reactive oxygen species (ROS), which aggravate endothelial damage and necrosis of the tissue [4]. It makes brain cannot function properly [6].
Increasing the number of white blood cells, especially neutrophils in peripheral blood are related to severity of stroke, tends to have worsen clinical outcome and had possibility for stroke reccurrent [7, 8]. These effector cells modulate cellular activation and attachment (between one another and with endothelial cells), release cytokines, and synthesize and also release other factors which support the progression of atherosclerosis and the formation of acute thromboembolism [9].
Platelets and white blood cells count can be converted into Platelet to-White Blood Cell Ratio (PWR), which is a comparison between the number of platelets and white blood cells [10].
Neurological deficits in ischemic stroke patients can be assessed using the National Institute of Health Stroke Scale (NIHSS). NIHSS is not only used to assess severity of stroke, but can also determine appropriate treatment, initial prognosis and intervention required [11, 12].
Based on the description above, we examined correlation PWR and clinical outcome in acute ischemic stroke using NIHSS tools.
2. Methods
The study was conducted with a restrospective analytic method for acute ischemic stroke patients treated at the General Hospital Dr. Hasan Sadikin Bandung between 1 January 2015 to 31 December 2017. The research sample was determined by total sampling. The data obtained are secondary data from medical records. Inclusion criteria were stroke patients who were diagnosed based on CT scans and were inpatients who had laboratory results on platelet counts and white blood cells counts in their medical records. Ischemic stroke divided into 2 groups: cardioembolic stroke and atherothrombotic stroke based on medical records. We calculated PWR and National Institute of Health Stroke Scale (NIHSS) for assessing clinical outcome. Statistical significance calculated with Spearman rank test.NIHSS examined on admission and 7th day hospitalization. Exclusion criteria were ischemic stroke patients accompanied by hematologic disease, immunosuppressant drug users, had a history of infection within two weeks before the symptoms of stroke or during treatment, and had a history of stroke in the past six months.
This study is approved by Research Ethics Committee of Universitas Padjadjaran (no. 1123/UN6.KEP/EC/2018). This study had complied with all relevant ethical regulations (including The Declaration of Helsinki).
3. Results
After collecting 503 medical records of acute ischemic stroke patients, 391 research subjects were found that met the inclusion criteria. Table 1 illustrates that the majority of research subjects were 213 females (54.5%). The mean age of the subjects was 57.24 years. The most risk factor is hypertension with 321 subjects (83.2%) having more than one risk factor.
Table 1.
Clinical characteristic research subjects.
| Variable | Total (n = 391) |
|---|---|
| Gender | |
| Men (n,%) | 179 (45.5) |
| Women (n,%) | 213 (55.5) |
| Age (year), mean ± SD | 57.14 ± 11.55 |
| Risk factors∗ | |
| Hypertension | 321 (83.2) |
| Diabetes Mellitus | 68 (17.4) |
| Smoking | 60 (15.3) |
| Cardiac problem | 102 (26.1) |
| Mean white blood cells, n (uL) ± SD | 11026.94 ± 12026.94 |
| Mean thrombocyte, n (uL) ± SD | 291321.23 ± 88954.77 |
| Mean PWR, n ± SD | 30.81 ± 11.95 |
| Mean NIHSS, n ± SD | 7.52 ± 3.86 |
Risk factors can be >1.
The mean number of white blood cells in the study subjects was 11026.94/uL. The mean platelet count of study subjects was 291321.21/uL. The mean PWR for all subjects is 30.81. The mean NIHSS for all subjects is 7.52 (Table 2).
Table 2.
Mean white blood cells, thrombocyte, PWR, NIHSS based on type of ischemic stroke.
| Type of ischemic stroke | n (%) | Mean White blood cells n (uL) ± SD |
Mean thrombocyte n (uL) ± SD |
Mean PWR n (uL) ± SD |
Mean NIHSS n (uL) ± SD |
r(p) |
|---|---|---|---|---|---|---|
| Cardioembolic stroke | 118 (30.2) | 12572.71 ± 19343.97 | 265394.92 ± 86126.96 | 26.74 ± 11.28 | 10.31 ± 3.78 | -0.9603 (0.001∗) |
| Atherothrombotic stroke | 273 (67.8) | 10161.67 ± 6605.65 | 303317.39 ± 89313.49 | 33.02 ± 11.77 | 5.81 ± 2.71 |
∗Significance p<0.05.
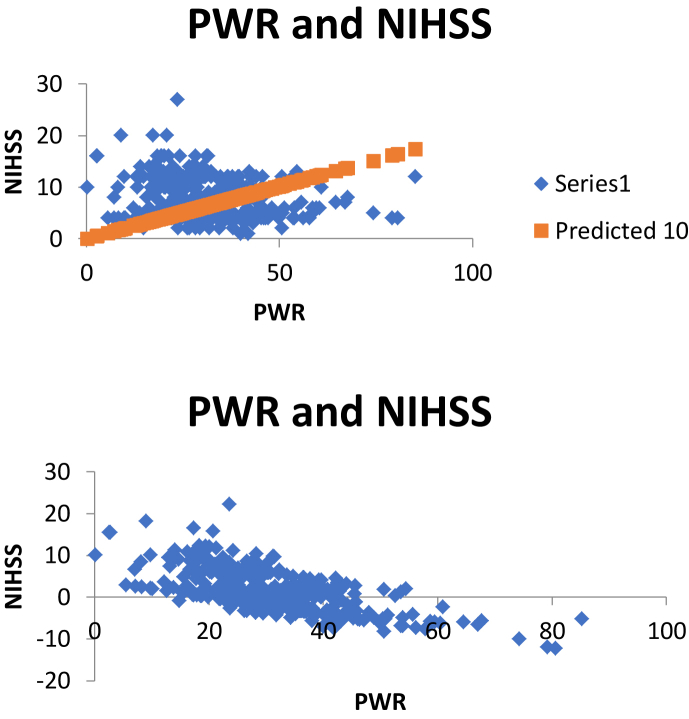
Atherothrombotic stroke was the most subjects in this study (58.8%). The highest mean white blood cells count was found in the stroke group of cardioembolic stroke (12572.71/uL); and mean white blood cells count atherothrombotic stroke group was 10161.67/uL. The mean platelet count of atherothrombotic stroke group was 303317.39/uL and the cardioembolic stroke group was 265394.92/uL. Mean PWR of atherothrombotic stroke group had higher than cardioembolic stroke (33.02 vs 26.74). Mean NIHSS of atherothrombotic stroke group was lower than cardioembolic stroke group (5.81 vs 10.31). There was a strong negative significant correlation between PWR and NIHSS (r = -0.9603; p < 0.001). The complete data can be seen in Table 2. The sum of squared errors was 22247.91 and total sum squared errors was 27833 (Table 3), so we found R-Square 0.7993. It mean 79% of total variation in clinical outcome (NIHSS) across 390 acute ischemic stroke patients (Table 4).
Table 3.
Analysis of variant.
| df | SS | MS | F | Significance F | |
|---|---|---|---|---|---|
| Regression | 5 | 22247.91 | 4449.582 | 306.7256 | 1.2E-131 |
| Residual | 385 | 5585.088 | 14.50672 | ||
| Total | 390 | 27833 |
Table 4.
Regression statistic of independent variable.
| Regression Statistics | |
|---|---|
| Multiple R | 0.894056 |
| R Square | 0.799336 |
| Adjusted R Square | 0.794654 |
| Standard Error | 3.808769 |
| Observations | 390 |
From logistic regression, we found that PWR and platelet was statistically significance correlate with NIHSS (p < 0.05). The coefficient if PWR is the highest (absolute value) among other independent variables (Table 5). It shows that PWR has positive effect on clinical outcome using NIHSS tools in acute ischemic stroke patients (Figure 1).
Table 5.
Confidence interval for PWR, patelet, WBC, age and onset.
| Coefficients | Standard Error | t Stat | P-value | Lower 95% | Upper 95% | Lower 95.0% | Upper 95.0% | |
|---|---|---|---|---|---|---|---|---|
| PWR | -0.05984 | 0.020252 | -2.95449 | 0.003324 | -0.09965 | -0.02002 | -0.09965 | -0.02002 |
| Platelet | 9.18E-06 | 2.37E-06 | 3.880126 | 0.000123 | 4.53E-06 | 1.38E-05 | 4.53E-06 | 1.38E-05 |
| WBC | 3.08E-05 | 1.68E-05 | 1.830088 | 0.06801 | -2.3E-06 | 6.39E-05 | -2.3E-06 | 6.39E-05 |
| Age | 0.113981 | 0.009866 | 11.55316 | 1.04E-26 | 0.094583 | 0.133378 | 0.094583 | 0.133378 |
| Onset | -0.00999 | 0.005606 | -1.7817 | 0.075586 | -0.02101 | 0.001034 | -0.02101 | 0.001034 |
Bold signifies p<0.05.
Figure 1.
Correlation PWR and NIHSS.
4. Discussion
Based on the results of the study above, it is known that research subjects with female gender are 213 subjects or 54.5% of the whole subject. These results are consistent with research by Cintya Agreayu with more female subjects than male subjects [13]. The results of this study contradict the results in other studies which concluded that stroke infarction should have a higher prevalence in males [10]. The subjects of this study were 17–92 years old with an mean age was 57.14 years. The results of this age demographic approach the mean age of the subjects of the study conducted by Ibrahim Ilker with the results of mean age of the subjects was 58.76 years [14].
This study shows that the risk factors for hypertension occur in 83.2% of subjects who are the highest risk factors when compared with other risk factors such as diabetes, smoking, and cardiac abnormalities. These results are consistent with research on stroke risk factors conducted in Asia in 2014 found that there are six risk factors that influence the incidence of stroke, body mass index ≥25 kg/m2, smoking, diabetes, cardiac abnormalities, alcohol consumption, and hypertension. The study shows that hypertension is the most common risk factor found in stroke patients [15].
As a major component to prevent bleeding, platelets are crucial in the pathogenesis of infarction strokes because they play a role in the formation of atherosclerosis and thromboembolism that initiate stroke symptoms. Activated platelets initiate the production of hemostatic plaques and play an important role in the activation of the coagulation system and the formation of atherosclerosis [16]. Atherosclerosis is the most frequent pathogenesis and results in cerebral infarction. Vascular collagen will enlarge the area in contact with platelets in areas that have endothelial damage. It contributes and plays an important role in the formation of thrombosis, which is the process of platelet aggregation. Platelets will be activated by Platelet Activating Factor (PAF) which is produced by ischemic parenchymal tissue, thereby impacting on the activation and aggregation of sustained platelets. In addition, PAF is also a toxic agent against nerves [10].
A description of the function and platelet activity can be drawn from the Mean Platelet Volume (MPV) and platelet count calculation. High platelet count increases the risk of stroke infarction. This increase is believed to affect the ease of thrombus formation whereas if the number is lower it can have implications for the lack of good coagulation function which is at risk of cerebral hemorrhage [16]. High platelet counts can be detected in infarct stroke patients with poor prognosis and subjects who die of infarction stroke [17]. White blood cells play an important role in the inflammatory process, namely in vascular damage and secondary parenchymal damage after the onset of an ischemic stroke. Cytokines and adhesion molecules that regulate increased migration of white blood cells into the infarct area [10]. The number of circulating neutrophils increases several hours after the onset of a stroke and related to the severity of the stroke, infarct volume, and worsening neurological outcomes [18]. Increasing white blood cells after the onset of a stroke occurs due to increased production, increased release of bone marrow and spleen, and may also be influenced by a reduction in neutrophil apoptosis. Releasing factors during cerebral infarction such as cytokines, chemokines, and Damage-Associated Molecular Patterns (DAMPs) which play a role in activating and recruiting neutrophils to infarct locations. After neutrophils are recruited and activated regarding exposure of these factors, neutrophils will release proinflammatory molecules, Reactive Oxygen Species (ROS), cytokines, chemokines, and proteases [19].
Blockages due to emboli usually occur in branching of arteries such as bifurcations from carotid internal arteries to cerebral arteries media and anterior sebaceous arteries, or at the bifurcation of the cerebral artery media. Emboli rarely clogs the penetrating artery in the media cerebral artery such as the lenticulostriate artery because this artery is almost perpendicular to the source artery and it makes large infarction area [20]. The greater the number and activation of white blood cells proinflammation after a stroke, is related to the size of the infarction area, damage to the Blood Brain Barrier (BBB), transformation of infarction into bleeding, and worsening neurological outcomes [19].
This study showed that cardioembolic stroke group had higher mean white blood cells count than atherothrombotic stroke group (12572.71 vs 10161.67/uL) and cardioembolic stroke group had lower PWR than atherothrombotic stroke group (26.74 vs 33.02).
The degree of neurological deficits in stroke infarction patients can be assessed using the National Institute of Health Stroke Scale (NIHSS). NIHSS is not only used to assess the degree of neurological deficit, but also to facilitate communication between patients with medical personnel, evaluate, determine appropriate treatment and predict outcomes of stroke patients, determine the initial prognosis and complications and interventions needed. NIHSS is also widely used to assess the severity of patients with ischemic stroke. Currently NIHSS is widely used routinely to assess the severity of strokes in stroke service centers. The NIHSS assessment includes eleven components which include: level of consciousness, best gaze, visual field testing, facial paresis, arm and leg motor functions, limb ataxia, sensory, language, dysarthria, extinction, and inattention. NIHSS has a maximum score of 42 and a minimum score of 0. The interpretations of NIHSS are: score> 25 is very heavy, 14–25 severe, 5–14 moderate, and <5 mild [[11], [21]].
The interaction between platelets and white blood cells is associated with inflammation and onset of stroke infarction. White blood cells-Platelet Aggregates (LPAs) in the periphery are believed to be one of the signs of platelets that have been activated as a bridge for other cells in blood vessels, especially white blood cells whose recruitment is influenced by components released by platelets including some chemokines and membrane ligands. The formation of LPA becomes a very important factor when there is damage to infarction reperfusion by accumulating in blood vessels with infarction conditions. The location of the infarction will double the amount of LPA after infarction and reperfusion stroke compared to preischemic values leading to microvascular plaque formation and decreased ischemic perfusion. This process is accompanied by further white blood cells recruitment by platelet aggregation and activation. That is why PWR can represent the degree of inflammation and thrombosis as well as illustrate the severity of ischemic stroke [10].
In this study, mean NIHSS in cardioembolic stroke group was higher compared with atherotrombotic stroke group (10.31 vs 5.81). There was a strong negative significant correlation (r = -0.9603; p < 0.001) between PWR and NIHSS. It means, higher PWR will provide higher NIHSS than the lower PWR. From logistic regression, we found that PWR and platelet was statistically significance correlate with NIHSS (p < 0.05). The coefficient of PWR is the highest (absolute value) among other independent variables. It shows that PWR has positive effect on clinical outcome using NIHSS tools in acute ischemic stroke patients.
This study had several limitation. This study used retrospective data and we did not compare with another marker for inflammatory responses, such as C-Reactive Protein (CRP), Interleukin (IL), or the proinflammatory molecules, Reactive Oxygen Species (ROS), cytokines, chemokines, and proteases, and we purposed cohort design in future study to examine Odds Ratio and association of PWR to predict clinical outcome in acute ischemic stroke patients.
5. Conclusion
PWR was associated with severity of acute ischemic stroke, cardioembolic stroke had higher PWR compared with atherothrombotic stroke. PWR had a strong correlation with NIHSS. The higher PWR will provide higher NIHSS and has positive effect on clinical outcome using NIHSS tools in acute ischemic stroke patients.
Declarations
Author contribution statement
L. Amalia: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
N. Dalimonthe: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Johnson W., Onuma O., Owolabi M., Sachdev S. Stroke: a global response is needed. Bull. World Health Organ. 2016;94(9):634A–635A. doi: 10.2471/BLT.16.181636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badan Penelitian dan Pengembangan . 2013. RISET KESEHATAN DASAR. [Google Scholar]
- 3.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation. 2016;133(4):e38–48. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Barker-collo S., Norrving B., Mensah A., Taylor S., Johnson C.O., Murray C.J.L. 2015. Stroke Prevalence , Mortality and Disability-Adjusted Life Years in Adults Aged 20 – 64 Years in 1990 – 2013 : Data from the Global Burden of Disease 2013 Study; pp. 190–202. [DOI] [PubMed] [Google Scholar]
- 5.Alexandru N., Andrei E., Dragan E., Georgescu A. Interaction of platelets with endothelial progenitor cells in the experimental atherosclerosis: role of transplanted endothelial progenitor cells and platelet microparticles. Biol. Cell. 2015;107(6):189–204. doi: 10.1111/boc.201400071. [DOI] [PubMed] [Google Scholar]
- 6.Balami J.S., Chen R.L., Grunwald I.Q., Buchan A.M. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2013;10(4):357–371. doi: 10.1016/S1474-4422(10)70313-6. [Internet] [DOI] [PubMed] [Google Scholar]
- 7.Hug A., Dalpke A., Wieczorek N., Giese T., Lorenz A., Auffarth G. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2013;40(10):3226–3232. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- 8.Furlan J.C., Vergouwen M.D.I., Fang J., Silver F.L. White blood cell count is an independent predictor of outcomes after acute ischaemic stroke. Eur. J. Neurol. 2014;21(2):215–222. doi: 10.1111/ene.12233. [Internet] [DOI] [PubMed] [Google Scholar]
- 9.Franks Z.G., Campbell R.A., Weyrich A.S., Rondina M.T. Platelet – white blood cells interactions linkinflammatory and thromboembolic events in ischemic stroke. 2013;1207:11–17. doi: 10.1111/j.1749-6632.2010.05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Huang Y., Li S., Lin J., Liu W., Ding Z. Platelet-to-White blood cell Ratio: a prognostic predictor for 90-day outcomes in ischemic stroke patients with intravenous thrombolysis. J. Stroke Cerebrovasc. Dis. 2016;25(10):2430–2438. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.015. [Internet] [DOI] [PubMed] [Google Scholar]
- 11.Jojang H., Runtuwene T., JM P.S. Perbandingan NIHSS pada pasien stroke hemoragik dan NonHemoragik yang rawat inap di bagian neurologi RSUP prof. Dr. R. D. Kandou Manado. J eClinic. 2016;4(1):3–6. [Google Scholar]
- 12.Abdul-Rahim A.H., Fulton R.L., Sucharew H., Kleindorfer D., Khatri P., Broderick J.P. National institutes of health stroke scale item profiles as predictor of patient outcome: external validation on safe implementation of thrombolysis in stroke-monitoring study data. Stroke. 2015;46(10):2779–2785. doi: 10.1161/STROKEAHA.115.010380. [DOI] [PubMed] [Google Scholar]
- 13.Dinata C.A., Safrita Y., Sastri S. Artikel penelitian gambaran faktor risiko dan tipe stroke pada pasien rawat inap di Bagian penyakit dalam RSUD kabupaten solok selatan periode. 2013;2(2):57–61. 1 January 2010 - 31 June 2012. [Google Scholar]
- 14.Oz I.I., Yucel M., Bilici M., Ilikhan S.U. Is mean platelet volume a reliable marker to predict ischemic stroke in the follow-up of patients with carotid stenosis ? 2015;1–6 doi: 10.1016/j.jstrokecerebrovasdis.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Chen X., Zhou L., Zhang Y., Yi D., Liu L., Rao W. 2014. Risk Factors of Stroke in Western and Asian Countries : A Systematic Review and Meta-Analysis of Prospective Cohort Studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J., Wang Q., He B., Liu P., Chen J., Quan H. 2016. Association of Mean Platelet Volume and Platelet Count with the Development and Prognosis of Ischemic and Hemorrhagic Stroke; pp. 233–239. [DOI] [PubMed] [Google Scholar]
- 17.Das D., Bhutia C.T., Lamichaney R., Chettri B. 2017. Platelet Size in Stroke : A Study in a Tertiary Hospital; pp. 533–535. i(December) [Google Scholar]
- 18.Kumar A.D., Boehme A.K., Siegler J.E., Gillette M., Albright K.C., Martin-schild S. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J. Stroke Cerebrovasc. Dis. 2013;1:7. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jickling G.C., Liu D., Ander B.P., Stamova B., Zhan X., Sharp F.R. 2015. Targeting Neutrophils in Ischemic Stroke : Translational Insights from Experimental Studies; pp. 1–14. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim B.J., Kim J.S. 2014. Ischemic stroke subtype classification: an asian viewpoint. J. Stroke. 2014;16(1):8–17. doi: 10.5853/jos.2014.16.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westrick R., Fredman G. Platelets: context-dependent vascular protectors or mediators of disease. Arterioscler. Thromb. Vasc. Biol. 2015;35(7):e25–e29. doi: 10.1161/ATVBAHA.115.305898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.