Abstract
Objective:
REM sleep behavior disorder (RBD) affects 33-46% of patients with Parkinson’s Disease (PD) and may be a risk factor for neuropsychological and functional deficits. However, the role of RBD on neuropsychological functioning in PD has yet to be fully determined. We, therefore, examined differences in neurocognitive performance, functional capacity, and psychiatric symptoms among non-demented PD patients with probable RBD (PD/pRBD+) and without (PD/pRBD−), and healthy comparison participants (HC).
Methods:
172 participants (58 PD/pRBD+; 65 PD/pRBD−; 49 HC) completed an RBD sleep questionnaire, psychiatric/clinical questionnaires, performance-based and self-reported functional capacity measures, and underwent a comprehensive neuropsychological battery assessing attention/working memory, language, visuospatial function, verbal and visual learning and memory, and executive function.
Results:
Controlling for psychiatric symptom severity, the PD/pRBD+ group had poorer executive functioning and learning performance than the PD/pRBD− group and poorer neuropsychological functioning across all individual cognitive domains than the HCs. In contrast, PD/pRBD− patients had significantly lower scores than HCs only in the language domain. Moreover, PD/pRBD+ patients demonstrated significantly worse medication management skills compared to HCs. Both PD groups reported greater depressive and anxiety severity compared to HCs; PD/pRBD+ group also endorsed greater severity of apathy compared to HCs.
Conclusions:
The presence of pRBD is associated with worse neuropsychological functioning in PD such that PD patients with pRBD have poorer cognitive, functional, and emotional outcomes compared to HC participants and/or PD patients without pRBD. Our findings underscore the importance of RBD assessment for improved detection and treatment of neuropsychological deficits (e.g., targeted cognitive interventions).
Keywords: executive functioning, learning, neuropsychology, functional capacity, apathy, cognition
Although Parkinson’s disease (PD) is classified as a movement disorder (Postuma et al., 2015), it frequently includes a broad range of cognitive (e.g., executive dysfunction), emotional (e.g., depression), and somatic (e.g., sleep) non-motor symptoms. Non-motor symptoms are often harbingers of the classic hypokinetic syndrome, and they confer severe disability and are associated with increased mortality and decreased quality of life (Chaudhuri, Healy, & Schapira, 2006; Poewe, 2008; Schapira, Chaudhuri, & Jenner, 2017). Consequently, many researchers have begun focusing their efforts on the assessment and treatment of non-motor symptoms. For example, mild cognitive impairment in PD has received more attention as an outcome in recent years (e.g., Aarsland et al., 2017; Pedersen, Larsen, Tysnes, & Alves, 2017; Riedel et al., 2008), as it has been shown to predict later conversion to PD-dementia (Kehagia, Barker, & Robbins, 2010) and to be associated with lower quality of life (Lawson et al., 2016; Schiehser et al., 2009). Much of the cognitive literature in parkinsonian disorders has highlighted a profile characterized by poor attention (Génier Marchand et al., 2018; Lawson et al., 2016) and dysexecutive performance (Chahine et al., 2016; Dirnberger & Jahanshahi, 2013; Kudlicka, Clare, & Hindle, 2011; Zgaljardic, Borod, Foldi, & Mattis, 2003; Zgaljardic et al., 2006), but the neuropsychological impairments can be quite heterogeneous (Kehagia et al., 2010). Relatedly, performance-based measures of functional abilities (i.e., medication and financial management) are objective, ecologically valid lab-based assessments of daily living skills that have proven utility in detecting functional deficits in those with PD-mild cognitive impairment (PD-MCI; Pirogovsky-Turk et al., 2014)
Two other classes of impactful non-motor symptoms in PD are psychiatric and sleep syndromes. Depression, anxiety, apathy, and visual hallucinations are all common in PD (Aarsland, Marsh, & Schrag, 2009; den Brok et al., 2015; Zgaljardic et al., 2003) and are often associated with cognitive deficits (Dissanayaka et al., 2017; Goldman et al., 2018; Hu et al., 2014). Sleep is frequently disrupted (Tandberg, Larsen, & Karlsen, 1998) and poor quality of sleep is related to reduced mental health (Borek, Kohn, & Friedman, 2006). Although there are dozens of distinct sleep syndromes (Walker, 2017), rapid eye movement (REM) sleep behavior disorder (RBD) is particularly relevant, as it is disproportionately prevalent in PD, with estimates ranging from 33 to 47% (Gagnon et al., 2002; Rolinski et al., 2014; Sixel-Döring, Trautmann, Mollenhauer, & Trenkwalder, 2011). RBD is a parasomnia, characterized by a lack of muscle atonia during the REM sleep phase, resulting in unwanted and occasionally violent motor behavior during dreams. RBD has been classified as an early non-motor prodromal marker of both PD (Berg et al., 2015; Poewe, 2008) and Lewy body dementia (LBD; Berg et al., 2014) – a disease with substantial neurobiological and phenotypic overlap with PD. Therefore, there is substantial clinical utility in the timely detection of RBD in order to inform early intervention. Moreover, the RBD syndrome itself (i.e., idiopathic RBD) is associated with cognitive deficits (Ferini–Strambi et al., 2004; Marques et al., 2010), and it may account for a portion of the overall PD syndrome, as PD patients with RBD (PD/RBD+) have an altered motor profile (Postuma, Gagnon, Vendette, Charland, & Montplaisir, 2008), different patterns of neural activity on EEG (Gagnon et al., 2004), and a unique autonomic presentation (Postuma, Lang, Gagnon, Pelletier, & Montplaisir, 2012) compared to PD patients without RBD (PD/RBD−), who can resemble healthy comparison (HC) participants across these variables.
With respect to cognitive outcomes in PD, RBD is a risk factor for dementia (Anang et al., 2014; Nomura, Inoue, Kagimura, & Nakashima, 2013; Postuma et al., 2019) and is associated with a higher prevalence of MCI (Gagnon et al., 2009; Jozwiak et al., 2017; Zhang et al., 2016). A number of studies have also examined overall cognitive performance in PD/RBD+ and many (Chahine, Amara, & Videnovic, 2017; Gong et al., 2014; Jozwiak et al., 2017; Marques et al., 2010; Rolinski et al., 2014; Sinforiani et al., 2006; Vendette et al., 2007; Zhang et al., 2016), but not all (Bugalho & Paiva, 2011; Lavault et al., 2010; Plomhause et al., 2013; Sixel-Döring, Trautmann, Mollenhauer, & Trenkwalder, 2014), investigators have found that PD/RBD+ patients score lower on neuropsychological tests than PD/RBD− patients. However, these studies include a variety of methodological limitations including small sample sizes (e.g., Plomhause et al., 2013), the absence of a HC group (e.g., Bugalho & Paiva, 2011), the lack of performance-based measures of functional capacity (e.g., Zhang et al., 2016), and the use of screening instruments rather than full neuropsychological batteries to assess cognition (e.g., (Lavault et al., 2010). Those researchers that did assess multiple cognitive domains reported that PD/RBD+ underperformed relative to PD/RBD− patients in a variety of cognitive areas, most predominantly in visuospatial/visuoconstructional skills (Jozwiak et al., 2017; Marques et al., 2010; Vendette et al., 2007; Zhang et al., 2016), verbal learning (Erro et al., 2012; Jozwiak et al., 2017; Vendette et al., 2007), delayed memory (Erro et al., 2012; Jozwiak et al., 2017; Vendette et al., 2007; Zhang et al., 2016), and executive functions (Jozwiak et al., 2017; Sinforiani et al., 2006; Vendette et al., 2007; Zhang et al., 2016). A final limitation of the RBD/cognition literature in PD is that, although psychiatric comorbidities are related to cognitive deficits, and RBD can be associated with higher levels of depression and anxiety (Jozwiak et al., 2017; Rolinski et al., 2014; Sixel-Döring et al., 2011), no investigations to our knowledge measured and controlled for important psychiatric symptoms such as apathy when assessing RBD and cognition in PD.
We are only aware of two studies that have examined the relationship between RBD and cognition in a large PD sample compared to a HC sample using a full neuropsychological battery and measures of psychiatric functioning (Jozwiak et al., 2017; Zhang et al., 2016). Zhang and colleagues (2016) recruited 32 PD/RBD+ patients, 42 PD RBD− patients, and 36 HC participants from hospitals affiliated with Soochow University in Suzhou, China. RBD was diagnosed with the Mayo Sleep Questionnaire (Boeve et al., 2011) and the RBD Screening Questionnaire (RBDSQ). PD/RBD+ performed worse than PD/RBD− and HCs, and PD/RBD− performed worse than HC participants across a variety of cognitive tests including on tests of attention, episodic memory, visuospatial abilities, and executive functions; depression and anxiety did not differ between the two PD groups. Importantly, the authors did not administer any performance-based measures of functional capacity and did not measure apathy, which is a common psychiatric correlate of PD (Oguru, Tachibana, Toda, Okuda, & Oka, 2010). Jozwiak and colleagues (2017) reported on 53 PD/RBD+ patients, 40 PD/RBD− patients, and 69 HC participants recruited from Montreal General Hospital and nearby medical centers. RBD was diagnosed with polysomnography. PD/RBD+ performed worse on neuropsychological tests than PD/RBD− and a comparison group but, contrary to expectations, PD/RBD− did not differ cognitively from HC participants. Moreover, the authors reported no differences between PD/RBD+ and PD/RBD− on sleep quality, depression, and anxiety measures, and there were no significant associations between cognitive performance and a) sleep quality, b) depression, and c) anxiety. Finally, the study did not include performance-based tests of functional capacity and did not measure apathy in their sample.
Overall, the literature on the association between RBD and cognition in PD includes a variety of methodological limitations. Therefore, it is not surprising that findings are mixed in PD and a consensus has not been reached on the a) cognitive profile and deficits associated with RBD, b) influence of RBD on functional status, and c) potential influence of important psychiatric comorbidities (such as apathy) on cognition in RBD. Therefore, the purpose of the current study was to address these issues by analyzing data from a large, well-characterized U.S. sample divided into three groups – PD/RBD+, PD/RBD−, and HC participants. Although the role of RBD in LBD is also of interest (Berg et al., 2014), we focused our efforts on nonmotor symptoms in PD.
All participants completed a comprehensive neuropsychological evaluation, including performance-based tests of functional abilities, and we accounted for the potential mitigating influence of psychiatric symptoms on cognition. Based on the literature reviewed above, we aimed to examine the association between RBD symptomatology and cognitive, functional, and psychiatric outcomes. Specifically, we hypothesized that 1) PD/RBD+ patients would perform worse than PD/RBD− patients and HCs on tests of visuospatial/visuoconstructional skills, learning and memory, and executive functions. We also hypothesized that 2) the PD/RBD+ group would earn lower scores on performance-based measures of functional status compared to the PD/RBD− and HC groups. Finally, we hypothesized that 3) depression, anxiety, and apathy would be higher in the PD/RBD+ group compared to the PD/RBD− and HC groups, but that the relationship between RBD and cognitive performance and functional capacity would remain significant after controlling for psychiatric symptoms.
Method
Participants and Procedures
This retrospective investigation included 123 PD patients and 49 HC participants drawn from a longitudinal parent study on cognition in PD (results to be published in a subsequent paper). Study participants were recruited from the community and the Movement Disorders Clinic at the University of California, San Diego (UCSD) and VA San Diego Healthcare System (VASDHS), between 2009 and 2017. Participants provided informed consent and the study was approved by the local institutional review board.
PD patients were diagnosed by board-certified neurologists specializing in movement disorders based on the United Kingdom Parkinson’s Disease Society Bank Criteria (Hughes, Ben-Shlomo, Daniel, & Lees, 1992). PD patients were excluded from the larger study if: 1) there were plausible secondary causes of PD, 2) they met criteria for dementia according to DSM-IV-TR (see Emre et al., 2007), or 3) they received scores of <124 on the Mattis Dementia Rating Scale (MDRS; Schmidt et al., 1994), as this cut-off score has been specifically recommended for screening dementia in PD (Llebaria et al., 2008). No participants for this present study were excluded based on this criterion. Furthermore, both PD and HC participants were excluded in the presence of other neurodegenerative disorders, a history of psychosis and/or untreated major depression, as well as history of treatment for substance abuse. PD patients receiving dopaminergic therapy (n=118) during study participation were tested while on medication; levodopa equivalent dosage (LED) is presented in Table 1 (Tomlinson et al., 2010). Participants also self-reported any current clonazepam or other benzodiazepine use. Motor symptoms were assessed using Part III of the Unified Parkinson’s Disease Rating Scale (UPDRS; (Goetz et al., 2008) and a modified version of the Hoehn and Yahr rating scale (Goetz et al., 2004) to denote the disease stage. Moreover, PD-MCI status was also determined based on Level II criteria of the Movement Disorder Society Task Force Guidelines (Litvan et al., 2012). Participants completed a comprehensive neuropsychological test battery and additional neuropsychiatric and clinical measures at baseline and 2-3 years later for follow-up. Baseline neuropsychological, clinical, and functional assessments were used in the current analyses.
Table 1.
Participant demographics and group statistics
| PD/RBD+ Mean/% (SD) (n = 58) (a) |
PD/RBD− Mean/% (SD) (n = 65) (b) |
HC Mean/% (SD) (n = 49) (c) |
F, t, or χ2 | p-value | Effect Size | Post-hoc Comparisons |
|
|---|---|---|---|---|---|---|---|
| Demographic Characteristics | |||||||
| Age (years) | 68.40 (8.31) | 66.88 (8.42) | 67.14 (8.21) | .56 | .571 | -- | |
| Education (years) | 16.13 (2.35) | 16.77 (2.10) | 16.16 (2.37) | 1.54 | .271 | -- | |
| Gender (% male) | 74 | 66 | 43 | 11.75 | .003 | φ=.261 | a,b>c |
| Race/Ethnicity ( % White) |
93 | 94 | 98 | 7.63 | .665 | φ=.212 | -- |
| Disease Characteristics | |||||||
| Duration of PD (years) | 5.58 (4.82) | 4.75 (4.83) | -- | .92 | .340 | d=.172 | -- |
| UPDRS Part III* | 17.93 (10.33) | 20.13 (12.83) | -- | 1.04 | .308 | d=.189 | -- |
| Modified Hoehn and Yahr stage |
1.83 (0.66) | 1.89 (0.89) | -- | .13 | .720 | d=.077 | -- |
| Levodopa dosage equivalence | 831.59 (747.07) | 622.43 (817.55) | -- | 2.13 | .147 | d=.267 | -- |
| PD-MCI status (%) | 21 | 20 | 10 | 6.66 | .155 | φ=.197 | -- |
|
Self-reported Antidepressant Use** | |||||||
| % taking antidepressants | 14 | 7 | 8 | .780 | .460 | --- | |
| Types of Antidepressants (n) | |||||||
| Celexa/Citalopram | 2 | 0 | 0 | --- | --- | --- | --- |
| Effexor/Venlafaxine | 0 | 0 | 1 | --- | --- | --- | --- |
| Elavil/Amitriptyline | 0 | 0 | 0 | --- | --- | --- | --- |
| Klonopin/Clonazepam | 0 | 0 | 0 | --- | --- | --- | --- |
| Lamictal/Lamotrigine | 0 | 0 | 0 | --- | --- | --- | --- |
| Lexapro/Escitalopram | 0 | 1 | 0 | --- | --- | --- | --- |
| Oleptro/Trazodone | 0 | 0 | 0 | --- | --- | --- | --- |
| Pamelor/Nortriptyline | 0 | 0 | 0 | --- | --- | --- | --- |
| Paxil/Paroxetine | 4 | 0 | 1 | --- | --- | --- | --- |
| Pristiq/Desvenlafaxine | 0 | 0 | 0 | --- | --- | --- | --- |
| Prozac/Fluoxetine | 0 | 0 | 1 | --- | --- | --- | --- |
| Seroquel/Quetiapine | 0 | 0 | 0 | --- | --- | --- | --- |
| Wellbutrin/Bupropion | 0 | 1 | 0 | --- | --- | --- | --- |
| Zoloft/Sertraline HCI | 1 | 0 | 0 | --- | --- | --- | --- |
| More than one | 0 | 2 | 1 | --- | --- | --- | --- |
| Mood/Sleep Characteristics | |||||||
| Geriatric Depression Scale |
7.65 (5.48) | 5.82 (5.20) | 2.60 (3.49) | 14.09 | <.001 | a,b>c+ | |
| Apathy Scale | 12.16 (5.23) | 10.92 (5.61) | 9.18 (4.81) | 4.25 | .016 | a>c+ | |
| State Anxiety (STAI) | 36.13 (10.82) | 33.57 (9.45) | 26.67 (8.12) | 13.46 | <.001 | a,b>c+ | |
| Trait Anxiety (STAI) | 36.54 (10.43) | 33.84 (9.10) | 27.59 (8.34) | 12.43 | <.001 | a,b>c+ | |
| RBDSQ total score | 8.45 (3.22) | 3.05 (1.48) | 2.14 (1.34) | 242.43 | <.001 | a,b>c; a>b+ | |
Bold font denotes p<.05. UPDRS=Unified Parkinson’s Disease Rating Scale; STAI=State/Trait Anxiety Inventory; RBDSQ= RBD Screening Questionnaire; PD-MCI=Parkinson’s disease-Mild Cognitive Impairment status.
No on vs. off medication differences between groups
PD/RBD+: n = 52; PD/RBD−: n = 59; HC: n = 49
Tukey’s HSD
Measures
RBD
The REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ; Stiasny-Kolster et al., 2007) was used to assess self-reported presence and severity of RBD symptoms in all participants and to classify participants into clinically probable RBD−positive (pRBD+) and RBD-negative (pRBD−) groups, as detailed below. The RBDSQ has demonstrated validity in PD (sensitivity = 0.842, specificity = 0.962; Nomura, Inoue, Kagimura, Uemura, & Nakashima, 2011; Wang et al., 2015).
Cognition
All participants completed a comprehensive neuropsychological assessment battery, which included measures commonly used for cognitive assessment of PD patients (Pirogovsky-Turk et al., 2017). The battery measured cognition in the following domains: attention/working memory, executive functioning, language, learning, memory, and visuospatial functioning. All raw scores were converted to standardized T-scores using published normative procedures and demographically corrected norms when available (see Table 2 for list of measures by cognitive domains). A global T-score composite was also created by averaging across all individual test scores.
Table 2.
Neuropsychological assessment battery
| Cognitive domain | Tests within each domain | Normative data | Demographic adjustments |
|---|---|---|---|
| Attention/Working Memory | CVLT-II Trial 1 | Delis et al., 2000 | Age, gender |
| DOT-A | Unpublished norms (VJF) | Gender | |
| DKEFS Color Naming | Delis et al., 2001 | Age | |
| Executive Functioning | WCST Total Errors | Heaton & Staff, 1993 | Age, education |
| DKEFS Letter Fluency | Delis et al., 2001 | Age | |
| DKEFS Color-Word Inhibition | Delis et al., 2001 | Age | |
| DKEFS Inhibition/Switching | Delis et al., 2001 | Age | |
| Language | MDRS Similarities | Schiehser et al., in preparation | -- |
| DKEFS Category Fluency | Delis et al., 2001 | Age | |
| Learning | CVLT II Trials 1-5 Total | Delis et al., 2000 | Age, gender |
| WMS-III Logical Memory I Recall | Wechsler, 1997 | Age, education | |
| WMS-III Visual Reproduction I Recall | Wechsler, 1997 | Age, education | |
| Memory | CVLT II Long Delay Free Recall | Delis et al., 2000 | Age, gender |
| WMS-III Logical Memory II Recall | Wechsler, 1997 | Age, education | |
| WMS-III Visual Reproduction II Recall | Wechsler, 1997 | Age, education | |
| Visuospatial Functioning | JLOT | Schiehser et al., in preparation | Gender |
| WMS-III Visual Reproduction Copy | Wechsler, 1997 | Age, education |
CVLT-II= California Verbal Learning Test -Second Edition; DOT-A=Ascending Digit Order Test; DKEFS=Delis-Kaplan Executive Function System; WCST=Wisconsin Card Sorting Test; MDRS=Mattis Dementia Rating Scale; WMS-III=Wechsler Memory Scale-Third Edition; JLOT=Judgment of Line Orientation Test.
Functional skills
The Medication Management Abilities Assessment (MMAA; Patterson et al., 2002) was used as a performance-based assessment of participants’ capacity to carry out a prescribed medication regimen. The UCSD Performance-Based Skills Assessment (UPSA; Patterson, Goldman, McKibbin, Hughs, & Jeste, 2001) finance subtest was used to measure performance-based functional capacity in the domain of financial management. Caregivers of PD patients completed the Instrumental Activities of Daily Living Scale (IADL; Lawton & Brody, 1969). Total scores were used for all functional skills measures; higher scores reflect better performance and functioning.
Neuropsychiatric Symptoms
State Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) Trait and State total scores were used to measure anxiety (Barnes, Harp, & Jung, 2002). Severity of depressive symptoms and apathy were measured using the total scores of the Geriatric Depression Scale (GDS; Yesavage et al., 1982) and the Apathy Scale (Starkstein et al., 1992), respectively. All measures have demonstrated reliability and validity in PD (Dissanayaka et al., 2017; Leentjens et al., 2008a,b). Higher scores on all neuropsychiatric measures reflect higher symptomatology.
Statistical Analyses
PD patients were classified into pRBD+ (n=58) and pRBD− (n=65) groups using an established cut-off score of 6 on the RBDSQ (Nomura et al., 2011). Six HCs met criteria for pRBD based on this cut-off and were excluded from the analyses. Thus, a final sample of 172 (n=49 HCs) participants were included. Given that item 6 on the RBDSQ directly assesses the cardinal symptom of RBD (i.e., movements while dreaming), we examined the utility of this item in predicting probable RBD status. Indeed, item 6 on the RBDSQ pertaining to dream enactment behavior distinguished the PD groups; odds of a probable RBD diagnosis increase by a factor of 4.71 given a positive response to item 6 (OR=4.714, Wald z=15.82, df=1, p<0.001).
Independent samples t-test, univariate analyses of variance (ANOVA), nonparametric Kruskal-Wallis one-way ANOVA (for non-normally distributed variables), and chi-square analyses were conducted to examine differences in demographic and clinical characteristics between groups. Partial correlations, with cognitive diagnosis as a covariate, demonstrated comparable associations between pRBD status and individual cognitive domains to correlations that did not include cognitive status as a covariate. Therefore, more parsimonious models unadjusted for cognitive diagnosis are reported. We used univariate analysis of covariance (ANCOVA) and multivariate ANCOVA (MANCOVA) to examine differences between PD/pRBD+, PD/pRBD−, and HC participants on global neurocognitive functioning and individual cognitive domains, controlling for the effects of psychiatric symptom severity (i.e., simultaneously covarying for scores on the GDS, Apathy Scale, and trait anxiety component of the STAI; note, given the strong association between state and trait anxiety total scores, we only chose to covary for trait anxiety). Of note, we also performed univariate analysis of covariance (ANCOVA) to compare groups on psychiatric and clinical variables given the significant differences in sex distribution between the PD and HC groups. However, analyses with and without sex as a covariate did not differ; thus, the results from analyses without sex as a covariate are reported. Tukey’s HSD or Bonferroni adjusted alpha levels were used to evaluate statistical significance of pair-wise comparisons within each familywise comparison across groups, (i.e., cognition, functional capacity, and psychiatric symptom severity.)
Results
Demographic and clinical comparison
The PD/pRBD+, RD/pRBD−, and HC groups did not significantly differ in age, education, MCI status, and race/ethnicity (ps>.10). There were significant differences, however, in sex distribution (χ2(2)=11.75, p=.003), with a significantly greater proportion of women in the HC group compared to both PD groups. There were significant differences between the groups on the RBDSQ (F(2, 169)=242.43, p<.001, ), with both PD/pRBD+ and PD/pRBD− groups reporting greater RBD symptoms compared to the HC group, and the PD/pRBD+ group also reporting greater RBD symptoms compared to the PD/pRBD− group. None of the participants in the study reported any current use of clonazepam and there were no group differences in self-reported antidepressant use.
Within the PD group, there was no difference between RBD groups in duration of PD (t (121)=−.96, p=.340), motor symptoms, as assessed by Part III of UPDRS (t (117)=1.02, p=.308), the modified Hoehn and Yahr rating scale (t (116)=.36, p=.720), and levodopa equivalent dosage (t (118)= −1.46, p=.147). Of note, there were also no significant differences between PD RBD groups in their medication status (on vs. off) while completing the UPDRS (χ2 (1)=.024, p=.878). Table 2 shows the demographic comparison between the groups and descriptive statistics for significant outcomes of interest.
PD and RBD effects on neuropsychiatric symptoms
State anxiety (F(2, 165)=13.46, p<.001, ), trait anxiety (F(2, 165)=12.43, p<.001, ), and depression severity (F(2, 167)=14.09, p<.001, ) differed significantly between PD patients and HCs. Tukey’s post-hoc analyses indicated that the PD/pRBD+ and PD/pRBD− groups reported greater current depression and anxiety compared to the HCs. Similarly, there were significant differences between the groups on their scores on the apathy scale (F(2, 169)=4.25, p=.016, ), with Tukey’s post-hoc comparison indicating that the PD/pRBD+ group endorsed greater severity of symptoms of apathy as compared to the HCs. No significant differences were found between the PD/pRBD+ and PD/pRBD− groups on psychiatric outcomes.
PD and RBD effects on neuropsychological performance
A 3-group ANOVA, controlling for psychiatric symptom severity (depression, trait anxiety, apathy), demonstrated a significant difference between the groups on global neurocognitive performance (F(2, 160)=10.73, p<001, ; see Table 3 for statistics). Post-hoc analyses using Bonferroni adjustments for multiple comparisons demonstrated that PD/pRBD+ patients exhibited poorer global neurocognitive functioning compared to the PD/pRBD− patients (p=.017) and HCs (p<.001). MANCOVA demonstrated significant differences among the groups on individual cognitive domains (F(12, 310)=2.30, p=.008, Λ=.84, ), with effects across domains as follows: attention/working memory (F(2, 160)=7.46, p=.001, ), executive functioning (F(2, 160)=5.19, p=.007, ), language (F(2, 160)=6.67, p=.002, ), learning (F(2, 160)=7.82, p=.001, ), memory (F(2, 160)=7.28, p=.001, ), and visuospatial functioning (F(2, 160)=4.10, p=.018, ; see Figure 1). Specifically, Bonferroni-adjusted post-hoc analyses demonstrated that the PD/pRBD+ group had poorer executive functioning and learning performance compared to the PD/pRBD− group (ps<.05). Moreover, PD/pRBD+ patients had poorer neuropsychological functioning, generalized across all individual cognitive domains, as compared to HCs (ps<.03); while the PD/pRBD− patients had significantly lower scores compared to the HCs only in the language domain (p=.018), and marginally significantly lower scores compared to the HCs in attention/working memory (p=.05). Although not statistically significant, the PD/pRBD+ group had marginally significantly lower visuospatial functioning score compared to the PD/pRBD− group (p=.066). No other significant differences in cognitive performance were found.
Table 3.
Group differences on cognitive and functional outcomes
| PD/RBD+ (n = 58) (a) |
PD/RBD− (n = 65) (b) |
HC (n = 49) (c) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean/% (SD) | Range | Mean/% (SD) | Range | Mean/% (SD) | F | p | Effect Size |
Post-hoc Comparisons+ |
|
| Cognitive Functioning a | ||||||||||
| MDRS total score | 124-144 | 137.93 (4.23) | 127-144 | 138.92 (3.17) | 129-144 | 140.71 (3.00) | 4.87 | .009 | a<c | |
| Global NP | 25.93-63.29 | 49.41 (7.05) | 34.41-65.48 | 52.90 (5.50) | 43.56-66.41 | 56.29 (6.13) | 10.73 | <.001 | a<b,c | |
| Attention/Working memory | 20-57.61 | 44.23 (8.15) | 30-64.20 | 47.18 (7.54) | 34.48-69.94 | 51.47 (8.38) | 7.46 | .001 | a<c | |
| Executive Functioning | 23.33-65.75 | 50.05 (8.93) | 38-70 | 53.92 (6.61) | 35.75-70 | 56.02 (7.79) | 5.19 | .007 | a<b,c | |
| Language | 34.40-68.72 | 50.44 (8.49) | 25.85-68.72 | 52.24 (8.85) | 40.17-75.67 | 56.97 (6.99) | 6.67 | .002 | a,b<c | |
| Learning | 28.67-65.67 | 49.82 (9.60) | 39.33-72 | 54.31 (7.71) | 40.33-72 | 57.85 (8.33) | 7.82 | .001 | a<b,c | |
| Memory | 29.33-67.67 | 51.30 (8.04) | 20-70.67 | 54.84 (7.83) | 41-75.67 | 58.80 (8.45) | 7.28 | .001 | a<c | |
| Visuospatial functioning |
15.92-66.53 | 51.43 (9.03) | 15.05-69.25 | 55.60 (9.51) | 36.52-69.25 | 57.28 (8.52) | 4.10 | .018 | a<c | |
| Functional Capacity | ||||||||||
| MMAAb | 0-33 | 28.58 (6.60) | 4-33 | 30.06 (5.03) | 27-33 | 31.57 (1.73) | H = 6.24 | .044 | a<c | |
| UPSA (Finance subscale)b | 1-11 | 9.79 (1.62) | 7-11 | 10.03 (0.93) | 7-11 | 10.24 (0.72) | H = 2.18 | .336 | -- | |
| IADLa | 3-16 | 13.45 (2.90) | 8-16 | 14.41 (1.99) | 12.73-20 | -- | 2.66 | .106 | -- | |
Bold font denotes p<.05. MDRS=Mattis Dementia Rating Scale; Global NP=global neuropsychological performance (calculated as an average of all individual cognitive measures); MMAA= Medication Management Abilities Assessment; UPSA= UCSD Performance-Based Skills Assessment; IADL=Instrumental Activities of Daily Living Scale. All cognitive domains report results using T-scores (M±SD=50±10, range 20–80), except for MDRS total score, which is expressed as a total raw score (range 0-144).
Statistics reported from models controlling for psychiatric symptom severity (depression, trait anxiety, apathy).
Non-parametric Kruskal-Wallis Test
Bonferroni adjustment for multiple comparisons
Figure 1.
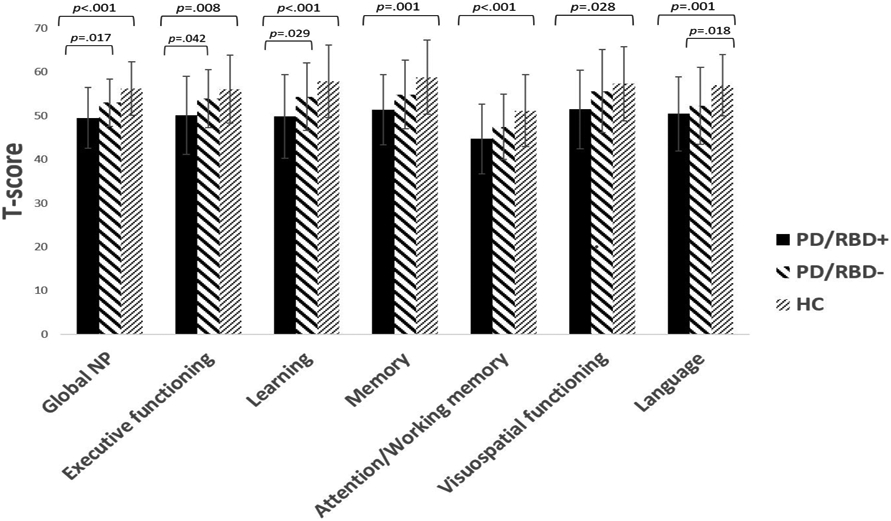
PD/RBD status effects on neurocognitive performance.
PD and RBD effects on functional capacity
Nonparametric Kruskal-Wallis one-way ANOVA demonstrated no statistically significant group differences on performance-based functional capacity, as measured by the UPSA-Financial subscale (H(2)=2.18, p=.336). However, there were significant group differences on MMAA (H(2)=6.24, p=.044), such that the PD/pRBD+ group demonstrated significantly poorer performance as compared to the HCs using Bonferroni correction (p=.038). No other significant group differences in financial functional capacity were found. A 2-group ANCOVA, controlling for psychiatric symptom severity, demonstrated no significant difference on IADL scores between PD/pRBD+ and PD/pRBD− patients F(1, 100)=2.66, p=.11, ).
Discussion
The primary objective of the current study was to characterize PD patients neuropsychologically based upon the presence or absence of RBD by examining differences in cognitive performance, psychiatric symptoms, and functional capacity between PD patients with and without probable RBD as well as compared to HCs. Our results indicated that the presence of RBD is associated with poorer neuropsychological functioning in PD. Specifically, pRBD in PD is associated with greater severity of apathy, worse cognition across all domains, and poorer functional capacity compared to healthy adults. Moreover, individuals with PD and pRBD demonstrated worse overall cognition, executive functioning, and learning compared to PD patients without RBD. Importantly, our study examined the unique variance in cognitive functioning accounted for by pRBD by controlling for psychiatric symptom severity; this suggests that the association between self-reported symptoms of RBD and cognition cannot be explained by symptoms of depression, anxiety, or apathy.
The current findings indicated that the presence of pRBD is not only associated with the severity of neurocognitive symptoms, but also the neuropsychological profile. In this non-demented sample, PD patients without pRBD performed similarly to HC participants, with the exception of worse performance in the language domain. However, in those non-demented PD patients with pRBD, a striking pattern of worse performances emerged across multiple domains, including executive functioning and verbal learning. Moreover, compared to PD patients without pRBD, PD/pRBD+ patients had poorer global neurocognitive functioning, with poorer performance on executive functioning and learning domains driving this relationship. Of note, PD/pRBD+ participants’ lowest cognitive performance was evidenced within the domain of attention and working memory. Differences in cognitive performance between the PD groups may partly be explained by the PD/RBD+ group evidencing their lowest scores within the domain of attention/working memory, which is known to subserve performance across higher-order functions such as executive functioning and learning. Moreover, previous studies have identified a profile characterized by poor attention in parkinsonian disorders as an important early cognitive feature of patients with RBD who later develop MCI and dementia (Génier Marchand et al., 2018). Together, these results support our hypothesis of a unique cross-sectional association between pRBD and worse cognitive performance in non-demented PD patients, although the direction of this relationship remains unclear. These findings are consistent with previous studies associating RBD in PD with poorer verbal learning (Erro et al., 2012; Jozwiak et al., 2017; Vendette et al., 2007) and executive functioning (Jozwiak et al., 2017; Sinforiani et al., 2006; Vendette et al., 2007; Zhang et al., 2016) compared to HCs and/or PD patients without RBD, as well as imaging studies implicating abnormalities in frontal and temporal regions and microstructural pathways subserving these cognitive functions (Ansari, Rahmani, Dolatshahi, Pooyan, & Aarabi, 2017; Ford et al., 2013; Lim et al., 2016).
Though the presence of pRBD in PD was associated with lower scores across multiple cognitive domains in the current study, the average performance of the PD/pRBD+ group across these domains was not impaired. Furthermore, prevalence of PD-MCI was similar across the pRBD groups, which is inconsistent with previous studies (Gagnon et al., 2009; Jozwiak et al., 2017; Zhang et al., 2016). Compared to participants in these previous studies, participants within the current study endorsed higher levels of education (≥16 years on average), which may partially explain these findings. Similarly, average neuropsychiatric symptom severity scores do not indicate clinically elevated levels of depression or apathy within the PD groups.
With respect to functional skills, significant differences in performance-based medication management abilities were revealed between the PD/pRBD+ and HCs, but not between PD/pRBD− and HCs. These findings suggest that there is a subgroup of non-demented individuals with PD – those with pRBD – with suboptimal functional medication management skills. This is important given that suboptimal medication adherence has been noted extensively in PD literature and linked to negative prognoses (Grosset, Bone, & Grosset, 2005; Kulkarni et al., 2008; Leopold, Polansky, & Hurka, 2004). Given that cognitive impairment is associated with medication adherence in PD (Daley, Myint, Gray, & Deane, 2012), findings from the current study highlight the clinical and scientific importance of evaluating PD patients for RBD to identify those at greatest risk for both cognitive and functional deficits related to medication management.
Regarding financial functional skills, results did not support the hypothesis that the PD/pRBD+ group would perform worse than the PD/pRBD− group. It may be that RBD mediated cognitive deficits in PD are not severe enough to have a measurable impact on overlearned behaviors such handling routine financial transactions (e.g., counting change, writing checks). Alternatively, it is possible that RBD affects financial skills in PD, but our functional assessment was not sensitive enough to detect this effect. Future research that focuses on RBD in relation to additional performance-based measures of functional capacity may yield important information.
Overall, the limited differences between the non-demented PD patients without pRBD and healthy adults across the majority of study outcomes are consistent with prior studies suggesting the presence of pRBD in individuals with PD as an indicator of a more severe subtype and greater risk for poorer outcomes (Fereshtehnejad et al., 2015). In a future paper, we will report on a longitudinal investigation examining the enduring effects of these pRBD-related outcomes in PD. The difference in language functioning between the PD groups, regardless of pRBD status, and HCs, however, suggests that performance in this domain is linked to non-RBD pathology in PD.
There are certain limitations of the current study that need to be considered. The study utilized convenience sampling and participants were primarily White and highly educated, thereby limiting the generalizability of the findings. There was greater representation of women in the HC group as compared to the PD groups; however, this is consistent with the well-documented gender difference noted within the PD literature (Van Den Eeden et al., 2003). Polysomnography was not used to confirm a definitive diagnosis of RBD and determination of probable RBD was not verified by a bed partner; however, the RBDSQ cutoff score used to identify RBD+ patients (≥6) has been shown to have high sensitivity and specificity in studies validating the measure in PD patients (Nomura et al., 2011; Wang et al., 2015). Our results add to the evidence base supporting the utility of the RBDSQ as a practical and unobtrusive alternative to polysomnography and reports from informants—who may have limited availability or knowledge of the patient’s sleep behaviors—in assessing RBD in PD patients. Although Zhang and colleagues (2016) also utilized the RBDSQ to classify their sample, they applied a different cutoff score (7) than the established cutoff of 6 (Nomura, Inoue, Kagimura, Uemura, & Nakashima, 2011) utilized in our study. Moreover Zhang et al. (2016) did not adjust for multiple comparisons, which may partially explain any inconsistencies between the two studies.
Despite these limitations, our findings suggest that PD/pRBD+ patients have a more impaired cognitive profile than PD/pRBD− patients and healthy adults and evidence poorer functioning compared to healthy adults. Specifically, compared to PD patients without pRBD, PD/pRBD+ patients have greater levels of cognitive impairment specific to learning and executive functioning. These findings underscore the importance of RBD assessment for improved detection and treatment of neuropsychological impairment. Recognition of the association between RBD and cognition, as well as adopting targeted interventions (e.g., cognitive training), could positively influence cognition, functional status, and mood in PD.
Acknowledgements/ Study Funding
This work was supported by VA CSR&D (Grant #: I01 CX000813; PI: Filoteo) and RR&D (Grant #: I01 RX001691; PI: Schiehser) Merit Awards. Zanjbeel Mahmood, M.S., and Ryan Van Patten, Ph.D. are supported by the National Institute of Mental Health Institutional Training Grant in Geriatric Mental Health (Grant #: T32 MH 19934, PIs: Dilip V. Jeste, M.D.; Elizabeth W. Twamley, Ph.D.)
Footnotes
Disclosure of Interest
Authors report no conflict of interests or financial disclosures.
References
- Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, & Ballard C (2017). Cognitive decline in Parkinson disease. Nature Reviews Neurology, 13(4), 217–231. 10.1038/nrneurol.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Marsh L, & Schrag A (2009). Neuropsychiatric symptoms in Parkinson’s disease. Movement Disorders, 24(15), 2175–2186. 10.1002/mds.22589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anang JBM, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, … Postuma RB (2014). Predictors of dementia in Parkinson disease: A prospective cohort study. Neurology, 83(14), 1253–1260. 10.1212/WNL.0000000000000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M, Rahmani F, Dolatshahi M, Pooyan A, & Aarabi MH (2017). Brain pathway differences between Parkinson’s disease patients with and without REM sleep behavior disorder. Sleep and Breathing, 21(1), 155–161. 10.1007/s11325-016-1435-8 [DOI] [PubMed] [Google Scholar]
- Barnes LLB, Harp D, & Jung WS (2002). Reliability generalization of scores on the Spielberger State-Trait Anxiety Inventory. Educational and Psychological Measurement, 62(4), 603–618. 10.1177/0013164402062004005 [DOI] [Google Scholar]
- Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, … Deuschl G (2015). MDS research criteria for prodromal Parkinson’s disease. Movement Disorders, 30(12), 1600–1611. 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, … Lang AE (2014). Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Movement Disorders, 29(4), 454–462. 10.1002/mds.25844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Molano JR, Ferman TJ, Lin SC, Bieniek K, Tippmann-Peikert M, … & Silber MH (2013). Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. Journal of Clinical Sleep Medicine, 9(05), 475–480. 10.1212/01.WNL.0000106460.34682.E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek LL, Kohn R, & Friedman JH (2006). Mood and sleep in Parkinson’s disease. The Journal of Clinical Psychiatry, 67(6), 958–963. [DOI] [PubMed] [Google Scholar]
- Bugalho P, da Silva JA, & Neto B (2011). Clinical features associated with REM sleep behavior disorder symptoms in the early stages of Parkinson’s disease. Journal of Neurology, 258(1), 50–55. 10.1007/s00415-010-5679-0 [DOI] [PubMed] [Google Scholar]
- Bugalho P, & Paiva T (2011). Dream features in the early stages of Parkinson’s Disease. Journal of Neural Transmission, 118(11), 1613 10.1007/s00702-011-0679-5 [DOI] [PubMed] [Google Scholar]
- Chahine LM, Amara AW, & Videnovic A (2017). A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Medicine Reviews, 35, 33–50. 10.1016/j.smrv.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine LM, Weintraub D, Hawkins KA, Siderowf A, Eberly S, Oakes D, … & PARS Investigators. (2016). Cognition in individuals at risk for Parkinson's: Parkinson associated risk syndrome (PARS) study findings. Movement Disorders, 31(1), 86–94. 10.1002/mds.26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, & Schapira AHV (2006). Non-motor symptoms of Parkinson’s disease: Diagnosis and management. The Lancet Neurology, 5(3), 235–245. [DOI] [PubMed] [Google Scholar]
- Daley DJ, Myint PK, Gray RJ, & Deane KHOL (2012). Systematic review on factors associated with medication non-adherence in Parkinson’s disease. Parkinsonism and Related Disorders, 18(10), 1053–1061. 10.1016/j.parkreldis.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan Executive Function System®(D-KEFS®): Examiner's Manual: Flexibility of Thinking, Concept Formation, Problem Solving, Planning, Creativity, Impulse Control, Inhibition. Pearson. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). CVLT-II: California Verbal Learning Test: Adult Version. Psychological Corporation. [Google Scholar]
- den Brok MGHE, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RMA, & Richard E (2015). Apathy in Parkinson’s disease: A systematic review and meta-analysis. Movement Disorders, 30(6), 759–769. 10.1002/mds.26208 [DOI] [PubMed] [Google Scholar]
- Dirnberger G, & Jahanshahi M (2013). Executive dysfunction in Parkinson’s disease: A review. Journal of Neuropsychology, 7(2), 193–224. 10.1111/jnp.12028 [DOI] [PubMed] [Google Scholar]
- Dissanayaka NNW, Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, … Burn DJ (2017). Anxiety is associated with cognitive impairment in newly-diagnosed Parkinson’s disease. Parkinsonism and Related Disorders, 36, 63–68. 10.1016/j.parkreldis.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, … Dubois B (2007). Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders, 22(12), 1689–1707. 10.1002/mds.21507 [DOI] [PubMed] [Google Scholar]
- Erro R, Santangelo G, Picillo M, Vitale C, Amboni M, Longo K, … Barone P (2012). Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. Journal of Neurology, 259(9), 1808–1813. 10.1007/s00415-011-6407-0 [DOI] [PubMed] [Google Scholar]
- Fereshtehnejad SM, Romenets SR, Anang JBM, Latreille V, Gagnon JF, & Postuma RB (2015). New clinical subtypes of Parkinson disease and their longitudinal progression a prospective cohort comparison with other phenotypes. JAMA Neurology, 72(8), 863–873. 10.1001/jamaneurol.2015.0703 [DOI] [PubMed] [Google Scholar]
- Ferini–Strambi L, Di Gioia MR, Castronovo V, Oldani A, Zucconi M, & Cappa SF (2004). Neuropsychological assessment in idiopathic REM sleep behavior disorder (RBD): Does the idiopathic form of RBD really exist?. Neurology, 62(1), 41–45. 10.1212/01.WNL.0000101726.69701.FA [DOI] [PubMed] [Google Scholar]
- Ford AH, Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Burn DJ, & O’Brien JT (2013). Rapid eye movement sleep behavior disorder in Parkinson’s disease: Magnetic resonance imaging study. Movement Disorders, 28(6), 832–836. 10.1002/mds.25367 [DOI] [PubMed] [Google Scholar]
- Gagnon J-F, Bédard M-A, Fantini ML, Petit D, Panisset M, Rompré S, … Montplaisir J (2002). REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology, 59(4), 585 LP – 589. 10.1212/WNL.59A585 [DOI] [PubMed] [Google Scholar]
- Gagnon JF, Fantini ML, Bédard MA, Petit D, Carrier J, Rompré S, … Montplaisir J (2004). Association between waking EEG slowing and REM sleep behavior disorder in PD without dementia. Neurology, 62(3), 401–406. 10.1212/01.WNL.0000106460.34682.E9 [DOI] [PubMed] [Google Scholar]
- Gagnon J-F, Vendette M, Postuma RB, Desjardins C, Massicotte-Marquez J, Panisset M, & Montplaisir J (2009). Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Annals of Neurology, 66(1), 39–47. 10.1002/ana.21680 [DOI] [PubMed] [Google Scholar]
- Génier Marchand D, Postuma RB, Escudier F, De Roy J, Pelletier A, Montplaisir J, & Gagnon J (2018). How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Annals of Neurology, 83(5), 1016–1026. 10.1002/ana.25239 [DOI] [PubMed] [Google Scholar]
- Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, y Seidl L (2004). Movement Disorder Society Task Force Report on the Hoehn and Yahr Staging Scale: Status and recommendations. Movement Disorders, 19(9), 1020–1028. https://doi-org.libproxy.sdsu.edu/10.1002/mds.20213 [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, … LaPelle N (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders: Official Journal of the Movement Disorder Society, 23(15), 2129–2170. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- Goldman JG, Holden SK, Litvan I, McKeith I, Stebbins GT, & Taylor JP (2018). Evolution of diagnostic criteria and assessments for Parkinson’s disease mild cognitive impairment. Movement Disorders, 33(4), 503–510. 10.1002/mds.27323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Xiong K, Mao C, Shen Y, Hu W, Huang J, … Liu C (2014). Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep Medicine, 15(6), 647–653. [DOI] [PubMed] [Google Scholar]
- Grosset KA, Bone I, & Grosset DG (2005). Suboptimal medication adherence in Parkinson’s disease. Movement Disorders, 20(11), 1502–1507. 10.1002/mds.20602 [DOI] [PubMed] [Google Scholar]
- Hu MTM, Szewczyk-Królikowski K, Tomlinson P, Nithi K, Rolinski M, Murray C, … Ben-Shlomo Y (2014). Predictors of cognitive impairment in an early stage Parkinson’s disease cohort. Movement Disorders, 29(3), 351–359. 10.1002/mds.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, & Lees AJ (1992). What features improve the accuracy of clinical diagnosis in Parkinson’s disease: A clinicopathologic study. Neurology, 42(6), 1142 LP – 1142. 10.1212/WNL.42.6.1142 [DOI] [PubMed] [Google Scholar]
- Jozwiak N, Montplaisir J, Latreille V, Bourgouin P-A, Gagnon J-F, Postuma RB, … Chouinard S (2017). REM Sleep Behavior Disorder and cognitive impairment in Parkinson’s disease. Sleep, 40(8). 10.1093/sleep/zsx101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, & Robbins TW (2010). Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. The Lancet Neurology, 9(12), 1200–1213. [DOI] [PubMed] [Google Scholar]
- Kudlicka A, Clare L, & Hindle JV (2011). Executive functions in Parkinson’s disease: Systematic review and meta-analysis. Movement Disorders, 26(13), 2305–2315. 10.1002/mds.23868 [DOI] [PubMed] [Google Scholar]
- Kulkarni AS, Balkrishnan R, Anderson RT, Edin HM, Kirsch J, & Stacy MA (2008). Medication adherence and associated outcomes in medicare health maintenance organization-enrolled older adults with Parkinson’s disease. Movement Disorders, 23(3), 359–365. 10.1002/mds.21831 [DOI] [PubMed] [Google Scholar]
- Lavault S, Leu-Semenescu S, du Montcel S, de Cock V, Vidailhet M, & Arnulf I (2010). Does clinical rapid eye movement behavior disorder predict worse outcomes in Parkinson’s disease? Journal of Neurology, 257(7), 1154–1159. 10.1007/s00415-010-5482-y [DOI] [PubMed] [Google Scholar]
- Lawton MP, & Brody EM (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9(3_Part_1), 179–186. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, Williams-Gray CH, … ICICLE-PD Study Group (2016). Cognitive decline and quality of life in incident Parkinson’s disease: The role of attention. Parkinsonism and Related Disorders, 27, 47–53. 10.1016/j.parkreldis.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leentjens AFG, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, … Goetz CG (2008a). Anxiety rating scales in Parkinson’s disease: Critique and recommendations. Movement Disorders, 23(14), 2015–2025. 10.1002/mds.22233 [DOI] [PubMed] [Google Scholar]
- Leentjens AFG, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, … Goetz CG (2008b). Apathy and anhedonia rating scales in Parkinson’s disease: Critique and recommendations. Movement Disorders, 23(14), 2004–2014. 10.1002/mds.22229 [DOI] [PubMed] [Google Scholar]
- Leopold NA, Polansky M, & Hurka MR (2004). Drug adherence in Parkinson’s disease. Movement Disorders, 19(5), 513–517. 10.1002/mds.20041 [DOI] [PubMed] [Google Scholar]
- Lim JS, Shin SA, Lee JY, Nam H, Lee JY, & Kim YK (2016). Neural substrates of rapid eye movement sleep behavior disorder in Parkinson’s disease. Parkinsonism and Related Disorders, 23, 31–36. 10.1016/j.parkreldis.2015.11.027 [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, … & Aarsland D (2012). Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Movement Disorders, 27(3), 349–356. 10.1002/mds.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llebaria G, Pagonabarraga J, Kulisevsky J, García-Sánchez C, Pascual-Sedano B, Gironell A, & Martínez-Corral M (2008). Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson’s disease. Movement Disorders, 23(11), 1546–1550. 10.1002/mds.22173 [DOI] [PubMed] [Google Scholar]
- Marques A, Dujardin K, Boucart M, Pins D, Delliaux M, Defebvre L, … Monaca C (2010). REM sleep behaviour disorder and visuoperceptive dysfunction: A disorder of the ventral visual stream? Journal of Neurology, 257(3), 383–391. 10.1007/s00415-009-5328-7 [DOI] [PubMed] [Google Scholar]
- Nomura T, Inoue Y, Kagimura T, Uemura Y, & Nakashima K (2011). Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson’s disease patients. Sleep Medicine, 12(7), 711–713. 10.1016/j.sleep.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Nomura T, Inoue Y, Kagimura T, & Nakashima K (2013). Clinical significance of REM sleep behavior disorder in Parkinson’s disease. Sleep Medicine, 14(2), 131–135. 10.1016/j.sleep.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Oguru M, Tachibana H, Toda K, Okuda B, & Oka N (2010). Apathy and depression in Parkinson disease. Journal of Geriatric Psychiatry and Neurology, 23(1), 35–41. 10.1177/0891988709351834 [DOI] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, & Jeste DV (2001). UCSD Performance-Based Skills Assessment: Development of a new measure of everyday functioning for severely mentally ill adults. Schizophrenia Bulletin, 27(2), 235–245. 10.1093/oxfordjournals.schbul.a006870 [DOI] [PubMed] [Google Scholar]
- Patterson TL, Lacro J, McKibbin CL, Moscona S, Hughs T, & Jeste DV (2002). Medication Management Ability Assessment: Results from a performance-based measure in older outpatients with schizophrenia. Journal of Clinical Psychopharmacology, 22(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Pedersen KF, Larsen JP, Tysnes O-B, & Alves G (2017). Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study. Neurology, 88(8), 767–774. 10.1212/WNL.0000000000003634 [DOI] [PubMed] [Google Scholar]
- Pirogovsky-Turk E, Moore RC, Filoteo JV, Litvan I, Song DD, Lessig SL, & Schiehser DM (2017). Neuropsychiatric predictors of cognitive decline in parkinson disease: A longitudinal study. American Journal of Geriatric Psychiatry, 25(3), 279–289. 10.1016/j.jagp.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Pirogovsky-Turk E, Schiehser DM, Obtera KM, Burke MM, Lessig SL, Song DD, … Filoteo JV (2014). Instrumental activities of daily living are impaired in Parkinson’s disease patients with mild cognitive impairment. Neuropsychology, Vol. 28, pp. 229–237. 10.1037/neu0000045 [DOI] [PubMed] [Google Scholar]
- Plomhause L, Dujardin K, Duhamel A, Delliaux M, Derambure P, Defebvre L, & Monaca Charley C (2013). Rapid eye movement sleep behavior disorder in treatment-naïve Parkinson disease patients. Sleep Medicine, 14(10), 1035–1037. [DOI] [PubMed] [Google Scholar]
- Poewe W (2008). Non-motor symptoms in Parkinson’s disease. European Journal of Neurology, 15(s1), 14–20. 10.1111/j.1468-1331.2008.02056.x [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, … Deuschl G (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders: Official Journal of the Movement Disorder Society, 30(12), 1591–1601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- Postuma RB, Iranzo A, Hu M, Högl B, Boeve BF, Manni R, … Puligheddu M (2019). Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain, 142(3), 744–759. 10.1093/brain/awz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Gagnon J-F, Vendette M, Charland K, & Montplaisir J (2008). Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Movement Disorders, 23(12), 1665–1672. 10.1002/mds.22099 [DOI] [PubMed] [Google Scholar]
- Postuma RB, Lang AE, Gagnon JF, Pelletier A, & Montplaisir JY (2012). How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain, 135(6), 1860–1870. 10.1093/brain/aws093 [DOI] [PubMed] [Google Scholar]
- Riedel O, Klotsche J, Spottke A, Deuschl G, Förstl H, Henn F, … Wittchen H-U (2008). Cognitive impairment in 873 patients with idiopathic Parkinson’s disease. Journal of Neurology, 255(2), 255–264. 10.1007/s00415-008-0720-2 [DOI] [PubMed] [Google Scholar]
- Rolinski M, Szewczyk-Krolikowski K, Tomlinson PR, Nithi K, Talbot K, Ben-Shlomo Y, & Hu MTM (2014). REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry, 85(5), 560–566. 10.1136/jnnp-2013-306104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR, & Jenner P (2017). Non-motor features of Parkinson disease. Nature Reviews Neuroscience, 18(7), 435 10.1038/nrn.2017.62 [DOI] [PubMed] [Google Scholar]
- Schiehser DM, Han SD, Lessig S, Song DD, Zizak V, & Filoteo JV (2009). Predictors of health status in nondepressed and nondemented individuals with Parkinson’s disease. Archives of Clinical Neuropsychology, 24(7), 699–709. 10.1093/arclin/acp064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Freidl W, Fazekas F, Reinhart B, Grieshofer P, Koch M, … Lechner H (1994). The Mattis Dementia Rating Scale: Normative data from 1,001 healthy volunteers. Neurology, 44(5), 964 – 964. 10.1212/WNL.44.5.964 [DOI] [PubMed] [Google Scholar]
- Sinforiani E, Zangaglia R, Manni R, Cristina S, Marchioni E, Nappi G, … Pacchetti C (2006). REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson’s disease. Movement Disorders, 21(4), 462–466. 10.1002/mds.20719 [DOI] [PubMed] [Google Scholar]
- Sixel-Döring F, Trautmann E, Mollenhauer B, & Trenkwalder C (2011). Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology, 77(11), 1048 LP – 1054. 10.1212/WNL.0b013e31822e560e [DOI] [PubMed] [Google Scholar]
- Sixel-Döring F, Trautmann E, Mollenhauer B, & Trenkwalder C (2014). Rapid Eye Movement Sleep behavioral events: A new marker for neurodegeneration in early Parkinson disease? Sleep, 37(3), 431–438. 10.5665/sleep.3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Starkstein SE, Mayberg HS, Preziosi T, Andrezejewski P, Leiguarda R, & Robinson RG (1992). Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 4(2), 134–139. 10.1176/jnp.4.2.134 [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, & Oertel WH (2007). The REM sleep behavior disorder screening questionnaire—A new diagnostic instrument. Movement Disorders, 22(16), 2386–2393. 10.1002/mds.21740 [DOI] [PubMed] [Google Scholar]
- Tandberg E, Larsen JP, & Karlsen K (1998). A community-based study of sleep disorders in patients with Parkinson’s disease. Movement Disorders, 13(6), 895–899. 10.1002/mds.870130606 [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, & Clarke CE (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement Disorders, 25(15), 2649–2653. 10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, & Nelson LM (2003). Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. American Journal of Epidemiology, 157(11), 1015–1022. 10.1093/aje/kwg068 [DOI] [PubMed] [Google Scholar]
- Vendette M, Gagnon JF, Décary A, Massicotte-Marquez J, Postuma RB, Doyon J, … Montplaisir J (2007). REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology, 69(19), 1843–1849. 10.1212/01.wnl.0000278114.14096.74 [DOI] [PubMed] [Google Scholar]
- Walker M (2017). Why we sleep: Unlocking the power of sleep and dreams. New York, NY: Scribner. [Google Scholar]
- Wang Y, Wang ZW, Yang YC, Wu HJ, Zhao HY, & Zhao ZX (2015). Validation of the rapid eye movement sleep behavior disorder screening questionnaire in China. Journal of Clinical Neuroscience, 22(9), 1420–1424. 10.1016/j.jocn.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997). Wechsler Memory Scale–Third Edition, San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Zhang JR, Chen J, Yang ZJ, Zhang HJ, Fu YT, Shen Y, … Liu CF (2016). Rapid eye movement sleep behavior disorder symptoms correlate with domains of cognitive impairment in Parkinson’s disease. Chinese Medical Journal, 129(4), 379–385. 10.4103/0366-6999.176077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, & Mattis P (2003). A review of the cognitive and behavioral sequelae of Parkinson’s disease: Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology, 16(4). 10.1097/00146965-200312000-00001 [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Feigin A, & Eidelberg D (2006). An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology, 28(7), 1127–1144. 10.1080/13803390500246910 [DOI] [PMC free article] [PubMed] [Google Scholar]


