Abstract
Background:
While desensitization and sustained unresponsiveness (SU) have been shown with egg oral immunotherapy (OIT), the benefits of baked egg (BE) therapy for egg allergy have not been well studied.
Objective:
To evaluate the safety and efficacy of BE ingestion compared to egg OIT in participants allergic to unbaked egg but tolerant to BE.
Methods:
BE tolerant but unbaked egg reactive children ages 3-16 years were randomized to 2 years of treatment with either BE or egg OIT. Double-blind, placebo-controlled food challenges (DBPCFC) were conducted after 1 and 2 years of treatment to assess for desensitization, and after 2 years of treatment followed by 8-10 weeks off of treatment to assess for SU. Mechanistic studies were conducted to assess for immune modulation. A cohort of BE reactive participants underwent egg OIT and identical DBPCFCs as a comparator group.
Results:
Fifty participants (median age 7.3 years) were randomized and initiated treatment. SU was achieved in 3 of 27 (11.1%) BE participants versus 10 of 23 (43.5%) egg-OIT participants (p=0.009). In the BE reactive comparator group, 7 of 39 (17.9%) participants achieved SU. More BE tolerant participants withdrew from BE versus egg OIT (29.6% versus 13%). Dosing symptom frequency in BE tolerant participants was similar with BE and egg OIT, but more frequent in BE reactive participants. Egg white-specific IgE, skin testing and basophil activation decreased similarly after BE and egg OIT.
Conclusion:
Among children allergic to unbaked egg but tolerant to BE, those treated with egg OIT were significantly more likely to achieve SU compared to children ingesting BE.
Keywords: Egg allergy, baked egg, extensively heated egg, desensitization, sustained unresponsiveness, oral immunotherapy
Capsule Summary:
In egg-allergic children tolerant of BE, egg OIT induced SU in a greater proportion of patients than BE therapy and may be an effective treatment option.
Introduction
The rise in food allergies over the past few decades has been well documented.1 While much of the recent focus has been on peanut allergy, egg allergy has remained one of the most common food allergies in childhood with a prevalence as high if not higher than peanut.2, 3 The ubiquitous nature of egg in the Western diet has made avoidance extremely difficult leading to a constant risk of allergic reactions and negative effects on quality of life.4 While the majority of egg-allergic children are expected to outgrow their allergy, in about 20% it will persist into adulthood.
Recent data have suggested that the majority of egg-allergic children can tolerate egg incorporated into baked foods such as muffins and cakes while still being reactive to unbaked egg products such as scrambled, fried or boiled eggs, French toast, or custards.5, 6 The ability to tolerate extensively heated or “baked egg” represents a phenotype of the allergy that is more likely to result in natural resolution.7, 8 Furthermore, a small study suggested that regular ingestion of baked egg (BE) products may hasten the eventual resolution of egg allergy.9
Extensive heating of egg denatures the allergenic proteins disrupting conformational epitopes while leaving linear epitopes intact.10 This may support the finding that IgE to the more heat resistant ovomucoid is typically lower in patients tolerant of BE.11 An additional theory has suggested that key egg allergenic epitopes may be masked from the immune system when heated with gluten containing foods.10 However, the actual impact of this so called “matrix effect” has remained questionable.12
Oral immunotherapy (OIT) using egg white powder has demonstrated successful desensitization for most egg-allergic children. A subset of these desensitized children further demonstrated sustained unresponsiveness (SU) by maintaining the non-reactive state for up to 6 weeks off of therapy13. The SU outcome has particular clinical relevance as it has been associated with more frequent and larger quantity ingestions of BE and unbaked egg after treatment.14 In clinical trials, longer durations of SU could also represent a proxy for tolerance. Unfortunately, adverse events from OIT have made the treatment difficult for some.13 Because BE is tolerated by the majority of egg-allergic patients, it has been considered as a potential treatment; however the immunological effects of these BE products has not been well studied. A small open-label study by Bird, et al. investigated the ability of dietary BE to induce desensitization in children reactive to both BE and lightly cooked egg15, suggesting favorable immune modulation with daily BE ingestion and supporting its potential role as a treatment for egg allergy in that subset of patients.
The Consortium for Food Allergy Research (CoFAR) developed this protocol to evaluate the safety and efficacy of BE ingestion compared to egg white powder OIT in participants allergic to unbaked egg but tolerant to BE.
Methods
Study design
This multicenter, randomized, open-label study compared the induction of SU in BE tolerant but unbaked egg-allergic participants after 2 years of treatment with daily BE ingestion versus egg OIT. The primary endpoint was the development of SU defined as passing a cumulative dose of 7444 mg egg white protein in a double-blind, placebo-controlled food challenge (DBPCFC) 8 to 10 weeks after discontinuation of 2 years of BE or egg OIT therapy. Secondary endpoints included desensitization after 1 and 2 years of treatment (successfully consuming at least 4444 mg egg white protein), the safety of BE and egg OIT treatments, and changes in mechanistic biomarkers throughout therapy.
Participant selection and randomization
Participants were recruited from 5 US sites (Icahn School of Medicine at Mount Sinai, New York, NY; Johns Hopkins University School of Medicine, Baltimore, MD; Arkansas Children’s Hospital, Little Rock, AR; National Jewish Health, Denver, CO; and University of North Carolina School of Medicine, Chapel Hill, NC). Inclusion criteria included the following: 1) age 3-16 years; 2) egg white-specific IgE (EW-sIgE) ≥ 5 kUA/L; 3) negative BE DBPCFC, and 4) positive unbaked egg DBPCFC with dose-limiting symptoms to 1444 mg of egg white protein or less. Participants with a history of severe anaphylaxis, history of eosinophilic gastrointestinal disease within the past 2 years, or poorly controlled asthma were excluded. Qualifying participants were randomized 1:1 to the “Baked egg-randomized” (BE-R) or “Egg-OIT-randomized” (OIT-R) treatment arms. To facilitate comparison between BE tolerant and BE reactive participants treated with egg OIT, the first 40 participants meeting eligibility criteria, but with a positive BE DBPCFC were also enrolled as a separate “Egg OIT-assigned” (OIT-A) comparison group.
Study product and dosing protocol
BE therapy used commercially available food ingredients and was prepared according to prespecified recipes developed at the Jaffe Food Allergy Institute.9 A typical BE dose consisted of a muffin or equivalent (1/3 of whole egg in serving or approximately 2000 mg of egg white protein). Egg OIT was prepared from dried standard egg white powder (pasteurized, uncooked egg; 80% protein by weight) which was purchased from a commercial manufacturer (Deb-El Food Products, Elizabeth, NJ). Measured doses were provided in individual vials for doses <25 mg egg white powder and capsules for doses between 50-225 mg of egg white powder. For doses of 260-2500 mg egg white powder, a container of bulk powder and a dose-specific scoop were dispensed to the participant with instructions to use 1 scoop per day.
Participants in the BE-R arm ingested 2000 mg of egg white protein as a single BE dose or divided throughout the day. BE-R participants were also allowed to ingest commercial baked egg products and provided a list of safe options. BE dosing continued daily for the duration of the study with a DBPCFC at year 1 and year 2. OIT-R participants began treatment with an initial escalation day during which increasing egg OIT doses were administered as tolerated starting with 0.1 mg up to a maximum of 25 mg egg white powder. Participants tolerating at least 3 mg egg white powder then began daily egg OIT home dosing with return clinic visits every 2 weeks for further dose escalations up to the target maintenance egg OIT dose of 2500 mg egg white powder (2000 mg egg white protein). The minimum maintenance dose per protocol was 350 mg egg white powder (280 mg egg white protein). Maintenance dosing was continued for at least 8 weeks prior to the year 1 DBPCFC. Maintenance dosing was then continued for an additional 12 months; escalation up to the target dose of 2500 mg egg white powder could also continue after the year 1 DBPCFC if the 2500 mg dose had not been achieved prior to this DBPCFC. After this additional 12 months of maintenance dosing, the year 2 DBPCFC was conducted. The OIT-A group completed identical egg OIT therapy except for the initial escalation day which was capped at a maximum of 12 mg egg white powder. All participants passing the year 2 DBPCFC were then instructed to discontinue BE or egg OIT for 8 to 10 weeks after which a final DBPCFC was conducted to assess for SU.
Adherence and safety
Dosing adherence and dosing symptoms were recorded by participants on daily home diary logs. Phone follow-up was conducted 1 week after each dose escalation and participants were seen in clinic every 3 months during year 1 and every 4 months during year 2 to review adherence and safety data. Adverse events (AEs), serious adverse events (SAEs) and accidental exposures to egg were reported throughout the study and graded using the CoFAR grading system for allergic reactions (supplemental table 1)
Double-blind, placebo-controlled food challenges
The baseline BE food challenge was performed as a DBPCFC with the active portion consisting of a muffin with 2000 mg of egg white protein. For ease of measurement of a non-powder food, the muffin was administered over 6 steps per expert guidelines: 5%, 10%, 15%, 20%, 25%, 25%.16 For the placebo portion, Egg Replacer (Ener-G Foods, Seattle, WA) was substituted for egg in the muffin recipe. The unbaked egg DBPCFC consisted of increasing doses of egg white powder identical to the egg OIT material. The cumulative dose of the baseline unbaked egg DBPCFC was 1444 mg of egg white protein and was administered over 7 steps specified in the PRACTALL guidelines17 on DBPCFC: 1, 3, 10, 30, 100, 300, and 1000 mg egg white protein. The year 1, year 2 and SU unbaked egg DBPCFC had a cumulative dose of 7444 mg (1, 3, 10, 30, 100, 300, 1000, 3000, 3000 mg egg white protein). Successfully consumed dose (SCD) was defined as the cumulative dose of egg white protein ingested prior to the dose causing objective or persistent subjective gastrointestinal symptoms requiring treatment and discontinuation of the challenge.
Long-term follow-up
After completing the protocol, participants were contacted by phone annually starting at 3 years from enrollment to complete a long-term follow-up questionnaire (LFQ) to assess incorporation of egg into the diet, frequency of egg consumption, and any allergic reactions due to egg. Participants completed up to three questionnaires listed as Year 3, Year 4, and Year 5 in reference to the time since enrollment.
Immune studies
Mechanistic assays were conducted at baseline, every 3 months during year 1, every 4 months during year 2, and at the SU DBPCFC and 6-month long-term follow-up visits. Skin prick testing (SPT) was performed using commercially available egg white extract (1:20 dilution) from Stallergenes Greer Laboratories (Lenoir, NC). SPT score was calculated as the egg wheal size minus the saline control wheal size. IgE and IgG4 specific to egg white, ovomucoid, and ovalbumin were measured by ImmunoCAP (Thermo-Fisher, Uppsala, Sweden). Whole blood was stimulated with 4 dilutions of egg white (1 mcg, 0.1 mcg, 0.01 mcg, 0.001 mcg) to evaluate for basophil reactivity. Activated basophils were identified as CD123+ CD203c+ Lin- (CD3, CD14, CD19, and CD41) events, with CD63 as the primary marker of activation.
Ethics
Institutional review boards at each clinical site approved the protocol and consent forms. The study was registered on clinicaltrials.gov (NCT01846208), conducted under a US Food and Drug Administration investigational new drug application and monitored by a National Institute of Allergy and Infectious Diseases (NIAID) Data and Safety Monitoring Board (DSMB). Written informed consent was obtained from parents/guardians, with assent from those 7 years of age and older.
Statistical methods
The sample size of 96 participants randomized 1:1 to BE or egg OIT was determined to have 85% power to detect a difference of 40% in SU in the two arms using a 5% level two-sided Barnard’s exact unconditional test. The pre-specified analysis population included all participants initiating treatment. Summary statistics included means and standard deviations or medians and interquartile ranges (IQRs) for continuous variables and proportions for categorical variables. The primary endpoint and desensitization were compared in a pairwise fashion between BE-R and OIT-R as well as OIT-R and OIT-A using Barnard’s exact unconditional test. SCD was compared using Wilcoxon Rank-Sum test. Other comparisons used the Wilcoxon Rank Sum and chi-square, Barnard’s exact unconditional or Fisher’s Exact as appropriate.
Changes over time in mechanistic endpoints were assessed using mixed effect models accounting for within participant correlation across visits up through the year 2 DBPCFC. Separate mixed effects models were considered to compare treatment effects and SU successes.
The safety analysis included reporting of unsolicited AEs/SAEs, dosing symptoms, and DBPCFC reactions. Percent of doses per participant were compared between treatment groups using the Wilcoxon Rank Sum test.
For mechanistic outcomes, a p-value of 0.01 was considered significant to control for multiple comparisons while a p-value of 0.05 was considered significant for all other endpoints.
Results
Study Participants
A target enrollment of 96 participants over an 18-month period was planned. However, accrual was slowed by a higher than expected rate of failure during the BE DBPCFC. The enrollment period was extended, but capped at 26 months to ensure study completion. From July 2013 through August 2015 (Figure 1), 187 participants were screened and 169 proceeded to the baseline BE DBPCFC. Fifty-seven of the 169 (33.7%) passed the BE DBPCFC, 54 (32.0%) failed the egg white DBPCFC, and 52 (30.8%) of the planned 96 participants were randomized to treatment (BE-R=28, OIT-R=24). The initial 40 of the 112 participants who failed the BE DBPCFC were enrolled in the OIT-A group and subsequent failures were considered screen failures per protocol. Three participants (1 BE-R, 1 OIT-R, 1 OIT-A) did not initiate treatment and were not included in the analysis population.
Figure 1: Subject Disposition:
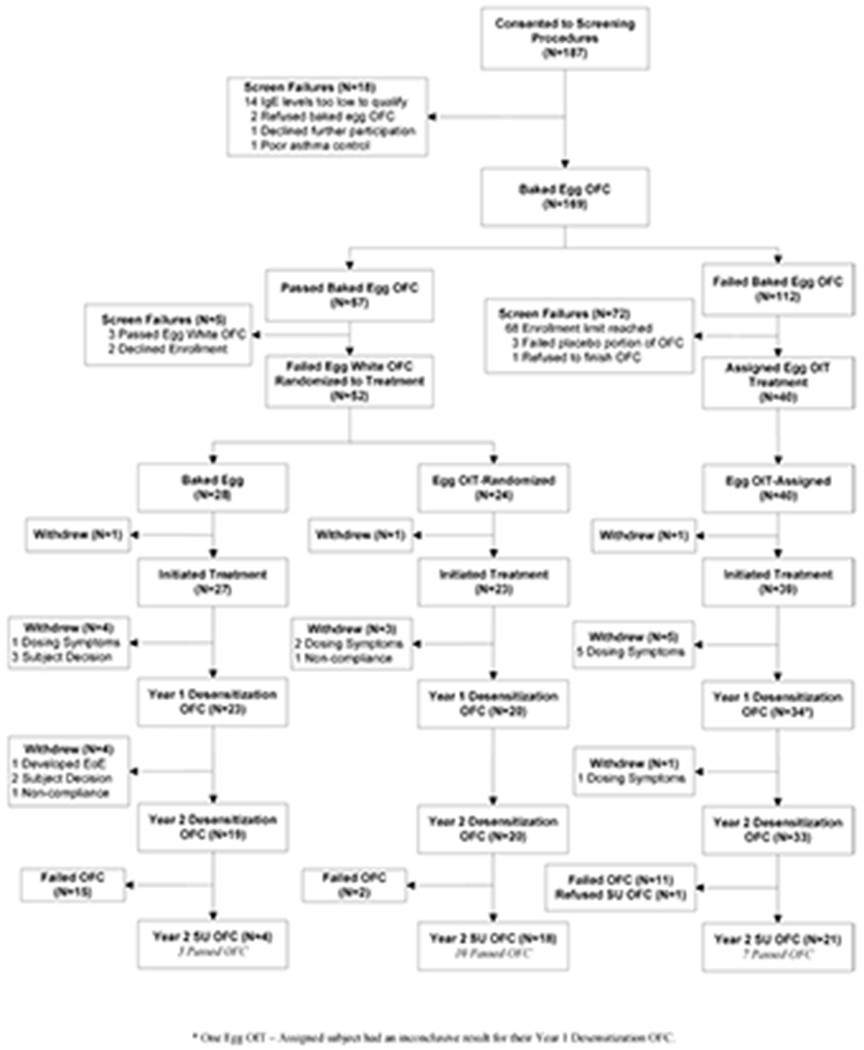
Participants passing a BE DBPCFC and subsequently failing an unbaked egg DBPCFC were randomized 1:1 to BE-R or OIT-R groups. Participants failing a BE DBPCFC were presumed reactive to unbaked egg without a DBPCFC and assigned to the OIT-A group. Enrollment in the OIT-A group was limited to 40 participants. All active participants underwent the year 1 and year 2 unbaked egg DBPCFCs. Subjects passing the year 2 DBPCFC discontinued BE or OIT therapy for 8-10 weeks before undergoing the SU DBPCFC. *One OIT-A participant had an inconclusive result for the year 1 DBPCFC.
Demographic and other baseline characteristics are summarized in Table 1. A majority of the study participants were male (57/89, 64%), and the median age was 7.7 years (range 3.5–16.8 years). The treatment groups were overall similar with regard to gender, race, ethnicity, and other baseline characteristics, except that the mean age at enrollment was lower in the BE-R group compared to the OIT-R group. In addition, when comparing the OIT-R and OIT-A groups, the median EW-sIgE (p=0.015), ovalbumin IgE (OVA-sIgE) (p=0.0015), and ovomucoid IgE (OVM-sIgE) (p=0.011) were all higher in the OIT-A compared to the OIT-R group.
Table 1:
Demographics and Baseline Characteristics
| BE-R (N=27) | OIT-R (N=23) | OIT-A (N=39) | Total (N=89) | P-value1 BE-R vs OIT-R | P-value1 OIT-R vs OIT-A | |
|---|---|---|---|---|---|---|
| Gender - n (%) | ||||||
| Male | 15 (55.6) | 17 (73.9) | 25 (64.1) | 57 (64.0) | 0.1777 | 0.4247 |
| Female | 12 (44.4) | 6 (26.1) | 14 (35.9) | 32 (36.0) | ||
| Race - n (%) | ||||||
| Asian | 2 (7.4) | 2 (8.7) | 4 (10.3) | 8 (9.0) | 0.9185 | 0.3307 |
| Black/African American | 1 (3.7) | 2 (8.7) | 0 (0.0) | 3 (3.4) | ||
| White | 23 (85.2) | 18 (78.3) | 31 (79.5) | 72 (80.9) | ||
| Other | 1 (3.7) | 1 (4.3) | 4 (10.3) | 6 (6.7) | ||
| Age at enrollment (years) - n | 27 | 23 | 39 | 89 | ||
| Mean (SD) | 7.2 (3.0) | 9.1 (3.1) | 8.8 (2.4) | 8.4 (2.8) | 0.0187 | 0.9537 |
| Median (IQR) | 6.8 (5.3, 8.4) | 9.0 (6.3, 10.7) | 8.6 (6.9, 10.2) | 7.7 (6.2, 10.0) | ||
| Other food allergy history – n (%) | ||||||
| No/Unknown | 1 (3.7) | 3 (13.0) | 8 (20.5) | 12 (13.5) | 0.2876 | 0.5714 |
| Yes | 26 (96.3) | 20 (87.0) | 31 (79.5) | 77 (86.5) | ||
| Asthma history – n (%) | ||||||
| No | 9 (33.3) | 10 (43.5) | 13 (33.3) | 32 (36.0) | 0.5889 | 0.4595 |
| Yes | 18 (66.7) | 13 (56.5) | 26 (66.7) | 57 (64.0) | ||
| Allergic rhinitis history – n (%) | ||||||
| No | 3 (11.1) | 5 (21.7) | 8 (20.5) | 16 (18.0) | 0.3554 | 0.9765 |
| Yes | 24 (88.9) | 18 (78.3) | 31 (79.5) | 73 (82.0) | ||
| Atopic dermatitis history – n (%) | ||||||
| No | 9 (33.3) | 10 (43.5) | 15 (38.5) | 34 (38.2) | 0.5889 | 0.7208 |
| Yes | 18 (66.7) | 13 (56.5) | 24 (61.5) | 55 (61.8) | ||
| Baseline egg white OFC (mg) – n | 27 | 23 | NA | 50 | 0.8798 | NA |
| Median (IQR) | 144 (44, 444) | 144 (44, 444) | NA | 144 (44,444) | NA | |
| Min, Max | 4, 444 | 1, 444 | 1, 444 | |||
| Egg white SPT score (mm) – n | 27 | 23 | 39 | 89 | 0.2763 | 0.2785 |
| Median (IQR) | 7.5 (5.5, 13.5) | 11.0 (6.5, 15.0) | 12.0 (7.5, 16.5) | 11.0 (6.5, 15.0) | ||
| Min, Max | 0, 27.0 | 1.5, 23.0 | 0, 28.5 | 0, 28.5 | ||
| Total IgE – n | 27 | 23 | 38 | 88 | 0.5619 | 0.4778 |
| Median (IQR) | 809.0 (389.0, 1870.0) | 873.0 (269.0, 1671.0) | 960.0 (390.0, 1659.0) | 885.5 (366.5, 1699.0) | ||
| Min, Max | 122.0, 5001.0 | 79.7, 3075.0 | 126.0, 5001.0 | 79.7, 5001.0 | ||
| Egg white-specific IgE – n | 27 | 23 | 38 | 88 | 0.6287 | 0.0149 |
| Median (IQR) | 9.77 (6.54, 27.10) | 12.30 (9.09, 31.10) | 26.25 (11.70, 68.80) | 15.60 (8.22, 37.65) | ||
| Min, Max | 4.26, 239.00 | 5.99, 50.30 | 4.31, 497.00 | 4.26, 497.00 | ||
| Ovalbumin-specific IgE - n | 27 | 23 | 38 | 88 | 0.7275 | 0.0015 |
| Median (IQR) | 5.53 (3.20, 20.20) | 5.85 (3.41, 10.40) | 16.10 (7.30, 29.70) | 8.88 (4.11, 20.20) | ||
| Min, Max | 1.79, 102.00 | 1.91, 26.70 | 3.12, 163.00 | 1.79, 163.00 | ||
| Ovomucoid-specific IgE - n | 27 | 23 | 38 | 88 | 0.6355 | 0.0114 |
| Median (IQR) | 6.94 (1.52, 13.40) | 6.93 (3.27, 13.20) | 16.40 (6.11, 35.50) | 9.76 (3.48, 18.95) | ||
| Min, Max | 0.73, 141.00 | 1.45, 33.30 | 0.08, 176.00 | 0.08, 176.00 | ||
| Egg white-specific IgG4 – n | 27 | 23 | 38 | 88 | 0.8013 | 0.0381 |
| Median (IQR) | 0.84 (0.08, 7.31) | 0.81 (0.26, 3.71) | 0.28 (0.13, 1.02) | 0.64 (0.17, 1.84) | ||
| Min, Max | 0, 40.20 | 0.07, 11.60 | 0, 6.04 | 0, 40.20 |
Pearson’s chi-square tests, Fisher’s Exact test, or the Wilcoxon Rank Sum Test were utilized as appropriate.
Clinical Outcomes
Of the 27 BE-R participants, 4 (14.8%) withdrew in the first year (one due to dosing symptoms),and 4 (14.8%) withdrew in the second year (one due to EoE) (Figure 1). Of the 23 OIT-R participants, 3 (13.0%) withdrew in the first year (2 due to dosing symptoms). The remaining 20 OIT-R participants successfully reached the 2500 mg OIT maintenance dose, 18 prior to and 2 after the year 1 DBPCFC. Of the 39 participants in the OIT-A group, 5 (12.8%) withdrew in the first year and 1 (2.6%) withdrew in the second year due to dosing symptoms. Thirty-two of 33 OIT-A participants successfully reached the 2500 mg OIT maintenance dose, 22 prior to and 10 after the year 1 DBPCFC. One participant could not escalate beyond 1320 mg egg white powder and completed therapy at this maintenance dose. The median (IQR) time until reaching the 2500 mg maintenance dose for the OIT-R group was 281.5 (243, 301) days and 292 (275, 301) days for the OIT-A group.
DBPCFC outcomes in the three groups are represented in Table 2 and Figure 2. Nineteen of 27 BE-R participants (70.4%) completed the year 2 DBPCFC, 4 (14.8%) passed the year 2 DBPCFC and proceeded to the SU DBPCFC and 3 (11.1%) passed. Twenty of 23 OIT-A participants (87.0%) completed the year 2 DBPCFC, 18 (78.3%) passed the year 2 DBPCFC and 10 (43.5%) passed the SU DBPCFC. Thirty-three of 39 (84.6%) OIT-A participants completed to the year 2 DBPCFC, 22 (56.4%) passed the year 2 DBPCFC and 7 (17.9%) passed the SU DBPCFC. In addition to the primary endpoint, passing the SU DBPCFC, a pre-specified secondary endpoint defined treatment success at the year 1 and year 2 DBPCFC as successfully consuming at least 4444 mg egg white protein. When comparing results of the participants randomized to BE-R or OIT-R, outcomes were significantly better in the OIT-R group at the year 1 DBPCFC (treatment success: p=0.002) and the year 2 DBPCFC (treatment success: p<0.0001), as well as the SU DBPCFC (BE-R 11.1% passed vs OIT-R 43.5% passed, p=0.009). When comparing the OIT-R and OIT-A groups, results were not statistically significantly different at year 1 (treatment success: p=0.18) or year 2 (treatment success: p=0.15) but were superior for the OIT-R group for the SU DBPCFC (DBPCFC passed in 43.5% OIT-R vs 17.9%, OIT-A, p=0.031).
Table 2:
DBPCFC Outcomes
| Outcome | BE-R (N=27) | OIT-R (N=23) | OIT-A (N=39) | Total (N=89) | P-value3 |
|---|---|---|---|---|---|
| Year 1 Desensitization DBPCFC - n (%) | |||||
| Treatment success | |||||
| Passed DBPCFC | 2 (7.4) | 13 (56.5) | 15 (38.5) | 30 (33.7) | |
| Failed, SCD >= 4444 mg | 6 (22.2) | 4 (17.4) | 7 (17.9) | 17 (19.1) | |
| Treatment failure | |||||
| Failed, SCD < 4444 mg | 15 (55.6) | 3 (13.0) | 11 (28.2) | 29 (32.6) | |
| Did not complete1 | 4 (14.8) | 3 (13.0) | 6 (15.4) | 13 (14.6) | |
| p-value OIT-R vs BE-R2 | 0.0019 | ||||
| p-value OIT-R vs OIT-A2 | 0.1809 | ||||
| Year 2 Desensitization DBPCFC – n (%) | |||||
| Treatment success | |||||
| Passed DBPCFC | 4 (14.8) | 18 (78.3) | 22 (56.4) | 44 (49.4) | |
| Failed, SCD >= 4444 mg | 2 (7.4) | 2 (8.7) | 5 (12.8) | 9 (10.1) | |
| Treatment failure | |||||
| Failed, SCD < 4444 mg | 13 (48.1) | 0 (0.0) | 6 (15.4) | 19 (21.3) | |
| Did not complete | 8 (29.6) | 3 (13.0) | 6 (15.4) | 17 (19.1) | |
| p-value OIT-R vs BE-R2 | <.0001 | ||||
| p-value OIT-R vs OIT-A2 | 0.1507 | ||||
| Year 2 Sustained Unresponsiveness DBPCFC - n (%)4 | |||||
| Passed DBPCFC5 (Treatment success) | 3 (11.1) | 10 (43.5) | 7 (17.9) | 20 (22.5) | |
| Treatment failure | |||||
| Failed DBPCFC | 1 (3.7) | 8 (34.8) | 14 (35.9) | 23 (25.8) | |
| Did not complete | 23 (85.2) | 5 (21.7) | 18 (46.2)6 | 46 (51.7) | |
| p-value OIT-R vs BE-R7 | 0.0093 | ||||
| p-value OIT-R vs OIT-A7 | 0.0308 | ||||
One OIT-A participant had an inconclusive result during the Year 1 DBPCFC and was considered not completing.
P-value was comparing those who were treatment successes to those who were treatment failures.
Barnard’s test was used for comparison between treatment groups.
Only participants passing the Year 2 DBPCFC underwent the SU DBPCFC after withholding BE or OIT treatment for 8–10 weeks.
3 participants did not have an open feeding (1 OIT-R and 1 OIT-A due to site error, 1 OIT-R due to investigator decision due to minor symptoms during DBPCFC), 1 OIT-A participant completed an open feeding but vomited after 1 hour and received antihistamine treatment.
One OIT-A participant passing the year 2 DBPCFC refused the SU DBPCFC.
P-value was comparing those who passed the DBPCFC to those who failed/did not complete the DBPCFC.
Figure 2: Successfully Consumed Dose of Egg White Protein in the DBPCFCs by Treatment Group:
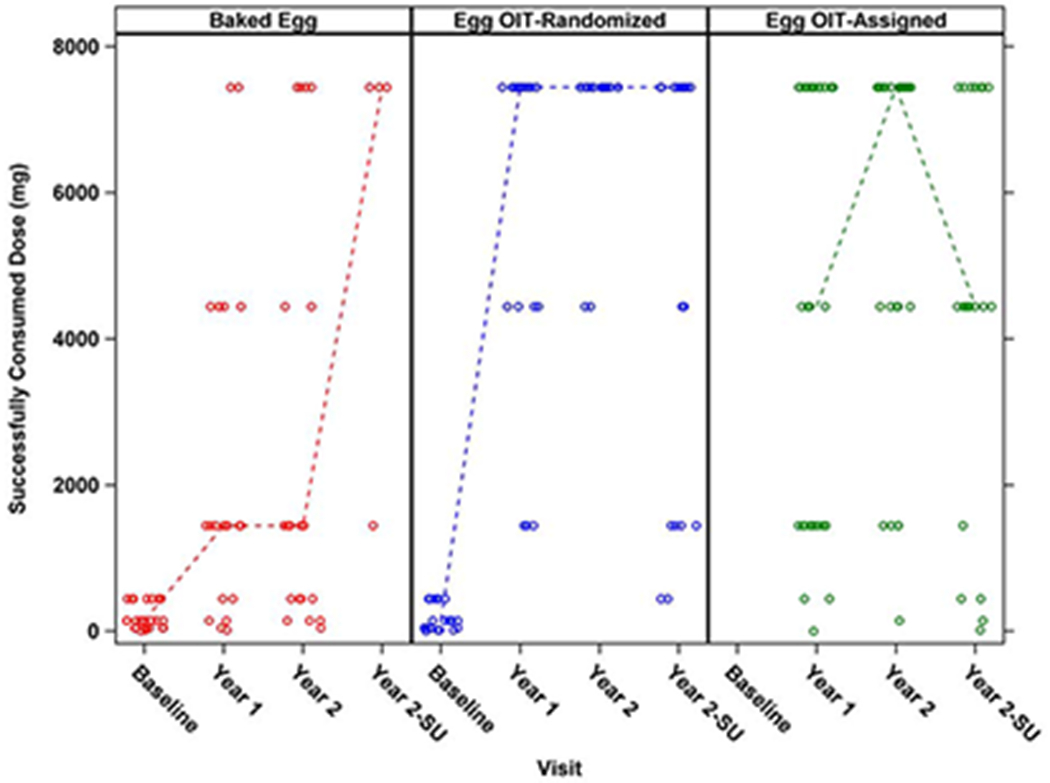
Circles represent individual participants and dotted lines represent median. OIT-A participants failed the baseline BE DBPCFC and did not undergo a baseline unbaked egg DBPCFC. A significantly greater percentage of treatment successes were seen in OIT-R versus BE-R at year 1 and year 2 with treatment success defined as SCD ≥4444 mg egg white protein (p=0.0019, p<0.0001 respectively). A significantly greater percentage of SU was seen in OIT-R versus BE-R with SU defined as passing the year 2 DBPCFC and SU DBPCFC (p=0.0093). No significant difference in treatment successes was seen in OIT-R versus OIT-A at year 1 and year 2. A significantly greater percentage of SU was seen in OIT-R versus OIT-A (p=0.0308).
Similar differences were seen with regard to the SCD in the three DBPCFCs (Figure 2). Because the OIT-A group did not have an egg white DBPCFC at baseline, no change from baseline results could be calculated. The median SCD and median change from baseline in SCD was significantly higher in the OIT-R group compared to the BE-R group at the year 1 (median SCD of 7444 vs 1444 mg protein, p=0.0002; median change in SCD 7000 vs 1300 mg protein, p=0.0001) and year 2 DBPCFCs (median SCD of 7444 vs 1444 mg protein, p<0.0001; median change in SCD 7350 vs 1300 mg protein, p=0.0002) (Supplemental table 2).
Immunologic Outcomes
The OIT-A group had higher baseline levels of EW-sIgE, OVA-sIgE and OVM-sIgE, but these levels decreased to levels similar to those of the other treatment groups over the course of treatment (Figure 3, Supplemental Figure 1). Overall, all groups showed a decrease in EW-sIgE from baseline through the end of treatment (median change from baseline to year 2: BE-R= −8.68 kUA/L, OIT-R= −8.02 kUA/L, OIT-A= −20.01 kUA/L). The decrease was largest in the OIT-A group, however, the change from baseline was not significantly different between the OIT-R and OIT-A groups at any time point (year 2, OIT-R vs OIT-A: p=0.13).
Figure 3: Egg White-Specific IgE Levels by Treatment Group and Visit:
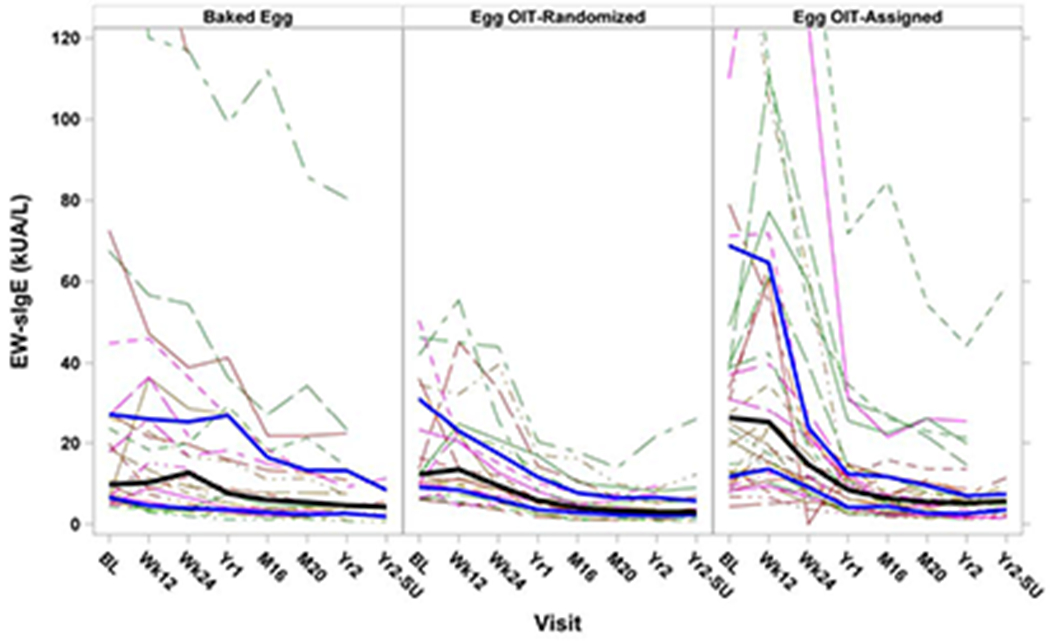
Solid black lines represent median values with the solid blue lines representing the interquartile range; dashed lines represent individual participants. Decreases were seen in egg sIgE from baseline to year 2 in all groups. Changes from baseline were not significant at any time points between BE-R and OIT-R or between OIT-R and OIT-A groups.
IgG4 responses differed significantly between treatment groups over time for log10 egg white-specific IgG4 (EW-sIgG4), log10 ovalbumin-specific IgG4 (OVA-sIgG4), log10 ovomucoid-specific IgG4 (OVM-sIgG4), and log10 ratio EW-sIgG4/EW-sIgE (p<0.0001 for a global treatment effect for all of these IgG4 measurements) (Figure 4, Supplemental Figure 2). In general, the OIT-R and OIT-A groups developed an earlier and larger rise in EW-sIgG4 and egg component IgG4 than BE-R participants.
Figure 4: Egg White-Specific IgG4 Levels by Treatment Group and Visit:
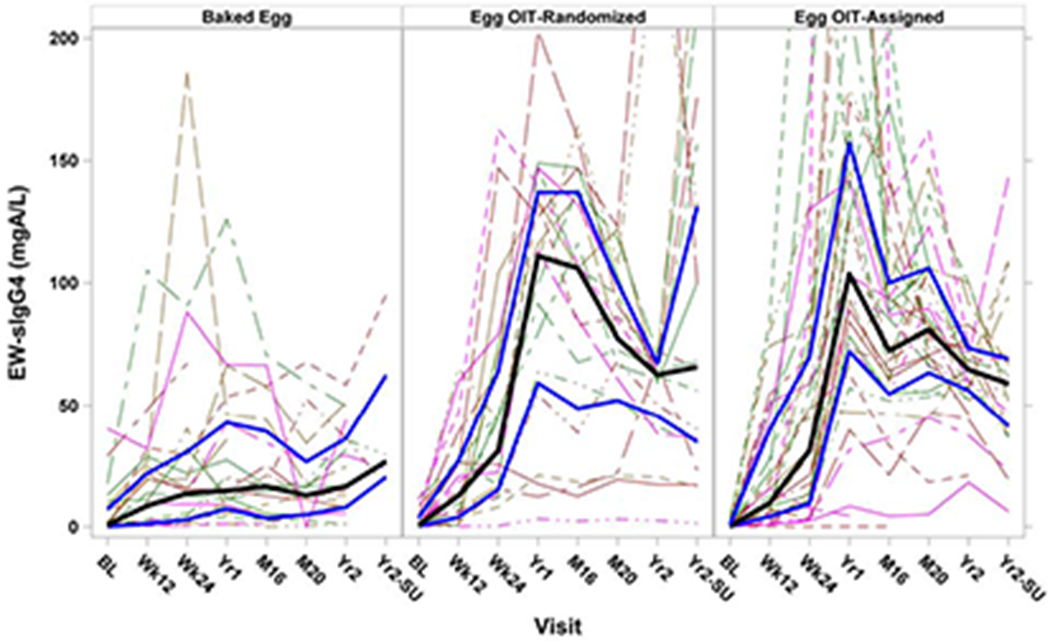
Solid black lines represent median values with the solid blue lines representing the interquartile range; dashed lines represent individual participants. Significant increases were seen in EW-sIgG4 overtime in BE-R versus OIT-R (p<0.0001) and BE-R versus OIT-A (p<0.0001). No significant differences were seen between OIT-R and OIT-A groups.
Egg white SPT results showed median reductions in score from baseline to year 2 of 3.5 mm for BE-R, 5.8 mm for OIT-R, and 8.5 for OIT-A (Supplemental Figure 3). These differences at year 2 were not significantly different between treatment groups (OIT-R vs. BE-R, p=0.16; OIT-R vs. OIT-A, p=0.067).
Basophil activation test results demonstrated no significant differences between treatment groups through year 2 (SU visit not included); however, a global treatment effect over time approached significance for the 0.1 μg egg stimulant (p=0.015) (Supplemental Figure 4).
Safety Outcomes
Overall compliance with treatment was high, although BE-R participants had slightly but significantly lower compliance (89.8%) than the OIT-R and OIT-A groups (95.1% and 95.4%) (Supplemental Table 3). In year 1, the OIT-A participants had more dosing symptoms as well as doses that required treatment than the BE-R or OIT-R participants (Table 3). The median percent of doses per participant with any reaction in year 1 was 2.8% for BE-R, 3.9% for OIT-R, and 12.6% for OIT-A (OIT-A vs BE-R: p=0.0008, OIT-A vs OIT-R: p=0.014) (Table 3), which dropped to less than 1% in all three groups in the second year (data not shown). In year 1, the median percent of doses per participant with oropharyngeal symptoms was 0.8% for BE-R participants compared to 0.3% and 2.2% in the OIT-R and OIT-A participants, respectively. The median percent of doses per participant with symptoms other than oropharyngeal in year 1 was 1.3% for BE-R participants compared to 2.4% of OIT-R participants and 11.4% of OIT-A participants (OIT-A vs BE-R: p<0.0001, OIT-A vs OIT-R: p=0.008) (Table 3). Symptoms were usually mild and only 3 were categorized as severe (one in the OIT-R group and 2 in the OIT-A group) (Supplemental Table 4). Very few reactions required any treatment but 8 required treatment with epinephrine (2 OIT-R and 6 OIT-A) (Supplemental Table 4). After year 1, there were approximately 25,000 doses administered with a lower percentage of doses causing symptoms in all participants, but more in the OIT-A group compared to the BE-R and OIT-R groups (<1% BE-R, <1% OIT-R, 4.2% OIT-A). No symptoms were severe and only 1 reaction in the OIT-A group needed to be treated with epinephrine (Supplemental Table 4). One OIT-A participant reported a serious adverse event (hospitalization due to Pneumonia) that was considered unrelated to study treatment. Three TEAEs related to study treatment occurred in the OIT-R group (mild vomiting, moderate abdominal pain, and a reaction to the egg powder getting in the eye); all other TEAEs were not related (Supplemental Table 5).
Table 3:
Dosing Symptoms By Treatment Group (Percent of Doses per Participant Prior to Year 1 DBPCFC)
| P-value1 | |||||||
|---|---|---|---|---|---|---|---|
| Dosing Symptom [Median (IQR)] | BE-R (N=27) | OIT-R (N=23) | OIT-A (N=39) | Total (N=89) | BE-R vs OIT-R | OIT-R vs OIT-A | OIT-A vs BE-R |
| Number of doses | 328.0 (283.0, 353.0) | 357.0 (339.0, 372.0) | 360.0 (345.0, 369.0) | 353.0 (325.0, 365.0) | 0.0029 | >0.999 | 0.0006 |
| Any reaction | 2.8 (1.1, 7.0) | 3.9 (0.6, 16.6) | 12.6 (3.9, 41.6) | 5.5 (1.9, 18.3) | 0.7202 | 0.0138 | 0.0008 |
| Symptom-free doses | 97.2 (93.0, 98.9) | 96.1 (83.4, 99.4) | 87.4 (58.4, 96.1) | 94.5 (81.7, 98.1) | 0.7202 | 0.0138 | 0.0008 |
| Oropharyngeal symptoms | 0.8 (0, 5.8) | 0.3 (0, 6.4) | 2.2 (0.6, 5.2) | 0.9 (0.3, 5.8) | 0.7301 | 0.0354 | 0.1370 |
| Symptoms other than oropharyngeal | 1.3 (0.3, 2.7) | 2.4 (0.6, 9.9) | 11.4 (2.9, 33.3) | 3.3 (0.8, 14.8) | 0.2071 | 0.0082 | <0.0001 |
| Mild | 1.3 (0.3, 2.7) | 2.4 (0.6, 9.9) | 9.7 (2.9, 33.3) | 2.9 (0.6, 14.3) | 0.1971 | 0.0189 | 0.0002 |
| Moderate | 0.0 (0, 0) | 0.0 (0, 0) | 0.0 (0, 0.6) | 0.0 (0, 0.3) | 0.1572 | 0.0665 | 0.0025 |
| Severe | 0.0 (0, 0) | 0.0 (0, 0) | 0.0 (0, 0) | 0.0 (0, 0) | 0.3019 | 0.8913 | 0.2488 |
| Skin | 0.0 (0, 1.2) | 0.0 (0, 1.3) | 0.5 (0, 1.4) | 0.3 (0, 1.3) | 0.8227 | 0.1179 | 0.1679 |
| GI | 0.0 (0, 0.8) | 0.5 (0, 3.1) | 3.5 (0.5, 22.9) | 0.6 (0, 3.7) | 0.0986 | 0.0070 | <0.0001 |
| Respiratory | 0.0 (0, 0.8) | 0.3 (0, 1.2) | 0.8 (0.3, 3.1) | 0.4 (0, 1.6) | 0.3607 | 0.0582 | 0.0055 |
| Symptoms > 0.5 hours | 0.6 (0, 1.3) | 0.6 (0.3, 2.2) | 1.4 (0.3, 11.8) | 0.8 (0.3, 2.5) | 0.5061 | 0.1217 | 0.0157 |
| Treated | 0.4 (0, 1.2) | 0.5 (0.3, 1.4) | 1.9 (0.6, 4.1) | 0.8 (0.3, 2.9) | 0.7380 | 0.0035 | 0.0042 |
| Treated with oral antihistamines | 0.4 (0, 1.2) | 0.5 (0.3, 1.4) | 1.3 (0.6, 3.8) | 0.6 (0.3, 2.4) | 0.7752 | 0.0081 | 0.0106 |
| Treated with epinephrine2 | 0.0 (0, 0) | 0.0 (0, 0) | 0.0 (0, 0) | 0.0 (0, 0) | 0.1351 | 0.4332 | 0.0391 |
The Wilcoxon Rank Sum Test was used for comparison between treatment groups.
Values below 0.05% were rounded down to 0. OIT-R reported 2/7923 doses = 0.025%; OIT-A reported 6/12305 doses = 0.049%
Study discontinuation due to dosing symptoms occurred for 1/27 (3.7%) in the BE-R group, 2/23 (8.7%) in the OIT-R group, and 6/39 (15.4%) in the OIT-A group. The BE-R participant developed EoE leading to withdrawal while the 2 OIT-R and 1 of the OIT-A participants reported recurrent abdominal pain without a diagnosis of EoE. Five of the OIT-A participants went not able to proceed beyond the initial escalation day and were withdrawn.
Over the course of the study, there were a total of 14 accidental ingestions of egg, 3 in the BE-R, 6 in the OIT-R and 5 in the OIT-A groups respectively. Three episodes were purposeful ingestions of egg-containing foods that were thought to have undercooked egg in them and the remaining episodes were unintended exposures. Three episodes resolved without medication and the remaining 11 episodes required oral antihistamine therapy. One of these episodes, in an OIT-R participant, involved rash, chest tightness and chest pain and after beta-agonist and epinephrine treatment resulted in an emergency department visit with full resolution of symptoms.
Long Term Follow-up
Questionnaires regarding dietary egg consumption were administered 1–3 years after treatment and were completed (Table 4) with a protocol specified end date of September 30, 2018. Differences were observed in time between enrollment and the last LFQ completed between the 3 groups [median (IQR): 48.0 (36.7, 48.5) months for BE-R, 47.7 (36.8, 48.4) months for OIT-R, 50.1 (48.3, 59.6) months for OIT-A] as the OIT-A group accrued more rapidly. At year 3, 14 of 19 (73.7%) OIT-R participants were consuming unbaked egg in their diet compared to 4 of 17 (23.5%) BE-R and 12 of 30 (40%) OIT-A participants (OIT-R vs BE: p=0.003, OIT-R vs. OIT-A: p=0.023). By year 4, 5 of 11 (45.5%) BE-R participants were consuming unbaked egg compared to 7 of 12 (58.3%) OIT-R participants and the difference between these treatment groups was no longer significant (p=0.60). Completion of the questionnaire declined between years 3 and 4 in the BE-R and OIT-R groups (BE-R: year 3: N=17, year 4: N=11; OIT-R: year 3: N=19, year 4: N=12), but did not in the OIT-A group (year 3: N=30, year 4: N=31). The OIT-A group enrolled earlier in the study and completed the year 4 time point prior to the September 2018 data collection cutoff compared to the randomized groups where 5 BE-R and 6 OIT-R participants did not complete the year 4 LFQ due to this cutoff. Of 6 BE-R participants who were not eating unbaked egg at year 3 and 6 OIT-R participants who were eating unbaked egg at year 3 with no data at year 4, 5 in each group did not complete the year 4 LFQ due to this cutoff. There were 2 BE-R participants and 1 OIT-R participant not eating unbaked egg at year 3 who were eating at year 4, and 1 BE-R participant and 2 OIT-R participants eating unbaked egg at year 3 who were no longer eating it at year 4. The number of participants completing the long-term follow-up questionnaire at year 5 was small (BE-R: N=2; OIT-R: N=2; OIT-R: N=14). For participants who achieved SU, 19 of 20 (95.0%) at year 3 and 12 of 16 (75%) at year 4 were consuming unbaked egg in their diet, compared to 11 of 46 (23.9%) and 11 of 38 (28.9%) at year 3 and 4 respectively for those who did not achieve SU (Year 3: p<0.0001, Year 4: p=0.002) (Supplemental Table 6).
Table 4:
Long Term Follow-up of Egg Consumption by Treatment Group
| BE-R (N=27) |
OIT-R (N=23) |
OIT-A (N=39) |
Total (N=89) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yr3 | Yr4 | Yr5 | Yr3 | Yr4 | Yr5 | Yr3 | Yr4 | Yr5 | Yr3 | Yr4 | Yr5 | |
| Total participants completing questionnaire1 – n | 17 | 11 | 2 | 19 | 12 | 2 | 30 | 31 | 14 | 66 | 54 | 18 |
| Fully restricting egg – n (%) | ||||||||||||
| No | 17 (100.0) | 11 (100.0) | 2 (100.0) | 18 (94.7) | 11 (91.7) | 2 (100.0) | 28 (93.3) | 30 (96.8) | 14 (100.0) | 63 (95.5) | 52 (96.3) | 18 (100.0) |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (8.3) | 0 (0.0) | 2 (6.7) | 1 (3.2) | 0 (0.0) | 3 (4.5) | 2 (3.7) | 0 (0.0) |
| Reason not restricting egg – n (%) | ||||||||||||
| Passed a tolerance DBPCFC on study and cleared to eat egg | 3 (17.6) | 3 (27.3) | 2 (100.0) | 10 (52.6) | 6 (50.0) | 1 (50.0) | 7 (23.3) | 7 (22.6) | 4 (28.6) | 20 (30.3) | 16 (29.6) | 7 (38.9) |
| Passed a non-study DBPCFC since the last visit | 0 (0.0) | 1 (9.1) | 0 (0.0) | 4 (21.1) | 1 (8.3) | 0 (0.0) | 8 (26.7) | 9 (29.0) | 4 (28.6) | 12 (18.2) | 11 (20.4) | 4 (22.2) |
| Added egg to the diet on their own | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (6.7) | 2 (6.5) | 1 (7.1) | 2 (3.0) | 2 (3.7) | 1 (5.6) |
| Did not pass DBPCFC but study doctor still added egg to the diet | 14 (82.4) | 7 (63.6) | 0 (0.0) | 4 (21.1) | 4 (33.3) | 1 (50.0) | 11 (36.7) | 12 (38.7) | 5 (35.7) | 29 (43.9) | 23 (42.6) | 6 (33.3) |
| Form of egg in diet – n (%) | ||||||||||||
| Both unbaked & baked egg | 4 (23.5) | 5 (45.5) | 2 (100.0) | 14 (73.7) | 7 (58.3) | 2 (100.0) | 12 (40.0) | 11 (35.5) | 6 (42.9) | 30 (45.5) | 23 (42.6) | 10 (55.6) |
| Unbaked egg only | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Baked egg only | 13 (76.5) | 6 (54.5) | 0 (0.0) | 4 (21.1) | 4 (33.3) | 0 (0.0) | 16 (53.3) | 19 (61.3) | 8 (57.1) | 33 (50.0) | 29 (53.7) | 8 (44.4) |
| Neither unbaked or baked egg | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (8.3) | 0 (0.0) | 2 (6.7) | 1 (3.2) | 0 (0.0) | 3 (4.5) | 2 (3.7) | 0 (0.0) |
| Quantity of egg intake – n (%) | ||||||||||||
| Limited | 6 (35.3) | 9 (81.8) | 1 (50.0) | 7 (36.8) | 5 (41.7) | 2 (100.0) | 18 (60.0) | 17 (54.8) | 11 (78.6) | 31 (47.0) | 31 (57.4) | 14 (77.8) |
| Unlimited | 11 (64.7) | 2 (18.2) | 1 (50.0) | 11 (57.9) | 6 (50.0) | 0 (0.0) | 10 (33.3) | 13 (41.9) | 3 (21.4) | 32 (48.5) | 21 (38.9) | 4 (22.2) |
| None | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (8.3) | 0 (0.0) | 2 (6.7) | 1 (3.2) | 0 (0.0) | 3 (4.5) | 2 (3.7) | 0 (0.0) |
| Reason for limited egg intake – n (%) | ||||||||||||
| History of reactions | 4 (23.5) | 3 (27.3) | 0 (0.0) | 5 (26.3) | 1 (8.3) | 0 (0.0) | 8 (26.7) | 8 (25.8) | 5 (35.7) | 17 (25.8) | 12 (22.2) | 5 (27.8) |
| Anxiety or fear of reactions | 0 (0.0) | 1 (9.1) | 0 (0.0) | 2 (10.5) | 2 (16.7) | 1 (50.0) | 4 (13.3) | 1 (3.2) | 0 (0.0) | 6 (9.1) | 4 (7.4) | 1 (5.6) |
| Taste/preference | 2 (11.8) | 5 (45.5) | 1 (50.0) | 0 (0.0) | 2 (16.7) | 1 (50.0) | 6 (20.0) | 8 (25.8) | 6 (42.9) | 8 (12.1) | 15 (27.8) | 8 (44.4) |
| Eating unbaked egg in diet – n (%) | ||||||||||||
| No | 13 (76.5) | 6 (54.5) | 0 (0.0) | 5 (26.3) | 5 (41.7) | 0 (0.0) | 18 (60.0) | 20 (64.5) | 8 (57.1) | 36 (54.5) | 31 (57.4) | 8 (44.4) |
| Yes | 4 (23.5) | 5 (45.5) | 2 (100.0) | 14 (73.7) | 7 (58.3) | 2 (100.0) | 12 (40.0) | 11 (35.5) | 6 (42.9) | 30 (45.5) | 23 (42.6) | 10 (55.6) |
| Frequency of eating unbaked egg – n (%) | ||||||||||||
| Daily | 1 (5.9) | 0 (0.0) | 0 (0.0) | 5 (26.3) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 0 (0.0) | 7 (10.6) | 0 (0.0) | 0 (0.0) |
| Weekly | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (15.8) | 1 (8.3) | 1 (50.0) | 5 (16.7) | 1 (3.2) | 1 (7.1) | 8 (12.1) | 2 (3.7) | 2 (11.1) |
| Several days/week | 2 (11.8) | 2 (18.2) | 1 (50.0) | 5 (26.3) | 4 (33.3) | 1 (50.0) | 4 (13.3) | 4 (12.9) | 4 (28.6) | 11 (16.7) | 10 (18.5) | 6 (33.3) |
| Monthly | 1 (5.9) | 2 (18.2) | 0 (0.0) | 1 (5.3) | 2 (16.7) | 0 (0.0) | 1 (3.3) | 4 (12.9) | 1 (7.1) | 3 (4.5) | 8 (14.8) | 1 (5.6) |
| Less than monthly | 0 (0.0) | 1 (9.1) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 2 (6.5) | 0 (0.0) | 1 (1.5) | 3 (5.6) | 1 (5.6) |
| None | 13 (76.5) | 6 (54.5) | 0 (0.0) | 5 (26.3) | 5 (41.7) | 0 (0.0) | 18 (60.0) | 20 (64.5) | 8 (57.1) | 36 (54.5) | 31 (57.4) | 8 (44.4) |
| Amount of unbaked egg eaten at one time – n (%) | ||||||||||||
| A few bites | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.2) | 1 (7.1) | 1 (1.5) | 1 (1.9) | 2 (11.1) |
| 1/2 serving | 2 (11.8) | 1 (9.1) | 0 (0.0) | 1 (5.3) | 1 (8.3) | 0 (0.0) | 2 (6.7) | 2 (6.5) | 1 (7.1) | 5 (7.6) | 4 (7.4) | 1 (5.6) |
| Full serving | 1 (5.9) | 2 (18.2) | 0 (0.0) | 11 (57.9) | 6 (50.0) | 2 (100.0) | 7 (23.3) | 8 (25.8) | 3 (21.4) | 19 (28.8) | 16 (29.6) | 5 (27.8) |
| More than a full serving | 1 (5.9) | 2 (18.2) | 1 (50.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 2 (6.7) | 0 (0.0) | 1 (7.1) | 5 (7.6) | 2 (3.7) | 2 (11.1) |
| None | 13 (76.5) | 6 (54.5) | 0 (0.0) | 5 (26.3) | 5 (41.7) | 0 (0.0) | 18 (60.0) | 20 (64.5) | 8 (57.1) | 36 (54.5) | 31 (57.4) | 8 (44.4) |
| Eating baked egg in diet – n (%) | ||||||||||||
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (8.3) | 0 (0.0) | 2 (6.7) | 1 (3.2) | 0 (0.0) | 3 (4.5) | 2 (3.7) | 0 (0.0) |
| Yes | 17 (100.0) | 11 (100.0) | 2 (100.0) | 18 (94.7) | 11 (91.7) | 2 (100.0) | 28 (93.3) | 30 (96.8) | 14 (100.0) | 63 (95.5) | 52 (96.3) | 18 (100.0) |
| Frequency of eating baked egg – n (%) | ||||||||||||
| Daily | 9 (52.9) | 3 (27.3) | 0 (0.0) | 5 (26.3) | 5 (41.7) | 0 (0.0) | 10 (33.3) | 11 (35.5) | 5 (35.7) | 24 (36.4) | 19 (35.2) | 5 (27.8) |
| Weekly | 1 (5.9) | 2 (18.2) | 0 (0.0) | 1 (5.3) | 2 (16.7) | 2 (100.0) | 4 (13.3) | 2 (6.5) | 1 (7.1) | 6 (9.1) | 6 (11.1) | 3 (16.7) |
| Several days/week | 7 (41.2) | 5 (45.5) | 2 (100.0) | 9 (47.4) | 4 (33.3) | 0 (0.0) | 13 (43.3) | 16 (51.6) | 8 (57.1) | 29 (43.9) | 25 (46.3) | 10 (55.6) |
| Monthly | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.2) | 0 (0.0) | 3 (4.5) | 1 (1.9) | 0 (0.0) |
| Less than monthly | 0 (0.0) | 1 (9.1) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.5) | 1 (1.9) | 0 (0.0) |
| None | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (8.3) | 0 (0.0) | 2 (6.7) | 1 (3.2) | 0 (0.0) | 3 (4.5) | 2 (3.7) | 0 (0.0) |
| Amount of baked egg eaten at one time n (%) | ||||||||||||
| A few bites | 0 (0.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (6.7) | 1 (3.2) | 0 (0.0) | 2 (3.0) | 3 (5.6) | 0 (0.0) |
| 1/2 serving | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (15.8) | 2 (16.7) | 0 (0.0) | 2 (6.7) | 3 (9.7) | 3 (21.4) | 5 (7.6) | 5 (9.3) | 3 (16.7) |
| Full serving | 16 (94.1) | 6 (54.5) | 2 (100.0) | 13 (68.4) | 9 (75.0) | 2 (100.0) | 17 (56.7) | 23 (74.2) | 11 (78.6) | 46 (69.7) | 38 (70.4) | 15 (83.3) |
| More than a full serving | 1 (5.9) | 3 (27.3) | 0 (0.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 6 (20.0) | 1 (3.2) | 0 (0.0) | 9 (13.6) | 4 (7.4) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 2 (6.5) | 0 (0.0) | 1 (1.5) | 2 (3.7) | 0 (0.0) |
| None | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (8.3) | 0 (0.0) | 2 (6.7) | 1 (3.2) | 0 (0.0) | 3 (4.5) | 2 (3.7) | 0 (0.0) |
Six participants completed LFQs at Year 2.5, Year 3, and Year 4 post-enrollment; the LFQs from Year 2.5 were collected in error and were not included in the analysis.
Discussion:
Egg allergy has remained a significant public health problem not only due to its physical risks and its effect on quality of life for the patient, but also due to its impact on the family unit, the school systems, the food industry, and public policy. The optimism that most will outgrow the allergy has been tempered by the 5-15 years that many must live with the allergy prior to resolution, and the fact that it persists into adulthood in up to 20% of patients. BE is known to be less allergenic than unbaked egg and is tolerated by the majority of egg-allergic patients making it an attractive option for treatment. However, the immunogenicity of BE and its potential to speed up the natural resolution of egg allergy has not been well studied to date.
Universally accepted markers of natural resolution of egg allergy and tolerance have been lacking. Consequently, our multicenter study of BE therapy versus egg OIT utilized SU for its primary outcome as the broad range of dietary egg ingestion associated with SU and nondependence on daily egg ingestion was thought to suggest a tolerance-like state. After 2 years of treatment in BE tolerant but unbaked egg reactive children, 11% of BE-R participants achieved SU compared to 42% of participants on OIT-R. Furthermore, only 23.5% of BE-R participants versus 73.7% of OIT-R participants introduced unbaked egg into the diet 3 years after study completion. While the efficacy of egg OIT has previously been described13, our data suggest a much smaller effect with BE therapy than was hypothesized.
Compliance was generally strong in both BE-R and OIT-R groups but interestingly 95.1% of doses were taken in the OIT-R group and 95.4% in the OIT-A group versus 89.7% of doses in those receiving the baked egg products, i.e. the BE-R group. Guidelines for OIT dosing including dosing on a full stomach, restriction of exercise, and planning adequate observation time were not required with BE and would have been expected to make OIT compliance more difficult. The lower compliance rate in the BE-R group could be related to the additional effort required to prepare BE products with the proper amount of egg white protein and/or the participants tiring of the BE products offered on a daily basis.
In comparison to BE tolerant participants, SU after egg OIT was achieved in only 17.5% of the OIT-A group that was reactive to both BE and unbaked egg at baseline. Baseline EW-sIgE, OVA-sIgE and OVM-sIgE were lower and baseline EW-sIgG4 and OVM-sIgG4, and baseline EW-sIgG4/EW-sIgE ratio were all significantly greater in the BE tolerant patients compared to those reactive to BE. Whereas symptoms related to dosing, symptoms requiring treatment, and study discontinuation due to dosing symptoms were similar in the BE-R and OIT-R groups that were both tolerant of BE; these AEs were all higher in the OIT-A group that was reactive to BE. These data support the perception that patients tolerant to BE have a distinct, less severe phenotype of egg allergy.
Previous research on BE introduction5, 9 demonstrated an increase in EW-sIgG4 and decrease in egg white SPT with BE ingestion suggesting an immunological effect similar to that which has been seen with OIT with egg and other foods. Our data demonstrated similar findings with decreases in egg white SPT and increases in EW-sIgG4 in addition to decreases in EW-sIgE. While the egg white SPT and EW-sIgE decreases were not statistically different between the BE-R, OIT-R and OIT-A groups, the increase in EW-sIgG4 appeared to be earlier and greater after egg OIT when compared to BE therapy. The clinical relevance of this earlier IgG4 effect is not understood, however, these data support an immunological effect of BE.
Although the current data do not support a strong short-term effect of BE ingestion on the induction of SU to egg, there are additional benefits of BE ingestion that would support the current practice of BE introduction. These benefits include but are not limited to a more flexible diet, improved nutrition, and improved quality of life for the patient and family. However, an important consideration is that patients tolerant to BE but allergic to unbaked egg remain at risk for allergic reactions to accidental exposures to unbaked egg. This raises the question of whether egg OIT, which desensitizes patients to all forms of egg, should be considered not only in patients reactive to all forms of egg, but also in patients tolerant of BE. Compared to BE reactive participants, BE tolerant participants tolerated the egg OIT treatment with significantly less AEs, especially gastrointestinal symptoms, and significantly less need for treating AEs. In addition, the likelihood of achieving SU after egg OIT appeared to be greater in the BE tolerant group. The decision to initiate egg OIT in BE tolerant patients would likely be best considered on a patient by patient basis. Patient and family goals regarding risk reduction and quality of life would need to be considered against the physical risk, financial cost, and time constraints of treatment within shared decision making between the patient and provider.
Strengths of the current study were its multi-center approach at experienced food allergy centers across the United States and the inclusion of a BE reactive group as a comparator to provide insight into the differing responsiveness and treatment tolerance of the BE tolerant and BE reactive phenotypes. A major limitation of the study was the larger than expected number of participants failing the BE OFC which ultimately led to the termination of enrollment prior to reaching the prespecified sample size. The low number of randomized participants could have led the study to be underpowered for the null hypothesis of detecting no difference in SU induction between BE and egg OIT treatments. However, despite the limited sample size, a statistically significant difference was found between the groups demonstrating a higher rate of SU after egg OIT over BE therapy. The study may have been underpowered for secondary outcomes. This high screen fail rate may have been related to the higher baseline median EW-sIgE for the current cohort (BE-R 9.77 kUA/L, OIT-R 12.3 kA/L)) compared to previously reported cohorts by Leonard, et al9 (2.5 kA/L) and Lemon-Mule, et al5 (between 1.3–5.1 kA/L) investigating BE tolerance. The higher titer EW-sIgE of this cohort may suggest a more severe phenotype negatively affecting the final results and limiting the generalizability of the results. Compliance data may have been limited by the difference in accountability in the BE-R versus the OIT groups. In addition to participant home diaries, OIT groups had compliance monitored by actual counts of drug dispensation and used dose returns, whereas BE compliance was only tracked using participant home diaries. Furthermore, a stronger emphasis on compliance may have been impressed upon the OIT groups due to the perceived increased risks of missed doses with OIT. Additional limitations included a lack of racial diversity in the study population and the lack of a placebo group to assess for the natural resolution of egg allergy. The removal of BE from the diet through the use of a placebo was not felt to be ethical and unlikely to be acceptable to patients and their families. Finally, a difference in median age between the BE-R and OIT-R groups was noted. Further analysis did not show a significant correlation between age of enrollment and year 2 DBPCFC results. There was also no association between age and development of SU after adjusting for treatment group. The appropriate amount of time necessary for food immunotherapy to induce tolerance has remained an outstanding question and it is possible that 2 years of treatment was not long enough to observe an effect with BE therapy. In the Leonard, et al. study of BE therapy, a considerable treatment effect for BE compared to avoidance was seen between 2 and 4 years of treatment.9 The immunological changes noted with BE in our study supported a treatment effect that could possibly have become more clinically detectable with longer therapy.
In conclusion, in egg-allergic patients who were tolerant of BE but reactive to unbaked egg, SU was achieved in a limited subset of patients after 2 years of BE immunotherapy. However, egg OIT in BE tolerant participants induced SU in a statistically greater percentage of patients and was associated with an acceptable rate of AEs suggesting a possible role for egg OIT even in patients tolerant of BE.
Supplementary Material
Clinical Implications:
In BE tolerant patients, egg OIT appears superior to BE ingestion for inducing SU. Egg OIT may also be safer and more effective in BE tolerant versus BE reactive children.
Acknowledgements:
The following persons provided physician oversight, study coordination and support: D Bailey, A Bell, M Boguniewicz, C Bronchick, J Fishman, D Fitzgerald, D Fleischer, J French, E Gibson, M Groetch, D Hamilton, P Hauk, L Herlihy, S House, S Leung, A Liu, K Mudd, R Pesek, J Ross, J Slinkard, P Steele, L Talarico, and M Taylor. We thank A Grishin, M Mishoe, G Grishina, and J Grabowska at the Icahn School of Medicine at Mount Sinai for their contributions to mechanistic studies. We thank the staff of the clnical research units at each participating center, and the Statistical and Clinical Coordinating Center and Krisy Peyton, the SACCC Project Manager. We thank J Poyser, Project Manager for the CoFAR Program (NIH/NIAID). The study was designed by the investigators of the CoFAR, with Dr. Sampson as study chair. The data were gathered by the investigators, and managed and analyzed by the Statistical and Clinical Coordinating Center at Emmes. The manuscript was written collaboratively by Dr. Kim and reviewed and edited by the authors. The decision and approval to publish was made by the authors, as investigators in CoFAR, Emmes and the NIH/NIAID leadership.
Sources of support: NIH-NIAID U19AI066738 and U01AI066560. The project was also supported by grant numbers UL1 TR-002535 (National Jewish Health), UL1 TR-000067 (Icahn School of Medicine at Mount Sinai), UL1 TR-003107 (University of Arkansas for Medical Sciences), UL1 TR-000083 (U North Carolina School of Medicine) and UL1 TR-000424 (Johns Hopkins University School of Medicine) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Disclosure statement:
EH Kim reports clinical medical advisory board membership with DBV Technologies; consultancy with Aimmune Therapeutics, DBV Technologies, AllerGenis, Allakos, Ukko, and Vibrant America; and receives grant support to his institution from the National Institute of Allergy and Infectious Diseases (NIH/NIAID), National Center for Complementary and Integrative Health (NIH/NCCIH), FARE and the Wallace Research Foundation.
TT Perry receives grant support to her institution from National Institute of Nursing Research (NIH/NINR); National Heart, Lung, and Blood Institute (NIH/NHLBI); and National Center for Advancing Translational Sciences (NIH/NCATS).
RA Wood reports royalty payments from UpToDate and receives grant support to his institution from the NIH/NIAID, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, and Sanofi.
DYM Leung receives grant support to his institution from the NIH/NIAID, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) and is Chairman of the DSMC for Aimmune Therapeutics.
MC Berin reports scientific advisory board membership with Prota Therapeutics.
AW Burks reports being a minority stock holder in Allertein, Mastcell pharmaceuticals; scientific advisory board membership with Aimmune Therapeutics, Consortia TX Inc, Prota therapeutics; consultancy for DBV technologies, N-fold LLC, kaleo, Ukko Inc; royalties with UpToDate; and receives grant support to his institution from the NIH/NIAID, NIH/NCCIH, FARE and the Wallace Research Foundation.
SM Jones reports research advisory board membership with FARE; reports consultancy with Aimmune Therapeutics and Astellas Pharma Global Development, Inc.; has received grant support to her institution from the NIH/NIAID, Immune Tolerance Network, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, Genentech, and FARE; and has performed CSR review/preparation for DBV Technologies on behalf of Emmes.
AM Scurlock reports clinical medical advisory board membership with DBV Technologies and receives grant support to her institution from NIH/NIAID, Immune Tolerance Network, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, and FARE.
SH Sicherer reports royalty payments from UpToDate and the American Academy of Allergy, Asthma and Immunology, and receives grant support to his institution from the NIH/NIAID and HAL Allergy.
AK Henning is employed by Emmes which received support for this work via grant from the NIH/NIAID through Johns Hopkins University.
P Dawson is employed by Emmes which received support for this work via grant from the NIH/NIAID through Johns Hopkins University.
RW Lindblad is employed by Emmes which received support for this work via grant from the NIH/NIAID through Johns Hopkins University.
HA Sampson reports being a part-time employee of DBV Technologies; receiving consultant fees from N-Fold LLC, and royalties for various textbooks; holding stock options in DBV Technologies and N-FOLD; and receives grant support to his institution from the NIH/NIAID. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations
- AE
Adverse event
- BE
Baked egg
- BE-R
Baked egg-randomized arm
- DBPCFC
Double-blind, placebo-controlled food challenge
- DSMB
Data and safety monitoring board
- EW-sIgE
Egg white-specific IgE
- EW-sIgG4
Egg white-specific IgG4
- IQR
Interquartile range
- OFC
Oral food challenge
- OIT-A
Oral immunotherapy-assigned arm
- OIT-R
Oral immunotherapy-randomized arm
- OIT
Oral immunotherapy
- OVA-sIgE
Ovalbumin-specific IgE
- OVA-sIgG4
Ovalbumin-specific IgG4
- OVM-sIgE
Ovomucoid-specific IgE
- OVM-sIgG4
Ovomucoid-specific IgG4
- SAE
Serious adverse event
- SCD
Successfully consumed dose
- SPT
Skin prick test
- SU
Sustained unresponsiveness
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Tang ML, Mullins RJ. Food allergy: is prevalence increasing? Intern Med J. 2017;47(3):256–61. [DOI] [PubMed] [Google Scholar]
- 2.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–46. [DOI] [PubMed] [Google Scholar]
- 3.Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol. 2017;140(1):145–53 e8. [DOI] [PubMed] [Google Scholar]
- 4.Stensgaard A, Bindslev-Jensen C, Nielsen D, Munch M, DunnGalvin A. Quality of life in childhood, adolescence and adult food allergy: Patient and parent perspectives. Clin Exp Allergy. 2017;47(4):530–9. [DOI] [PubMed] [Google Scholar]
- 5.Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122(5):977–83 e1. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman JA, Huang FR, Sampson HA, Nowak-Wegrzyn A. Outcomes of 100 consecutive open, baked-egg oral food challenges in the allergy office. J Allergy Clin Immunol. 2012;129(6):1682–4 e2. [DOI] [PubMed] [Google Scholar]
- 7.Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133(2):492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters RL, Dharmage SC, Gurrin LC, Koplin JJ, Ponsonby AL, Lowe AJ, et al. The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study. J Allergy Clin Immunol. 2014;133(2):485–91. [DOI] [PubMed] [Google Scholar]
- 9.Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012;130(2):473–80 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak-Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9(3):234–7. [DOI] [PubMed] [Google Scholar]
- 11.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159(4):2026–32. [PubMed] [Google Scholar]
- 12.Miceli Sopo S, Greco M, Cuomo B, Bianchi A, Liotti L, Monaco S, Dello Iacono I. Matrix effect on baked egg tolerance in children with IgE-mediated hen’s egg allergy. Pediatr Allergy Immunol. 2016;27(5):465–70. [DOI] [PubMed] [Google Scholar]
- 13.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367(3):233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA, et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol. 2016;137(4):1117–27 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird JA, Clark A, Dougherty I, Brown LS, Arneson A, Crain M, et al. Baked egg oral immunotherapy desensitizes baked egg allergic children to lightly cooked egg. J Allergy Clin Immunol Pract. 2019;7(2):667–9 e4. [DOI] [PubMed] [Google Scholar]
- 16.Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123(6 Suppl):S365–83. [DOI] [PubMed] [Google Scholar]
- 17.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Tueber SS, Burks AW, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130(6):1260–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


