Abstract
BACKGROUND:
High rates of cerebrovascular disease (CVD) have previously been described in pediatric Human Immunodeficiency Virus (HIV). However, little is known about pediatric CVD in the era of antiretroviral therapy (ART), or about the contribution of CVD to HIV-associated neurocognitive disorders.
METHODS:
We completed a neuroimaging sub-study of the HIV-Associated Neurocognitive Disorders in Zambia study, a prospective cohort study of neurocognitive complications of pediatric HIV. Brain MRI (1.5 T) was acquired for 34 HIV+ children on ART and 17 HIV-exposed uninfected (HEU) children (8-17 years old). Demographics, medical history, neurologic exam, and neuropsychological testing results were also collected. Two neuroradiologists, blinded to HIV status and clinical course read and annotated the scans.
RESULTS:
CVD was identified in 7/34 children with HIV (HIV+ CVD+) and no HEU children (21% vs. 0%, p = 0.05). Three participants had white matter changes suggestive of small vessel disease, four had ischemic infarcts, and two also had evidence of intracranial artery stenosis. Age of ART initiation and exposure to protease inhibitors or efavirenz was not significantly different between children with and without CVD. HIV+ CVD+ children had significantly worse scores on a summary measure of cognition than the HIV+ CVD− group (NPZ8 score −0.57 vs. 0.33, p = 0.04).
CONCLUSIONS:
This study demonstrates high rates of CVD in children with HIV despite treatment with ART, and worse cognitive performance in children with CVD. Longitudinal studies are necessary to determine the mechanisms and incidence of new onset CVD in children with HIV.
Keywords: Cerebrovascular Disease, HIV, Global Health, Pediatric Neurology, Africa
Introduction
Symptomatic stroke affects between 0.1% and 3% of adults [1,2] with Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Syndrome (AIDS) [3,4]. The prevalence of symptomatic stroke in children with perinatally infected HIV is unknown in the era of widespread use of antiretroviral therapy (ART), but in older studies has been reported to be between 1.3 and 2.6% (Hammond et al 2016). Neuroimaging and autopsy studies reveal an even higher incidence of stroke when clinically-silent events are included. These studies report an incidence of stroke between 5% and 29% in adults with HIV [5,6] and between 1.5% and 35% in children with HIV [7,8]. When neuroimaging evidence of clinically-silent cerebrovascular disease (CVD) is considered in addition to stroke, the rates of CVD in children with HIV have been reported as high as 59% [9]. CVD in individuals with HIV includes white matter abnormalities suggestive of small vessel ischemic disease or cerebrovascular insufficiency [10,11], aneurysmal dilatation or stenosis of cerebral vasculature [1,12], and moyamoya-like phenomenon [13], in addition to infarcts. When combination antiretroviral therapy (ART) was first introduced, it was believed that there would be a decrease in stroke burden among individuals with HIV [14]. While ART reduces the risk of stroke caused by opportunistic infections or HIV-associated malignancies, there are data to suggest that the incidence of CVD in individuals with HIV infection has not changed in the ART era [15] and may, in fact, be increasing [16]. This may be because HIV infection of the central nervous system occurs early in the disease [17], prior to initiation of ART, and can be a primary cause of stroke, either through vasculopathy or inflammation [18-21]. In addition, some studies suggest that ART may increase the risk of stroke [22,23], either through neurotoxicity [24] and the metabolic side effects of efavirenz [25], or the metabolic side effects [18,26] and platelet activation [27] of protease inhibitors, although the data here are mixed [28,29]. Few studies have investigated the incidence of CVD among children with HIV in the ART era [21]. To date, only five studies detail cases of CVD among children with HIV in sub-Saharan Africa [4,17,30-32]. The contribution of CVD to HIV-associated neurocognitive disorders has not been formally investigated in children. In a neuroimaging sub-study of the HIV-associated Neurocognitive Disorders in Zambia (HANDZ) study cohort, we noted that 21% of children with HIV (vs. 0% of HIV-exposed uninfected children) had neuroimaging evidence of CVD (Dean et al 2019). In the current study, we evaluate the clinical associations, neurological examination findings, neuropsychological testing results, and neuroimaging findings of these children. In addition, we compare their performance on a battery of neuropsychological tests to both children with HIV who do not have imaging evidence of CVD and to HIV exposed, uninfected (HEU) children. We hypothesized that CVD would be associated with poorer neurocognitive performance.
Methods
Overview:
The HANDZ study is a prospective longitudinal study of cognition in children with HIV infection in Zambia. Protocols and methods were described previously [33]. Briefly, children with HIV between the ages of 8 and 17 were recruited from the HIV clinic at the Pediatric Center of Excellence (PCOE) in Lusaka, Zambia. In order to account for HIV exposure and socioeconomic status, HEU children of the same age range and sociodemographic background were recruited from community health clinics in the same neighborhoods where the HIV-positive children resided. The first 34 children with HIV meeting eligibility criteria for the neuroimaging sub-study were enrolled, followed by 17 age-matched HEU controls.
Inclusion and Exclusion Criteria:
Children with laboratory-confirmed HIV (Western blot or DNA PCR) were enrolled if they had been taking ART for at least one year prior to entering the study. Children in the HEU group were enrolled if they were exposed to, but not infected with HIV in utero. Children in each group were excluded if they had a known history of central nervous system infection, apart from HIV. For the neuroimaging sub-study, children were required to be older than 8 years old, not pregnant, and without medical or psychiatric conditions that would contraindicate a nonsedated MRI scan, including indwelling permanent metal objects or claustrophobia. A comprehensive list of exclusion criteria can be found in our published protocol [33].
Data Collection:
Chart review, standardized questionnaires with the parent(s) and child, physical examination, and standardized neuropsychological tests were completed for each child. Variables extracted from chart review included: past medical history, current and prior CD4 counts, viral loads, WHO stage, and ART regimens. Standardized questionnaires included: the UNICEF Multiple Indicator Cluster Survey 5 adapted for an urban setting (MICS5, http://mics.unicef.org/tools), which evaluates socioeconomic status (SES) based on factors including access to running water and electricity, toilet facilities, food security, parental education, income, and wealth; and the Brief Impairment Scale, which assess the child’s interpersonal, school, and social functioning [34]. At least one standardized comprehensive neurologic exam was conducted by a neurologist or medical student on all children having positive MRI findings. The neurologic exam included an assessment of cranial nerves, motor strength and tone, abnormal movements, reflexes, sensation, gait, coordination, language, attention, behavior, and affect.
Complete details of our neuropsychological testing battery are available in Adams et al., 2019. Briefly, neuropsychological testing was conducted with traditional measures as well as a customized cognition and motor battery utilizing the NIH Toolbox for Assessment of Neurological and Behavioral Function [35], delivered via an iPad-based testing platform. Performance was evaluated across 8 domains (executive function/response inhibition, set shifting, attention/working memory, processing speed, immediate recall, verbal fluency, nonverbal reasoning, and motor speed). Individual domain scores from all children were averaged and used to create an age-and-sex adjusted Z-score (NPZ8 score) for each child as a summary measure of cognition. Performance on both the NIH toolbox and the formal neuropsychological tests in each domain was classified on a 5-point scale (0 = no impairment, 5 = severe impairment) based on standard deviations from normalized scores [36]. In the motor domain, a fine motor laterality index was calculated as the difference in performance on the 9-hole peg-board test (part of the NIH toolbox) with the dominant vs. the non-dominant hand. Finally, a global deficit score (GDS) consisted of the average of the domain scores, with a GDS greater than 0.5 indicative of cognitive impairment [37].
Neuroimaging acquisition and interpretation:
Neuroimaging was performed on a 1.5T Siemens Magnetom Essenza scanner (Erlangen, Germany) located at the Cancer Disease Hospital, in Lusaka, Zambia using a 4-channel head coil. All participants were scanned with the following sequences: 1) 3-plane localizer, 2) whole-brain sagittal three-dimensional (3D) T1 weighted gradient echo sequence, 3) axial T2 and FLAIR sequences, 4) DWI and ADC sequences, and 5) time of flight, non-contrast enhanced Magnetic Resonance Angiography sequences (MRA). Each scan was anonymized and reviewed by two neuroradiologists who were blinded to HIV status and clinical characteristics (including cognitive status) of the child. Consensus was obtained in cases where there was disagreement among the neuroradiologists. Scans were annotated using the Neurointerp system, a database and annotation system used to standardize and quantify MRI findings [38].
Statistical Analysis:
All statistical analyses were performed in R studio. Pairwise deletion was used to deal with missing data. Descriptive statistics included mean and standard deviation (SD) for normally distributed variables and median and interquartile range (IQR) for variables that were not normally distributed. Differences between groups were analyzed with a Fisher exact test for dichotomous variables where the number of observations was less than 10 and a chi square test where the number of observations were greater than 10, a Student’s T test for normally distributed variables, and a Mann- Whitney U test for variables that were not normally distributed. Tests were two-tailed for determining differences in demographics and baseline characteristics between groups (Table 2) and, were one-tailed for determining differences in cognitive abilities between groups (Table 3), based on the hypothesis that cognition should be negatively affected by CVD. The neuroimaging sub-study was powered to detect volumetric differences between HIV+ and HIV- participants; therefore, for the purposes of this analysis, this should be considered a convenience sample.
Table 2:
Differences in demographics, past medical history, and HIV course for HIV-infected children with and without neuroimaging evidence of CVD.
| CVD+ (n = 7) | CVD− (n = 24) | p value | |
|---|---|---|---|
| Demographics | |||
| Age | 13.2 (1.6) | 13.3 (2.4) | 0.85 |
| Sex male | 2 (29%) | 13 (54%) | 0.39 |
| Socioeconomic status index | 6.8 (4.8) | 7.5 (2.5) | 0.77 |
| Health indicators | |||
| Poor health by self-report | 1 (14%) | 2 (9%) | 1.00 |
| History of malnutrition | 1 (14%) | 5 (21%) | 1.00 |
| History of severe malnutrition | 1 (14%) | 2 (8%) | 0.55 |
| Height % | 10 (6-27.5) | 4 (2-15) | 0.24 |
| Weight % | 24 (10-42.5) | 4 (2-12.5) | 0.01** |
| Number of hospitalizations | 1 (1-2) | 1 (1-2) | 0.92 |
| History of malaria | 3 (43%) | 16 (67%) | 0.38 |
| Cerebral malaria | 0 (0%) | 2 (8%) | 1.00 |
| History of TB | 2 (29%) | 9 (38%) | 1.00 |
| Opportunistic infections | 1 (0.5-1) | 0.5 (0-1.3) | 0.70 |
| HIV specific factors | |||
| Age of ART initiation | 5 (4-6) | 4 (2-7) | 0.61 |
| Initiation of ART before 1 yr | 0 (0%) | 3 (13%) | 1.00 |
| ART treatment interrupted | 1 (14%) | 2 (9%) | 1.00 |
| ART adherence issues | 0 (0%) | 3 (13%) | 1.00 |
| Worst WHO stage 4 | 2 (29%) | 11 (46%) | 0.67 |
| Most recent CD4 < 200 | 0 (0%) | 1 (4%) | 1.00 |
| Lowest CD4 | 372.1 (205.3) | 455.3 (210.2) | 0.37 |
| Viral load ever detected | 3 (43%) | 11 (48%) | 1.00 |
| Protease inhibitor any use | 2 (29%) | 13 (54%) | 0.39 |
| Efavirenz any use | 6 (86%) | 14 (58%) | 0.37 |
Table 3:
Differences in cognition for HIV-infected children with and without neuroimaging evidence of CVD and all children with normal neuroimaging. All tests are one-tailed. Motor laterality is the difference in z-normalized peg board scores for the dominant versus the non-dominant hand (positive numbers show a dominant hand advantage).
| HIV+ CVD+ (n = 7) |
HIV+ CVD− (n = 24) |
p value | All CVD− (n = 40) |
p value | |
|---|---|---|---|---|---|
| In school | 6 (100%) | 20 (87%) | 1.00 | 32 (91%) | 1.00 |
| Brief impairment scale school sub-score | 4 (1-5.5) | 1 (0-3) | 0.01** | 1 (0-2.3) | 0.01** |
| Global deficit score | 0.25 (0.13-0.88) | 0.06 (0-0.41) | 0.10 | 0 (0-0.25) | 0.04* |
| Globally impaired | 3 (43%) | 6 (25%) | 0.32 | 7 (18%) | 0.15 |
| NPZ8 | −0.57 (1.04) | 0.13 (1.19) | 0.08 | 0.33 (1.12) | 0.04* |
| Attention/working memory | −0.12 (0.74) | 0.25 (1.08) | 0.16 | 0.40 (1.01) | 0.07 |
| Inhibition | −0.55 (0.78) | −0.11 (0.8) | 0.11 | 0.05 (0.73) | 0.05* |
| Motor | −0.59 (1.11) | −0.25 (0.71) | 0.24 | −0.05 (0.75) | 0.13 |
| Motor laterality | −0.86 (13.97) | 7.00 (12.31) | 0.11 | 4.72 (12.8) | 0.18 |
| Non-verbal reasoning | −0.55 (1.15) | 0.20 (1.26) | 0.08 | 0.25 (1.23) | 0.06 |
| Processing speed | −0.29 (1.09) | −0.17 (0.98) | 0.40 | 0.16 (0.97) | 0.17 |
| Recall | −0.16 (0.91) | 0.19 (1.07) | 0.20 | 0.29 (1.05) | 0.13 |
| Set shifting | −0.50 (1.00) | 0.32 (1.33) | 0.05* | 0.43 (1.31) | 0.03* |
| Verbal fluency | −0.41 (0.60) | 0.30 (1.24) | 0.02* | 0.31 (1.24) | 0.01** |
Results
Demographics
Neuroimaging was obtained on 34 children with HIV (15 male) and 17 HEU controls (10 male) between the ages of 8 and 17 years. The HIV+ children who participated in the neuroimaging sub-study were significantly older (mean = 13.09, SD = 2.4 vs. mean = 12.06, SD = 2.27, p = 0.03), and had higher SES scores (mean = 7.26, SD = 2.98 vs. mean = 5.71, SD = 2.60, p = 0.01), but were no different from the rest of the HIV+ HANDZ cohort in terms of sex, history of malaria or TB, and measures of cognition including the global deficit score and the NPZ8 score. There were significantly more male HEU children (58% vs 46%, p = 0.03) and more HEU children with a history of malaria (71% vs 41%, p = 0.02) who participated in the neuroimaging sub-study compared to the rest of the HEU HANDZ cohort, but the imaged HEU children did not significantly differ from the rest of the HEU HANDZ cohort in terms of age, SES score, history of TB, or measures of cognition including the global deficit score and the NPZ8 score. As reported previously, children with HIV and HEU controls who participated in the neuroimaging sub-study were similar in age, sex, socioeconomic status, and malaria history, but the children with HIV had higher rates of tuberculosis and cognitive impairment and lower NPZ8 scores compared to the HEU controls [39]. All of the children with HIV had been on a stable ART regimen for at least one year, with most children (74%) having been treated with ART for 5 or more years. Neuroimaging evidence of CVD was identified in 7/34 (21%) children with HIV and 0/17 (0%) HEU controls (p = 0.05). Neuroimaging evidence of neurocysticercosis was an incidental finding in 3 children with HIV and 1 HEU control, as previously described [40]. The children with neurocysticercosis were excluded from the current analyses.
Past medical history, abnormal neurologic exam findings, and abnormal neuroimaging findings are reported in Table 1 for the 7 children with HIV who had imaging evidence of CVD (HIV+ CVD+ group). Of note, in the HIV+ CVD+ group, participant 2 had upgoing toes on Babinski testing bilaterally, which was consistent with the subcortical location of the hyperintensities. Participant 5 had mild difficulties on the left with rapid alternating movements and displayed mild satelliting of the left hand on the forearm rolling test, which was consistent with an old infarct in the right MCA territory. Participant 6 had a positive Babinski finding on the left, consistent with an old infarct in the right basal ganglia and corona radiata. Participant 7 had mildly diminished finger strength and mild difficulties with rapid alternating movements on the right, consistent with an old infarct in the left MCA territory. All of these findings were consistent with the laterality of the neuroimaging findings (Figure 1).
Table 1:
Past medical history, neurologic exam findings and imaging findings for HIV-infected children with neuroimaging evidence of CVD.
| ID | Age | Gender | Significant medical history |
Neurological exam findings |
MRI findings | MRA findings |
|---|---|---|---|---|---|---|
| 1 | 13 | F | History of pulmonary tuberculosis and typhoid fever | None/normal | Mild cortical atrophy and an old ischemic lesion in the right cerebellum | Normal |
| 2 | 14 | F | History of syncope | Positive Babinski bilaterally | Bilateral multifocal punctate periventricular and subcortical white matter hyperintensities | Normal |
| 3 | 13 | M | Exposed to alcohol in utero; recurrent fevers | Hypoactive reflexes consistent with HIV-associated peripheral neuropathy | Punctate subcortical white matter foci of hyperintensity in the left greater than right frontal lobes | Normal |
| 4 | 12 | F | Born premature at 30 weeks | None/normal | Multifocal punctate white matter foci of hyperintensity in the frontal lobes bilaterally | Normal |
| 5 | 12 | M | History of learning disability | Mild difficulties with rapid alternating movements on the left side; mild satelliting of the left hand on forearm rolling test. | Small foci of encephalomalacia scattered in the right corona radiata, right putamen, and right frontal lobe, old right MCA territory infarct | Narrowing of the right M1 segment of the MCA and small collaterals from the external carotid artery to the more peripheral MCA territory |
| 6 | 14 | F | History of pulmonary tuberculosis | Positive Babinski on the left | Old infarct in the right basal ganglia and right corona radiata | Normal |
| 7 | 9 | F | History of severe malnutrition; episode of right hemiparesis two years prior that largely resolved within a month. | 4+/5 right finger strength and mild slowing of rapid alternating movements with right hand. | Old infarct in the territory of the left MCA | Stenosis of the left internal carotid artery with small collaterals in this region |
Figure 1:
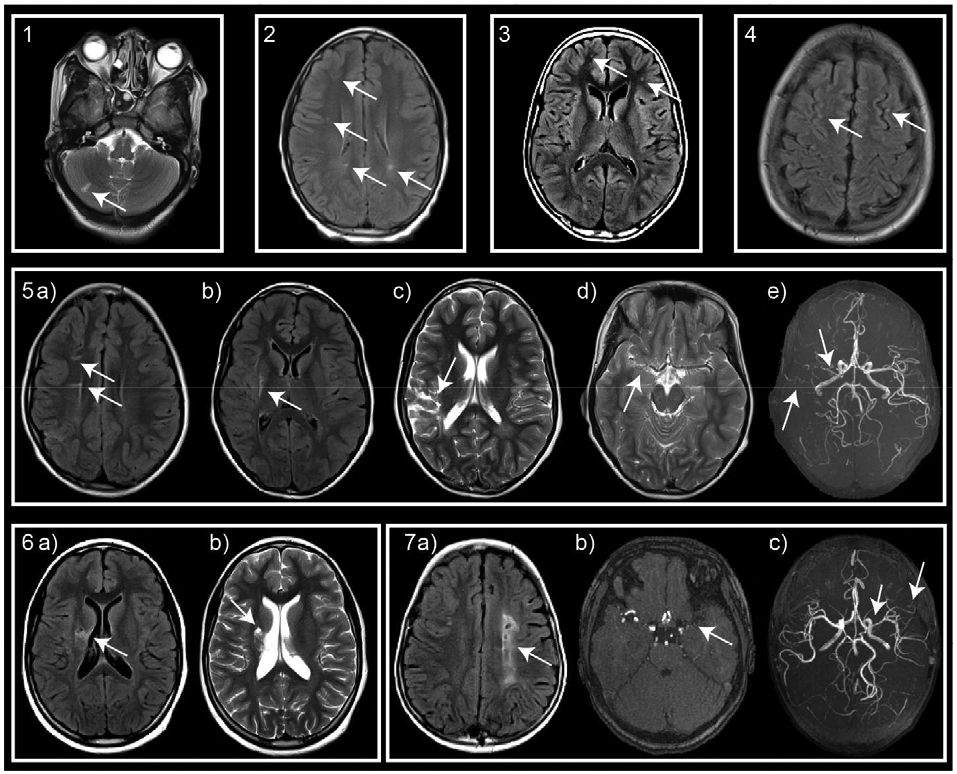
Neuroimaging evidence of CVD in HIV-infected patients. T2, T2 FLAIR, and reconstructed time of flight images demonstrate: participant 1: cerebellar infarct; participants 2-4: small foci of hyperintensities; participant 5: small infarcts in the a) right corona radiata, b) right putamen, c) right MCA territory, and vessel abnormalities consisting of d) high grade narrowing of the right M1 segment. e) MRA confirms M1 stenosis and demonstrates a paucity of distal MCA flow signal and net-like collateralization; participant 6: infarcts in the right basal ganglia and corona radiata; and participant 7: a) extensive infarct in the left centrum semiovale, b) left supraclinoid internal carotid artery stenosis and b,c) proximal M1 stenosis, and c) net-like collaterals in the left distal MCA territory.
Group level analyses
As shown in Table 2, the HIV+ CVD+ children did not significantly differ from the children with HIV who did not have MRI evidence of CVD (HIV+ CVD−) in demographic characteristics, past or current indicators of health, or HIV-specific factors. In particular, there was no significant difference between the two groups in terms of age at initiation of ART (5 vs. 4, p = 0.61) or any exposure to protease inhibitors (29% vs. 54%, p = 0.39) or efavirenz (86% vs. 58%, p = 0.37). The only significant difference between the two groups among these variables was with respect to weight percentile: the HIV+ CVD+ group was, on average, at a higher weight percentile than the HIV+ CVD− group, however, both groups were below the 50th percentile (24th percentile vs. 4th percentile, p = 0.01).
Cognitive abilities
Neuropsychological testing revealed differences between children with and without CVD (Table 3). Compared to both the HIV+ CVD− children and the entire group of CVD− children (HIV+ CVD− and HEU CVD−), the HIV+ CVD+ children scored significantly worse on the school sub-score of the Brief Impairment assessment (BISS; HIV+ CVD+ BISS = 4, HIV+ CVD− BISS = 1, CVD− BISS = 1, p = 0.01 in both comparisons) and also had significantly worse global deficit scores (HIV+ CVD+ GDS = 0.25, HIV+ CVD− GDS = 0.06, p = 0.10, CVD− GDS = 0, p = 0.04). There were numerically more patients in the HIV+ CVD+ group meeting global deficit score criteria for cognitive impairment, although this did not reach the threshold for statistical significance (HIV+ CVD+ 43% vs all CVD− 18%, p = 0.15). HIV+ CVD+ children also had significantly worse NPZ8 scores compared to all CVD− children (−0.57 vs 0.33, p = 0.04). When the tests that contributed to the NPZ8 score were analyzed by their eight cognitive domain sub-scores, HIV+ CVD+ children performed significantly worse in inhibition (−0.55 vs. 0.05, p = 0.05), set shifting (−0.5 vs. 0.43, p = 0.03), and verbal fluency (−0.41 vs. 0.31, p = 0.01) than all of the CVD− children. Compared to the HIV+ CVD− children, the HIV+ CVD+ children performed significantly worse in the set shifting (−0.5 vs. 0.32, p = 0.05) and verbal fluency tasks (−0.41 vs. 0.30, p = 0.02). Excluding the two HIV+ CVD+ children with other possible causes of poor cognitive outcome (fetal alcohol exposure in participant 3 and prematurity in participant 4) did not alter the effect size or statistical significance of any of these findings.
Since the extent and type of CVD varied across children with HIV, we then compared each HIV+ CVD+ child’s performance to the group of HIV+ CVD− children (Table 4). All of the children with old infarcts performed greater than 1 SD below the HIV+ CVD− children in at least one cognitive assessment. The BISS and motor laterality scores for participant 1 were both greater than 2 SDs worse than the mean and. her GDS, motor sub-score, processing speed sub-score, and verbal fluency sub-score were all greater than 1 SD worse than the mean. The motor laterality score for participant 5 was 1 SD worse than the mean. The BISS score for participant 6 was greater than 1 SD worse than the mean, and her motor sub-score was on the border of being abnormal. The BISS, GDS, and motor sub-score for participant 7 were greater than 2 SDs worse than the mean and her NPZ8, attention/working memory, inhibition, non-verbal reasoning, processing speed, recall, and set shifting sub-scores were all greater than 1 SD worse than the mean. In addition, to those with infarcts, the child with the largest burden of white matter abnormalities, participant 2, also scored greater than 2 SDs below the mean on the GDS and non-verbal reasoning and her NPZ8, inhibition, set shifting, and verbal fluency scores were greater than 1 SD worse than the mean.
Table 4:
Individual performance on cognitive testing
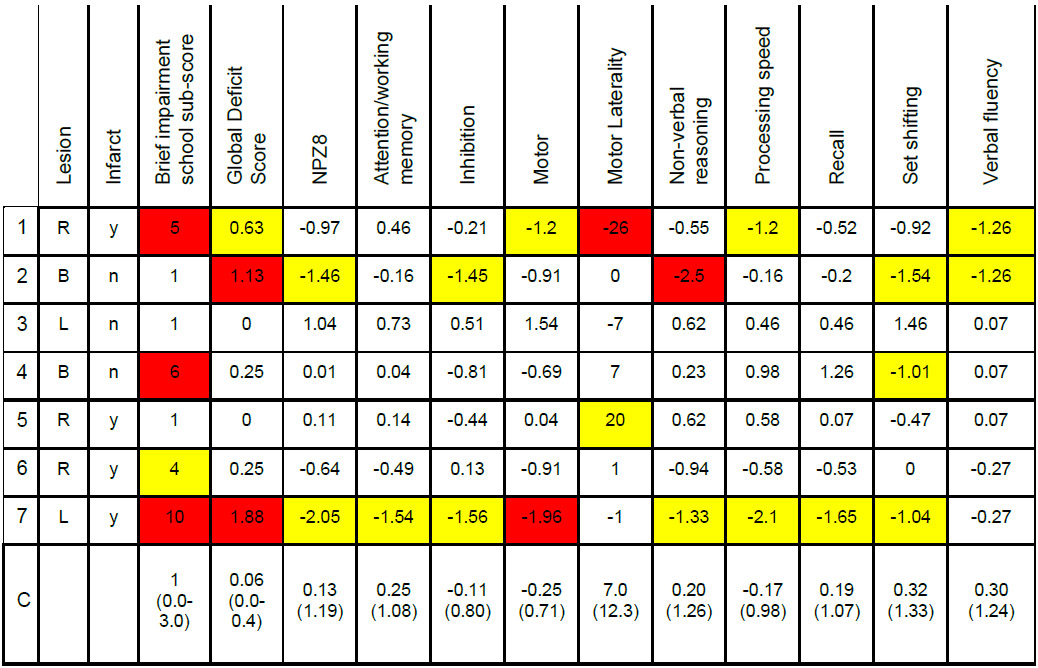 |
Yellow: > 1 SD; Red > 2 SD from the HIV+ CVD− group (row C). Motor laterality is the difference in z-normalized peg board scores for the dominant versus the non-dominant hand (positive numbers show a dominant hand advantage). All individuals were right handed. R: right; L: left; B: bilateral.
Discussion
In this neuroimaging sub-study of an investigation of HIV-associated neurocognitive disorders in Zambian children, we compare the neurological findings and neuropsychological testing results of 7 children with HIV who had MRI evidence of CVD with HIV+ CVD− and HEU controls. Importantly, CVD in these children occurred despite adequate viral suppression and sustained ART treatment. MRI evidence of CVD included infarcts, white matter T2 hyperintensities, large vessel stenosis, and small net-like collaterals. Only one of the children with CVD (patient 4, who was born prematurely) had known risk factors for CVD, and none had risk factors such as hypertension, diabetes, or sickle cell disease (based on clinical history and laboratory studies). Only one of the 7 HIV+ CVD+ children had a clinically identifiable cerebrovascular event, even though infarcts were detected on MRI in 4 of the children. Despite a majority of the CVD cases being clinically-silent, subtle neurologic exam findings were present and formal neuropsychological testing revealed deficits in the HIV+ CVD+ children compared to both the HIV+ CVD− children and the HEU controls. Children with CVD scored lower on indicators of global cognitive functioning (BISS, GDS, NPZ8) compared to children without CVD. When their performance on neuropsychological testing was analyzed by cognitive domain, the children with CVD specifically performed worse on inhibition, set-shifting, and verbal fluency tasks compared to children without CVD. In addition, 3 of the 4 children with infarcts had significantly worse performance in the motor domain (either significant laterality on the peg-board test consistent with the location of the infarct, or poor performance in general). Finally, the child with the largest burden of white matter T2 hyperintensities performed poorly in 4 out of the 8 cognitive domains.
The incidence of CVD in our study cohort is within the range previously reported in the literature among pediatric patients infected with HIV both in the era before [7,8] and after the introduction of combination ART [9,17]. Interestingly, none of the children with HIV in our study had aneurysmal dilatation of the cerebral vessels even though this has commonly been reported as a vascular finding in individuals with HIV [12]. Our findings highlight that CVD is a common complication of HIV among Zambian children, adding to the already recognized problem of stroke in Zambian adults infected with HIV where 24% of stroke patients had HIV compared to a 14% prevalence of HIV in the population [41]. Furthermore, the preponderance of clinically-silent infarcts among the children in our study is in line with the literature [42].
To our knowledge, we are the first to report that the presence of CVD is associated with worse cognitive performance in children perinatally infected with HIV compared to HIV+ CVD− and HEU controls. While the children in the neuroimaging sub-study had higher SES scores than the overall HIV+ HANDZ cohort, higher SES was not protective against cognitive impairment in the HIV+ CVD+ children. Two prior studies reported on cognitive abilities and CVD in children with HIV [4,17]. Govender and colleagues (2011) looked at CVD and administered the Denver II Developmental Screening Test to children with HIV who were between 1 month and 12 years old, however, they did not compare Denver Development scores between those with and without CVD. Ackermann and colleagues (2014) used the Griffiths mental development scales (general quotient, locomotor subscale, and language subscale) in children between 8 months and 4.5 years who acquired HIV perinatally, but did not detect a correlation between white matter disease burden and developmental delays. Their negative finding could be explained by the possibility that cognitive deficits in these children may not emerge until later in childhood, or deficits may not have been evaluable, but could have been detected with tests that were not administered, such as those assessing domains of practical reasoning, hand-eye coordination, or fine motor abilities.
The increased prevalence of CVD in individuals with HIV has been attributed to a variety of factors [21,43]. HIV can directly infect the vessel wall [44] or induce an inflammatory or autoimmune vasculitis [21,43,45]. Either directly or indirectly, the presence of HIV in the cerebral vasculature can cause fibrosis, weakening, or thinning of the artery wall, which increases the risk of aneurysms and/or stroke [18,19]. This process may occur early in the infection, before ART is prescribed [17], or may continue to be an issue in the case of CNS escape from ART [46,47]. In the era of combination ART, opportunistic infections are less likely to be a cause of CVD in HIV patients; however, ART itself is a possible contributor to CVD [22]. For example, one study has reported a 26% increase in the risk of myocardial infarction for every additional year on ART [48].
Limitations and generalizability:
The primary limitations of this study are its relatively small sample size and crosssectional design. As a result of the cross sectional design, we were unable to determine the timing of occurrence of CVD and whether it is static or progressive over time. In addition, we acknowledge that it is possible that the radiologic finding of white matter disease consistent with small vessel ischemic disease is actually secondary to other etiologies such as infection. In particular, both large vessel vasculopathy and small vessel ischemic disease can be secondary to varicella zoster infection (VZV) [49]. VZV vasculopathy may be clinically indistinguishable from HIV vasculopathy, although VZV may be more likely to include MRA evidence of vasculitis [50] or aneurysmal dilation [42,51], neither of which were present in any of the HIV+ children in our study. In addition, none of the HIV+ children in the neuro-imaging sub-study had a clinical history of chicken pox or shingles. However, given that the time of occurrence of CVD was unknown in most of our participants, a lumbar puncture to ascertain the presence of VZV in the cerebrospinal fluid was not possible. Finally, this study was not designed to evaluate the etiology of CVD. Future studies should prospectively determine the contribution of CVD to neurocognitive disorders in children with HIV and over the course of the child’s development. A longitudinal imaging study with initial imaging conducted in early childhood would help determine timing of CVD, whether CVD worsens over time, and whether the burden of CVD is mediated by ART. We would anticipate these findings to be moderately generalizable to other urban locations in Sub-Saharan Africa, but would strongly suspect that contemporary HIV+ populations in high-resource settings would have lower rates of CVD.
The high prevalence of CVD in children with HIV raises concern for the developmental trajectory of these children. The potential contribution of CVD to cognitive impairment suggests that mitigating the risk factors for CVD in these children will play an important role in addressing the issue of HIV-associated neurocognitive disorders in the future. Furthermore, even children who escape cerebrovascular events in childhood may be at increased risk for cerebrovascular events later in life. These findings suggest that stroke risk factor reduction in children with HIV may be particularly important to address in the era of combination ART.
Acknowledgments
Funding Source: This work was supported by the University of Rochester Center for AIDS Research (CFAR), an NIH funded program (P30 AI 78498); by a pilot grant from the McGowan Foundation; and by the University of Rochester Medical Center Office of Medical Education International Research Grant. Preparation of this manuscript was supported, in part, by NIH grant F30EY027988 (C.L.S.)
Research reported in this publication was supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number K23NS117310 and R01NS094037. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schieffelin JS, Williams PL, Djokic D, Anderson JP, Nachman S, Oleske JM, et al. Central nervous system vasculopathy in HIV-infected children enrolled in the pediatric AIDS clinical trials group 219/219C study. J Pediatric Infect Dis Soc 2013; 2:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of Ischemic Stroke Incidence in HIV-Infected and Non-HIV-Infected Patients in a U.S. Health Care System. J Acquir Immune Defic Syndr 2012; 60:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izbudak I, Chalian M, Hutton N, Baskaran V, Jordan L, Siberry GK, et al. Perinatally HIV-infected youth presenting with acute stroke: Progression/evolution of ischemic disease on neuroimaging. J Neuroradiol 2013; 40:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govender R, Eley B, Walker K, Petersen R, Wilmshurst JM. Neurologic and neurobehavioral sequelae in children with human immunodeficiency virus (HIV-1) infection. J Child Neurol 2011; 26:1355–1364. [DOI] [PubMed] [Google Scholar]
- 5.Connor MD, Lammie GA, Bell JE, Warlow CP, Simmonds P, Brettle RD. Cerebral infarction in adult AIDS patients: Observations from the Edinburgh HIV Autopsy Cohort. Stroke 2000; 31:2117–2126. [DOI] [PubMed] [Google Scholar]
- 6.Mizusawa H, Hirano A, Llena JF, Shintaku M. Cerebrovascular lesions in acquired immune deficiency syndrome (AIDS). Acta Neuropathol 1988; 76:451–457. [DOI] [PubMed] [Google Scholar]
- 7.Philippet P, Blanche S, Sebag G, Rodesch G, Griscelli C, Tardieu M. Stroke and cerebral infarcts in children infected with human immunodeficiency virus. Arch Pediatr Adolesc Med 1994; 148:965–70. [DOI] [PubMed] [Google Scholar]
- 8.Kure K, Llena JF, Lyman WD, Soeiro R, Weidenheim KM, Hirano A, et al. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Hum Pathol 1991; 22:700–10. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Caan MWA, Mutsaerts HJ, Scherpbier HJ, Kuijpers TW, Reiss P, et al. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology 2016; 86:19–27. [DOI] [PubMed] [Google Scholar]
- 10.Blokhuis C, Mutsaerts HJMM, Cohen S, Scherpbier HJ, Caan MWA, Majoie CBLM, et al. Higher subcortical and white matter cerebral blood flow in perinatally HIV-infected children. Medicine (Baltimore) 2017; 96:e5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brilla R, Nabavi DG, Schulte-Altedorneburg G, Kemény V, Reichelt D, Evers S, et al. Cerebral vasculopathy in HIV infection revealed by transcranial doppler: A pilot study. Stroke 1999; 30:811–813. [DOI] [PubMed] [Google Scholar]
- 12.Baeesa SS, Bakhaidar M, Almekhlafi MA, Madani TA. Human Immunodeficiency Virus-Associated Cerebral Aneurysmal Vasculopathy: A Systematic Review. World Neurosurg 2016; 87:220–229. [DOI] [PubMed] [Google Scholar]
- 13.Hammond CK, Shapson-Coe A, Govender R, Van Toorn R, Ndondo A, Wieselthaler N, et al. Moyamoya Syndrome in South African Children with HIV-1 Infection. J Child Neurol 2016; 31:1010–1017. [DOI] [PubMed] [Google Scholar]
- 14.Johann-Liang R, Lin K, Cervia J, Stavola J, Noel G. Neuroimaging findings in children perinatally infected with the human immunodeficiency virus. Pediatr Infect Dis J 1998; 17:753–754. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, et al. Risk of cerebrovascular events in persons with and without HIV: A Danish nationwide population-based cohort study. Aids 2011; 25:1637–1646. [DOI] [PubMed] [Google Scholar]
- 16.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology 2011; 76:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermann C, Andronikou S, Laughton B, Kidd M, Dobbels E, Innes S, et al. White matter signal abnormalities in children with suspected HIV-related neurologic disease on early combination antiretroviral therapy. Pediatr Infect Dis J 2014; 33:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Biagio A, Rosso R, Maggi P, Mazzei D, Bernardini C, Nulvesu L, et al. Inflammation Markers Correlate with Common Carotid Intima-Media Thickness in Patients Perinatally Infected with Human Immunodeficiency Virus 1. J Ultrasound Med 2013; 32:763–768. [DOI] [PubMed] [Google Scholar]
- 19.Ross AC, Storer N, Ann O’Riordan M, Dogra V, McComsey GA. Longitudinal changes in carotid intima-media thickness and cadiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J 2010; 29:634–638. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez J, Glenn M, Isaacson RS, Marr AD, Mash D, Petito C. Thinning of the arterial media layer as a possible preclinical stage in HIV vasculopathy: A pilot study. Stroke 2012; 43:1156–1158. [DOI] [PubMed] [Google Scholar]
- 21.Hammond CK, Eley B, Wieselthaler N, Ndondo A, Wilmshurst JM. Cerebrovascular disease in children with HIV-1 infection. Dev Med Child Neurol 2016; 58:452–460. [DOI] [PubMed] [Google Scholar]
- 22.D’Arminio A, Sabin C, Phillips A, Reiss P, Weber R, Kirk O, et al. Cardio- and cerebrovascular events in HIV-infected persons. Aids 2004; 18:1811–1817. [DOI] [PubMed] [Google Scholar]
- 23.Thakur KT, Boubour A, Saylor D, Das M, Bearden DR, Birbeck GL. Global HIV neurology: a comprehensive review. AIDS 2019; 33:163–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: A systematic review. Expert Opin Drug Saf 2013; 12:841–846. [DOI] [PubMed] [Google Scholar]
- 25.Tripathi A, Liese AD, Winniford MD, Jerrell JM, Albrecht H, Rizvi AA, et al. Impact of clinical and therapeutic factors on incident cardiovascular and cerebrovascular events in a population-based cohort of HIV-infected and non-HIV-infected adults. Clin Cardiol 2014; 37:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsen S, Dolan SE, Fitch K V., Kanter JR, Hemphill LC, Connelly JM, et al. Carotid intimal medial thickness in human immunodeficiency virus-infected women: Effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab 2006; 91:4916–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurence J, Elhadad S, Ahamed J. HIV-associated cardiovascular disease: Importance of platelet activation and cardiac fibrosis in the setting of specific antiretroviral therapies. Open Hear 2018; 5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med 2003; 348:702–710. [DOI] [PubMed] [Google Scholar]
- 29.Ances BM, Bhatt A, Vaida F, Rosario D, Alexander T, Marquie-Beck J, et al. Role of metabolic syndrome components in human immunodeficiency virusassociated stroke. J Neurovirol 2009; 15:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariam AG, Assefa G. Clinical and neuroimaging profile of HIV-1 Encephalopathy in infancy and childhood in a sub-Saharan African Country. Ethiop Med J 2012; 50:337–347. [PubMed] [Google Scholar]
- 31.Alemayehu T, Moges A, Mekuanint A. Ischemic stroke and disseminated tuberculosis in a child living with human immunodeficiency virus: a case report and review of the literature. J Med Case Rep 2019; 13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilmshurst JM, Burgess J, Hartley P, Eley B. Specific Neurologic Complications of Human Immunodeficiency Virus Type 1 (HIV-1) Infection in Children. J Child Neurol 2006; 21:788–794. [DOI] [PubMed] [Google Scholar]
- 33.Adams HR, Mwanza-Kabaghe S, Mbewe EG, Kabundula PP, Potchen MJ, Maggirwar S, et al. The HIV-Associated Neurocognitive Disorders in Zambia (HANDZ) Study: Protocol of a Research Program in Pediatric HIV in Sub- Saharan Africa. medRxiv 2019; :19003590. [Google Scholar]
- 34.Bird HR, Canino GJ, Davies M, Ramírez R, Chávez L, Duarte C, et al. The Brief Impairment Scale (BIS): A multidimensional scale of functional impairment for children and adolescents. J Am Acad Child Adolesc Psychiatry 2005; 44:699–707. [DOI] [PubMed] [Google Scholar]
- 35.Gershon RC, Wagster M V., Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013; 80:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackstone K, Moore DJ, Franklin DR Jr, Clifford DB, Collier AC, Marra CM, et al. Defining Neurocognitive Impairment in HIV: Deficit Scores versus Clinical Ratings Diego Joint Doctoral Program in Clinical Psychology. Clin Neuropsychol Author Manuscr Clin Neuropsychol 2012; 26694479:619–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: Charter Study. Neurology 2010; 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potchen MJ, Kampondeni SD, Ibrahim K, Bonner J, Seydel KB, Taylor TE, et al. Neurointerp: A method for facilitating neuroimaging research on cerebral malaria. Neurology 2013; 81:585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dean O, Buda A, Adams HR, Mwanza-kabaghe S. Brain Magnetic Resonance Imaging Findings Associated with Cognitive Impairment in Children and Adolescents with Human Immunodeficiency Virus in Zambia. Pediatr Neurol 2019; :1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buda A, Dean O, Adams HR, Mwanza-Kabaghe S, Potchen MJ, Mbewe EG, et al. Neurocysticercosis Among Zambian Children and Adolescents With Human Immunodeficiency Virus: A Geographic Information Systems Approach. Pediatr Neurol 2019; :1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atadzhanov M Stroke Characteristics and Outcomes of Adult Patients Admitted to the University Teaching Hospital, Lusaka, Zambia. Open Gen Intern Med J 2012; 5:3–8. [Google Scholar]
- 42.Patsalides AD, Wood L V., Atac GK, Sandifer E, Butman JA, Patronas NJ. Cerebrovascular disease in HIV-infected pediatric patients: Neuroimaging findings. Am J Roentgenol 2002; 179:999–1003. [DOI] [PubMed] [Google Scholar]
- 43.Singer EJ, Valdes Sueiras M, Commins DL, Yong W, Carlson M. HIV stroke risk: Evidence and implications. Ther Adv Chronic Dis 2013; 4:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park YD, Belman AL, Kim TS, Kure K, Llena JF, Lantos G, et al. Stroke in pediatric acquired immunodeficiency syndrome. Ann Neurol 1990; 28:303–11. [DOI] [PubMed] [Google Scholar]
- 45.Legido A, Lischner HW, de Chadarevian JP, Katsetos CD. Stroke in pediatric HIV infection. Pediatr Neurol 1999; 21:588. [DOI] [PubMed] [Google Scholar]
- 46.Joseph J, Cinque P, Colosi D, Dravid A, Ene L, Fox H, et al. Highlights of the Global HIV-1 CSF Escape Consortium Meeting, 9 June 2016, Bethesda, MD, USA. J virus Erad 2016; 2:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kugathasan R, Collier DA, Haddow LJ, El Bouzidi K, Edwards SG, Cartledge JD, et al. Diffuse white matter signal abnormalities on magnetic resonance imaging are associated with human immunodeficiency virus type 1 viral escape in the central nervous system among patients with neurological symptoms. Clin Infect Dis 2017; 64:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DAD Study Group. Combination Antiretroviral Therapy and the Risk of Myocardial Infarction. N Engl J Med 2003; 349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 49.Nagel M, Cohers R, Mahalingam R, Wellish M, Forghani B, Schiller A, et al. The varicella zoster virus vasculopathies: Clinical, CSF, imaging, and virologic features. Neurology 2008; 70:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frank Y, Lim W, Kahn E, Farmer P, Gorey M, Pahwa S. Multiple ischemic infarcts in a child with AIDS, varicella zoster infection, and cerebral vasculitis. Pediatr Neurol 1989; 5:64–67. [DOI] [PubMed] [Google Scholar]
- 51.Dubrovsky T, Curless R, Scott G, Chaneles M, Post MJD, Altman N, et al. Cerebral aneurysmal arteriopathy in childhood AIDS. Neurology 1998; 51:560–565. [DOI] [PubMed] [Google Scholar]


