Abstract
Isoquinoline alkaloids, an important class of N-based heterocyclic compounds, have attracted considerable attention from researchers worldwide since the early 19th century. Over the past 200 years, many compounds from this class were isolated, and most of them and their analogs possess various bioactivities. In this review, we survey the updated literature on bioactive alkaloids and highlight research achievements of this alkaloid class during the period of 2014–2018. We reviewed over 400 molecules with a broad range of bioactivities, including antitumor, antidiabetic and its complications, antibacterial, antifungal, antiviral, antiparasitic, insecticidal, anti-inflammatory, antioxidant, neuroprotective, and other activities. This review should provide new indications or directions for the discovery of new and better drugs from the original naturally occurring isoquinoline alkaloids.
Keywords: isoquinoline alkaloids, biological activities, berberine, antitumor
1. Introduction
Isoquinoline alkaloids, an important class of N-heterocyclic bioactive natural products, are common throughout the plant kingdom1. They are likely derived from tyrosine or phenylalanine building blocks and show a wide range of structural diversity2. Since the first bioactive isoquinoline alkaloid, morphine, was isolated from the opium plant in the early 19th century3, this compound class has attracted considerable scientific attention. Increasing numbers of isoquinoline alkaloids have been isolated and identified from natural sources, and various studies have reported their antitumor, antimalarial, antibacterial, antifungal, antiparasitic and insecticidal, antiviral, anti-inflammatory, antiplatelet and other activities4-12. As lead compounds in the drug discovery and development process, isoquinoline alkaloids have high probabilities of success,13 as reflected by several revolutionary drugs, such as the analgesic morphine, the antibacterial berberine, the antitussive codeine14, the antirheumatic sinomenine15, and the acetylcholinesterase inhibitor galanthamine16 (Figure 1). Therefore, the search for novel isoquinolines as promising drug leads remains an active area of study in natural product chemistry.
Figure 1.
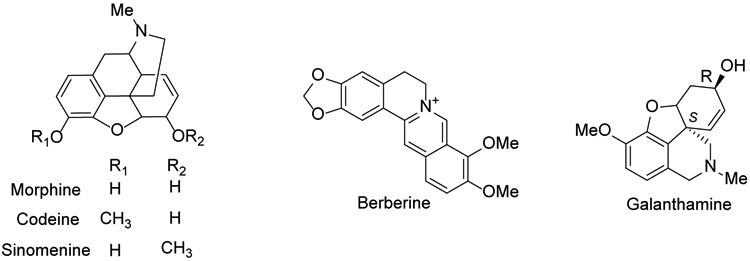
The chemical structures of five major isoquinoline molecules
In view of the importance and significant biological activities of isoquinoline alkaloid natural products, several thousand publications (journal articles, books and patents) on isoquinoline alkaloids have been recorded over the past 200 years. The increasing numbers of publications reflect the research intensity and importance of this field, as well as the bright prospect for drug development from these compounds. Some excellent earlier reviews on the chemical structures and biological properties of isolated isoquinoline alkaloids have contributed significantly to the general scientific understanding of this kind of compounds5,6,8,9,10,11,12,17-21. However, during the past five years, significant studies and novel technologies, such as metabolomics, were widely reported and used to identify alkaloids from plants. Many new compounds were isolated, and novel pharmacological activities and comprehensive mechanism of actions were investigated by researchers worldwide. Hence, a more comprehensive and up-to-date review is merited. Therefore, this review combines newer literature reports as well as presents the developments in this field particularly from the perspective of biological activities. It covers not only the chemical structures of isolated isoquinoline alkaloids (Table 1), but also their biological activities and mechanism of actions. We hope that this review will provide new indications or directions for the development of these compounds as new clinically useful therapeutic agents.
Table 1.
Isolated isoquinoline alkaloids between 2014 to 2018
| No. | Names | Species | Year | Ref. |
|---|---|---|---|---|
| Simple isoquinoline alkaloids | ||||
| 1 | 3,8-Diolisoquinoline | Scolopendra subspinipes mutilans | 22 | |
| 2 | 1-Methoxy-4,5-diolisoquinoline | Scolopendra subspinipes mutilans | 22 | |
| 3 | 1,5-Dihydroxy-4-methoxyisoquinoline | Centipede species | 23 | |
| 4 | Carnegine | Hammada scoparia | 24 | |
| 5 | N-Methylisosalsoline | Hammada scoparia | 24 | |
| 6 | N-Methylcorydaldine |
Fumaria officinalis Michelia champaca |
25 27 |
|
| 7 | 6,7-Dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid | Mucuna pruriens | 26 | |
| 8 | 7-Methoxy-1,2,3,4-tetrahydroisoquinolin-1-one, thalifoline | Michelia champaca | 27 | |
| 9 | Thalifoline |
Corydalis tomentella Plumula nelumbinis |
27-30 | |
| 10 | Corydaldine |
Corydalis tomentella
Corydalis hendersonii |
27-30 | |
| 11 | Oxohydrastinine | Corydalis tomentella | 28 | |
| 12 | 6,7-Methylenedioxy-1(2H)-isoquinolinone | Corydalis tomentella | 28 | |
| Corydalis hendersonii | 29 | |||
| 13 | Oxyhydrastinine | Corydalis hendersonii | 29 | |
| 14 | 6,7-Dihydroxy-1-methyl-3,4-dihydroisoquinolone, | Portulaca oleracea | 31 | |
| 15 | (S)-(−)-Salsolinol | Portulaca oleracea | 31 | |
| 16 | 6,7-Dihydroxy-3,4-dihydroisoquinolone | Portulaca oleracea | 31 | |
| 17 | (R)-(+)-1-Isobutyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline | Portulaca oleracea | 31 | |
| 18 | Ealaine A | Ancistrocladus ealaensis | 32 | |
| 19 | Ealaine B | Ancistrocladus ealaensis | 32 | |
| 20 | Ealaine C | Ancistrocladus ealaensis | 32 | |
| 21 | Ealaine D | Ancistrocladus ealaensis | 32 | |
| 22 | Noroxyhydrastinine | Phellodendron amurense | 33 | |
| Benzylisoquinoline alkaloids | ||||
| 23 | Reticuline |
Litsea cubeba Cryptocarya densiflora, Cryptocarya infectoria, Cryptocarya griffithiana Unonopsis floribunda Dehaasia longipedicellata Bocageopsis pleiosperma |
34 35 36 37 38 39 |
|
| 24 | (+)-N-Methylisococlaurine |
Cryptocarya species
Plumula nelumbinis |
37 30 |
|
| 25 | (−)-N-Methylcoclaurine |
Sinomenium acutum Plumula nelumbinis |
40 30 |
|
| 26 | Berbithine | Coptis chinensis | 41 | |
| 27 | 6-([1,3]Dioxolo[4,5-g]isoquinoline-5-carbonyl)-2,3-dimethoxybenzoic acid methyl ester | Coptis chinensis | 41 | |
| 28 | Norcolaurine-4′-O-glucoside | Plumula nelumbinis | 30 | |
| 29 | N-Methylhigenamine | Plumula nelumbinis | 30 | |
| 30 | Norcoclaurine-6-O-glucoside | Plumula nelumbinis | 30 | |
| 31 | Norcoclaurine | Plumula nelumbinis | 30 | |
| 32 | Argemexirine | Plumula nelumbinis | 30 | |
| 33 | Lotusine | Plumula nelumbinis | 30 | |
| 34 | Isococlaurine | Plumula nelumbinis | 30 | |
| 35 | Armepavine | Plumula nelumbinis | 30 | |
| 36 | 6-Demethy-4′-methyl-N-methylcoclaurine | Plumula nelumbinis | 30 | |
| 37 | Coclaurine | Plumula nelumbinis | 30 | |
| 38 | N-Nor-O-methylarmepavine | Plumula nelumbinis | 30 | |
| 39 | Isococlaurine-5′-O-pentoside | Plumula nelumbinis | 30 | |
| 40 | Coclaurine-5′-O-pentoside | Plumula nelumbinis | 30 | |
| 41 | Juzirine | Leonurus japonicus | 42 | |
| 42 | (R)-(+)-1-Benzyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline | Portulaca oleracea | 31 | |
| 43 | Laudanosine | Thalictrum cirrhosum | 31 | |
| 44 | Pseudolaudanine | Thalictrum cirrhosum | 43 | |
| 45 | Rugosinone | Thalictrum cirrhosum | 43 | |
| 46 | Hendersine B methyl ester | Corydalis tomentella | 28 | |
| 47 | Bicucullinine | Corydalis tomentella | 28 | |
| 48 | Hendersine B | Corydalis tomentella | 28 | |
| 49 | 6,6′,7′,12-Tetramethoxy-5′-hydroxy-2,2′-dimethyloxycanthan | Thalictrum foliolosum | 44 | |
| 50 | 6,5′,6′,7′,12-Pentamethoxy-2,2′-dimethoxyethane | Thalictrum foliolosum | 44 | |
| 51 | Hernandezine | Thalictrum flavum | 45 | |
| 52 | 6,7,12-Trimethoxy-2-methyl-13-hydroxy-11-(4′-formylphenoxy) benzylisoquinoline | Thalictrum wangii | 46 | |
| 53 | 5,6-(Methylenedioxy)-7,12-dimethoxy-2-methyl-10-(4′-formylphenoxy) benzylisoquinoline | Thalictrum wangii | 46 | |
| 54 | Tiliamosine | Thalictrum racemosa | 47 | |
| 55 | (−)-Pseudocurine | Stephania abyssinica | 48 | |
| 56 | (−)-Pseudoisocurine | Stephania abyssinica | 48 | |
| 57 | Tetrandrine | Stephania tetrandra | 49 | |
| 58 | Tangchinoline | Stephania tetrandra | 49 | |
| 59 | (−)-O-O-Dimethylgrisabine | Dehaasia longipedicellata | 38 | |
| 60 | Berbamine | Mahonia aquifolium | 50 | |
| 61 | Neferine |
Nelumbo nucifera Plumula nelumbinis |
59 30 |
|
| 62 | Liensinine | Plumula nelumbinis | 30 | |
| 63 | Isoliensinine | Plumula nelumbinis | 30 | |
| 64 | Norisoliensinine | Plumula nelumbinis | 30 | |
| 65 | 6-Hydroxynorisoliensinine | Plumula nelumbinis | 30 | |
| 66 | (−)-Gyrolidine | Alseodaphne corneri | 60 | |
| 67 | (+)-O-Methyllimacusine | Alseodaphne corneri | 60 | |
| 68 | (+)-2-Norobaberine | Alseodaphne corneri | 60 | |
| 69 | Norstephasubine | Alseodaphne corneri | 60 | |
| 70 | (+)-Stephasubine | Alseodaphne corneri | 60 | |
| 71 | Coptichic aldehyde |
Coptidis Rhizoma- Euodiae Fructus couple |
61 | |
| 72 | Fumaranine | Fumaria officinalis | 24 | |
| 73 | (−)-Fumaricine | Fumaria officinalis | 24 | |
| 74 | (+)-Dihydrofumariline | Fumaria officinalis | 24 | |
| 75 | (−)-Fumaritine | Fumaria officinalis | 24 | |
| 76 | (−)-O-Methylfumarophycine | Fumaria officinalis | 24 | |
| 77 | (−)-Fumarophycine | Fumaria officinalis | 24 | |
| 78 | (+)-Fumariline | Fumaria officinalis | 24 | |
| 79 | (+)-Parfumidine | Fumaria officinalis | 24 | |
| 80 | (+)-Parfumine | Fumaria officinalis | 24 | |
| 81 | Hendersine C | Corydalis hendersonii | 29 | |
| 82 | Hendersine D | Corydalis hendersonii | 29 | |
| 83 | Hendersine E | Corydalis hendersonii | 29 | |
| 84 | Hendersine F | Corydalis hendersonii | 29 | |
| Aporphine alkaloids | ||||
| 85 | Boldine |
Litsea cubeba
Dehaasia longipedicellata |
67
60, 38 |
|
| 86 | (−)-Norboldine | Dehaasia longipedicellata | 38 | |
| 87 | (+)-Laurotetanine | Alseodaphne corneri | 68 | |
|
Cryptocarya densiflora,
Cryptocarya infectoria Cryptocarya griffithiana Bocageopsis pleiosperma |
37 39 |
|||
| 88 | (+)-Nornantenine |
Cryptocarya densiflora,
Cryptocarya infectoria Cryptocarya griffithiana |
37 | |
| 89 | (+)-N-Methyllaurotetanine |
Cryptocarya densiflora,
Cryptocarya infectoria Cryptocarya griffithiana Thalictrum cirrhosum Bocageopsis pleiosperma |
37 43 39 |
|
| 90 | Corydine | Croton echinocarpus | 69 | |
| 91 | Norisoboldine | Croton echinocarpus | 69 | |
| 92 | Isocorydine | Alseodaphne corneri | 60 | |
| 93 | Norisocorydine | Alseodaphne corneri | 60 | |
| Unonopsis floribunda | 35, 36 | |||
| 94 | 1,2-Methylenedioxy-3-methoxyaporphine | Aconitum carmichaelii | 70 | |
| 95 | N-Formyl-asimilobine-2-O-β-D-glucoside | Stephania succifera | 71 | |
| 96 | Isoboldine | Annona hypoglauca | 72 | |
| Bocageopsis pleiosperma | 39 | |||
| 97 | Anonaine | Annona hypoglauca | 72 | |
| Plumula nelumbinis | 30 | |||
| Unonopsis floribunda | 35, 36 | |||
| Unonopsis duckei | 75 | |||
| Bocageopsis pleiosperma | 39 | |||
| 98 | Nornuciferine | Annona hypoglauca | 72 | |
| Plumula nelumbinis | 30 | |||
| Unonopsis floribunda | 35, 36 | |||
| Unonopsis duckei | 75 | |||
| 99 | Actinodaphnine | Annona hypoglauca | 72 | |
| 100 | Magnoflorine |
Mahonia aquifolium
Coptis japonica Sinomenium acutum |
50
73 40 |
|
| 101 | Norpurpureine | Annona purpurea | 74 | |
| 102 | Purpureine | Annona purpurea | 74 | |
| 103 | Nornuciferidine | Plumula nelumbinis | 30 | |
| 104 | N-Nornuciferine | Plumula nelumbinis | 30 | |
| 105 | O-Nornuciferine | Plumula nelumbinis | 30 | |
| 106 | Nuciferine | Plumula nelumbinis | 30 | |
| 107 | Roemerine | Plumula nelumbinis | 30 | |
| 108 | Oxidation-nuciferine | Plumula nelumbinis | 30 | |
| 109 | Asimilobine | Unonopsis floribunda | 35, 36 | |
| Unonopsis duckei | 75 | |||
| Bocageopsis pleiosperma | 39 | |||
| 110 | Isopiline | Unonopsis floribunda | 35, 36 | |
| 111 | O-Methylisopiline | Unonopsis floribunda | 35, 36 | |
| 112 | Glaucine | Unonopsis floribunda | 35, 36 | |
| Unonopsis duckei | 75 | |||
| 113 | Norglaucine | Unonopsis floribunda | 35, 36 | |
| 114 | (+)-N-Formylnorglaucine | Unonopsis stipitata | 76 | |
| 115 | 6aR-2′-Methoxycarbonyl-thaliadin | Thalictrum cirrhosum | 43 | |
| 116 | 6aR-2′-Carboxylthaliadin | Thalictrum cirrhosum | 43 | |
| 117 | 6aR-3-Methoxy-hernandalinol | Thalictrum cirrhosum | 43 | |
| 118 | 6aS-1,3,10-Trimethoxy-natalamine | Thalictrum cirrhosum | 43 | |
| 119 | Predicentrine | Thalictrum cirrhosum | 43 | |
| 120 | Thaliadine |
Thalictrum cirrhosum
Thalictrum wangii |
43 | |
| 121 | Glaucine | Corydalis turtschaninovii | 77 | |
| 122 | (+)-8-(4′-Formylphenoxy)glaucine | Thalictrum wangii | 46 | |
| 123 | (+)-8-(4′-Hydroxymethylphenoxy) glaucine | Thalictrum wangii | 46 | |
| 124 | (+)-3-Methoxy-8-(4′-formylphenoxy) glaucine | Thalictrum wangii | 46 | |
| 125 | 4-Methoxyoxohernandaline | Thalictrum wangii | 46 | |
| 126 | Dactyllactone A | Dactylicapnos scandens | 78 | |
| 127 | Sallisonine E | Sinomenium acutum | 39 | |
| 128 | Dauriporphine | Sinomenium acutum | 81 | |
| 129 | Isomoschaltoline | Guatteria blepharophylla | 237 | |
| 130 | O-Methylmoschatoline | Guatteria blepharophylla | 237 | |
| 131 | Liriodenine |
Guatteria blepharophylla Unonopsis floribunda Unonopsis duckei |
237 35 75 |
|
| 132 | Subsessiline | Guatteria blepharophylla | 237 | |
| 133 | Lysicamine |
Guatteria blepharophylla Unonopsis floribunda Unonopsis duckei |
237 35 75 |
|
| 134 | 7-Hydroxyguatteriopsiscine | Guatteria friesiana | 82 | |
| 135 | (R)-Dihydroguatteriscine | Guatteria friesiana | 82 | |
| 136 | Guatterfriesidine | Guatteria friesiana | 82 | |
| 137 | Iso-9-methoxyguatterfriesine | Guatteria friesiana | 82 | |
| 138 | Norushinsunine | Unonopsis floribunda | 35 | |
| 139 | Oxoglaucine | Unonopsis floribunda | 35 | |
| 140 | Lanuginosine | Unonopsis floribunda | 35 | |
| 141 | 3-Methoxy-2′-methoxycarbonyl-oxohernandalincin | Thalictrum cirrhosum | 43 | |
| 142 | 3-Methoxy-oxohernandaline | Thalictrum cirrhosum | 43 | |
| 143 | Oxopurpureine | Thalictrum cirrhosum | 43 | |
| 144 | Oxophoebine | Thalictrum cirrhosum | 43 | |
| 145 | 1,2,3,9,10-Pentamethoxy-11-(4′-formylphenoxy)-7-oxoaporphine | Thalictrum wangii | 46 | |
| 146 | 1,2,9,10-Tetramethoxy-11-(4′-formylphenoxy)-7-oxoaporphine | Thalictrum wangii | 46 | |
| 147 | Dehydrocrebanine | Stephania venosa | 85 | |
| 148 | Crebanine | Stephania venosa | 85 | |
| 149 | Stephanine | Stephania venosa | 85 | |
| 150 | O-Methylbulbocapnine | Stephania venosa | 85 | |
| 151 | 6-Formyl-1,2,9,10-tetramethoxy-6α,7-dehydroaporphine | Annona crassiflora | 70 | |
| 152 | Glaziovine | Unonopsis duckei | 75 | |
| 153 | (+)-Oridine | Cryptocarya densiflora, Cryptocarya infectoria Cryptocarya griffithiana | 37 | |
| 154 | (−)-10-O-Acetyl prodensiflorin A | Thalictrum wangii | 46 | |
| 155 | (−)-10-O-Acetyl prodensiflorin B | Thalictrum wangii | 46 | |
| 156 | Prodensiflorin B | Thalictrum wangii | 46 | |
| 157 | Dihydroglaziovine | Thalictrum cirrhosum | 43 | |
| 158 | Linearisine | Thalictrum cirrhosum | 43 | |
| 159 | Pronuciferine | Plumula nelumbinis | 30 | |
| 160 | Stepharine |
Unonopsis genus Bocageopsis pleiosperma |
35, 36 39 |
|
| Berberines and tetrahydroberberines isoquinoline alkaloids | ||||
| 161 | Berberine |
Berberis sp. Thalictrum foliolosum Chelidonium majus Mahonia aquifolium Mahonia bealei Coptis chinensis Corydalis turtschaninovii Ancistrocladus tectorius |
33 44 123 50 87 41 89 77 127 |
|
| 162 | Jatrorrhizine | Corydalis turtschaninovii | 77 | |
| 163 | Epiberberine |
Chelidonium majus Mahonia aquifolium Mahonia bealei |
50 87 |
|
| 164 | Demethyleneberberine |
Chelidonium majus Mahonia aquifolium Mahonia bealei |
50 87 |
|
| 165 | Coptisine | Corydalis turtschaninovii | 77 | |
| 166 | Palmatine | Corydalis turtschaninovii | 77 | |
| 167 | Pseudodehydrocorydaline | Corydalis turtschaninovii | 77 | |
| 168 | Dehydrocorybulbine | Corydalis turtschaninovii | 77 | |
| 169 | Pseudocoptisine | Corydalis turtschaninovii | 77 | |
| 170 | Dehydroisoapocavidine | Corydalis tomentella | 28 | |
| 171 | Dehydrocheilanthifoline | Corydalis tomentella | 28 | |
| 172 | Corydamine | Fumaria officinalis | 24 | |
| 173 | 5-Hydroxyl-8-oxyberberine | Coptis chinensis | 41, 89 | |
| 174 | 8,13-Dioxocoptisine hydroxide | Coptis chinensis | 41, 89 | |
| 175 | 8-Oxyberberine | Coptis chinensis | 41, 89 | |
| 176 | 8-Oxo-epiberberine | Coptis chinensis | 41, 89 | |
| 177 | 8-Oxocoptisine |
Coptis chinensis Coptis pallida Coptidis Rhizoma-Euodiae Fructus couple |
41, 89 90 61 |
|
| 178 | 8-Oxyberberrubine | Coptis chinensis | 41, 89 | |
| 179 | Tetrahydroberberine | Coptis chinensis | 41, 89 | |
| 180 | Corydaline |
Coptis chinensis Corydalis turtschaninovii |
41, 89
77 |
|
| 181 | Orydalidzine | Coptis pallida | 90 | |
| 182 | (−)-Corybulbine | Coptis pallida | 90 | |
| 183 | (−)-Yuanhunine | Coptis pallida | 90 | |
| 184 | (−)-Ophiocarpine | Coptis pallida | 90 | |
| 185 | Dehydrocorydaline | Coptis pallida | 90 | |
| 186 | Dihydrocoptisine | Corydalis tomentella | 28 | |
| 187 | Trans-Protopinium |
Corydalis tomentella Fumaria parviflora |
28 91 |
|
| 188 | Cis-Protopinium |
Corydalis tomentella Fumaria parviflora |
28 91 |
|
| 189 | Thalictrifoline | Corydalis tomentella | 28 | |
| 190 | Tetrahydrocoptisine | Corydalis turtschaninovii | 77 | |
| 191 | 13-Carboxaldehyde-8-oxocoptisine | Coptidis Rhizoma-Euodiae Fructus couple | 61 | |
| 192 | Tetrahydropalmatine | Corydalis hendersonii | 29 | |
| 193 | 8-Hydroxy-7, 8-dihydrocoptisine | Coptis japonica | 73 | |
| 194 | Cavidine | Corydalis impatiens | 92 | |
| 195 | (−)-Stylopine |
Fumaria officinalis Corydalis rupestris |
24 98 |
|
| 196 | (−)-Sinactine | Fumaria officinalis | 24 | |
| 197 | Cheilanthifoline |
Fumaria officinalis Sinomenium acutum |
24 81 |
|
| 198 | Phellodendrine | Phellodendri chinensis | 93 | |
| 199 | (−)-1-O-β-D-Glucoside-8-oxotetrahydropalmatine | Stephania succifera | 71 | |
| 200 | N-Methylcanadine | Zanthoxylum tingoassuiba | 94 | |
| 201 | Demethylalangiside | Ophiorrhiza nutans | 95 | |
| 202 | Alangiside | Ophiorrhiza nutans | 95 | |
| 203 | Isoalangiside |
Ophiorrhiza nutans Alangium longiflorum |
95 97 |
|
| 204 | Scoulerine | Corydalis dubia | 96 | |
| 205 | 2′-O-Trans-Sinapoylisoalangiside | Alangium longiflorum | 97 | |
| 206 | Rupestrine A | Corydalis rupestris | 98 | |
| 207 | Rupestrine B | Corydalis rupestris | 98 | |
| 208 | Rupestrine C | Corydalis rupestris | 98 | |
| 209 | Rupestrine D | Corydalis rupestris | 98 | |
| Protopine isoquinoline alkaloids | ||||
| 210 | Protopine |
Fumaria officinalis Corydalis mucronifera |
24
99 |
|
| 211 | Cryptopine | Fumaria officinalis | 24 | |
| Naphthylisoquinoline alkaloids | ||||
| 212 | Ancistectorine D | Ancistrocladus tectorius | 105, 107 | |
| 213 | 6-O-Demethyl ancistectorine D | Ancistrocladus tectorius | 105 | |
| 214 | Ancistrotectoriline A |
Ancistrocladus tectorius Unidentified Ancistrocladus plant Ancistrocladus ealaensis |
105 109 113 |
|
| 215 | Ancistrotanzanine B | Ancistrocladus tectorius | 105 | |
| 216 | Ancistroealaine A | Ancistrocladus tectorius | 105 | |
| 217 | 6-O-Methylancistectorine B1 | Ancistrocladus tectorius | 105 | |
| 218 | Ancistectorine B2 | Ancistrocladus tectorius | 105 | |
| 219 | 6-O-Demethyl-8-O-methyl-7-epi-ancistrobrevine D | Ancistrocladus tectorius | 105 | |
| 220 | Ancistrobenomine B | Ancistrocladus tectorius | 106 | |
| 221 | Ancistrobenomine C | Ancistrocladus tectorius | 106 | |
| 222 | 6-O-Methylancistectorine A3 | Ancistrocladus tectorius | 106 | |
| 223 | 4′-O-Demethylancistectorine A2 | Ancistrocladus tectorius | 106 | |
| 224 | Ancistectorine A3 | Ancistrocladus tectorius | 106 | |
| 225 | Ancistrocladine |
Ancistrocladus tectorius Ancistrocladus ileboensis |
106 108 |
|
| 226 | Hamatine | Ancistrocladus tectorius | 106 | |
| Ancistrocladus congolensis | 110 | |||
| 227 | 5′-O-Demethylhamatine | Ancistrocladus tectorius | 106 | |
| 228 | Ancistrocline | Ancistrocladus tectorius | 106 | |
| 229 | Ancistrocladinine | Ancistrocladus tectorius | 106 | |
| 230 | Hamatinine | Ancistrocladus tectorius | 106 | |
| 231 | Ancistectorine A2 | Ancistrocladus tectorius | 106 | |
| 232 | 5-Epi-ancistectorine A2 | Ancistrocladus tectorius | 106 | |
| 233 | Ancistrobenomine A | Ancistrocladus tectorius | 106 | |
| 234 | 6-O-Methylancistrocladine | Ancistrocladus tectorius | 106 | |
| 235 | 6-O-Methylhamatine |
Ancistrocladus tectorius Unidentified Ancistrocladus plant |
106 109 |
|
| Ancistrocladus congolensis | 110 | |||
| 236 | 4′-O-Demethylancistrocladine |
Ancistrocladus tectorius Unidentified Ancistrocladus plant |
106 109 |
|
| 237 | 5′-O-Demethylhamatine |
Ancistrocladus tectorius Ancistrocladus congolensis |
106 110 |
|
| 238 | 6-O-Methylhamatinine |
Ancistrocladus tectorius Ancistrocladus congolensis |
106
110 |
|
| 239 | 5′-O-Demethylhamatinine | Ancistrocladus tectorius | 106 | |
| 240 | Korupensamine D | Ancistrocladus congolensis | 110 | |
| 241 | Ancistrocyclinone A | Ancistrocladus tectorius | 107 | |
| 242 | Ancistrocyclinone B | Ancistrocladus tectorius | 107 | |
| 243 | Ancistrocladinium A (a/b) |
Ancistrocladus tectorius Unidentified Ancistrocladus plant Ancistrocladus ealaensis |
107
109 113 |
|
| 244 | 4′-O-Demethylancistrocladinium A (a/b) | Ancistrocladus tectorius | 107 | |
| 245 | 6,4′-O,O-Didemethylancistrocladinium A (a/b) |
Ancistrocladus tectorius Ancistrocladus ealaensis |
107 113 |
|
| 246 | Ancistrotectorine B1 | Ancistrocladus tectorius | 107 | |
| 247 | Shuangancistrotectorine C | Ancistrocladus tectorius | 107 | |
| 248 | Ancistrotectoquinone B (a/b) | Ancistrocladus tectorius | 107 | |
| 249 | Dioncophylline F | Ancistrocladus ileboensis | 108 | |
| 250 | Dioncophylline C2 | Ancistrocladus ileboensis | 108 | |
| 251 | Dioncophylline D2 | Ancistrocladus ileboensis | 108 | |
| 252 | 5′-O-Methyldioncophylline D | Ancistrocladus ileboensis | 108 | |
| 253 | Dioncophylline A | Ancistrocladus ileboensis | 108 | |
| 254 | 4′-O-Demethyldioncophylline A | Ancistrocladus ileboensis | 108 | |
| 255 | Ancistrocladisine B | Ancistrocladus ileboensis | 108 | |
| 256 | Ancistrobrevine C | Ancistrocladus ileboensis | 108 | |
| 257 | Ancistrocladisine A | Ancistrocladus ileboensis | 108 | |
| 258 | Ancistrobertsonine D | Ancistrocladus ileboensis | 108 | |
| 259 | Ancistroyafungine A | Unidentified Ancistrocladus plant | 109 | |
| 260 | Ancistroyafungine B | Unidentified Ancistrocladus plant | 109 | |
| 261 | Ancistroyafungine C | Unidentified Ancistrocladus plant | 109 | |
| 262 | Ancistroyafungine D | Unidentified Ancistrocladus plant | 109 | |
| 263 | Ancistroguineine A | Unidentified Ancistrocladus plant | 109 | |
| 264 | Ancistrobertsonine A | Unidentified Ancistrocladus plant | 109 | |
| 265 | Ancistrobrevine B | Unidentified Ancistrocladus plant Ancistrocladus congolensis |
109 110 |
|
| 266 | 6,5′-O,O-Didemethylancistroealaine A | Unidentified Ancistrocladus plant | 109 | |
| 267 | 6-O-Demethylancistroealaine A | Unidentified Ancistrocladus plant | 109 | |
| 268 | 7-Epi-ancistrobrevine D | Unidentified Ancistrocladus plant | 109 | |
| 269 | Ancistrocladinium B | Unidentified Ancistrocladus plant | 109 | |
| 270 | Michellamine A2 |
Ancistrocladus congolensis Unidentified Ancistrocladus plant |
110 111 |
|
| 271 | Michellamine A3 | Ancistrocladus congolensis | 110 | |
| 272 | Michellamine A4 | Ancistrocladus congolensis | 110 | |
| 273 | Michellamine B2 | Ancistrocladus congolensis | 110 | |
| 274 | Michellamine B3 | Ancistrocladus congolensis | 110 | |
| 275 | Michellamine A | Ancistrocladus congolensis | 110 | |
| 276 | Michellamine B | Ancistrocladus congolensis | 110 | |
| 277 | Michellamine A6 | Unidentified Ancistrocladus plant | 111 | |
| 278 | Michellamine A7 | Unidentified Ancistrocladus plant | 111 | |
| 279 | Michellamine B4 | Unidentified Ancistrocladus plant | 111 | |
| 280 | Michellamine B5 | Unidentified Ancistrocladus plant | 111 | |
| 281 | Ancistrobonsoline A1 | Unidentified Ancistrocladus plant | 111 | |
| 282 | Ancistrobonsoline A2 | Unidentified Ancistrocladus plant | 111 | |
| 283 | Ancistroealaine C | Unidentified Ancistrocladus plant Ancistrocladus ealaensis |
111 113 |
|
| 284 | Korupensamine A | Unidentified Ancistrocladus plant | 111 | |
| Ancistrocladus ealaensis | 113 | |||
| 285 | Korupensamine B | Unidentified Ancistrocladus plant | 111 | |
| 286 | Michellamine E | Unidentified Ancistrocladus plant | 111 | |
| 287 | Ealapasamine A | Ancistrocladus ealaensis | 112 | |
| 288 | Ealapasamine B | Ancistrocladus ealaensis | 112 | |
| 289 | Ealapasamine C | Ancistrocladus ealaensis | 112 | |
| 290 | Mbandakamine A | Ancistrocladus ealaensis | 107 | |
| 291 | Mbandakamine C | Ancistrocladus ealaensis | 113 | |
| 292 | Mbandakamine D | Ancistrocladus ealaensis | 113 | |
| 293 | Mbandakamine E | Ancistrocladus ealaensis | 113 | |
| 294 | Mbandakamine A | Ancistrocladus ealaensis | 113 | |
| 295 | Ancistroealaine D | Ancistrocladus ealaensis | 113 | |
| 296 | Ancistroealaine E | Ancistrocladus ealaensis | 113 | |
| 297 | Ancistroealaine F | Ancistrocladus ealaensis | 113 | |
| 298 | Ancistrolikokine B | Ancistrocladus ealaensis | 113 | |
| Phenanthridine alkaloids | ||||
| 299 | Sanguinarine | Chelidonium majus | 124 | |
| 300 | Chelidonine | Chelidonium majus | 125 | |
| 301 | Homochelidonine | Chelidonium majus | 125 | |
| 302 | (1′R,6R/1′S,6S)-1-(Dihydrochelerythrine-6-yl) ethanol | Chelidonium majus | 126 | |
| 303 | (1′S,6R/1′R,6S)-1-(Dihydrochelerythrine-6-yl) ethanol | Chelidonium majus | 126 | |
| 304 | (1′R,6R)/(1′S,6S)-1-(Dihydrosanguinarine-6-yl)ethanol | Chelidonium majus | 126 | |
| 305 | (1′S,6R)/(1′R,6S)-1-(Dihydrosanguinarine-6-yl)ethanol | Chelidonium majus | 126 | |
| 306 | (±)-Ethyl 2-(dihydrosanguinarine-6-yl) acetate | Chelidonium majus | 126 | |
| 307 | (±)-Ethyl dihydrosanguinarine-6- carboxylate | Chelidonium majus | 126 | |
| 308 | Heitziquinone | Zanthoxylum heitzii | 127 | |
| 309 | Dihydronitidine | Zanthoxylum heitzii | 127 | |
| 310 | Isoarnottianamide | Zanthoxylum heitzii | 127 | |
| 311 | Rhoifoline B | Zanthoxylum heitzii | 127 | |
| 312 | Dihydrocheleryhtrine | Zanthoxylum tingoassuiba | 94 | |
| 313 | Decarine | Zanthoxylum myriacanthum var. pubescens | 128 | |
| 314 | Corynoline | Corydalis bungeana | 129 | |
| 315 | Ambinine | Corydalis ambigua var. amurensis | 130 | |
| 316 | Norsanguinarine | Corydalis tomentella | 28 | |
| 317 | (−)-6-Acetonyldihydrisanguinarine |
Corydalis tomentella Corydali pallida |
28 90 |
|
| 318 | Cavidilinine | Corydalis tomentella | 28 | |
| 319 | 8-Methoxydihydrosanguinarine | Corydalis mucronifera | 99 | |
| 320 | Dihydrosanguinarine | Corydalis mucronifera | 99 | |
| 321 | Lycorine | Amaryllidaceae family | 136 | |
| 322 | Acetycaranine | Amaryllidaceae family | 136 | |
| 323 | Caranine | Amaryllidaceae family | 136 | |
| 324 | Galanthine | Amaryllidaceae family | 136 | |
| 325 | 9-O-Demthylgalanthine | Amaryllidaceae family | 136 | |
| 326 | Haemanthamine | Amaryllidaceae family | 136 | |
| Narcissus poeticus cv. Pink Parasol | 138 | |||
| 327 | Haemanthidine | Amaryllidaceae family | 136 | |
| 328 | Ambelline | Amaryllidaceae family | 136 | |
| 329 | 11-O-Acetylambelline | Amaryllidaceae family | 136 | |
| 330 | 1-O-Acetylbulbisine | Amaryllidaceae family | 136 | |
| 331 | Undulatine | Amaryllidaceae family | 136 | |
| 332 | Crinamidine | Amaryllidaceae family | 136 | |
| 333 | Buphanamine | Amaryllidaceae family | 136 | |
| 334 | Crinine | Amaryllidaceae family | 136 | |
| 335 | 6,7,11b,11c-Didehydrolycorinium salt |
Crinum firmifolium Crinum hardyi |
137 | |
| 336 | Seco-isopowellaminone | Narcissus poeticus cv. Pink Parasol | 138 | |
| 337 | Incartine | Narcissus poeticus cv. Pink Parasol | 138 | |
| Manzamine alkaloids | ||||
| 338 | Manzamine A | Acanthostrongylophora sp. sponge | 146 | |
| 339 | Kepulauamine A | Acanthostrongylophora sp. sponge | 146 | |
| 340 | Manzamine B N-oxide | Acanthostrongylophora sp. sponge | 146 | |
| 341 | 3,4-Dihydromanzamine B N-oxide | Acanthostrongylophora sp. sponge | 146 | |
| 342 | 11-Hydroxymanzamine J | Acanthostrongylophora sp. sponge | 146 | |
| 343 | 31-Hydroxymanzamine A | Acanthostrongylophora sp. sponge | 146 | |
| 344 | 32,33-Dihydro-31-hydroxymanzamine A | Acanthostrongylophora sp. sponge | 146 | |
| 345 | 6-Deoxymanzamine X | Acanthostrongylophora sp. sponge | 146 | |
| 346 | Manzamine B | Acanthostrongylophora sp. sponge | 146 | |
| 347 | neo-Kauluamine | Acanthostrongylophora sp. sponge | 146 | |
| Emetine isoquinoline alkaloids | ||||
| 348 | Emetine | Alangiaceae, Icacinaceae, and Rubiaceae | 149 | |
| 349 | 7′,10-Dide-O-methylcephaeline | Ophiorrhiza nutans | 95 | |
| 350 | 10-O-Demethylprotoemetine | Ophiorrhiza nutans | 95 | |
| 351 | 8-Hydroxytubulosine | Alangium longiflorum | 97 | |
| 352 | 9-Demethyltubulosine | Alangium longiflorum | 97 | |
| 353 | (+)-Sebiferine | Dehaasia longipedicellata | 38 | |
| 354 | (−)-Milonine | Dehaasia longipedicellata | 38 | |
| 355 | Sinomacutine A | Sinomenium acutum | 40 | |
| 356 | Sinomacutine B | Sinomenium acutum | 40 | |
| 357 | Sinomacutine C | Sinomenium acutum | 40 | |
| 358 | Cephalonine-2-O-β-D-glucopyranoside | Sinomenium acutum | 40 | |
| 359 | Sinomenine | Sinomenium acutum | 40 | |
| Sinomenium acutum | 81 | |||
| 360 | Sinoacutine | Sinomenium acutum | 40 | |
| 361 | 8-Demethoxycephatonine | Sinomenium acutum | 81 | |
| 362 | 7(R)-7,8-dihydrosinomenine | Sinomenium acutum | 81 | |
| 363 | 8-Demethoxyrunanine | Sinomenium acutum | 81 | |
| 364 | 14-Episinomenine | Sinomenium acutum | 81 | |
| 365 | Sinomenine N-oxide | Sinomenium acutum | 81 | |
| 366 | Salutaridine | Sinomenium acutum | 81 | |
| 367 | Acutumine | Sinomenium acutum | 81 | |
| 368 | Acutumidine | Sinomenium acutum | 81 | |
| 369 | Dauricumine | Sinomenium acutum | 81 | |
| 370 | Pallidine | Unonopsis floribunda | 36 | |
| 371 | O-Methylflavinantine | Thalictrum cirrhosum | 43 | |
| Phthalideisoquinoline alkaloids | ||||
| 372 | (+)-Bicuculline | Fumaria officinalis | 24 | |
| 373 | (+)-Corlumine | Viola tianschanica | 150 | |
| 374 | (9S, 7′S) Tomentelline A | Corydalis tomentella | 28 | |
| 375 | (9S, 7′R) Tomentelline A | Corydalis tomentella | 28 | |
| 376 | (9R, 7′S) Tomentelline B | Corydalis tomentella | 28 | |
| 377 | Adlumidine |
Corydalis tomentella
Corydalis mucronifera |
28 99 |
|
| 378 | (+)-Capnoidine | Corydalis tomentella | 28 | |
| 379 | Mucroniferanine A | Corydalis mucronifera | 99 | |
| 380 | Mucroniferanine B | Corydalis mucronifera | 99 | |
| 381 | Mucroniferanine C | Corydalis mucronifera | 99 | |
| 382 | Mucroniferanine D | Corydalis mucronifera | 99 | |
| 383 | Mucroniferanine E | Corydalis mucronifera | 99 | |
| 384 | Mucroniferanine F | Corydalis mucronifera | 99 | |
| 385 | Mucroniferanine G | Corydalis mucronifera | 99 | |
| 386 | (±)-Hypecorinine | Corydalis mucronifera | 99 | |
| 387 | (−)-7′-O-Methylegenine | Corydalis mucronifera | 99 | |
| 388 | Sibiricine | Corydalis mucronifera | 99 | |
| 389 | (+)-Humosine A | Corydalis mucronifera | 99 | |
| 390 | Capnoidine | Corydalis dubia | 151 | |
| Benzopyrroloisoquinoline Alkaloids | ||||
| 391 | Tengerensine | Ficus fistulosa var. tengerensis | 152 | |
| Phenylethyl tetrahydroisoquinoline alkaloids | ||||
| 392 | Fumarostrejdine | Fumaria officinalis | 24 | |
| 393 | (±)-O-Methylfumarofine | Fumaria officinalis | 24 | |
| Others | ||||
| 394 | Coptichine | Coptidis Rhizoma–Euodiae Fructus couple | 61 | |
| 395 | Coptisonine | Coptis chinensis | 89 | |
| 396 | Sallisonine D | Sinomenium acutum | 40 | |
| 397 | Alternamine A | Alternanthera littoralis | 153 | |
| 398 | (±)-7-Benzyloxy-1-(3-benzyloxy-4-methoxyphenethyl)-1,2,3,4-tetrahydro-6-methoxy-2-methylisoquinoline oxalate | Chemical library | 154 | |
| 399 | Tomentelline C | Corydalis tomentella | 28 | |
| 400 | Tomentelline D | Corydalis tomentella | 28 | |
| 401 | 6,7-Methylenedioxy-2-(6-acetyl-2,3-methylenedioxybenzyl)-1(2H)-isoquinolinone | Corydalis tomentella | 28 | |
| 402 | Oleracein E | Portulaca oleracea | 31 | |
| 403 | Pipermullesine B | Piper mullesua | 155 | |
| 404 | Pipermullesine C | Piper mullesua | 155 | |
| 405 | Delavatine A | Incarvillea delavayi | 156 | |
| 406 | Neotatarine | Acorus calamus | 158 | |
2. Structure and classification of isolated isoquinoline alkaloids
2.1. Simple isoquinoline alkaloids
The alkaloids in this classification have the simplest structures and are distributed mainly in the genera Papaver, Corydalis, Thalictrum and others. Eighteen isoquinoline alkaloids were identified and isolated from plants and animals between 2014 and 2018 (Figure 2).
Figure 2.
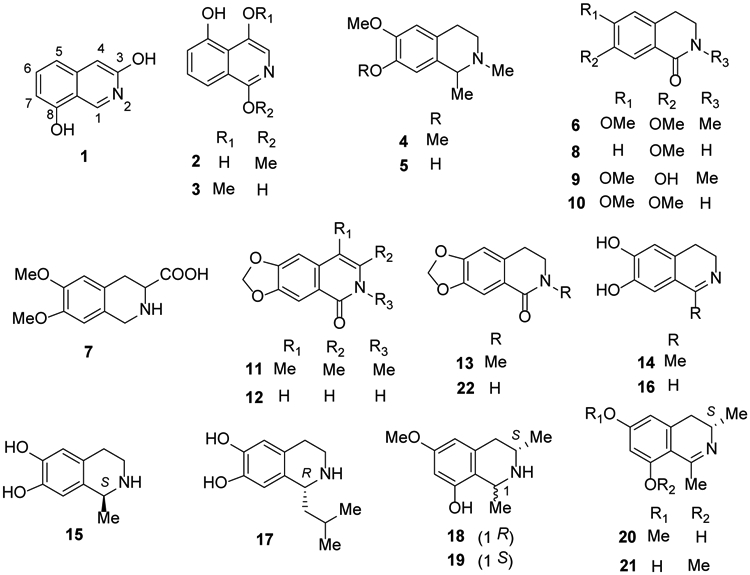
The Chemical Structures of Compounds 1-22
In 2016, two new isoquinoline alkaloids 3,8-diolisoquinoline (1) and 1-methoxy-4,5-diolisoquinoline (2) were isolated from an ethanol extract of the Chinese redheaded centipede Scolopendra subspinipes mutilans22. In another study in the following year, the new isoquinoline alkaloid 1,5-dihydroxy-4-methoxyisoquinoline (3), also isolated from this centipede species, showed moderate cytotoxicity against five cancer cells23.
Carnegine (4) and N-methylisosalsoline (5), isolated from the plant Hammada scoparia, exhibited antibacterial and antioxidant activities24. Also, in 2016, N-methylcorydaldine (6) was isolated from Fumaria officinalis25 and 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid (7) from Mucuna pruriens seeds26.
In 2018, the previously reported N-methylcorydaldine (6) together with two more isoquinoline alkaloids 7-methoxy-1,2,3,4-tetrahydroisoquinolin-1-one (8) and thalifoline (9) were isolated from Michelia champaca27. Other studies in same year described the isolation as well as hepatoprotective activities of the latter compound (9), N-methylisosalsoline (5), corydaldine (10), oxohydrastinine (11), 6,7-methylenedioxy-1(2H)-isoquinolinone (12) and oxyhydrastinine (13) from Corydalis tomentella, C. hendersonii and Plumula nelumbinis28-30.
6,7-Dihydroxy-1-methyl-3,4-dihydroisoquinolone (14), (S)-(−)-salsolinol (15), 6,7-dihydroxy-3,4-dihydroisoquinolone (16) and (R)-(+)-1-isobutyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (17), were isolated from the medicinal plant Portulaca oleracea31. These four simple isoquinoline alkaloids contained 6,7-dihydroxy substitution but different saturation or substituents at C-1. Four “naphthalene-devoid” tetra- and dihydroisoquinolines, named ealaines A–D (18-21), were isolated from the Congolese plant Ancistrocladus ealaensis32. Akihisa et al.33 isolated noroxyhydrastinine (22) from the bark of Phellodendron amurense (Figure 2).
2.2. Benzylisoquinoline alkaloids
2.2.1. Simple benzylisoquinoline alkaloids
Reticuline (23) exhibits significant pharmacological activities, leading to the search for and identification of alternate natural sources, such as Litsea cubeba, Unonopsis genus, Cryptocarya densiflora, C. infectoria, C. griffithiana and Dehaasia longipedicellata, over the past five years34-39. (+)-N-Methylisococlaurine (24) also was found in Cryptocarya species37 and (−)-N-methylcoclaurine (25) was identified in the rhizomes of Sinomenium acutum in 201440. Berbithine (26) and 6-([1,3]dioxolo[4,5-g]isoquinoline-5-carbonyl)-2,3-dimethoxybenzoic acid methyl ester (27) were isolated from the rhizome of Coptis chinensis41.
In 2018, several benzylisoquinoline alkaloids, including 24, 25, norcolaurine-4′-O-glucoside (28), N-methylhigenamine (29), norcoclaurine-6-O-glucoside (30), norcoclaurine (31), argemexirine (32), lotusine (33), isococlaurine (34), armepavine (35), 6-demethy-4′-methyl-N-methylcoclaurine (36), coclaurine (37), N-nor-O-methylarmepavine (38), isococlaurine-5′-O-pentoside (39), and coclaurine-5′-O-pentoside (40) were identified from Plumula nelumbinis through UPLC-ESI-QTOF-MS30. Subsequently, juzirine (41) was identified from the aerial parts of Leonurus japonicus42. (R)-(+)-1-Benzyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (42) from Portulaca oleracea showed anti-inflammatory and β2-adrenergic receptor agonist activities26. Laudanosine (43), pseudolaudanine (44) and rugosinone (45)were isolated from the whole herb of Thalictrum cirrhosum43 (Figure 3). Hendersine B methyl ester (46), bicucullinine (47) and hendersine B (48) were isolated from Corydalis tomentella28.
Figure 3.
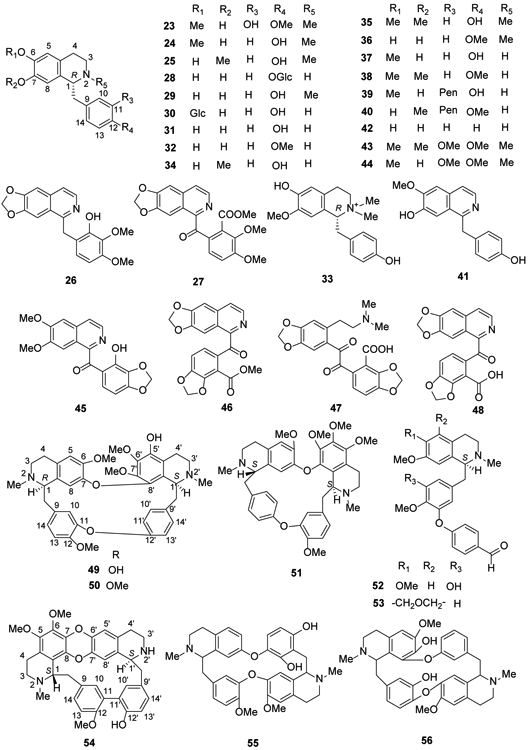
The Chemical Structures of Compounds 23-56
2.2.2. Bisbenzylisoquinoline alkaloids
Bisbenzyl isoquinoline alkaloids are one of the major phytochemicals reported from members of the plant families Menispermacea, Berberidaceae, Lauraceae, and Ranunculaceae, which grow in tropical and subtropical regions. They contain two benzylisoquinolines linked through diphenyl ether, benzyl phenyl ether, or biphenyl bonds5,19. In 2016, two new bisbenzylisoquinolines, 6,6′,7′,12-tetramethoxy-5′-hydroxy-2,2′-dimethyloxycanthan (49) and 6,5′,6′,7′,12-pentamethoxy-2,2′-dimethoxyethane (50), were isolated from the stems of Thalictrum foliolosum44. Meanwhile, hernandezine (51), a known alkaloid, was identified from T. flavum45. In 2018, two seco-bisbenzylisoquinolines, 6,7,12-trimethoxy-2-methyl-13-hydroxy-11-(4′-formylphenoxy)benzylisoquinoline (52) and 5,6-(methylenedioxy)-7,12-dimethoxy-2-methyl-10-(4′-formylphenoxy)benzylisoquinoline (53), were isolated from T. wangii46. Tiliamosine (54) was found from T. racemosa47 (Figure 3).
Bisbenzylisoquinoline alkaloids are also found in the genus Stephania. In 2014, two new compounds, (−)-pseudocurine (55) and (−)-pseudoisocurine (56), were isolated from a leaf extract of Stephania abyssinica48. Tetrandrine (57) and fangchinoline (58) were isolated from S. tetrandra, which has been used for 2,000 years as an antirheumatic herbal medicine in China49. In addition, (−)-O-O-dimethylgrisabine (59) from Dehaasia longipedicellata exhibited significant antiparasitic and antioxidant activities38. Using a 1H NMR-based metabolomics approach, berbamine (60), a bisbenzylisoquinoline-type compound, was identified from Mahonia aquifolium50 (Figure 4).
Figure 4.
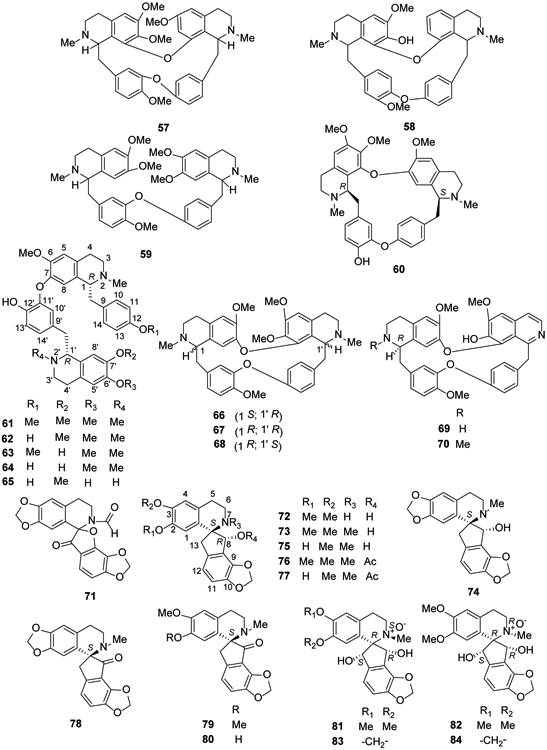
The Chemical Structures of Compounds 57-84
Neferine (61) is a well-known bisbenzylisoquinoline-type alkaloid due to its wide range of pharmacological activities, including antiarrhythmic, antihypertensive51,52, relaxant53, antidiabetic54, cholinesterase inhibitory55, antioxidant, anti-inflammatory, anti-amnesic56 and sedative57,58 effects. In addition to M. aquifolium, it is found in lotus (Nelumbo nucifera) seed embryos59. In 2018, compound 61 as well as four other bisbenzylisoquinoline alkaloids, liensinine (62), isoliensinine (63), norisoliensinine (64) and 6-hydroxynorisoliensinine (65) were found in Plumula nelumbinis30. Five alkaloids also were isolated from Alseodaphne corneri, including (−)-gyrolidine (66), (+)-O-methyllimacusine (67), (+)-2-norobaberine (68), (+)-norstephasubine (69) and (+)-stephasubine (70)60 (Figure 4).
2.2.3. Spirobenzylisoquinoline alkaloids
Spirobenzylisoquinoline alkaloids are isoquinoline alkaloids with a unique ‘spiro’ structure as shown in Figure 6. They have been found only within the plant family Fumariaceae, and more specifically within the genera Fumaria and Corydalis. In 2014, coptichic aldehyde (71) was isolated from the traditional Chinese medicine preparation Coptidis Rhizoma–Euodiae Fructus couple; it showed growth inhibitory activity against NCI-N87 cells with an IC50 value of 8.92 μM61. In 2016, the new isoquinoline alkaloid fumaranine (72) together with seven other alkaloids, (−)-fumaricine (73), (+)-dihydrofumariline (74), (−)-fumaritine (75), (−)-O-methylfumarophycine (76), (−)-fumarophycine (77), (+)-fumariline (78), (+)-parfumidine (79), and (+)-parfumine (80) were found from the aerial parts of F. officinalis24. Also, four new spirobenzylisoquinoline N-oxide alkaloids hendersines C-F (81-84) were identified from Corydalis hendersonii27 (Figure 4).
Figure 6.
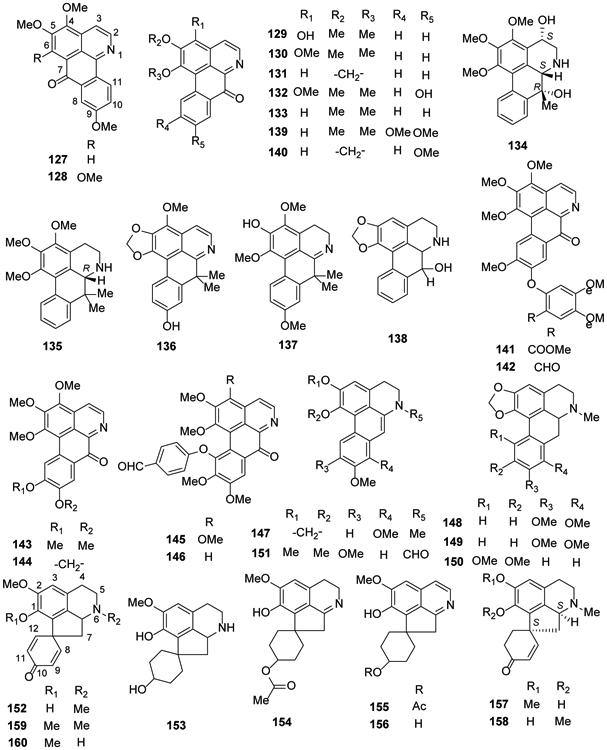
The Chemical Structures of Compounds 127-160
2.3. Aporphine isoquinoline alkaloids
Aporphine alkaloids are a large group of isoquinolines that generally possess a characteristic tetracyclic ring system (rings A-D) with a nitrogen in ring B62. The structures of the aporphine alkaloids can be classified into subtypes, including simple aporphines, their dehydro derivatives, oxoaporphines, miscellaneous aporphinoids, and dimeric aporphinoid alkaloids63-66.
2.3.1. Simple aporphines
Simple aporphines have a 5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline core substituted primarily with different numbers of hydroxy, methoxy, and methylenedioxy groups at various positions. The nitrogen is substituted most frequently with hydrogen or methyl, although other groups (e.g., formyl, acetyl, and others) are sometimes present. They are 1-benzylisoquinolines with one additional ring closure between the 2′-carbon in the pendant phenyl ring and the 1a-carbon in the isoquinoline ring junction, forming a non-linear tetracyclic (6-6-6-6) system.
Boldine (85), isolated from Litsea cubeba and Dehaasia longipedicellata, showed anti-inflammatory activity and potential synergistic effects in vivo67,60. Compound 85 and (−)-norboldine (86), also found in D. longipedicellata,38 showed moderate antioxidant and antiplasmodial activities38. In 2016, (+)-laurotetanine (87), isolated from Alseodaphne corneri68 and Bocageopsis pleiosperma39, was found to exhibit strong antiplasmodial activity (Figure 5).
Figure 5.
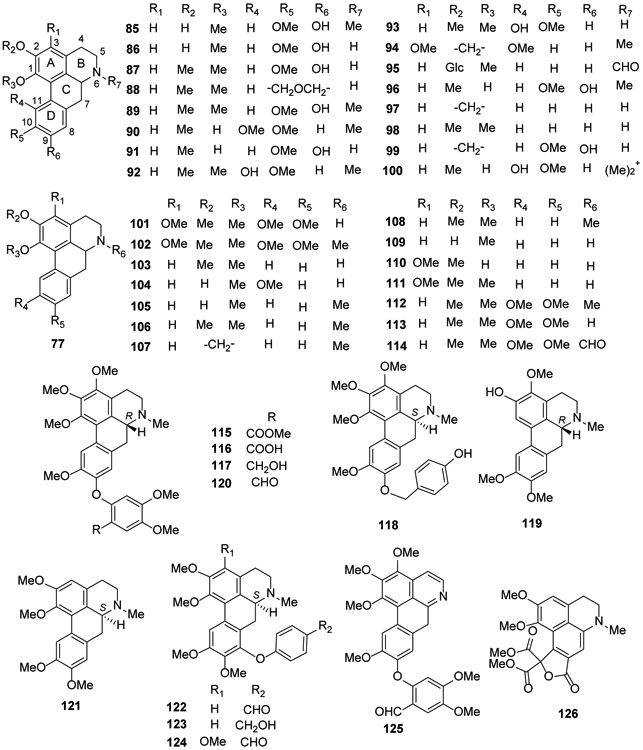
The Chemical Structures of Compounds 85-126
Compound 87, (+)-nornantenine (88) and (+)-N-methyllaurotetanine (89) were isolated from Cryptocarya densiflora, C. infectoria and C. griffithiana37. Corydine (90) and norisoboldine (91) were isolated from Croton echinocarpus leaves69, while isocorydine (92) and norisocorydine (93) were identified from Alseodaphne corneri60 (Figure 5).
The aporphine stephalagine (1,2-methylenedioxy-3-methoxyaporphine) (94) was isolated from the fruit peel of Annona crassiflora70. In 2017, N-formyl-asimilobine-2-O-β-d-glucoside (95), an amidic aporphine, was obtained from the tubers of Stephania succifera71. Four aporphine alkaloids, isoboldine (96), anonaine (97), nornuciferine (98) and actinodaphnine (99), were isolated from Annona hypoglauca72. Magnoflorine (100) was found in the rhizomes of Mahonia aquifolium, Coptis japonica and Sinomenium acutum40,50,73 (Figure 5).
Both norpurpureine (101) and purpureine (102) were isolated from Annona purpurea leaves74. In 2018, eight aporphine alkaloids [anonaine (97), N-nornuciferine (98), nornuciferidine (103), zenkerine (104), O-nornuciferine (105), nuciferine (106), roemerine (107) and oxidation-nuciferine (5-hydroxynuciferine) (108)] were identified from Plumula nelumbinis30. Compounds 93, 97 and 98 as well as asimilobine (109), isopiline (110), O-methylisopiline (111), glaucine (112), and norglaucine (113) were reported for the first time from Unonopsis floribunda35,36 (Figure 5). Compounds 97, 98, 109 and 112 also were found from Unonopsis duckei75, and compounds 89, 96, 97 and 109 also were reported from Bocageopsis pleiosperma40. (+)-N-Formylnorglaucine (114) was reported from Unonopsis stipitata76.
Four new phenyl-C1 substituted aporphine alkaloids, 6aR-2′-methoxycarbonylthaliadine (115), 6aR-2′-carboxylthaliadine (116), 6aR-3-methoxyhernandalinol (117), 6aS-1,3,10-trimethoxynatalamine (118), together with three known isoquinoline alkaloids 89, predicentrine (119), and thaliadine (120) were isolated from the whole herb of Thalictrum cirrhosum43.
Glaucine (121) from Corydalis turtschaninovii77, as well as three new analogs, (+)-8-(4′-formylphenoxy)glaucine (122), (+)-8-(4′-hydroxymethylphenoxy)glaucine (123), (+)-3-methoxy-8-(4′-formylphenoxy)glaucine (124), and two known alkaloids, 120 and its oxidized derivative 125, were isolated from the whole plant of Thalictrum wangii46 (Figure 5).
An unprecedented alkaloid, dactyllactone A (126), which contains a rearranged benzofuran lactone with a gemdimethoxycarbonyl unit and is derived from an 8,9;11,11a-bis-seco-aporphine skeleton, was isolated from Dactylicapnos scandens. It exhibited anti-inflammatory activity78 (Figure 5).
2.3.2. 7-Substituted Aporphines and Oxoaporphines
7-Oxygenated aporphines have a hydroxyl or methoxy group at C-7 or two such groups at C-4 and C-766,79. The oxoaporphines (7H-dibenzo[de,g]quinoline-7-one skeleton) and oxoisoaporphines (7H-dibenzo[de,h]quinoline-7-one skeleton) have an aromatic isoquinoline (aromatic ring B in the tetracyclic structure) and a carbonyl group at C-780.
Two oxoisoaporphines sallisonine E (127) and dauriporphine (128) were isolated from the rhizomes of Sinomenium acutum, in 2014 and 2016, respectively40,81. Five oxoaporphines isomoschatoline (129), O-methylmoschatoline (130), liriodenine (131), subsessiline (132) and lysicamine (133) were identified from Guatteria blepharophylla also in 201674 and compounds 131, 133 were reported from Unonopsis duckei in 201475.
One new 4,7-dihydroxy-7-methylaporphine alkaloid (7-hydroxyguatteriopsiscine (134)) and three new 7,7-dimethylaporphinoids [(R)-dihydroguatteriscine (135), guatterfriesidine (136), and iso-9-methoxyguatterfriesine (137)] were isolated from the stem bark of G. friesiana in 2018.75 Compound 136 exhibited antiglycation activity as determined by inhibiting the formation of advanced glycation end-products in both bovine serum albumin (BSA)/methylglyoxal and BSA/fructose assay systems82. In 2018, one 7-hydroxyaporphine [norushinsunine (138)] and four oxoaporphines [131, 133, oxoglaucine (139), and lanuginosine (140)] were reported for the first time from Unonopsis floribunda35 (Figure 6).
Another new oxoaporphine alkaloid 3-methoxy-2′-methoxycarbonyl-oxohernandalincin (141) as well as the known 3-methoxy-oxohernandaline (142), oxopurpureine (143), and oxophoebine (144) were isolated from the whole herb of Thalictrum cirrhosum43. 1,2,3,9,10-Pentamethoxy-11-(4′-formylphenoxy)-7-oxoaporphine (145) and 1,2,9,10-tetramethoxy-11-(4′-formylphenoxy)-7-oxoaporphine (146), two new oxoaporphines that, like 142, contain an ether-linked formylphenyl moiety were identified from T. wangii46 (Figure 6).
2.3.3. Dehydroaporphines
Dehydroaporphines are 5,6-dihydro-4H-dibenzo[de,g]quinolines with a double, rather than single, between C-6a and C-7. In the preceding subtypes, this bond is saturated or C-7 is substituted with hydroxy, methoxy, or methyl groups or part of a carbonyl unit19,83,84. Based on bioassay-guided fractionation against numerous cancer cells, Le et al.85 isolated one dehydroaporphine [dehydrocrebanine (147)] and three simple aporphines [crebanine (148), stephanine (149) and O-methylbulbocapnine (150)] from the tubers of Stephania venosa growing in Vietnam. Compound 149 was the most active among the four compounds with IC50 values of 3.33 μM, 5.66 μM and 6.49 μM against HeLa, MDA-MB231 and MCF-7 cells, respectively. In 2017, an amidic dehydroapophine (151, 6-formyl-1,2,9,10-tetramethoxy-6α,7-dehydroaporphine) was isolated from the aerial parts of Aconitum carmichaelii70 (Figure 6).
2.3.4. Proaporphine alkaloids
Proaporphine alkaloids are biogenetic precursors to certain aporphine alkaloids. The tetracyclic system (2’,3’,8’,8a’-tetrahydro-1’H-spiro[cyclohexane-1,7’-cyclopenta[ij]isoquinoline) is composed of a bicyclic isoquinoline fused to a five-membered ring that is also connected to a six-membered ring through a spiro carbon.
In 2014, glaziovine (152) was reported from Unonopsis duckei75. In 2016, the proaporpine (+)-oridine (153) was obtained from leaves of Cryptocarya densiflora37. In 2018, several proaporphines were identified from various plant species: two new [(−)-10-O-acetylprodensiflorins A (154) and B (155)] and one known [prodensiflorin B (156)] and from the whole plant of Thalictrum wangii46, dihydroglaziovine (157) and linearisine (158) from T. cirrhosum,43 pronuciferine (159) from Plumula nelumbinis30 and stepharine (160) from Unonopsis floribunda for the first time35,36, and compound 160 also was found Bocageopsis pleiosperma39. (Figure 6).
2.4. Berberine and protoberberine isoquinoline alkaloids
2.4.1. Berberine (quaternary protoberberine) alkaloids
Berberine (161) is a famous isoquinoline alkaloid from the rhizome, roots and stem bark of Berberis sp.; it exhibits various pharmacological effects, such as antitumor, antibacterial, antiviral, antiinflammatory, antidiabetic and myocardial protective activities. Berberine is a quaternary protoberberine alkaloid with a tetracyclic skeleton [5,6-dihydrobenzo[a,g]quinolizinium (C17H14N+) salt] with the nitrogen at the junction of the two middle rings (position 7). Structurally, it is a benzylisoquinoline with an additional ring formed between the 2′-carbon of the pendant phenyl ring and a methyl on the isoquinoline nitrogen. Various oxygenated substituents (hydroxy, methoxy, methylenedioxy) are present on the two outer rings, most often, although not exclusively, at positions 2,3,9,10 or 2,3,10,11, which is often designated as ‘pseudo’. Methylation at position 13 is commonly seen as well. Besides the genus Berberis, it has also been isolated from plants of the genera Coptis, Corydalis and Mahonia together with other known structurally related alkaloids, including jatrorrhizine (162), epiberberine (163), demethyleneberberine (164), coptisine (165) and palmatine (166)50,73,86-88.
Compounds 161, 162, 165, 166, pseudodehydrocorydaline (167), dehydrocorybulbine (168) and pseudocoptisine (169) were isolated from the roots of Corydalis turtschaninovii. They showed strong neuraminidase inhibitory activity (IC50, 12.8–65.2 μM)77. Dehydroisoapocavidine (170), dehydrocheilanthifoline (171), isolated from the related species C. tomentella, showed hepatoprotective activity28. Corydamine (172), a B-ring opened 3-phenyl isoquinoline analog of 159, was isolated from the aerial parts of Fumaria officinalis19 (Figure 7).
Figure 7.
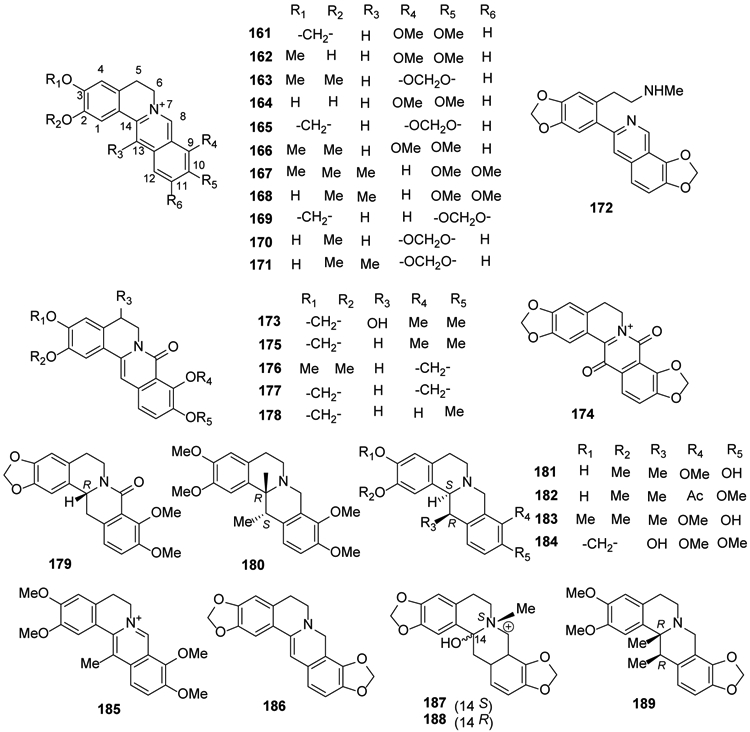
The Chemical Structures of Compounds 161-189
2.4.2. Protoberberine isoquinoline alkaloids
Other protoberberines include tetrahydroprotoberberines and dihydroprotoberberines. In 2014, several 8-oxo-protoberberines, including a pair of new enantiomeric isoquinoline alkaloids, (+)- and (−)-5-hydroxyl-8-oxyberberine (173), 8,13-dioxocoptisine hydroxide (174), 8-oxyberberine (175), 8-oxo-epiberberine (176), 8-oxocoptisine (177), and 8-oxoberberrubine (178), together with tetrahydroberberine (179) and corydaline (180) as well as the benzylisoquinoline alkaloid 26 were isolated from the rhizoma of Coptis chinensis. C2C12 cells exposed to 176 and 178 showed reduced glucose uptake41,89. The whole plant of Corydalis pallida also yielded 177 together with four tetrahydroprotoberberines, (−)-corydalidzine (181), (−)-corybulbine (182), (−)-yuanhunine (183) and (−)-ophiocarpine (184), as well as the quaternary protoberberine alkaloid dehydrocorydaline (185)90. Dihydrocoptisine (186), trans-protopinium (187), cis-protopinium (188), and thalictrifoline (189) from Corydalis tomentella displayed moderate hepatoprotective activities; the values of relative survival rates were 34.25–47.51% at a concentration of 10 μM28. The isomeric 187 and 188 obtained from roots of Fumaria parviflora also showed nematocidal activity91 (Figure 7).
In 2014, compound 180 and tetrahydrocoptisine (190) were isolated from the roots of Corydalis turtschaninovii77. A new compound 13-carboxaldehyde-8-oxocoptisine (191) together with 177 were isolated from the traditional Chinese preparation Coptidis Rhizoma-Euodiae Fructus couple, used to treat gastrointestinal disorders61. Corydalis hendersonii and Coptis japonica were found to contain tetrahydropalmatine (192) and 8-hydroxy-7,8-dihydrocoptisine (1 93), respectively27,73. Cavidine (194) was isolated from Corydalis impatiens92. In 2016, (−)-stylopine (195), (−)-sinactine (196) and (−)-cheilanthifoline (197) were isolated from aerial parts of Fumaria officinalis24. The latter compound also was found in Sinomenium acutum81. Phellodendrine (198) was identified from Phellodendri chinensis cortex93. In 2017, a new glycoalkaloid, (−)-1-O-β-d-glucoside-8-oxotetrahydropalmatine (199), isolated from tubers of Stephania succifera, exhibited antimicrobial activity against Staphylococcus aureus71. N-Methylcanadine (200) was isolated from Zanthoxylum tingoassuiba94, and demethylalangiside (201), alangiside (202) and isoalangiside (203) were identified and isolated from Ophiorrhiza nutans95. Subsequently, in 2018, it was shown that scoulerine (204) from Corydalis dubia exhibited promising suppression of cancer cell growth96. (Figure 8).
Figure 8.
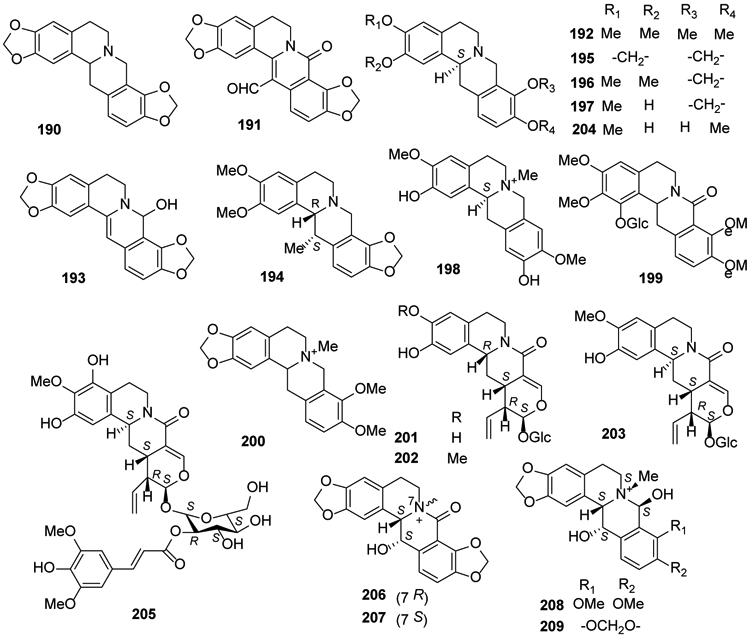
The Chemical Structures of Compounds 190-209
2′-O-trans-Sinapoylisoalangiside (205) was identified from Alangium longiflorum97. Four new isoquinoline alkaloids rupestrines A-D (206-209) and the known 195 were identified from Corydalis rupestris98 (Figure 8).
2.5. Protopine isoquinoline alkaloids
Compounds from this classification have a 5,6,7,8,13,14-hexahydrodibenzo[c,g]azecine skeleton. They lack the B/C bond and, thus, are tricyclic (6-10-6) with a 10-membered ring between two phenyl rings. Only two compounds of this type were identified during the past five years. Protopine (210) and cryptopine (211) were isolated from Fumaria officinalis24, and the former compound also was found in Corydalis mucronifera99 (Figure 9).
Figure 9.
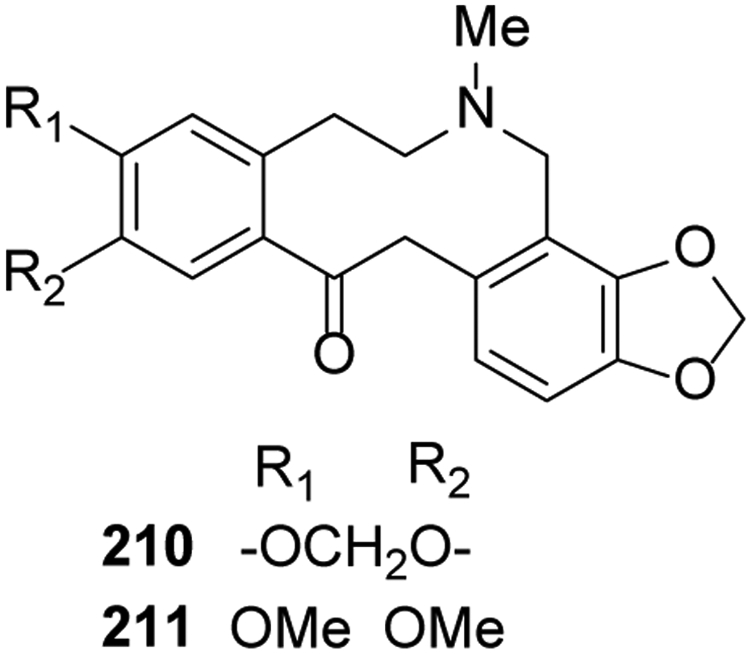
The Chemical Structures of Compounds 210-211
2.6. Naphthylisoquinoline alkaloids
Naphthylisoquinolines are a group of structurally diverse secondary metabolites containing both naphthalene and isoquinoline bicyclic systems connected by a C,C or C,N biaryl axis. These chiral compounds are mostly found only in two palaeotropic families, Dioncophyllaceae and Ancistrocladaceae. Dioncophyllaceae-type alkaloids have a R-configuration at C-3 and always lack an oxygen function at C-6. The structurally similar Ancistrocladaceae-type alkaloids are found in the closely related Ancistrocladaceae plant family. Among the studies over the past two decades on the isolation and bioactivity evaluation of naphthylisoquinoline alkaloids, extensive work has been published by Bringmann et al.21, 100-113.
From 2014 to the present, numerous new compounds were isolated in investigations by several research groups on Asian lianas. The approximately 60 structurally divergent monomeric and dimeric naphthylisoquinoline alkaloids exhibit all seven known C,C-coupling types (5,1′, 5,3′, 5,8′, 7,1′, 7,3′, 7,6′, and 7,8′). The twigs and stems of the Chinese liana Ancistrocladus tectorius contained five new 5,8′-coupled naphthylisoquinolines, ancistectorine D (212), its 6-O-demethyl derivative (213), ancistrotectoriline A (214), ancistrotanzanine B (215), and ancistroealaine A (216), three new 7,1′-linked alkaloids, 6-O-methylancistectorine B1 (217), ancistectorine B2 (218), and 6-O-demethyl-8-O-methyl-7-epi-ancistrobrevine D (219), and twenty 5,1′-linked naphthylisoquinoline alkaloids ancistrobenomines B (220) and C (221), 6-O-methylancistectorine A3 (222), 4′-O-demethylancistectorine A2 (223), ancistectorine A3 (224), ancistrocladine (225), hamatine (226), 5′-O-demethylhamatine (227), ancistrocline (228), ancistrocladinine (229), hamatinine (230), ancistectorine A2 (231) and its atropo-diastereomer 5-epi-ancistectorine A2 (232), ancistrobenomine A (233), 6-O-methylancistrocladine (234), 6-O-methylhamatine (235), 4′-O-demethylancistrocladine (236), 5′-O-demethylhamatine (237), 6-O-methylhamatinine (238) and 5′-O-demethylhamatinine (239)105,106. Although some compounds were already known from related Asian and African Ancistrocladus species, they were discovered from A. tectorius for the first time, such as a monomeric alkaloid, korupensamine D (240)110. From this species, two unique pentacyclic N,C-coupled naphthylisoquinolines, ancistrocyclinones A (241) and B (242), also were discovered, as well as six known N,C-coupled alkaloids, viz., ancistrocladinium A (a/b) (243), 4′-O-demethylancistrocladinium A (a/b) (244), 6,4′-O,O-didemethylancistrocladinium A (a/b) (245), ancistrotectorine B1 (246), shuangancistrotectorine C (247), ancistrotectoquinone B (a/b) (248) and compounds 161 and 222107 (Figures 10, 11).
Figure 10.
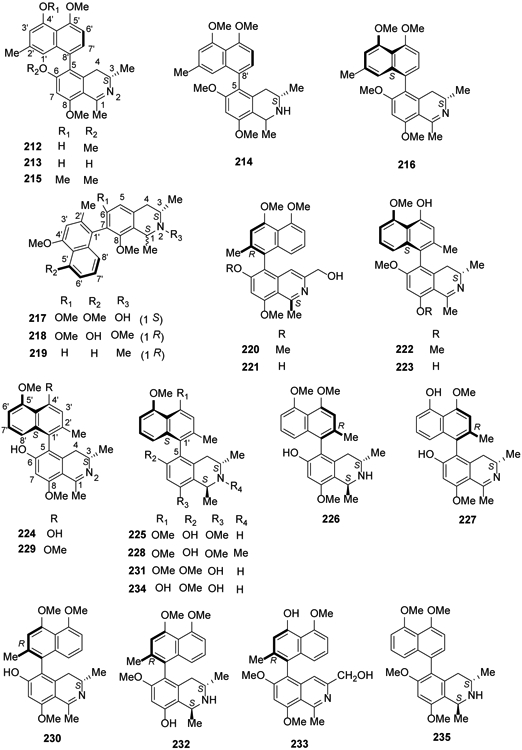
The Chemical Structures of Compounds 212-235
Figure 11.
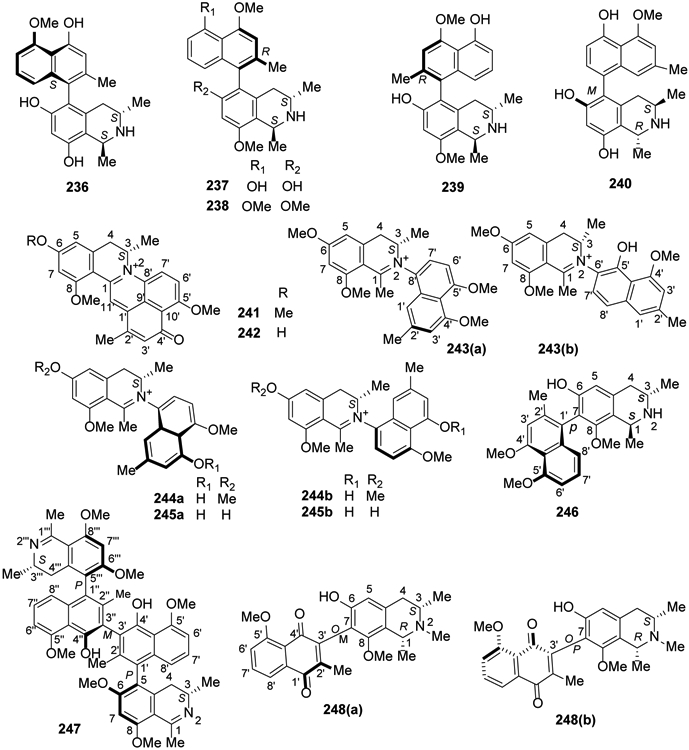
The Chemical Structures of Compounds 236-248
In 2017, the first 5,8′-coupled Dioncophyllaceous alkaloid, dioncophylline F (249), together with dioncophyllines C2 (250), D2 (251), and three known compounds, 5′-O-methyldioncophylline D (252), dioncophylline A (253) and 4′-O-demethyldioncophylline A (254) were isolated from the Congolese liana Ancistrocladus ileboensis108. Moreover, the Ancistrocladaceae-type compound ancistrocladisine B (255) (oxygenated at C-6 and S-configured at C-3), together with four known alkaloids, 225, ancistrobrevine C (256), ancistrocladisine A (257) and ancistrobertsonine D (258) also were identified. Four new C,C-coupled compounds, ancistroyafungines A-D (259-262), and eleven known C,C- and N,C-linked analogs, including compounds 214, 235, 236 and 243, ancistroguineine A (263), ancistrobertsonine A (264), ancistrobrevine B (265), 6,5′-O,O-didemethylancistroealaine A (266), 6-O-demethylancistroealaine A (267), 7-epi-ancistrobrevine D (268) and ancistrocladinium B (269), were isolated from an unidentified Ancistrocladus plant109 (Figure 12).
Figure 12.
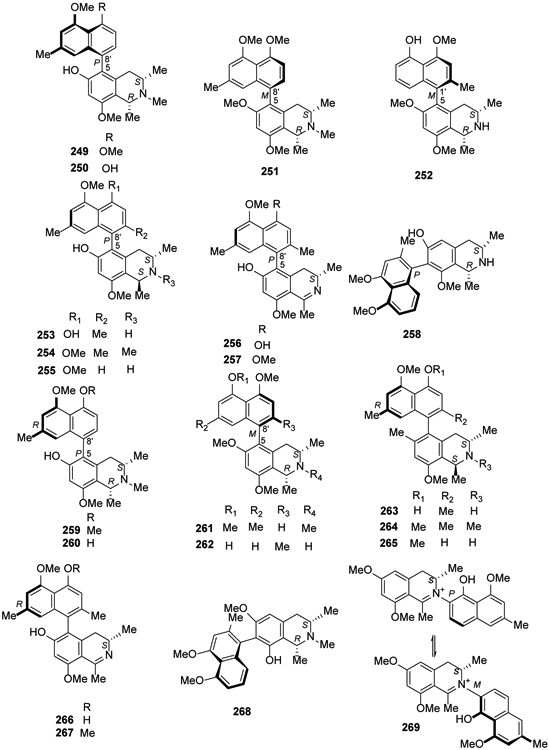
The Chemical Structures of Compounds 249-269
In 2016, five new michellamine-type dimeric naphthylisoquinoline alkaloids, named michellamines A2, A3, A4, B2, and B3 (270-274), were isolated from the root bark of the Central African liana Ancistrocladus congolensis, along with their two known parent compounds, michellamines A (275) and B (276)110. More recently in 2018, michellamines A6 (277) and A7 (278), the first dimeric 5,8′-coupled naphthylisoquinoline alkaloids with cis-configured stereocenters in both tetrahydroisoquinoline subunits, were isolated from the leaves of an unidentified Congolese Ancistrocladus liana together with two new dimeric analogs, michellamines B4 (279) and B5 (280)111 (Figure 13). In addition, ancistrobonsolines A1 (281) and A2 (282), unique naphthyldihydroisoquinolines with an M-configured biaryl axis and R-configuration at C-3, together with five known compounds, ancistroealaine C (283), korupensamines A (284) and B (285), 270 and michellamine E (286) were reported111 (Figure 14).
Figure 13.
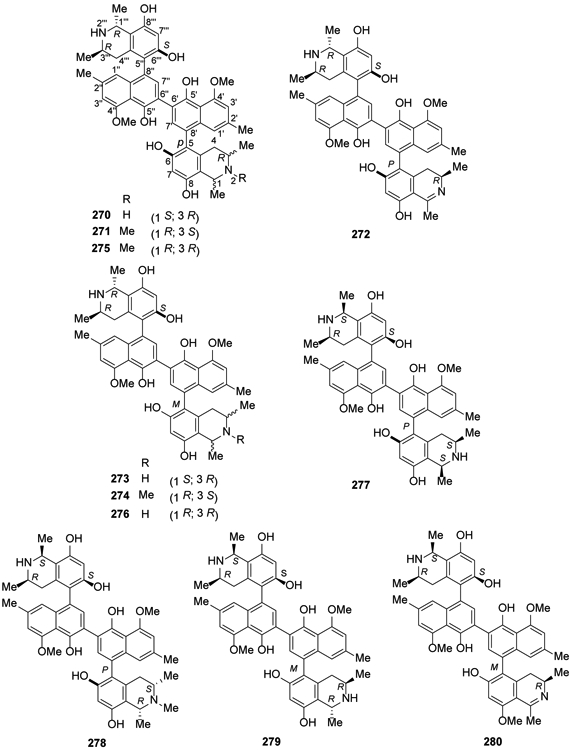
The Chemical Structures of Compounds 270-280
Figure 14.
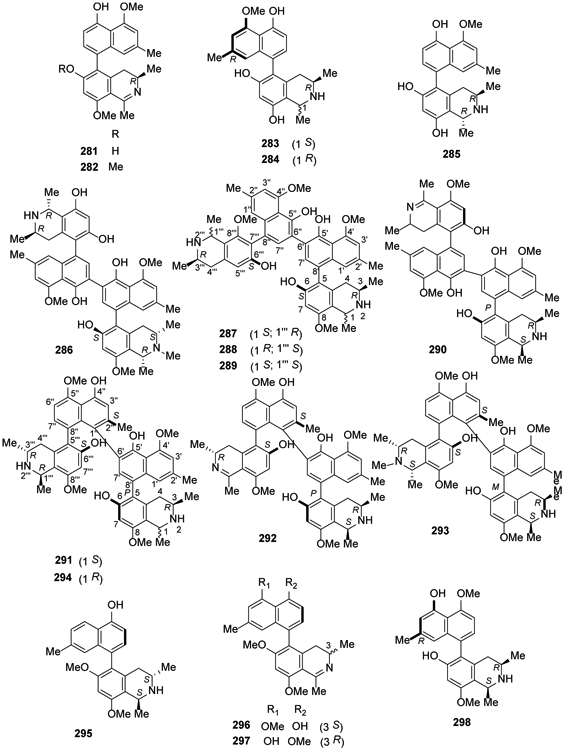
The Chemical Structures of Compounds 281-298
In 2017, ealapasamines A-C (287-289), three unusual new heterodimeric naphthylisoquinoline alkaloids, were obtained from the leaves of the Congolese Ancistrocladus ealaensis112 (Figure 14). These ‘mixed’, constitutionally unsymmetrical dimers are the first cross-coupled products of a 5,8′- and a 7,8′-coupled naphthylisoquinoline linked via C-6′ in both naphthalene segments. Previously, dimers with a central 6,6″-axis were found only from two African Ancistrocladus species112. The following year, four new [(michellamine A5 (290), mbandakamines C-E (291-293)] and one known [mbandakamine A (294)] dimeric naphthylisoquinoline alkaloids were isolated in another study on A. ealaensis32,113. Four new 5,8′-coupled monomeric naphthylisoquinolines, ancistroealaines C-F (283, 295-297) as well as five known compounds 214, 243, 245, 284 and ancistrolikokine B (298) were isolated from the same plant113 (Figure 14).
2.7. Phenanthridine alkaloid
2.7.1. Benzophenanthridine alkaloid
Benzophenanthridine isoquinoline compounds occur only in higher plants and show a wide spectrum of non-specific biological activities as well as multiple pharmacological properties. Sanguinarine (299) (Figure 15), the most extensively studied alkaloid of this group, exhibits many biological effects, such as antibacterial114, antifungal115,116, anti-inflammatory117, antioxidant118, antiviral119, nematicidal120, antitumor121, immunomodulatory122, and insecticidal123,124 activities.
Figure 15.
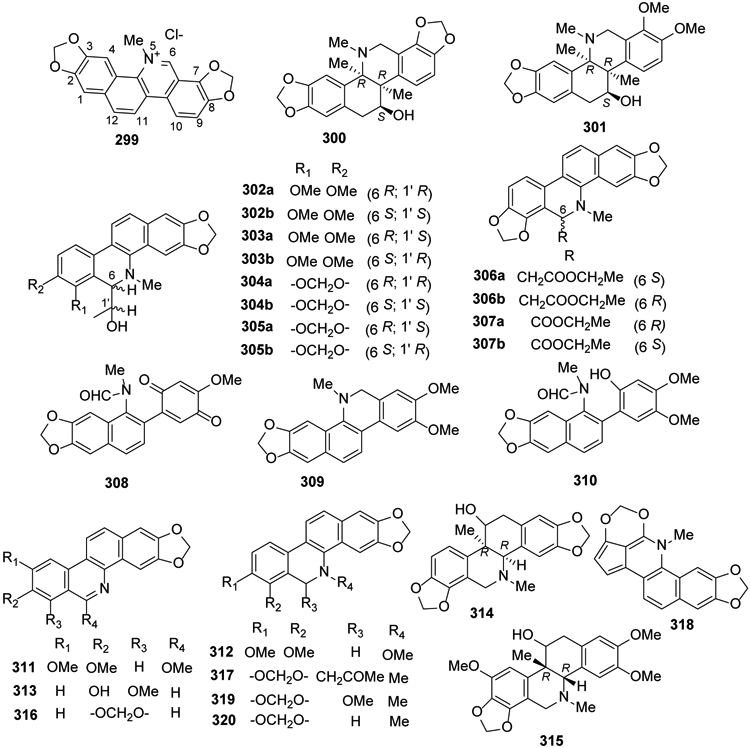
The Chemical Structures of Compounds 299-320
Chelidonine (300) and homochelidonine (301), two B/C-cis-11-hydroxyhexahydrobenzo[c]phenanthridine alkaloids classified as partially hydrogenated-type congeners, were isolated and described as the main natural constituents of Chelidonium majus125. From the same plant, six pairs of 6-monosubstituted dihydrobenzophenanthridine alkaloids were separated as corresponding six scalemic mixtures from the aerial parts. Two scalemic mixtures were assigned as (1′R,6R/1′S,6S)- and (1′S,6R/ 1′R,6S)-1-(dihydrochelerythrine-6-yl) ethanol (302, 303), two as (1′R,6R)/(1′S,6S)- and (1′S,6R)/ (1′R,6S)-1-(dihydrosanguinarine-6-yl)ethanol (304, 305), one as (±)-ethyl 2-(dihydrosanguinarine-6-yl) acetate (306), and one as (±)-ethyl dihydrosanguinarine-6-carboxylate (307) (Figure 15)126.
Heitziquinone (308), a new benzophenanthridine alkaloid, together with dihydronitidine (309), isoarnottianamide (310), rhoifoline B (311) were found as minor compounds from a hexane extract of Zanthoxylum heitzii stem bark127. Furthermore, dihydrocheleryhtrine (312) was isolated from Z. tingoassuiba94 and decarine (313) was identified from Z. myriacanthum var. pubescens bark128.
The genus Corydalis contains many benzophenanthridine alkaloids. Corynoline (314) from Corydalis bungeana possesses anti-inflammatory and antibacterial activities129. Ambinine (315), the major alkaloid of tuber C. ambigua var. amurensis tuber, produces protective effects on H9C2 myocardial cells130. Norsanguinarine (316), (−)-6-acetonyldihydrisanguinarine (317) and cavidilinine (318) were isolated from C. tomentella28, and compound 317 also was found in the whole plant of C. pallida90. 8-Methoxydihydrosanguinarine (319) and dihydrosanguinarine (320) were obtained from C. mucronifera99 (Figure 15).
2.7.2. Pyrrolophenanthridine alkaloids
The pyrrolephenanthridines have a non-linear tetracyclic structure (6-6-6-5) containing three six-membered rings (“phenanthridine”) and one five-membered ring (“pyrrole”). The N-atom and two carbons are common to the phenanthrene and pyrrole, while the points of fusion result in either a pyrrolo[3,2,1-de]phenanthridine (e.g., 321) or a 5,10b-ethanophenanthridine (e.g., 326, 328).
Lycorine-type alkaloids, including lycorine (321), acetycaranine (322), caranine (323), galanthine (324), 9-O-demethylgalanthine (325), as well as α-crinane types, haemanthamine (326), haemanthidine (327), and β-crinane types, ambelline (328), 11-O-acetylambelline (329), 1-O-acetylbulbisine (330), undulatine (331), crinamidine (332), buphanamine (333) and srinine (334), were isolated from Zephyranthes robusta, Chlidanthus fragrans, Nerine bowdenii and Narcissus poeticus cv. Brackenhurst by Cahlíková and collegaues, these compounds show moderate antitumor activities131-136. In 2018, a novel lycorine-related iminium salt, 6,7,11b,11c-didehydrolycorinium salt (335), as well as the above compounds were isolated from bulbs of both Crinum firmifolium and C. hardyi137. Seco-isopowellaminone (336), 326 and incartine (337) also were isolated from Narcissus poeticus cv. Pink Parasol138 (Figure 16).
Figure 16.
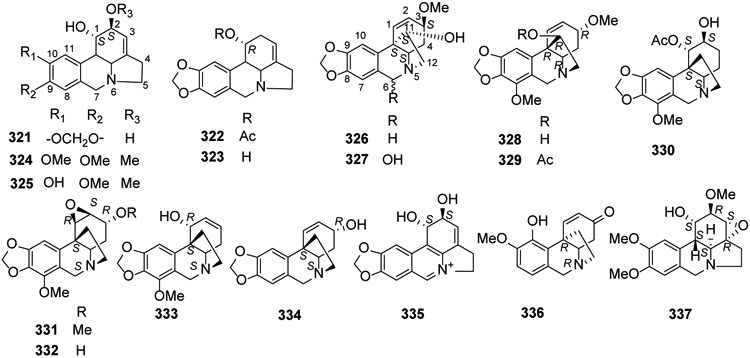
The Chemical Structures of Compounds 321-337
2.8. Manzamine alkaloids
The isoquinoline ring in manzamine alkaloids is both attached to a β-carboline (9H-pyrido[3,4-b]indole) heterocycle and fused with two polycyclic N-containing systems. Since manzamine A hydrochloride (keramamine A, 338) was initially isolated from an Okinawan sponge in 1986, almost 100 natural manzamines have been isolated from Indian and Pacific sponges9,139-145. In 2017, five new manzamine alkaloids, kepulauamine A (339), manzamine B N-oxide (340), 3,4-dihydromanzamine B N-oxide (341), 11-hydroxymanzamine J (342), and 31-hydroxymanzamine A (343), together with new hydrogen chloride salts of the known manzamine J N-oxide and 3,4-dihydromanzamine J N-oxide, as well as five known manzamine alkaloids, 32,33-dihydro-31-hydroxymanzamine A (344), 338, 6-deoxymanzamine X (345), manzamine B (346), and neo-kauluamine (347), a manzamine dimer, were isolated from an Indonesian Acanthostrongylophora sp. sponge146 (Figure 17).
Figure 17.
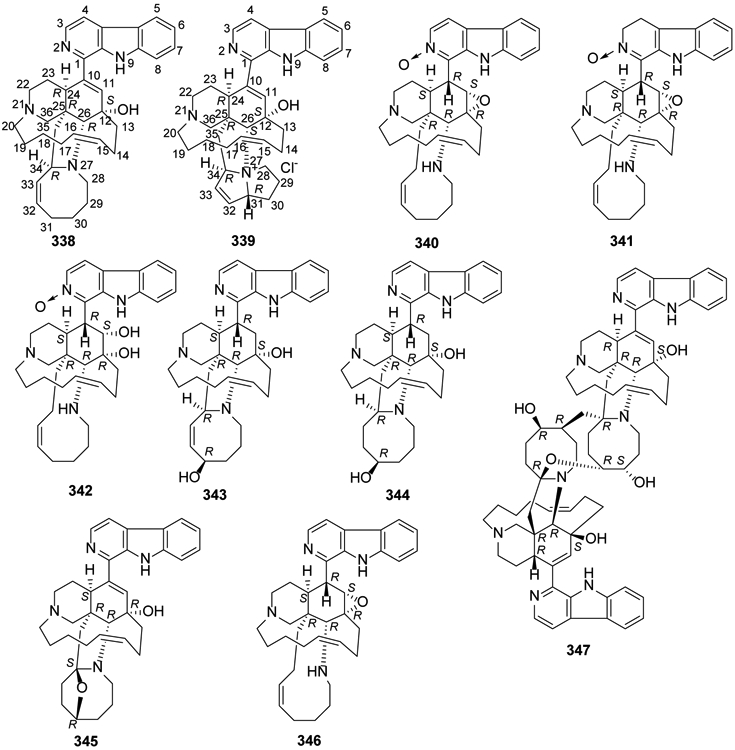
The Chemical Structures of Compounds 338-347
2.9. Emetine isoquinoline alkaloids
Emetine (348) as well as its analogs are present in three plant families, Alangiaceae, Icacinaceae, and Rubiaceae. Structurally, 348 contains both pyridoisoquinoline and isoquinoline heterocycles linked through a methylene bridge. Another heterocycle found in compounds from this classification is a 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole. Previous studies showed that 348 can be used as an emetic and expectorant,147 and recently, its antiviral and anti-trypanosomes activities were proved148,149. In 2017, a new emetine isoquinoline alkaloid, 7′,10-dide-O-methylcephaeline (349), as well as the known 10-O-demethylprotoemetine (350) were identified and isolated from Ophiorrhiza nutans95. In 2018, two new alkaloids of this type, 8-hydroxytubulosine (351) and 9-demethyltubulosine (352), were isolated from Alangium longiflorum97 (Figure 18).
Figure 18.
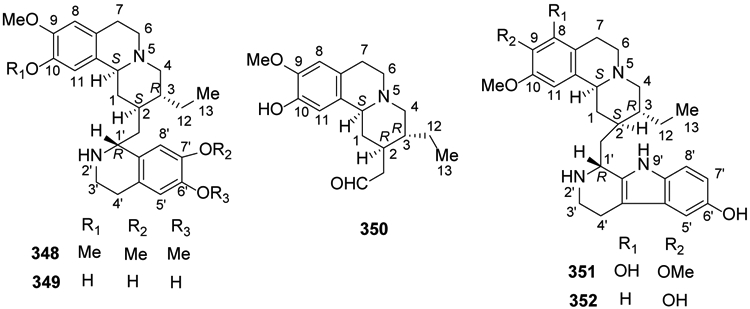
The Chemical Structures of Compounds 348-352
2.10. Morphine isoquinoline alkaloids
Like aporphine alkaloids, morphine alkaloids have a 1-benzylisoquinoline skeleton with one additional ring closure. However, the added bond is between the 2′-carbon in the pendant phenyl ring and carbon 4a, rather than 1a, at the isoquinoline ring junction. Morphinan or 1,3,4,9,10,10a-hexahydro-2H-10,4a-(azanoethano)phenanthrene is the prototype chemical skeleton of this alkaloid classification. However, compounds with several structural variations, including rearranged (e.g., spiro) or additional rings, are found as well.
In 2014, two morphinandienones, (+)-sebiferine (353) and (−)-milonine (354), were isolated from Dehaasia longipedicellata38. Also, new bistetrahydroisoquinolines with morphinane-proaporphine and morphinane-benzyltetrahydroisoquinoline types, sinomacutines A–C (355-357), and cephalonine-2-O-β-d-glucopyranoside (358), together with sinomenine (359) and sinoacutine (360) were isolated from the rhizomes of Sinomenium acutum40.
Subsequently, two new compounds, 8-demethoxycephatonine (361) and 7(R)-7,8-dihydrosinomenine (362), along with eight morphine alkaloids, 359, 8-demethoxyrunanine (363), 14-episinomenine (364), sinomenine N-oxide (365), salutaridine (366), acutumine (367), acutumidine (368) and dauricumine (369) were isolated from a rhizome extract of Sinomenium acutum81. Then in 2018, the morphinadienone pallidine (370) was found for the first time in Unonopsis floribunda36 and O-methylflavinantine (371) was isolated from Thalictrum cirrhosum43 (Figure 19).
Figure 19.
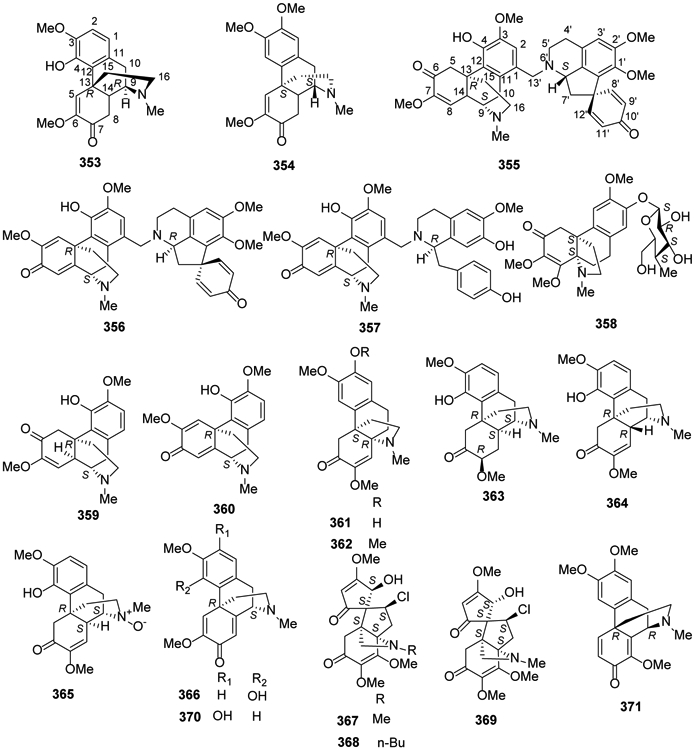
The Chemical Structures of Compounds 353-371
2.11. Phthalideisoquinoline alkaloids
As indicated by the classification’s name, tetracyclic phthalideisoquinoline alkaloids contain both bicyclic isoquinoline and bicyclic phthalide (fused benzene and gamma-lactone ring) systems. From the basic structure of a 1-benzylisoquinoline, the ester functionality (O-C=O) forming the lactone is inserted between the benzyl linking carbon and an alpha-carbon on the pendant phenyl ring.
Two phthalideisoquinoline alkaloids, (+)-bicuculline (372) and (+)-corlumine (373), were isolated from Fumaria officinalis and Viola tianschanica in 2016 and 2017, respectively24,150. Three undescribed isoquinolines, (9S,7′S) tomentelline A (374), (9S,7′R) tomentelline A (375), (9R,7′S) tomentelline B (376) together with adlumidine (377) and (+)-capnoidine (378) were isolated for the first time from Corydalis tomentella28. Five pairs of isoquinoline alkaloid enantiomers, mucroniferanines A–E (379-383), two inseparable epimeric pairs, mucroniferanines F (384) and G (385), and five known isoquinoline alkaloids, 377, (±)-hypecorinine (386), (−)-7′-O-methylegenine (387), sibiricine (388) and (+)-humosine A (389) were obtained from C. mucronifera99. Capnoidine (390) was isolated from a third related species, C. dubia151 (Figure 20).
Figure 20.
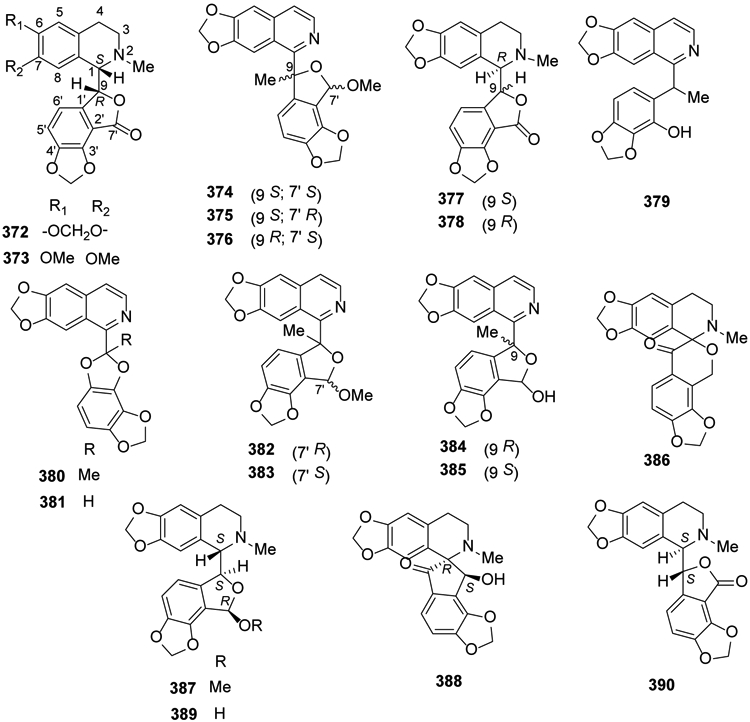
The Chemical Structures of Compounds 372-390
2.12. Benzopyrroloisoquinoline alkaloids
Seldom found in nature, the benzopyrroloisoquinolines have a linear tetracyclic structure (6-6-6-5) containing two aromatic six-membered rings, one non-aromatic six-membered heterocyclic ring and one five-membered heterocyclic ring. Thus, the alkaloid N-atom and one adjacent carbon are shared by benzopyrrole and isoquinoline systems. In 2017, a dimeric benzopyrroloisoquinoline alkaloid, tengerensine (391) with a rare unsymmetrical cyclobutane adduct was isolated from Ficus fistulosa var. tengerensis152 (Figure 21).
Figure 21.
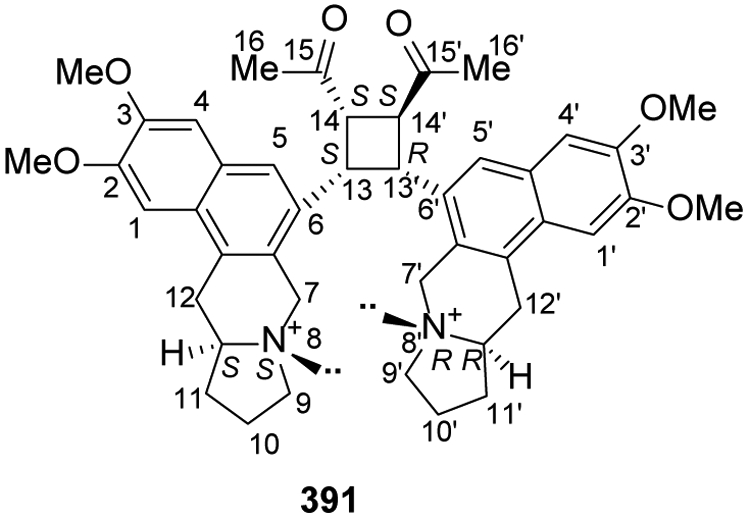
The Chemical Structure of Compound 391
2.13. Phenylethyltetrahydroisoquinoline alkaloids
The simplest compounds are 1-phenylethylisoquinolines (-CH2CH2C6H5) rather than 1-benzylisoquinolines (-CH2C6H5). However, more complex rearranged compounds, including those with a tetracyclic 6-7-5-6 system, also belong to this classification. In 2016, the new compound fumarostrejdine (392) and its known oxo-derivative (±)-O-methylfumarofine (393) were isolated from Fumaria officinalis24 (Figure 22).
Figure 22.
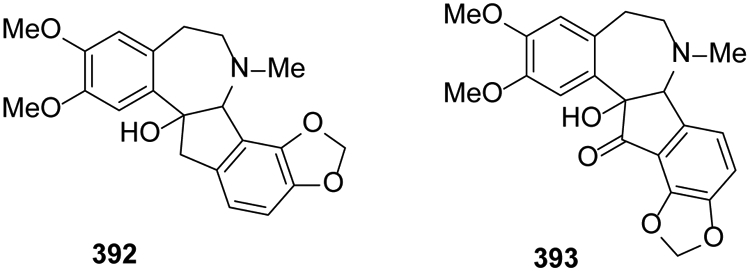
The Chemical Structures of Compounds 392-393
2.14. Various isoquinoline alkaloids
In 2014, a new alkaloid, coptichine (394), from the Coptidis Rhizoma-Euodiae Fructus couple showed significant cytotoxicity against NCI-N87 cells61. Coptisonine (395) from Coptis chinensis showed significant stimulation of glucose uptake89. Sallisonine D (396) was isolated from the rhizomes of Sinomenium acutum40.
A new compound, alternamine A (397) was isolated from the aerial parts of Alternanthera littoralis153. The phenethylisoquinoline alkaloid (±)-7-benzyloxy-1-(3-benzyloxy-4-methoxyphenethyl)-1,2,3,4-tetrahydro-6-methoxy-2-methylisoquinoline oxalate (398) was targeted as a novel ABCB1 inhibitor based on high-throughput screening of a chemical library154 (Figure 23).
Figure 23.
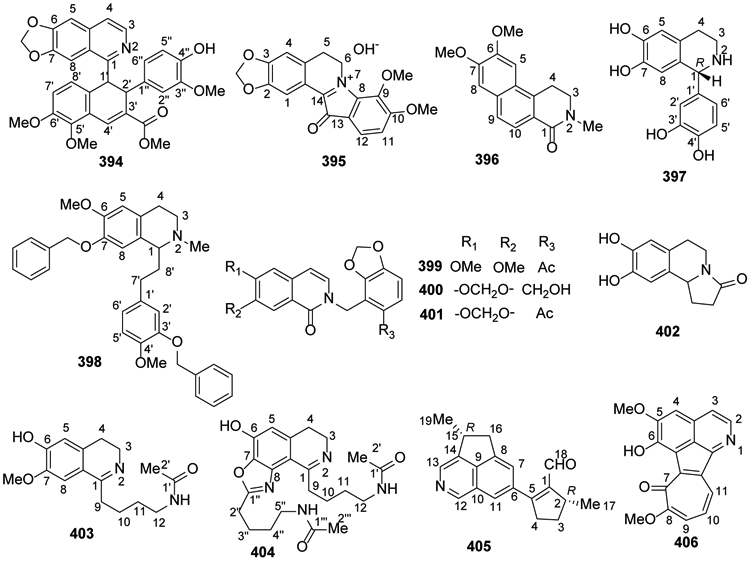
The Chemical Structures of Compounds 394-406
Tomentelline C (399), tomentelline D (400), and 6,7-methylenedioxy-2-(6-acetyl-2,3-methylenedioxybenzyl)-1(2H)-isoquinolinone (401) were obtained for the first time from Corydalis tomentella. They exhibited hepatoprotective activities28. Oleracein E (402) was isolated from the medicinal plant Portulaca oleracea31. Two undescribed isoquinoline alkaloids, pipermullesines B (403) and C (404), were isolated from the aerial parts of Piper mullesua155.
Zhang et al.156 isolated a structurally unusual cyclopenta[de]isoquinoline alkaloid, delavatine A (405), from Incarvillea delavayi. It exhibited substantial cytotoxicity and anti-inflammatory activities157. A novel tropoloisoquinoline alkaloid, neotatarine (406), was isolated from a 95% ethanol extract of the rhizome parts of Acorus calamus L in 2017158 (Figure 23).
3. Bioactivities
3.1. Antitumor activities
3.1.1. Cytotoxic activity
In the search to find potential antitumor agents from isoquinoline alkaloids, the most commonly studied bioactivity is the cytotoxicity of new isolated and known compounds from plants. In this review, we list in Table 2 the inhibitory rates and the IC50 values of compounds against various cancer cell lines corresponding to different human tumors, such as HL-60 (acute promyelocytic leukemia), Jurkat (acute T cell leukemia), MOLT-4 (acute lymphoblastic leukemia), A549 (lung carcinoma), H1299 (non-small cell lung cancer), COLO-201 (colorectal adenocarcinoma), AGS (gastric adenocarcinoma), PANC-1 (pancreas epithelioid carcinoma), A2780 (ovarian carcinoma), HeLa (cervix adenocarcinoma), MCF-7 (breast adenocarcinoma) and SAOS-2 (osteosarcoma). From this table, we found that most compounds exhibited moderate cytotoxicity with IC50 values ranging from 10 to 50 μM22, 25, 26, 33, 38, 40, 45, 46, 61, 85, 97, 105-109, 111, 113, 125, 136, 146, 152,156,159.
Table 2.
The cytotoxic activity of isoquinoline alkaloids
| Compound | Cell lines or organism | Biological results | Positive drug | Ref. |
|---|---|---|---|---|
| 3,8-Diolisoquinoline (1) | HT-29 U87 A549 Bel-7402 MGC-803 Hela cells |
4.40 μM (IC50) 3.46 μM (IC50) 6.20 μM (IC50) 8.05 μM (IC50) 25.75 μM (IC50) >30.00μM (IC50) |
Paclitaxel 0.77 μM (IC50), 2.74 μM (IC50), 2.67 μM (IC50), 1.98 μM (IC50), 3.87 μM (IC50), 0.90 μM (IC50) | 22 |
| 1-Methoxy-4,5-diolisoquinoline (2) | HT-29 U87 A549 Bel-7402 MGC-803 Hela cells |
1.19 μM (IC50) 2.14 μM (IC50) 2.46 μM (IC50) 4.10 μM (IC50) 9.73 μM (IC50) 16.15 μM (IC50) |
Paclitaxel 0.77 μM (IC50), 2.74 μM (IC50), 2.67 μM (IC50), 1.98 μM (IC50), 3.87 μM (IC50), 0.90 μM (IC50) | 22 |
| 4-Methoxy-1,5-dihydroisoquinoline (3) | HT29 A549 Bel7402 MGC803 U87 |
18.63 μM (IC50) 29.25 μM (IC50) 29.92 μM (IC50) 35.26 μM (IC50) 41.20 μM (IC50) |
-- | 23 |
| 6,7-Dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid (7) | Huh-7 | 13.97 μM (EC50) | 26 | |
| (−)-Reticuline (19) | A549 A375 BxPC-3 |
>200.00 μM (IC50) 97.60 μM (IC50) 82.57 μM (IC50) |
Cisplatin 17.52 μM, 35.9 μM, 26.86 μM (IC50) | 38 |
| Hernandezine (43) | pcDNA-HEK 293 (parental) MDR19-HEK293 (resistant) KB-3-1(parental) KB-V-1 (resistant) |
3.85, 27.25 nM (IC50, 500 nm + Doxorubicine); 0.11 and 0.10 μM (IC50, 500 nM + Doxorubicine) |
5.28 and 504.65 nM (IC50, Doxorubicine only) 0.15 and 5.07 μM (IC50, doxorubicine only) |
45 |
| 6,7,12-Trimethoxy-2-methyl-13-hydroxy-11-(4′-formylphenoxy) benzylisoquin oline (44) | GSC-3# | 43.15 μM (IC50) | Taxol IC50 15.92 μM; Temozolomide IC50 > 257.53 μM. | 46 |
| (−)-O-O-Dimethylgrisabine (51) | A549 A375 BxPC-3 |
>200.00 μM (IC50) 82.85 μM (IC50) >200.00 μM (IC50) |
Cisplatin 17.52 μM, 35.90 μM, 26.86 μM (IC50) | 38 |
| Coptichic aldehyde (63) | NCI-N87 Caco-2 |
30.14 μM (IC50) >100.00 μM (IC50) |
Vinorelbine 12.19 μM (IC50), 21.64 μM (IC50) | 61 |
| (−)-Boldine (77) | A549 A375 BxPC-3 |
117.57 μM (IC50) 112.53 μM (IC50) 45.50 μM (IC50) |
Cisplatin 17.52 μM, 35.90 μM, 26.86 μM (IC50) | 38 |
| (−)-Norboldine (78) | A549 A375 BxPC-3 |
>200.00 μM (IC50) 82.89 μM (IC50) 27.06 μM (IC50) |
Cisplatin 17.52 μM, 35.90 μM, 26.86 μM (IC50) | 38 |
| (+)-8-(4′-Formylphenoxy) glaucine (113) | GSC-3# | 40.48 μM (IC50) | Taxol IC50 15.92 μM; Temozolomide IC50 > 257.53 μM. | 46 |
| (+)-3-Methoxy-8-(4′-formylphenoxy) glaucine (115) | GSC-3# | 30.12 μM (IC50) | Taxol IC50 15.92 μM; Temozolomide IC50 > 257.53 μM. | 46 |
| 1,2,3,9,10-Pentamethoxy-11-(4′-formylphenoxy)-7-oxoaporphine (136) | GSC-3# | 32.52 μM (IC50) | Taxol IC50 15.92 μM; Temozolomide IC50 > 257.53 μM. | 46 |
| 1,2,9,10-Tetramethoxy-11-(4′-formylphenoxy)-7-oxoaporphine (137) | GSC-3# | 32.81 μM (IC50) | Taxol IC50 15.92 μM; Temozolomide IC50 > 257.53 μM. | 46 |
| Dehydrocrebanine (138) | HeLa MDA-MB231 MCF-7 |
18.73 μM (IC50) 14.52 μM (IC50) 10.64 μM (IC50) |
Paclitaxel 2.29 μM (IC50), 2.56 μM (IC50), 3.99 μM (IC50) | 85 |
| Crebanine (139) | HeLa MDA-MB231 MCF-7 |
48.13 μM (IC50) 38.94 μM (IC50) 30.50 μM (IC50) |
Paclitaxel 2.29 μM (IC50), 2.56 μM (IC50), 3.99 μM (IC50) | 85 |
| Stephanine (140) | HeLa MDA-MB231 MCF-7 |
3.33 μM (IC50) 5.66 μM (IC50) 6.49 μM (IC50) |
Paclitaxel 2.29 μM (IC50), 2.56 μM (IC50), 3.99 μM (IC50) | 85 |
| O-Methylbulbocapnine (141) | HeLa MDA-MB231 MCF-7 |
70.37 μM (IC50) 56.59 μM (IC50) 39.36 μM (IC50) |
Paclitaxel 2.29 μM (IC50), 2.56 μM (IC50), 3.99 μM (IC50) | 85 |
| Berberine (151) | HL60 AZ521 SK-BR-3 B16 melanoma |
29.40 μM (IC50) 2.60 μM (IC50) 21.00 μM (IC50) Melanin content 8.9% at 10 μM |
Cisplatin 4.20 μM (IC50), 9.50 μM (IC50), 18.80 μM (IC50) Melanin content 92.7% for arbutin at 10 μM |
33 |
| 8-Oxocoptisine (168) | NCI-N87 Caco-2 |
20.31 μM (IC50) >100.00 μM (IC50) |
Vinorelbine 12.19 μM (IC50), 21.64 μM (IC50) | 61 |
| 13-Carboxaldehyde-8-oxocoptisine (182) | NCI-N87 Caco-2 |
35.98 μM (IC50) >100.00 μM (IC50) |
Vinorelbine 12.19 μM (IC50), 21.64 μM (IC50) | 61 |
| Ancistectorine D (203) | CCRF-CEM CEM/ADR5000 |
4.50 μM (IC50) 25.83 μM (IC50) |
Doxorubicin 0.02 μM (IC50), 30.07 μM (IC50) | 105 |
| Ancistrotectoriline A (205) | PANC-1 | 67.80 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Ancistrobenomine B (211) | CCRF-CEM CEM/ADR5000 |
3.50 μM (IC50) 21.38 μM (IC50) |
Doxorubicin 0.02 μM (IC50), 30.07 μM (IC50) | 106 |
| 6-O-Methylhamatine (226) | PANC-1 | 31.90 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| 4′-O-Demethylancistrocladine (227) | PANC-1 | 11.20 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Ancistrocyclinone A (231) | CCRF-CEM CEM/ADR5000 | 16.21 μM (IC50) 25.32 μM (IC50) |
Doxorubicin 0.02 μM, 30.07 (IC50) | 107 |
| Ancistrocladinium A (233) | CCRF-CEM CEM/ADR5000 | 1.40 μM (IC50) >100.00 μM (IC50) |
Doxorubicin 0.02 μM, 30.07 (IC50) | 107 |
| 4′-O-Demethylancistrocladinium A (234) | CCRF-CEM CEM/ADR5000 | 1.50 μM (IC50) >100.00 μM (IC50) |
Doxorubicin 0.02 μM, 30.07 μM (IC50) | 107 |
| 6,4′-O,O-Didemethylancistrocladinium A (235) | CCRF-CEM CEM/ADR5000 | >100.00 μM (IC50) >100.00 μM (IC50) |
Doxorubicin 0.02 μM, 30.07 μM (IC50) | 107 |
| Dioncophylline F (239) | L6 cells INA-6 PMBCs |
14.52 μM (IC50) 21.00 μM (EC50) 16.00 μM (EC50) |
Podophyllotoxin 0.02 μM (IC50) Melphalan 2.00 μM (EC50); 3.00 μM (EC50) |
108 |
| Dioncophylline C2 (240) | L6 cells | 43.31 μM (IC50) | Podophyllotoxin 0.02 μM (IC50) |
108 |
| Dioncophylline D2 (241) | L6 cells INA-6 |
62.84 μM (IC50) 32.00 μM (EC50) |
Podophyllotoxin 0.02 μM (IC50) Melphalan 2.00 μM (EC50) |
108 |
| 5′-O-Methyldioncophylline D (242) | L6 cells INA-6 PMBCs |
4.02 μM (IC50) 2.60 μM (EC50) 19.00 μM (EC50) |
Podophyllotoxin 0.02 μM (IC50) Melphalan 2.00 μM (EC50); 3.00 μM (EC50) |
108 |
| Dioncophylline A (243) | INA-6 | 0.22 μM (EC50) | Melphalan 2.00 μM (EC50) | 108 |
| 4′-O-Demethyldioncophylline A (244) | INA-6 INA-6 PMBCs |
2.70 μM (EC50) P 16.00 μM (EC50) M 50.00 μM (EC50)M |
Melphalan 2.00 μM (EC50) 3.00 μM (EC50) |
108 |
| Ancistrobrevine C (246) | L6 cells | 34.85 μM (IC50) | Podophyllotoxin 0.02 μM (IC50) | 108 |
| Ancistrocladisine A (247) | L6 cells INA-6 |
30.01 μM (IC50) 4.80 μM (EC50) |
Podophyllotoxin 0.02 μM (IC50) Melphalan 2.00 μM (EC50) |
108 |
| Ancistroyafungine A (249) | PANC-1 | 22.70 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Ancistroyafungine B (250) | PANC-1 | 7.60 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Ancistroyafungine C (251) | PANC-1 | 15.00 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Ancistroyafungine D (252) | PANC-1 | 9.70 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Ancistroguineine A (253) | PANC-1 | 15.80 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Ancistrobertsonine A (254) | PANC-1 | 11.80 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Ancistrobrevine B (255) | PANC-1 | 20.20 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| 6,5′-O,O-Didemethylancistroealaine A (256) | PANC-1 | 9.80 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| 6-O-Demethylancistroealaine A (257) | PANC-1 | 14.00 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| 7-Epi-ancistrobrevine D (258) | PANC-1 | 29.90 μM (PC50) | Arctigenin 0.80 μM (PC50) | 109 |
| Michellamine A2 (260) | Hela PANC-1 |
32.10 μM (IC50) 19.30 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Michellamine A6 (268) | Hela PANC-1 |
14.80 μM (IC50) 54.20 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Michellamine A7 (269) | Hela PANC-1 |
20.60 μM (IC50) 24.30 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Michellamine B4 (270) | Hela PANC-1 |
46.30 μM (IC50) 50.30 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Michellamine B5 (271) | Hela PANC-1 |
29.80 μM (IC50) 60.20 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Ancistrobonsoline A1 (272) | Hela PANC-1 |
14.30 μM (IC50) 7.50 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Ancistrobonsoline A2 (273) | Hela PANC-1 |
21.50 μM (IC50) 12.10 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Ancistroealaine C (274) | Hela PANC-1 |
30.50 μM (IC50) >100.00 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Korupensamine A (275) | Hela PANC-1 |
48.30 μM (IC50) >100.00 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Korupensamine B (276) | Hela PANC-1 |
37.80 μM (IC50) 94.90 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Michellamine E (277) | Hela PANC-1 |
8.80 μM (IC50) 18.90 μM (IC50) |
5-Fluorouracil 13.90 μM; Arctigenin 0.80 μM (IC50) | 111 |
| Mbandakamine A (281) | CCRF-CEM CEM/ADR5000 | 7.40 μM (IC50) 23.88 μM (IC50) |
Doxorubicin 0.02 μM, 30.07 μM (IC50) | 113 |
| Mbandakamine C (282) | CCRF-CEM CEM/ADR5000 | 1.50 μM (IC50) 27.71 μM (IC50) |
Doxorubicin 0.02 μM, 30.07 μM (IC50) | 113 |
| Mbandakamine D (283) | CCRF-CEM, CEM/ADR5000 | 2.96 μM (IC50) 19.03 μM (IC50) |
Doxorubicin 0.02 μM, 30.07 μM (IC50) | 113 |
| Ancistroealaine F (288) | CCRF-CEM CEM/ADR5000 | 11.69 μM (IC50) 19.94 μM (IC50) |
Doxorubicin 0.02 μM, 30.07 μM (IC50) | 113 |
| Chelidonine (291) | MOLT-4, Jurkat, HL-60, Raji, U-937, HEL 92.1.7., PBMCs, MRC-5, WI-38 | 4.60, 2.20, 4.40, 3.20, 5.00, 3.40, >10.00, 1.8., >10.00 μM (IC50) | -- | 125 |
| Homochelidonine (292) | MOLT-4, Jurkat, HL-60, Raji, U-937, HEL 92.1.7., PBMCs, MRC-5, WI-38 | 4.80, 5.60, 8.30, 6.80, >10.00, >10.00, >10.00, >10.00, >10.00 μM (IC50) | 125 | |
| Lycorine (312) | HL-60, Jurkat, MOLT-4, A549, H1299, COLO- 201, HT-29, SW-480, AGS, PANC-1, A2780, HeLa, BT-549, MCF-7, MDA-MD-231, SAOS-2, SK-BR-3 | 0.80-1.40 μM (IC50) | -- | 136 |
| Haemanthamine (317) | HL-60, Jurkat, MOLT-4, A549, H1299, COLO-201, HT-29, SW-480, AGS, PANC-1, A2780, HeLa, BT-549, MCF-7, MDA-MD-231, SAOS-2, SK-BR-3 | 0.30-9.80 μM (IC50) | -- | 136 |
| Haemanthidine (318) | HL-60, Jurkat, MOLT-4, A549, H1299, COLO- 201, HT-29, SW-480, AGS, PANC-1, A2780, HeLa, BT-549, MCF-7, MDA-MD-231, SAOS-2, SK-BR-3 | 1.60-9.70 μM (IC50) | -- | 136 |
| Manzamine A (329) | A549 K562 |
8.30 μM (IC50) 11.00 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| Kepulauamine A (330) | A549 K562 |
4.60 μM (IC50) 7.20 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| Manzamine B N-oxide (331) | A549 K562 |
12.00 μM (IC50) 9.80 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| 3,4-Dihydromanzamine B N-oxide (332) | A549 K562 |
5.80 μM (IC50) 5.20 μM (IC50) |
Doxorubicin 0.92μM (IC50) and 1.1μM (IC50) | 146 |
| 11-Hydroxymanzamine J (333) | A549 K562 |
6.20 μM (IC50) 8.20 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| 31-Hydroxymanzamine A (334) | A549 K562 |
5.80 μM (IC50) 7.20 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| 32,33-Dihydro-31-hydroxymanzamine A (335) | A549 K562 |
8.20 μM (IC50) 8.40 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| 6-Deoxymanzamine X (336) | A549 K562 |
6.70 μM (IC50) 9.10 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| Manzamine B (337) | A549 K562 |
6.50 μM (IC50) 9.60 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| neo-Kauluamine (338) | A549 K562 |
13.00 μM (IC50) 12.00 μM (IC50) |
Doxorubicin 0.92 μM (IC50) and 1.10 μM (IC50) | 146 |
| 8-Hydroxytubulosine (342) | A549 MDA-MB-231 MCF-7 KB KB-VIN |
0.21 μM (IC50) 0.06 μM (IC50) 0.12 μM (IC50) 0.09 μM (IC50) 8.90 μM (IC50) |
Doxorubicin 0.48 μM, 0.78 μM (IC50), 0.72 μM (IC50), 0.82 μM (IC50), >1.00 μM (IC50) | 97 |
| 9-Demethyltubulosine (343) | A549 MDA-MB-231 MCF-7 KB KB-VIN |
0.36 μM (IC50) 0.19 μM (IC50) 0.25 μM (IC50) 0.29 μM (IC50) >10.00 μM (IC50) |
Doxorubicin 0.48 μM, 0.78 μM (IC50), 0.72 μM (IC50), 0.82 μM (IC50), >1.00 μM (IC50) | 97 |
| (+)-Sebiferine (344) | A549 A375 BxPC-3 |
>200.00 μM (IC50) >200.00 μM (IC50) 93.39 μM (IC50) |
Cisplatin 17.52 μM, 35.90 μM, 26.86 μM (IC50) | 38 |
| (−)-Milonine (345) | A549 A375 BxPC-3 |
>200.00 μM (IC50) >200.00 μM (IC50) >200.00 μM (IC50) |
Cisplatin 17.52 μM, 35.90 μM, 26.86 μM (IC50) | 38 |
| (+)-Tengerensine (382) | MDA-MB-468 | 7.40 μM (IC50) | Paclitaxel 0.01 μM (IC50) | 152 |
| Coptichine (385) | NCI-N87 Caco-2 |
8.92 μM (IC50) >100.00 μM (IC50) |
Vinorelbine 12.19 μM (IC50), 21.64 μM (IC50) | 61 |
| Noroxyhydrastinine (389) | B16 melanoma cells | Melanin content 76.1% at 10.00 μM | Melanin content 92.7% for arbutin at 10.00 μM | 33 |
| Delavatine A (401) | MCF7 HCT116 SKOV3 SMMC-7721 HeLa |
22.32 μM (IC50) 19.90 μM (IC50) 15.43 μM (IC50) 17.27 μM (IC50) 23.83 μM (IC50) |
Celastrol 2.04 μM (IC50), 3.20 μM (IC50), 3.93 μM (IC50), 1.31μM (IC50), 1.73 μM (IC50) | 156 |
| Neotatarine (402) | PC12 | At 2.00, 4.00, 8.00 μM inhibit Aβ25-35 induced cell death | -- | 158 |
INA-6 Multiple Myeloma Cells and Peripheral Mononuclear Blood Cells (PMBCs); Human breast cancer cell lines: MDA-MB-468, HL-60 (acute promyelocytic leukemia), Jurkat (acute T cell leukemia), MOLT-4 (acute lymphoblastic leukemia), A549 (lung carcinoma), H1299 (non-small cell lung cancer), COLO-201 (colorectal adenocarcinoma), HT-29 (colorectal adenocarcinoma, p53 mutant), SW-480 (colorectal adenocarcinoma), AGS (gastric adenocarcinoma), PANC-1 (pancreas epithelioid carcinoma), A2780 (ovarian carcinoma), HeLa (cervix adenocarcinoma), BT-549 (breast ductal carcinoma, triple negative), MCF-7 (breast adenocarcinoma), MDA-MD-231 (breast adenocarcinoma, triple negative), SAOS-2 (osteosarcoma) and SK-BR-3 (breast adenocarcinoma, p53-deficient), Huh-7 cells (human hepatic carcinoma cell line), Parental pcDNA3.1-HEK293, MDR19-HEK293 (HEK293 cells transfected with human ABCB1), GSC-3# (glioma stem cells); CCRF-CEM (human leukemia cells), CEM/ADR5000 (human multi-drug-resistant tumor cells).
NR: not reached.
--: No determination
Three Amaryllidaceae alkaloids, lycorine (321), haemanthamine (326) and haemanthidine (327), showed the best cytotoxicity against 17 human cell types with individual IC50 values in the range of 0.30-9.80 μM compared with other compounds listed in Table 2. Higher antiproliferative effects also were reported. Unfortunately, the cytotoxic activities of positive agents were not investigated in this reference136. The alkaloids caused cells to accumulate preferentially at G1 and G2 stages of the cell cycle with increased p16 expression and Chk1 Ser345 phosphorylation. Concerning a pro-apoptotic effect in the Jurkat leukemia cell line, compound 327 was more active than 326130. These results also provided a new clue for developing these alkaloids as potential antitumor agents.
8-Hydroxytubulosine (351) from Alangium longiflorum also exhibited remarkable antiproliferative activity. It presented better activities against A549, MDA-MB-231, MCF-7 and KB cell lines than the positive drug doxorubicin and the known alkaloid 9-demethyltubulosine (352). The IC50 values of 351 were 0.21, 0.06, 0.12 and 0.09 μM, respectively97.
The aporphine alkaloid (−)-norboldine (86) exhibited potent cytotoxicity towards pancreatic cancer cell line BxPC-3 with an IC50 value of 27.06 μM, but no toxicity towards the normal pancreatic cell line60. Two oxoaporphines, 145 and 146, showed cytotoxicity against glioma stem cells (GSC-3#) with IC50 values of 32.52 and 32.81 μM, respectively, while the IC50 of the antitumor drug taxol was 15.92 μM46. However, the antitumor activity in vivo and the mechanism underlying the cytotoxicity of the compounds are still unclear and should be studied further.
3.1.2. Mechanism of action
During the past five years, numerous studies have investigated and reported antitumor mechanism of known and new isolated isoquinoline alkaloids. In this section, we will briefly introduce the antitumor mechanisms of some prominent molecules.
Berberine (161) shows antitumor effects against various tumor cells. Noteworthy, it presents the strongest cytotoxicity against AZ521 cell with the IC50 value of 2.60 μM, while the IC50 of the antitumor drug cisplatin was 9.50 μM. Berberine inhibited cancer cell proliferation via several mechanisms of action, such as the positive regulation of reactive oxygen species and the apoptotic pathway as well as suppressed cancer metastasis by stopping transferase activity160-163. The potential targets also include mitochondrial function, DNA topoisomerase and arylamin N-acetyltransferase activity, NF-κB signal pathway, the EGF and the VEGF receptors, etc. In human hepatoma cells, the alkaloid’s antiproliferative effect on might be mediated via the CAR metabolic and the arachidonic acid pathways, cPLA2, COX-2 gene expression and mitochondria-mediated apoptosis also were suppressed in vitro and in vivo164-166, and the IC50 values against human hepatoma Bel-7404, H22 and HepG2 HCC cells were 9.21, 43.20 and 82.80 μM, respectively. However, the cytotoxicity of berberine against the normal hepatic embryonic cells was weak, the IC50 value was 122.4 μM for the HL-7702 at 72 h. The further report showed that it blocked the caspase 3-iPLA2-AA-COX-2-PGE2 pathway of ovarian cancer cells and reversed the repopulation, which was triggered by the chemotherapy drug VP16167. In breast tumors, berberine significantly down-regulated the expression of NF-κB and proliferating cell nuclear antigen (PCNA) In vivo168. However, the targets of berberine against the different breast cancer are different, it activated caspase-9/cytochrome c-mediated apoptosis to inhibit the growth of two triple negative breast cancer cell (TNBC) lines (IC50 43.28 μM for BT549 and 47.51 μM for MDA-MB-231 cells) in vitro 169 and inhibited the proliferation and migration of breast cancer ZR-75-30 cells (IC50 5.30 μM) by targeting Ephrin-B2170.
Reports indicated that berberine mediates epigenetic reprogramming via HDAC inhibition and regulates Bcl-2/Bax family proteins in the human lung cancer A549 cell line171. Furthermore, it inhibited the growth of intestinal polyps in animals and patients with the familial adenomatous polyposis and cell growth in colon cancer by down-regulating β-catenin signaling via binding RXRα172. In human glioblastoma cells, berberine induced senescence by down-regulating the EGFR-MEK-ERK signaling pathway173, and modulated the expression of epigenetic regulators in acute myelocytic leukemia cell lines HL-60/ADR and KG1-α174. The PI3K-Akt and mitogen-activated protein kinase (MAPK) signaling pathways in the treatment of thyroid carcinoma also were affected175,176. As an berberine isoquinoline alkaloid, coptisine (165) also affected PI3K/Akt and mitochondrial-associated apoptotic pathways177, it exhibited remarkably cytotoxic activities against HCT-116 cells by activating the caspase protease family, inducing G1-phase cell cycle arrest and increasing apoptosis178.
The protoberberine alkaloid palmatine (166) induced cell apoptosis in MCF-7 breast cancer cells, after the treatment (1 μM, 10.8 J/cm2) the early apoptotic and late apoptotic rates increased significantly up to 21.16% and 9.86% in photodynamic therapy179. Meanwhile, protoberberine stylopine (195) both functioned as an AKR1C3 inhibitor and significantly inhibited the AKR1C3-mediated reduction of the anthracycline drug daunorubicin within cells, the IC50 was 0.9 μM in DHO assay180. Liensinine (62) induced apoptosis and mitochondrial dysfunction, and significantly inhibited the proliferation and colony-forming ability of colorectal cancer cells accompanied by activation of the JNK signaling pathway in a dose-dependent manner181. However, the structurally related neferine (61) sensitized A549 cells to low doses of doxorubicin and inhibited human lung cancer cell growth through MAPK activation and cell cycle arrest182,183.
Through the mitochondria apoptosis pathway, 3,8-diolisoquinoline (1) and 1-methoxy-4,5-diolisoquinoline (2) induced apoptosis in U87 cells with the IC50 values of 3.46 and 2.14 μM, the Bcl-2/Bax protein ratio also was down-regulated22. 6,7-Dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid (7) had a significant anti-proliferative effect on human hepatoma (Huh-7) cells in vitro (EC50 13.97 μM) by inhibiting the action of caspase-8184, and blocked IL-6/JAK2/STAT3 oncogenic signaling in dimethylhydrazine-induced colorectal carcinoma185. Good in vivo anti-neoplastic properties were also found184.
Certain naphthylisoquinoline alkaloids are noteworthy due to their anti-pancreatic cancer activity. In one study109, ancistroyafungines A–D (259–262), 6-O-methylhamatine (235), 4′-O-demethylancistrocladine (236), ancistroguineine A (263), ancistrobertsonine A (264), ancistrobrevine B (265), ancistrotectoriline A (214), 6,5′-O,O-didemethylancistroealaine A (266), 6-O-demethylancistroealaine A (267), 7-epi-ancistrobrevine D (268), and ancistrocladiniums A (243) and B (269) showed moderate to strong anti-austerity activities against PANC-1 pancreatic cancer cells in a concentration-dependent manner. Their preferential cytotoxicity (PC)50 values ranged from 7.60 to 67.80 μM. Among of these compounds, compound 259 (PC50, 22.7 μM) was found to be almost three times less active than its 5′-O-demethyl analog compounds 260 and 262 (PC50, 7.60 μM and 9.70 μM, respectively), which has two methoxy functions at C-5′ and C-4′. Structure-activity relationship (SAR) analysis indicated that O-methylation in the naphthalene portion and the substitution pattern of the isoquinoline portion play a crucial role for the cytotoxic activities of the alkaloids, especially an OMe/OH pattern seems favorable for the activity. In a second study,111 ancistrobonsolines A1 (281) and A2 (282) also displayed significant PC against PANC-1 cells under nutrient-deprived conditions. Above reports suggest that the naphthylisoquinoline alkaloids are promising lead structures for the advancement of antitumor agents.
The benzophenanthridine alkaloids chelidonine (300) and homochelidonine (301) potently induced cell death in several blood cancer cell lines, such as MOLT-4, Jurkat, HL-60, Raji, PBMCs, MRC-5 and WI-38, their IC50 values ranged from 1.80 to >10 μM. For MOLT-4 and Jurkat cells, treatment with chelidonine induced cell cycle arrest at the G2/M cell cycle (IC50 4.60 and 2.20 μM, respectively); treated with homochelidonine underwent biphasic dose-dependent G1 and G2/M cell cycle arrest in MOLT-4 cells (IC50 4.80 μM), and an increase in G2/M cell population in Jurkat cells (IC50 5.60 μM). Both alkaloids inhibited tubulin polymerization in A549 cells125.
In addition, lycorine (321) presented the good therapeutic effect in a patient-derived glioblastoma xenograft by directly interacting with and inhibiting the activation of EGFR cancer cells186. The potent cytotoxicity against other various cancer cells, including HL-60, Jurkat, MOLT-4, A549, also were found with IC50 values from 0.80 to 1.40 μM. Noroxyhydrastinine (22) exhibited potent melanogenesis-inhibitory activities by inhibiting the expression of protein levels of tyrosinase, TRP-1, and TRP-2 partly in a-MSH-stimulated B16 melanoma cells, the melanin content was 76.10% at 10.00 μM33,159.
Multidrug-resistant (MDR) cancers present a critical clinical problem. Bringmann et al. have investigated the effects of naphthylisoquinolines. Mbandakamines C, D and F (291, 2923, 297) showed strong cytotoxic effects against human leukemia (CCRF-CEM) and MDR tumor cells (CEM/ADR5000) with IC50 values from 1.50 to 19.94 μM113. This result indicated that the axial chirality is necessary to the bioactivity. Ancistectorine D (212) and ancistrobenomine B (220) also demonstrated comparable cytotoxic effects against both cell lines (IC50 4.5 and 3.5 μM for 212; 25.83 and 21.38 μM for 220; 0.017 and 30.07 μM for positive control doxorubicin)105,106. The overexpression of ATP-binding cassette (ABC) transporters is a common mechanism leading to MDR cancer cells. Tetrandrine (57) and tangchinoline (58) from Stephania tetrandra reversed multidrug resistance by increasing the intracellular concentration of anticancer drugs and inhibiting P-glycoprotein activity in the MDR human cancer cells Caco-2 and CEM/ADR5000, the IC50 values were 19.38 and 24.98 μM respectively for compound 5749. Hernandezine (51) selectively inhibited the transport function of the ABC drug transporter ABCB1 and enhanced drug-induced apoptosis in cancer cells at nanomolar concentrations (IC50 3.85-27.25 nM). It could be further developed as a novel reversal agent for combination therapy in patients with MDR cancer due to its nontoxicity45.
Scoulerine (204) exhibited promising suppression of cancer cell growth and reduced the mitochondrial dehydrogenases activity of the evaluated leukemic cells with IC50 values ranging from 2.70 to 6.50 μM. Further study showed that it also interfered with microtubule elements of the cytoskeleton, checkpoint kinase signaling and p53 proteins96.
3.2. Effect on diabetes and its complications
Diabetes mellitus (DM) is mainly characterized by abnormal hyperglycemia, polydipsia, polyuria, polyphagia, and emaciation187,188, and persistent hyperglycemia can lead to several chronic diabetic complications, including neuropathy, nephropathy, cardiopathy, and retinopathy189. Currently, the global prevalence of DM is 8.5% among adults and is rising most rapidly in middle- and low-income countries190. Natural products have been increasingly applied to treat DM191,192. Over the past five years, the beneficial effects of berberines and protoberberine alkaloids on DM, atherosclerosis and hyperlipidemia have been proved in different animal models193,194.
Berberine (161) affects multiple pathways, including p38 MAPK-GLUT4, JNK, and PI3K-Akt, related to the metabolism of glucose and lipids89,195. For anti-diabetic activity, berberine regulated the glyco- and lipo-metabolism and stimulated of adenosine 5′-monophosphate-activated protein kinase (AMPK)196 in Zucker diabetic fatty (ZDF) rats, and also inhibited miR-106b/SIRT1 pathway by reversing miR-106b over-expression and up-regulating sirtuin 1 (SIRT1) both in islets of diabetic mice and pancreatic NIT-1 cells induced by high glucose88,197. By activating the AMPK pathway, the pioglitazone-induced bone loss in diabetic rats also were protected198. The toxic towards to mice or rats in vivo test is weak. Meanwhile, it may improve insulin resistance by increasing the expression of adiponectin receptors and the ratio of high-molecular weight to total adiponectin in rats fed a high fat diet (HFD)199, and improved glucose uptake and insulin-stimulated glucose consumption in palmitate-induced insulin-resistant H9c2 cardiomyocytes200-202.
DM is closely related to the development of cardiovascular diseases203. Vascular dysfunction is a distinctive phenotype in DM, and diabetic vascular complication is associated with impaired endothelial function, augmented vasoconstriction, and increased oxidative stress204. Berberine can exert a cardio-protective effect by attenuating myocardial apoptosis via Notch1/Hes1-PTEN/Akt signaling as well as inhibiting excessive autophagy in cardiomyocytes through the regulation of AMPK and mTOR signaling205,206. In addition, berberine relieved cerebral arterial contractility in a STZ-induced diabetic rat model by regulating intracellular Ca2+ management in smooth muscle cells and, thus, has an extra-protective effect on diabetic vascular dysfunction207.
Diabetic nephropathy (DN) is a major cause of morbidity and mortality in patients with diabetes and is highly prevalent in end-stage renal disease208. Many studies have reported that berberine exhibits renoprotective effects in DN rats via regulating the various pathways, such as the PGE2-EP1-Gαq-Ca2+ signaling pathway209, TLR4/NF-κB210 and S1P2/MAPK signaling pathway211. Tang et al.212 suggested that berberine (50-100 mg/kg) improved histopathological changes in the diabetic kidney, while it significantly reversed the diabetic-induced increases in the levels of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) as well as the decreases in the levels of β-arrestins 1 and 2. In NIT-1 pancreatic β cells, it inhibited PA-induced lipid accumulation by decreasing lipogenesis and increasing lipid oxidation213. In an experimental diabetic kidney model and high glucose-cultured glomerular mesangial cells (GMCs), berberine suppressed the expression of FN, ICAM-1, and TGF-β1 possibly by negatively regulating NF-κB and RhoA/ROCK214,215. Furthermore, it inhibited the Sphk1/S1P signaling pathway and MAPK activation, lowered AP-1 activity, and ultimately deceased fibronectin overproduction216-218. In 2016, Zhou et al.219 reported that berberine can positively affect DN by improving micro pathology and increasing neuritin expression via the MAPK pathway. In diabetic rats, the alkaloid exhibited renoprotective effects by changing the levels and regulation of the AGEs-RAGE-PKC-b-TGF-b1 signaling pathway220.
Obesity has become a worldwide public health problem. It is an established risk factor for metabolic diseases including type 2 diabetes221,222 and closely related to the metabolism of triacylglycerol (TG) in adipocytes. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase are rate-limiting enzymes that control the hydrolysis of TG. Berberine affects the metabolism of TG223 by increasing the expression of ATGL and therefore stimulating basal lipolysis in mature adipocytes through the coupled mechanisms linked to the AMPK pathway224. In addition, coptisine (165), a related alkaloid, inhibited obesity-related inflammation in Syrian golden hamsters through the LPS/TLR4-mediated signaling pathway225. The aporphine isoquinoline alkaloid stephalagine (94) could be used as a potential anti-obesity agent due to its significant pancreatic lipase inhibitory activity (IC50 8.35 μg/ml).70
In 2016, berberine (161), coptisine (165), palmatine (166), epiberberine (163), and jatrorrhizine (162) were evaluated for antihyperglycemic, antidyslipidemic and antidiabetic hyperlipidemic effects in HepG2 cells and diabetic KK-Ay mice226. All five alkaloids effectively modulated hyperglycemia and hyperlipidemia. Berberine and coptisine promoted glucose consumption in vitro as well as suppressed fasting blood glucose level and improved glucose tolerance in vivo. In the mice, the levels of serum total cholesterol and triglycerides were decreased by palmatine and jatrorrhizine. Moreover, diminished hepatomegaly was found in jatrorrhizine-treated mice226. SAR analysis showed that the methylene-dioxy groups at C2, C3, C9, and C10 positions are the key functional groups for the antihyperglycemic and antihyperlipidemic effects. Briefly, the oxidized form of methylene-dioxy group at the C-2 and C-3 positions and/or at C-9 and C-10 positions of compound 163 would inhibit the activities of rat lens aldose reductase and human recombinant aldose reductase activities, and the methylene-dioxy group is very important to the binding activity of coptisine to β-cell membranes. Berberine and coptisine had better antihyperglycemic effects than compounds 162, 163, 166 that may be associated with the methylene-dioxy group at the C-2 and C-3 positions, because the C-2 and C-3 positions of the latter three compounds were substituted by methoxy group or phenolic hydroxyl group.
In a subsequent study, a combination of the five alkaloids showed synergistic cholesterol-lowering in HepG2 cells and hypercholesterolemic hamsters, which was greater than that of the single alkaloids227. Activation of AMPK activation and alteration of neutral lipid metabolism may explain the hypoglycemic effect of berberine in differentiated cardiomyocytes228. Berberine and its metabolites exert lipid-lowering effects in human hepatoma cells metabolites likely by low density lipoprotein receptor up-regulation229. A meta-analysis of randomized clinical trials indicated that berberine can improve lipid profiles in dyslipidemia with acceptable safety230.
He et al.231 suggested that the related alkaloid coptisine might be used as an anti-hypercholesterolemia agent as it inhibited cholesterol synthesis by suppressing 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) expression and increasing the use and excretion of cholesterol through up-regulation of low-density lipoprotein receptor (LDLR) and CYP7A1 expression.
Atherosclerotic coronary artery disease is a leading cause of death and disability in diabetic patients, and diabetic patients with atherosclerosis usually show moderate hyperhomocysteinemia (HHCY). Berberine increased atherosclerotic plaque stability in Apoe−/− mice with HHCY by activating the peroxisome proliferator-activated receptor-γ (PPARG) and suppressing oxidative stress in endothelial cells232. It also protected rat retinal Müller cells from high-glucose-induced apoptosis by enhancing autophagy and activating the AMPK/mTOR signaling pathway233. Acting on the TGFβ1-PI3K/Akt pathway, berberine reduced injury to podocytes caused by exosomes derived from high glucose-induced mesangial cells234. Berberine showed good effects on bone parameters in the treatment of HFD-fed/streptozocin-induced diabetic rats and, thus, could have therapeutic potential in diabetic osteoporosis235.
3.3. Antibacterial and antifungal activities
Isoquinoline alkaloids exhibit good antibacterial and antifungal activities. A high content of berberine (161) is found in the well-known Chinese drug (Huangliansu) taken to treat intestinal infections caused by Escherichia coli, Bacillus dysteriae, and other microorganisms. The authors have recently described the significant antifungal activity of sanguinarine (299) and its possible use as a bio-fungicide for crop protection236. From 2014 to 2018, many publications have reported the antibacterial and antifungal activities of isoquinoline alkaloids; findings are briefly discussed below or listed in Table 3.23,71,77,237,94,146.
Table 3.
The antibacterial and antifungal activities of isoquinoline alkaloids
| Compound | Bacteria | Biological results | Positive drug | Ref. |
|---|---|---|---|---|
| Carnegine (4) | Gentamicine | 24 | ||
|
Staphylococcus aureus
Bacillus cereus Enterococcus faecalis Escherichia coli Pseudomonas aeruginosa Klebsiella pneumoniae Proteus vulgaris |
1129.69 μM (MIC) 2259.38 μM (MIC) 1129.69 μM (MIC) 564.84 μM (MIC) 2259.38 μM (MIC) 1129.69 μM (MIC) 1129.69 μM (MIC) |
1438.11 μM (MIC) 719.06 μM (MIC) 11504.91 μM (MIC) 1438.11 μM (MIC) 1438.11 μM (MIC) 1438.11 μM (MIC) 1438.11 μM (MIC) |
||
| N-Methylisosalsoline (5) | Gentamicine | 24 | ||
|
Staphylococcus aureus
Bacillus cereus Enterococcus faecalis Escherichia coli Pseudomonas aeruginosa Klebsiella pneumoniae Proteus vulgaris |
19298.50 μM (MIC) 9649.24 μM (MIC) 9649.24 μM (MIC) 19298.50 μM (MIC) 2412.31 μM (MIC) 19298.50 μM (MIC) 19298.50 μM (MIC) |
1438.11 μM (MIC) 719.06 μM (MIC) 11504.91 μM (MIC) 1438.11 μM (MIC) 1438.11 μM (MIC) 1438.11 μM (MIC) 1438.11 μM (MIC) |
||
| N-Formyl-asimilobine-2-O-β-D-glucoside (87) | Staphylococcus aureus MRSA strains | Diameters of inhibition zones 8.0 and 8.0 mm | Kanamycin sulfate was 40 and 34 mm | 71 |
| Isomoschatoline (120) |
Staphylococcus aureus
Staphylococcus epidermidis Escherichia coli Candida albicans Candida dubliniensis |
Non-irradiated (CFU/mL) 1.55×107; 9.10 ×107; 1.10×107; 1.03×107; 9.10×107 Irradiated (CFU/mL) 1.38×104; 4.05×104; 1.12×105; 8.53×103; 6.68×103 |
Methylene blue (0.01 mg/mL) Non-irradiated 2.61×105; 6.53×104; 0; 4.78×107; >1×108 Irradiated (CFU/mL) 0; 0; 0; 0; 0 |
237 |
| Berberine (151) |
Clostridium perfringens Candida albicans |
52.20 μM (MIC) 75.53 μM (MIC) |
Ampicillin 300 nM (MIC) |
77 238 |
| Palmatine (156) | Clostridium perfringens | 44.70 μM (MIC) | Ampicillin 300 nM (MIC) | 77 |
| (−)-1-O-β-D-Glucoside-8-oxotetrahydropalmatine (190) | Staphylococcus aureus | Diameters of inhibition zones 15 mm | Kanamycin sulfate was 34 mm | 71 |
| N-Methylcanadine (191) | ATCC 25923, Clinical isolates Staphylococcus aureus strains 1-4 | 307.80 μM (MIC) 76.90 μM (MIC) 153.90 μM (MIC) 153.90 μM (MIC) 307.80 μM (MIC) |
Chloramphenicol >49.51 μM (MIC); >198.10 μM (MIC); >24.76 μM (MIC); >24.76 μM (MIC); 198.1μM (MIC) |
94 |
| Michellamine B (263) | Bacillus subtilis | 21.14 μM (MIC) | -- | 110 |
| Dihydrocheleryhtrine (303) | Clinical isolates Staphylococcus aureus strains 1-4 | 76.9 - 307.8 μM (MIC) | -- | 94 |
| Manzamine A (329) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
>0.18 μM (MIC) 0.09 μM (MIC) 0.09 μM (MIC) 0.01 μM (MIC) >0.18 μM (MIC) >0.18 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| Kepulauamine A (330) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
0.014 μM (MIC) 0.055 μM (MIC) 0.028 μM (MIC) 0.028 μM (MIC) 0.014 μM (MIC) 0.110 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| Manzamine B N-oxide (331) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
>0.176 μM (MIC) 0.176 μM (MIC) 0.176 μM (MIC) 0.088 μM (MIC) >0.176 μM (MIC) >0.176 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| 3,4-Dihydromanzamine B N-oxide (332) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
0.023 μM (MIC) 0.011 μM (MIC) 0.005 μM (MIC) 0.005 μM (MIC) 0.044 μM (MIC) >0.176 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM( MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| 11-Hydroxymanzamine J (333) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
0.003 μM (MIC) 0.007 μM (MIC) 0.007 μM (MIC) 0.014 μM (MIC) 0.003 μM (MIC) 0.027 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| 31-Hydroxymanzamine A (334) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
0.044 μM (MIC) 0.011 μM (MIC) 0.011 μM (MIC) 0.003 μM (MIC) 0.023 μM (MIC) >0.176 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| 32,33-Dihydro-31-hydroxymanzamine A (335) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
0.088 μM (MIC) 0.044 μM (MIC) 0.023 μM (MIC) 0.003 μM (MIC) 0.176 μM (MIC) >0.176 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| 6-Deoxymanzamine X (336) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
0.177 μM (MIC) 0.177 μM (MIC) 0.089 μM (MIC) 0.005 μM (MIC) >0.177 μM (MIC) >0.177 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| Manzamine B (337) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
>0.182 μM (MIC) 0.182 μM (MIC) 0.182 μM (MIC) 0.091 μM (MIC) >0.182 μM (MIC) >0.182 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
||
| neo-Kauluamine (338) | Ampicillin | 146 | ||
|
Staphylococcus aureus Bacillus subtilis Kocuria rhizophila Salmonella enterica Proteus hauseri Escherichia coli |
>0.086 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) >0.086 μM (MIC) >0.086 μM (MIC) |
0.001 μM (MIC), 0.002 μM (MIC) 0.001 μM (MIC) 0.001 μM (MIC) 0.005 μM (MIC) 0.018 μM (MIC) |
Berberine (161) exhibited antibacterial activity against Candida albicans with an MIC value of 75.53 μM. It affected the synthesis of membrane ergosterol and induced increased membrane permeability causing loss of intracellular material to the outer space (DNA/protein leakage) as well as membrane depolarization and lipid peroxidation of membrane constituents238. Berberine also effectively protected mice infected with Salmonella typhimurium239. Further studies reported that this alkaloid could treat H. pylori-induced chronic gastritis by attenuating the BAFF-triggered Th17 response87. Berberine as well as palmatine (166), coptisine (165), epiberberine (163), and jatrorrhizine (162) acted as concentration-dependent inactivators of urease with IC50 values from 3.0 to 5087 μM for HPU (Helicobacter pylori urease) and 2.3 to >10,000 μM for JBU (jack bean urease). Epiberberine was the most potent inhibitor against both ureases with IC50 values of 3.0 μM for HPU and 2.3 μM for JBU and was more effective than the standard urease inhibitor acetohydroxamic acid (83 μM for HPU and 22 μM for JBU). The further studies showed that two methoxyl groups in the A ring as the polar systems and the dimethylene group in the D ring as the hydrophobic ring system of epiberberine are the functional structural groups for the potent urease inhibition. This alkaloid could be used in the treatment of diseases associated with ureolytic bacteria and could be further developed into a promising therapeutic approach for the treatment of urease-related diseases240. Meanwhile, berberine (161) and palmatine (166) suppressed Clostridium perfringens growth with MIC values of 44.7 and 52.2 μM, respectively77.
In 2017, some manzamine alkaloids from Acanthostrongylophora sp. sponge were found to show antibacterial activity146. neo-Kauluamine (347) showed the best activity (MIC ~0.001 μM) against Bacillus subtilis, Kocuria rhizophila and Salmonella enterica, and 11-hydroxymanzamine J (342) had the best activity (MIC 0.053 μM) of all isolated compounds against Staphylococcus aureus and Proteus hauseri. MIC values of the positive drug ampicillin were around 0.001 μM. 3,4-Dihydromanzamine B N-oxide (341) and 31-hydroxymanzamine A (343) demonstrated marked activities against some tested microorganisms. neo-Kauluamine and the hydrogen chloride salt of manzamine J N-oxide displayed mild inhibition against isocitrate lyase from Candida albicans, and the latter manzamine alkaloid was the only compound with activity against bacterial sortase A146.
The simple isoquinoline alkaloid carnegine (3) showed antibacterial activity with MIC ranging from 564-2259 μM against various strains. The time-kill curves indicated potent and rapid bactericidal activity23. Michellamine B (276), a dimeric naphthylisoquinoline alkaloid, inhibited E. coli MraY (IC50 456 μM) and B. subtilis MraY (IC50 386 μM) and showed antimicrobial activity against B. subtilis241.
Photodynamic therapy was discovered at the beginning of the last century and mostly used as a cancer therapy; however, it has emerged as a promising treatment alternative against infectious diseases. The oxoaporphine alkaloid isomoschatoline (129) had an absorption profile with bands at 600-700 nm, was positive for singlet oxygen production and exhibited photodynamic antimicrobial activity against both gram-positive and gram-negative bacteria and some Candida ssp. yeast strains at sub-inhibitory concentrations237.
Methicillin-resistant Staphylococcus aureus (MRSA) is a bacterium that is resistant to many antibiotics and can cause various problems ranging from skin infections to pneumonia to bloodstream infections242. Much effort has been put into the fight against this bacterium. Berberine (161) was active in vitro against clinical isolates of MRSA and lowered the MICs of ampicillin and oxacillin243. It also was synergistic with ceftazidime and cefepime against MRSA41. Bacteria do not develop resistance to berberine since its MIC within the same bacterial cultures (E. coli, S. aureus, B. subtilis, Proteus vulgaris, S. typhimurium and Pseudomonas aeruginosa) did not increase over 200 generations244.
Dihydrocheleryhtrine (312) and N-methylcanadine (200) from Zanthoxylum tingoassuiba presented anti-MRSA activity against the four tested clinical isolates S. aureus strains 1-4 (MIC ranging from 76.9 to 307.8 μM) and were more active than chloramphenicol against strain 4 and ATCC2592394. N-Formyl-asimilobine-2-O-β-D-glucoside (95) was equipotent against S. aureus and MRSA strains; the inhibition zone against both strains was 8.0 mm in diameter. The inhibitory diameters with the positive control kanamycin sulfate were 40 and 34 mm, respectively71. Bavarsadi et al.245 reported that the administration of incremental levels of sanguinarine (299) decreased microbial counts in the ileum and improved other intestinal health indices in laying hens.
3.4. Antiviral activity
Antiviral activity is one of the important bioactivities for berberine (161). In vitro, it regulated signaling pathways related to inflammation, such as NF-κB246 and AMPK/mTOR247 signaling pathways. In addition, berberine attenuated autophagy in adipocytes by targeting BECN1248 and inhibited the replication of respiratory syncytial virus (RSV), herpes simplex virus (HSV), human papillomavirus (HPV), and human cytomegalovirus (HCMV)249-251. Berberine suppressed viral infection-induced up-regulation in the TLR7 signaling pathway, such as TLR7, MyD88, and NF-κB (p65), at both the Mrna and protein levels, as well as significantly inhibited the viral-induced increases in Th1/Th2 and Th17/Treg ratios and inflammatory cytokine production252. In addition, berberine inhibited EV71 replication by down-regulating autophagy and the MEK/ERK signaling pathway, while the 50% toxicity concentration (TC50) was 73.10 μmol/L in Vero cells and the TC50 of the positive agent pirodavir was 27.49 μmol/L253.
Corydine (90) and norisoboldine (91), aporphine alkaloids from Croton echinocarpus leaves, displayed significant in vitro anti-HIV potential. The latter compound more potently inhibited HIV-1 reverse transcriptase enzyme activity69. Several michellamine-type dimeric naphthylisoquinoline alkaloids inhibited replication of HIV reference strain IIIB/LAI in A3.01 T lymphoblast cell cultures: michellamine A2 (270) (IC50, 29.6 μM), A3 (271) (IC50, 15.2 μM), A4 (272) (IC50, 35.9 μM), and B (276) (IC50, 20.4 μM). However, michellamines A (275) and B3 (274) were not active110.
Emetine (348) inhibits protein synthesis in mammalian, yeast and plant cells by inhibiting the aminoacyl-sRNA transfer reaction at the 40S ribosomal subunit254-256. It also inhibits DNA synthesis in mammalian cells257. Emetine inhibited replication of DNA viruses [buffalopoxvirus (BPXV) and bovine herpesvirus 1 (BHV-1)] as well as RNA viruses [peste des petits ruminants virus (PPRV) and Newcastle disease virus (NDV)]. After treatment, the syntheses of viral RNA (PPRV and NDV) and DNA (BPXV and BHV-1) as well as viral entry (NDV and BHV-1) were reduced and inhibited. Emetine significantly inhibited replication of NDV. Moreover, this alkaloid significantly inhibited BPXV-induced pock lesions on chorioallantoic membrane (CAM) along with associated mortality of embryonated chicken eggs. It significantly delayed NDV-induced mortality in chicken embryos associated with reduced viral titers. Hence, emetine could have significant therapeutic value against certain viruses by inhibiting viral RNA and DNA replication without producing an antiviral drug-resistant phenotype148.
Japanese encephalitis virus (JEV) is a major cause of severe encephalopathy. Huang et al.258 suggested that the protoberberine isoquinoline alkaloid (−)-tetrahydropalmatine (192) could be a strong drug candidate for the treatment of JEV infection, because it exhibited a neuroprotective effect in a JEV strain GP-78 infected mouse model.
3.5. Anti-inflammatory and immunosuppressive activities
The major isoquinoline alkaloid berberine (161) exerts significant anti-inflammatory activity259,260 and could be used to treat inflammation and other related diseases via different mechanisms of action, for example, ischemic stroke through downregulation of pro-inflammatory cytokines and upregulation of anti-inflammatory cytokines261 or acute pancreatitis via JNK deactivation262. Meanwhile, berberine suppressed Th17 responses and improved chronic relapsing colitis induced with dextran sulfate sodium (DSS) in C57BL/6 mice263. The alkaloid also improved the survival of septic and LPS-intoxicated mice and decreased inflammation and tissue injuries in the lung, spleen and gut as well as improved disrupted energy utilization, oxidative status, amino acid metabolism and nucleic acid metabolism264,265. In macrophages, berberine has no cellular toxicity on RAW264.7 cells at the concentration up to 5 μM, however, it inhibited M1 polarization via the AKT1/SOCS1/NF-κB signaling pathway266 and exerted anti-inflammatory effects by inhibiting NF-κB signaling via Sirt1-dependent mechanisms at the same concentration267.
Furthermore, by inhibiting TH17 cell response, berberine could exert an anti-arthritic effect and improve various autoimmune diseases, such as rheumatoid arthritis268. It suppressed NLRP3 (nucleotide-binding oligomerization domain-like receptor [NLR] pyrin domain-containing-3) inflammasome activation in monosodium urate (MSU) crystal-stimulated RAW 264.7 macrophages and pro-inflammatory cytokines through the upregulation of Nrf2 (nuclear factor erythroid-2-related factor 2) transcription factor and alleviated MSU crystal-induced inflammation in rats269. One mechanism of action against rheumatoid arthritis was inhibition of IL-21/IL-21R-mediated inflammatory proliferation via attenuation of the PI3K/Akt signaling pathway and amelioration of IL-21 mediated osteoclastogenesis270.
Norisoboldine (91) exerts anti-arthritic activity via anti-inflammatory and immune-regulatory effects. Mechanism of action studies showed that this alkaloid prevented both the infiltration of inflammatory cells and destruction of bone and cartilage in joints in adjuvant-induced arthritic rats, as a substrate of P-glycoprotein (P-gp)271-273. The anti-arthritic mechanism involved inhibition of inflammatory synovial hyperplasia by promoting the release of cytochrome C and regulating the expression of Bax and Bcl-2 proteins via a mitochondrial-dependent pathway274, as well as prevention of synovial angiogenesis by moderating the Notch1 pathway-related endothelial tip cell phenotype275,276. Tong et al.277 suggested that norisoboldine induced the generation of intestinal Treg cells by the activation of AhR (aryl hydrocarbon receptor) as well as promoted Treg differentiation and then reduced the development of colitis by regulating AhR/glycolysis axis and subsequent NAD+/SIRT1/SUV39H1/H3K9me3 signaling pathway278. Furthermore, norisoboldine reduced IL-1β production in LPS-stimulated RAW264.7 cells and decreased the serum level of IL-1β in collagen-induced arthritis273,279. Finally, the compound inhibited activation of the NLRP3 inflammasome by regulating the AhR/Nrf2/ROS signaling pathway and thereby improved the TNBS (2,4,6-trinitrobenzene sulfonic acid)-induced colitis in mice271.
Nuclear transcription factor-κB (NF-κB) plays an important role in inflammation, sepsis and immunity280. Hence, great attention has been focused on compounds that produce inhibitory effects on the NF-κB pathway. Demethyleneberberine (164) reduced inflammatory responses by inhibiting the NF-κB pathway and regulating the balance of Th cells281. Chelidonine (300) also significant inhibited NF-κB activity and related pathways at the concentrations of 5-20 μM, such as the TLR4/NF-κB signaling pathway, in HCT116 cells and RAW264.7 macrophages282, and it did not display cytotoxic effect with concentrations up to 20 μM. These results indicated that NF-κB pathway is very important to the anti-inflammatory activity of isoquinoline alkaloids. In addition, this alkaloid inhibited mitogen-activated protein kinase pathway activation by blocking c-Jun N-terminal kinase and p38 phosphorylation283. Salutaridine (366), dauricumine (369), dauriporphine (128) and cheilanthifoline (197) significantly inhibited receptor activator of NF-κB ligand-induced differentiation of mouse bone marrow-derived macrophages into multinucleated osteoclasts73. Zhang et al.156 reported that delavatine A (406) significantly decreased LPS-induced activation of NF-κB by suppressing the p65 subunits and the phosphorylation of IκBα157. Palmatine (166) promoted the proliferation of goat endometrial epithelial cells at the concentrations of 10–100 μg/mL, and reduced LPS-induced inflammatory responses through inhibition of the TRIF-dependent NF-κB pathway284.
Boldine (85) and reticuline (23) from Litsea cubeba exerted anti-inflammatory activity and potential synergistic effects in vivo partly by inhibiting the expression of pro-inflammatory cytokines, such as TNF-α and IL-6, perhaps resulting from interaction with JAK2/STAT3 and NF-κB pathways67. Tetrandrine (57) inhibited IκBα and NF-κB p65 phosphorylation in LPS-induced RAW 264.7 and chondrogenic ATDC5 cells285. Coptisine (165) inhibited IL-1β-induced inflammatory responses by suppressing the NF-κB and MAPK pathways signaling pathway, as well as suppressing the expression of iNOS, COX-2, matrix metalloproteinase-3 (MMP-3) and MMP-13, and NF-κB activation in IL-1β-induced human OA chondrocytes286,287.
Other anti-inflammatory mechanisms of action of isoquinoline alkaloids were also investigated. Palmatine (166), which has been used to treat abdominal pain, enteritis, gastritis, chronic endometritis, and pelvic inflammation, exerted protective effects on acute and chronic inflammation in experimental animal models288-290. Zhou et al.291 reported that this compound exerted chondroprotective effects in IL-1β-induced rabbit chondrocytes and an experimental OA model by inhibiting the Wnt/β-catenin and Hedgehog signaling pathways. As a potent IDO-1 inhibitor, palmatine improved dextran sulfate sodium-induced colitis by mitigating colonic injury, preventing gut microbiota dysbiosis, and regulating tryptophan catabolism292.
Compared with mice administered TNBS, mice treated with capnoidine (378) showed significantly improved clinical symptoms as well as reduced colon pathology and histological inflammation in the colon. Moreover, inflammatory cytokines profiles within the colon were altered and levels of p-IκB-α (Ser32) and p-NF-κB p65 (Ser536) were reduced151.
Alkaloids from Portulaca oleracea inhibited NO production in lipopolysaccharide-induced murine macrophage RAW 264.7 cells (EC50 18.0–498 μM). Among them, oleracein E (403) and (S)-(−)-salsolinol (15) were more potent (EC50 35.4 and 58.7 μM, respectively) than the positive control 3,4-dihydroxybenzohydroxamic acid. Additionally, some alkaloids showed β2-adrenergic receptor (β2-AR) agonist activity in the CHO-K1/GA15 cell line, which stably expresses β2-AR as detected by a calcium assay. The EC50 value of (R)-(+)-1-benzyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline was 87.9 nM31.
Corynoline (314) from Corydalis bungeana inhibited inflammatory mediators in LPS-stimulated RAW264.7 cells and attenuated LPS-induced acute lung injury in mice by activating Nrf2129,293,294. The unique alkaloid dactyllactone A (126) inhibited the expression of IL-1β and PGE2 in a dose-dependent manner in LPS-induced RAW264.7 cells78. Decarine (313) significantly inhibited IL-6 and IL-8 production in TNF-α + IL-1β-induced Caco-2 cells at a concentration of 20 μM128.
6aR-2′-Carboxylthaliadine (116), 3-methoxy-2′-methoxycarbonyl-oxohernandalincin (141), predicentrine (119), oxopurpureine (143) and laudanosine (43) from Thalictrum cirrhosum. significantly inhibited T lymphocytes with IC50 values of 43.90, 40.80, 43.70, 39.70 and 42.30 μM, respectively43.
3.6. Antioxidant activity
Berberine hydrochloride (161) has beneficial effects against cellular oxidative stress295,296. It reduced H2O2-induced growth inhibition and DNA damage as well as apoptosis in C2C12 cells by suppressing the accumulation of intracellular reactive oxygen species via activation of the Nrf2/HO-1 pathway297. Additionally, the alkaloid improved the antioxidant status of intestinal tissue in mice298. The related alkaloid coptisine (165) exerted an antioxidant activity against AAPH-induced toxicity by activating Akt and JNK/Nrf2/NQO1 pathways299.
The bisbenzylisoquinoline alkaloid (−)-O-O-dimethylgrisabine (36) exhibited potent antioxidant activity (44.3%) in the reducing power assay and IC50 values of 18.38 and 64.30 μg/mL in DPPH and metal chelating assays, respectively. Thus, it is a good reductant with the ability to chelate metals and prevent pro-oxidant activity38. By preventing NF-κB translocation, neferine (61) from the same compound classification protected muscle cells from oxidative stress. It also prevented apoptosis by decreasing the mitochondrial membrane potential and reactive oxygen species (ROS) production in cells subjected to hypoxia as well as inhibited the expression of the downstream regulator COX-259,300.
Two aporphine isoquinoline alkaloids, (−)-boldine (85) and (−)-norboldine (86), exhibited good to low potency in three antioxidant activity assays, DPPH (IC50 136.96, 255.21 μM), reducing power (34.37, 52.10%), and metal chelating (IC50 785.64, 501.55 μM)59. Two other aporphines, isocorydine (92) and norisocorydine (93), also showed antioxidant effects in these assays59.
The protoberberine phellodendrine (198) showed good antioxidant effects in vivo. It improved the decreased survival rate and abnormally elevated heart rate of zebrafish embryos. In vitro, the compound decreased the increased ROS production, lipid-peroxidation and cell death rate caused by AAPH-induced oxidative stress, most likely by down-regulation of AKT phosphorylation and NF-κB3 expression93.
Oleracein E (403) has a rare tetrahydroisoquinoline/pyrrolidone tricyclic skeleton and a catechol moiety301. It improved cognitive function, reversed abnormal brain antioxidant biomarkers (GSH, T-AOC, MDA and SOD) to normal levels, and inhibited hippocampal neuronal apoptosis in d-galactose/NaNO2-induced senescent mice and in some apoptotic indices induced by AlCl3302,303.
3.7. Antiparasitic and insecticidal activities
Parasitic diseases present a threat worldwide, particularly among developing countries, and cause considerable morbidity and mortality globally. Examples include trypanosomiasis, leishmaniasis and schistosomiasis304-310. Compared with synthetic molecules, natural products are believed to have significant advantages as lead compounds against these diseases, and some isoquinoline alkaloids have demonstrated antiparasitic activity.
The naphthylisoquinoline alkaloid ancistectorine D (212) showed the highest potency against the protozoan parasites Trypanosoma cruzi and Leishmania donovani with IC50 values of 4.40 μM and 1.20 μM, respectively, while ancistrobonsolines A1 and A2 (281, 282) showed weak-to-moderate antiprotozoal activities111. The bisbenzylisoquinoline 6,5′,6′,7′,12-pentamethoxy-2,2′-dimethyloxyacathan (50) effectively killed both wild type L. donovani (EC50 6.8 μM) and sodium antimony gluconate (SAG)-resistant promastigotes (EC50 8.2 μM). Also, at a concentration of 50 μM, it almost completely inhibited the protozoan DNA topoisomerase IB activity44. Alternamine A (397) showed moderate antiprotozoal effects against T. cruzi trypomastigotes and L. amazonensis amastigotes with IC50 values of 0.23 and 0.16 μM, respectively. The IC50 values of the positive drugs (crystal violet against T. cruzi and amphotericin B against L. amazonensis) were 0.18 and <0.01 μM153.
Ealapasamines A–C (287–289) exhibited excellent in vitro antimalarial activity against chloroquine-sensitive (NF54) and chloroquine-resistant (K1) strains of the malaria parasite P. falciparum; the IC50 values were 418, 210 and 34 nM (NF54) and 452, 138 and 6.3 nM (K1). Thus, ealapasamine C was the most active naphthylisoquinoline against the resistant strain K1. Its cytotoxicity was comparatively low (6.0 μM), giving a high selectivity index of nearly 1000106. Dioncophyllines F, C2 and D2 (249–251) showed high and specific activity against P. falciparum108, while mbandakamines C (291) and D (292) exhibited promising activity against the same parasite32.
Two morphinandienones, (+)-sebiferine (353) and (−)-milonine (354), from Dehaasia longipedicellata showed potent to moderate activity against a chloroquine-resistant strain of P. falciparum K1 with IC50 values ranging from 0.03 to 30.40 μM. (−)-Milonine exhibited potent activity with an IC50 value of 0.10 μM, comparable to that of the positive standard, chloroquine (0.09 μM). Meanwhile, they showed no toxicity towards the normal pancreatic cell line (hTERT-HPNE)60.
Bisbenzylisoquinolines also exhibit significant antiparasitic activity. In 2014, Omole et al.48 found that two new bisbenzylisoquinoline (−)-pseudocurine (55) and (−)-pseudoisocurine (56) exhibited strong to moderate anti-plasmodial activity against both strains of P. falciparum D6 (CQ-susceptible) (IC50 0.49 μM, 1.26 μM), and W2 (CQ resistant) (IC50 0.522 μM, 2.798 μM). (−)-O-O-Dimethylgrisabine (59), isolated from Dehaasia longipedicellata, exhibited significant activity against a chloroquine-resistant strain of P. falciparum K1 with an IC50 value of less than 0.031 μM and no toxicity towards the normal pancreatic cell line (hTERT-HPNE)38. (+)-Laurotetanine (87) and (+)-norstephasubine (69) exhibited strong antiplasmodial activity against P. falciparum strain K1 with IC50 values of 0.19 and 0.12 μM, respectively68.
Two aporphines, (−)-boldine (85) and (−)-norboldine (86), showed moderate activity against a chloroquine-resistant strain of P. falciparum K1 with IC50 values of 2.60 and 9.28 μM, respectively, while that of chloroquine was 0.09 μM. Isocorydine (92), norisocorydine (93) and boldine (85) bound free heme and neutralized the electrons produced during the P. falciparum-mediated hemoglobin destruction and prevented oxidative damage in the host60. Stephanine (149), a dehydroaporphine, displayed antiplasmodial activity against P. falciparum strains 3D7 and W2 with IC50 values of 0.69 μM and 1.32 μM, but its cytotoxicity against 184B5 cells led to low selectivity indexes (9.10 and 4.70). The positive drug chloroquine had IC50 values of 2628.3 and 134.2 against the two respective parasite strains.
Berberine chloride (161), coptisine chloride (165), palmatine chloride (166) and dehydrocorydaline nitrate showed strong anti-malarial effects (IC50 < 50 nM) against the P. falciparum 3D7 strain. Their cytotoxicity to host cells was low (cell viability > 90%)311. Berberine also displayed in vivo anticoccidial activity312.
Among four isoquinoline alkaloids, sanguinarine (299) presented the strongest insecticidal activity against 3rd instar Lymantria dispar with a LD50 value of 4.96 μg/larva. The rank order of insecticidal capacity was sanguinarine > chelidonine (300) > berberine hydrochloride (161) > coptisine (165), and the methylenedioxyphenyl(l,3-benzodioxole) group might play a key role in the larvicidal activity on L. dispar. Except for coptisine, the alkaloids also significantly reduced the food intake of the larvae and suppressed activity of digestive enzymes. Hence, the alkaloids induced antifeeding and larval lethality on L. dispar larvae124. Heitziquinone (308) from Zanthoxylum heitzii presented the weak or inactive toxicity against brine shrimp (Artemia salina) 127.
Cis- and trans-protopinium (187, 188) from Fumaria parviflora showed nematicidal activity against the southern root-knot nematode Meloidogyne incognita. In an in vitro study, the application of cis- and trans-protopinium at a concentration of 561.18 μM to nematode eggs and second stage juveniles for 120 h of incubation led to 100% values for the area under cumulative percent hatch inhibition and mortality. In the greenhouse and field settings during spring and autumn, application of the alkaloids at a concentration of 841.77 μM significantly reduced the nematode galling index, the number of females per gram of root, and the reproduction factor, as well as increased plant height, fresh and dry shoot weights, and root length91.
3.8. Neuroprotective Effects
Alzheimer’s disease (AD), a common neurodegenerative disease, is characterized by low levels of the neurotransmitter acetylcholine in the brain region involved with cognition. Berberine was highly tolerated when taken orally and freely blood-brain-barrier permeable313. Hence, it shows significant promise and activity for treating numerous neurodegenerative conditions, including Alzheimer’s, Huntington’s, and Parkinson’s diseases314,315. Berberine (161) provided neuroprotection via inhibition of the mTOR signaling pathways and activation of cell survival and antioxidative signaling pathways, such as up-regulated PI3K/AKT/Bcl-2 and Nrf2/HO-1 antioxidative signaling pathways246,316. Berberine reduced the accumulation of amyloid β (Aβ) and decreased the expression of β-site APP cleaving enzyme-1 by activating AMPK in N2a mouse neuroblastoma cells stably expressing human Swedish mutant APP695 (N2a/APP695sw), N2a cells316, and also inhibited Aβ-protein-induced apoptosis in primary cultured hippocampal neurons via the mitochondria-related caspase pathway317. Subsequently, Huang et al.318 suggested that berberine improves cognitive impairment by promoting autophagic clearance and inhibits production of Aβ in an APP/tau/PS1 mouse model of Alzheimer’s disease. Furthermore, berberine presented a neuroprotective effect against environmental heavy metal-induced neurotoxicity and Alzheimer’s-like disease in rats via its anti-inflammatory/antioxidant mechanisms319.
Postoperative cognitive dysfunction (POCD) is a significant cause of morbidity after surgery, especially in elderly patients. In an in vivo study, berberine alleviated POCD by suppressing neuroinflammation in aged mice and, in an in vitro study, it suppressed LPS stimulated production of TNF-α and IL-1β in BV2 cells320. Meanwhile, the alkaloid markedly improved aging-related reductions in cognitive ability and muscular function and activation of the AMPK/SIRT1/PGC-1α pathway in skeletal muscle321. Also, berberine is a potential alternative therapy for TDP-43-related neuropathology in frontotemporal dementia and amyotrophic lateral sclerosis322.
Depression is the most common mental disorder in humans. Berberine up-regulated BDNF expression in the hippocampus to lessen corticosterone-induced depressive-like behavior in mice 323 and enhanced dopamine expression to alleviate symptoms of anxiety in rats with post-traumatic stress disorder324. It also exerted antidepressant-like effects in ovariectomized mice and decreased immobility time in a dose-dependent manner. Berberine’s activation of the 5-HT2 receptor via the BDNF-CREB and eEF2 pathways activation may be partially related to these antidepressant-like effects. Furthermore, after berberine treatment, greater reduction was seen in c-Fos induced by ovariectomy325.
Epileptogenesis transforms a normal brain to an epileptic condition, eventually leading to seizures. Status epilepticus is a life-threatening neurologic condition with seizures of longer duration. Studies showed that berberine relieved status epilepticus and spontaneous recurrent seizures in an intrahippocampal kainite model of epilepsy and exerted neuroprotective effect via suppression of oxidative stress, neuroinflammation, and possibly apoptosis. Berberine also reduced cognitive deficits and hyperphosphorylation of tau by inhibiting the activation of the NF-κB signaling pathway326.
Cholinesterase (acetylcholinesterase, AChE; butyrylcholinesterase, BChE) inhibitors enhance cholinergic function by prolonging the availability of ACh released into the neuronal synaptic cleft. While several isoquinoline alkaloids, (+)-nornantenine (88), (+)-laurotetanine (79), (+)-N-methyllaurotetanine (89), (+)-oridine (153), (+)-N-methylisococlaurine (24) and (+)-reticuline (23), were inactive or exhibited poor inhibitory effects against AChE (IC50 > 200 μM), they exhibited a wide range of BChE inhibitory activity (IC50 3.95–288.34 μM)37. The protoberberine alkaloids (−)-stylopine (195) and (−)-sinactine (196) displayed weak inhibitory activity against AChE; however, sinactine potently inhibited the activity of prolyl oligopepetidase (POP), a neuronal enzyme involved in cognitive disorders, with IC50 of 53 μM24. Protopine (210) and cryptopine (211) showed weak inhibitory activities against AChE (IC50 230 and 209 μM) and BuChE (IC50 477 and 271 μM)24. Among phthalideisoquinoline alkaloids, (−)-mucroniferanine D (382), mucroniferanine F (384) and mucroniferanine G (385), exhibited AChE inhibitory activities with IC50 values of 28.3, 12.2 and 11.3 μM, respectively99, while (+)-bicuculline (372) was only weakly active against AChE, BuChE, and POP with higher IC50 values of 626 μM, 329 μM and 190 μM, respectively24,150. The pyrrolophenanthridine alkaloids seco-isopowellaminone (336), haemanthamine (326) and incartine (337) also exhibited weak anti-human AChE activity138. (+)-Parfumidine (79), a spirobenzylisoquinoline alkaloid, exhibited POP inhibitory activity with an IC50 value of 99 μM, compared with 3.27 μM for the positive drug (Z)-pro-prolinal24.
The known benzophenanthridine alkaloid chelerythrine potently and selectively inhibited an isoform of recombinant human monoamine oxidase A (MAO-A) with an IC50 value of 0.55 μM. It acted as a reversible competitive inhibitor (IC50 0.22 μM) and was more potent than the antidepressant drug toloxatone (IC50 1.10 μM), which also is a selective, reversible MOA inhibitor. Chelerythrine can be deemed a potential lead compound for the design of novel reversible MAO-A inhibitors327.
3.9. Hepatoprotective
Berberine (161) caused signification reduction in hepatic steatosis328,329. Although its bioavailability was less than 1% in some studies330,331, the alkaloid was typically concentrated in the liver after oral administration332. Berberine exerts anti-hyperglycemic and anti-dyslipidemic effects and can also ameliorate nonalcoholic fatty liver disease (NAFLD) via regulation of the hepatic SIRT1-UCP2 pathway332-334. By regulating cholesterol metabolism and inhibiting COX2-prostaglandin synthesis, the compound improved blockade of autophagic flux in the liver335. Moreover, berberine lessened the deposition of triglycerides in the liver following intraperitoneal injection or oral gavage336. It also protected against methotrexate-induced liver injury and attenuated oxidative stress and apoptosis, possibly through up-regulating the Nrf2/HO-1 pathway and PPARγ337. Moreover, berberine reduced staphylococcal enterotoxin B-mediated acute liver injury via regulation of HDAC expression338.
Wang et al.339 investigated the effects of tetrahydroberberine (179) and tetrahydropalmatine (192) on the expression of mouse liver cytochrome P450s and their liver toxicity in mice. Tetrahydroberberine induced mRNA expression of Cyp1a2, Cyp3a, and Cyp2e1, while tetrahydropalmatine inhibited Cyp1a2. While oral tetrahydroberberine increased mouse serum aspartate transaminase, total bilirubin, and liver malondialdehyde levels, as well as induced liver edema, tetrahydropalmatine did not cause such effects.
In 2018, study results showed that tiliamosine (54) could be used to treat non-alcoholic steatohepatitis53. Norsanguinarine (316), (−)-6-acetonyldihydrisanguinarine (317) and cavidilinine (318) from Corydalis tomentella exhibited moderate hepatoprotective activities at a concentration of 10 μM28. At a concentration of 10 μM, three stereoisomeric isoquinoline alkaloids (9S,7′S) tomentelline A (374), (9S,7′R) tomentelline A (375), (9R,7′S) tomentelline B (376), together with hendersine B methyl ester (46), bicucullinine (47), hendersine B (48), and (+)-capnoidine (378) presented moderate hepatoprotective activities with relative survival rates of 33.28–52.57%28.
3.10. Anti-platelets and myocardial protective effect
The aporphine alkaloid norpurpureine (101) exhibited activity (IC50 80 μM) against platelet aggregation stimulated by adenosine 5′-diphosphate (ADP), collagen and thrombin. It gradually inhibited granule secretion and adhesion of activated platelets to immobilized fibrinogen. At the intra-platelet level, norpurpureine prevented agonist-stimulated calcium mobilization and cAMP reduction. Its molecular target could be an effector common effector to Ca2+ and cAMP signaling, such as the PLC-PKC-Ca2+ pathway and phosphodiesterases74.
In addition, the isoquinoline alkalkaloid berberine (161) has been associated with myocardial protective effects340-342. By differentially modulating the activities of p-STAT1, p-STAT3 and p-STAT4 and, thus, suppressing Th17 and Th1 cell differentiation, the compound protected against myosin-induced myocardial injury in rats340. Subsequent reports showed that berberine protected the heart from ischemia/reperfusion injury induced by NaH2PO4 partly though reducing myocardial autophagy and apoptosis via the AMPK/mTOR and AK2/STAT3 signaling pathways in vivo and in vitro343,344 as well as reducing the striatum apoptosis via activation of the BDNF-TrkB-PI3K/Akt signaling pathway in the middle cerebral artery occlusion-induced cerebral ischemia/reperfusion model345. The related compound coptisine (165) also attenuated pro-inflammatory cytokines, including IL-1β, IL-6, and tumor necrosis factor-α, in heart tissue after myocardial ischemia/reperfusion injury346.
Neferine (61), a bisbenzylisoquinoline-type alkaloid, prevented hyperglycemia-induced endothelial cell apoptosis through suppression of ROS/Akt/NF-κB347. Also, it blocked Nav1.5 channels in myocardia under the open and inactivating states and, thus, was an open channel blocker of Nav1.5 channels348,349. Finally, ambinine (315), a benzophenanthridine alkaloid, had protective effects on H9C2 myocardial cells. It demonstrated anticoagulation and thrombolytic effects in vitro by significantly degrading blood clots and delaying plasma recalcification time in a dose-dependent manner (1.21-4.84 mM)228.
3.11. Anti-ulcer activity
Gastric ulcers are one of the most common gastrointestinal disorders. The anti-ulcer/gastroprotective effect of berberine (161) might involve positive regulation of antioxidant and anti-inflammatory status mediated, at least partially, through the Nrf2 signaling pathway and p38 MAPK translocation350. Palmatine (166) hydrochloride tablets have been used clinically to cure intestinal and gynecological inflammation, bacillary dysentery, respiratory and urinary tract infections, surgical infections and eye conjunctivitis. Wang et al.351 also reported that this alkaloid may protect the gastric mucosa by increasing PGE2 and decreasing PAF as well as against gastric ulcers, perhaps associated with anti-inflammatory status. After orally administrating palmatine for seven consecutive days, ulcer areas were significantly decreased with inhibitory rates of 51% to 62%.
Cavidine (194), a protoberberine isoquinoline alkaloid and potent inhibitor of COX-2, exerted a gastroprotective effect against gastric ulceration, which might be associated with the stimulation of PGE2, reduction of oxidative stress, suppression of NF-κB expression and subsequently reduced COX-2 and pro-inflammatory cytokines352,92. Pretreatment with this compound had a protective effect on acetic acid-induced ulcerative colitis in mice264.
3.12. Renoprotective effects
Berberine (161) is a good candidate to protect against the deleterious effect of chronic lead intoxication on the kidney353. Molecular, biochemical, and histopathological analysis indicated that this alkaloid exerted renoprotective effects in an animal model of lead-induced nephrotoxicity by inhibiting lipid peroxidation and enhancing antioxidant defense system mechanisms. Berberine also protected against renal ischemia/reperfusion injury by regulating the Sirt1/p53 pathway354.
3.13. Anti-muscle atrophy activity
Magnoflorine (100), an aporphine isoquinoline alkaloid, efficiently enhanced myoblast differentiation by activating the p38 MAP kinase and Akt pathways and increased the numbers of multinucleated and cylinder-shaped myotubes. It might be a promising lead compound for the development of a drug to combat muscle atrophy73.
3.14. Retinal effects
Berberine (161) exhibited a protective effect against light-induced photoreceptor degeneration associated with diminished oxidative stress in the mouse retina. Thus, it could provide protection against retinal diseases250.
3.15. Analgesic effects
Neuropathic pain is a major public health problem. Berberine (161) administration (i.p.) increased both mechanical and thermal pain thresholds in a dose-dependent manner. Moreover, berberine administration reversed the mRNA and protein expression of TRPV1 in dorsal root ganglion neurons after peripheral nerve injury and significantly inhibited capsaicin-induced pain behaviors. This action on neuropathic pain may be associated with the down-regulation of the heat and capsaicin receptor, TRPV1, in the dorsal root ganglia of rat neurons. Accordingly, berberine could be used to treat neuropathic pain originated in the peripheral nervous system355.
3.16. Others
Osteoarthritis, a common degenerative joint disease, is a major cause of joint dysfunction in the elderly356. Berberine (161) prevented articular degeneration cartilage by activating the Akt/p70S6K/S6 signaling pathway in interleukin-1β-induced rat chondrocytes as well as a rat model357. It also prevented NO-induced rat chondrocyte apoptosis and cartilage degeneration via AMPK and p38 MAPK signaling358. Moreover, the compound promoted sodium nitroprusside-stimulated chondrocyte proliferation by promotion of G1/S phase transition and synthesis of proliferating cell nuclear antigen in cartilage and bone marrow-derived mesenchymal stem cells as well as osteogenic differentiation through activation of the Wnt/β-catenin signaling pathway359,360.
In addition, berberine may be used to treat adenomyosis by inhibiting growth and inflammatory invasive phenotypes of ectopic stromal cells361 and acute respiratory distress syndrome (ARDS) by alleviating endothelial glycocalyx degradation and promoting glycocalyx restoration in LPS-induced ARDS362.
4. CONCLUSION
As an important class of alkaloids, isoquinoline alkaloids have various chemical structures and pharmacological activities. However, the potential of this promising and expanding platform of active natural compounds has only been partially developed by both the academic community and the pharmaceutical industry to date. Over the past century, the discovery of morphine opened a new area for the development of central analgesic agents, and the application of berberine in the clinic has inspired a new wave for the discovery of alternative, green antibacterial drugs. During the past five years, the identification of additional new compounds, significant biological activities or novel mechanism of actions will undoubtedly contribute to the continual development of future new drugs. Continued attention and long-lasting research on the isolation and identification of naturally occurring isoquinoline alkaloids will open the way to targeted pharmacological modelling and synthetic modifications, resulting in new and better drugs based on the original effects of these alkaloids.
Acknowledgements
This work was supported financially by the Key Program for International S&T Cooperation Projects of China Gansu Province (18YF1WA115) and the National Natural Science Foundation of China (21672092, 31371975, 31772790) and the National Key Research and Development Program of China (2017YFD0201404, 2016YFE0129000). Support also was supplied by NIH grant CA177584 from the National Cancer Institute awarded to K.H. Lee.
Biography
Xiaofei Shang received his B.S. degree in Bioscience from Londong University in 2007, and his M.S degree in Biochemistry from Lanzhou University in 2010, now he is Ph.D candidate in medicinal chemistry from Lanzhou University. He is currently an associate professor of Key Laboratory of Veterinary Pharmaceutical Development of Ministry of Agriculture, Lanzhou Institute of Husbandry and Pharmaceutical Sciences, CAAS. His research interests include the isolation, structural elucidation, and structural modification of bioactive compounds from natural products. He has published about 30 articles, applied for more than 10 patents and received several projects.
Cheng-Jie Yang received his B.S. degree in Pharmacy from Lanzhou University in 2016, and now he is Ph.D. candidate in medicinal chemistry from Lanzhou University. His research interests include the design and synthesis of bioactive compounds and natural products as potential anticancer agents, as well as exploring the potential mechanism.
Susan L. Morris-Natschke received her B.S. in chemistry from the University of Maryland-College Park in 1975 and her Ph.D. in organic chemistry from the University of North Carolina-Chapel Hill (UNC-CH) in 1982. She is currently Research Professor in the Division of Chemical Biology and Medicinal Chemistry, UNC Eshelman School of Pharmacy, UNC-CH, where she has been on the faculty since 1983. Her interests include scientific writing/editing, as well as the synthesis and structure-activity relationships of bioactive natural products.
Jun-Cai Li received his B.S. degree in Pharmaceutical engineering from School of Life Science and Engineering of Southwest Jiaotong University in 2016, and he is currently a M.S. student in School of Pharmacy of Lanzhou University. His research interests include the design and synthesis of bioactive compounds and natural products, and development of novel methodologies in medicinal chemistry.
Xiao-Dan Yin received her B.S. degree in pharmacy from Lan Zhou University in 2018 and she is currently a M.S. student in School of Pharmacy of Lanzhou University. Her research interests include the design and synthesis of bioactive compounds and natural products, as well as the discovery of antimicrobial lead compounds.
Ying-Qian Liu received his B.S. in chemistry from Yebei Normal University in 2002, and his Ph.D. degree in bioorganic chemistry from Lanzhou University in 2007. He worked as a postdoctoral scholar in School of Life Sciences of Lanzhou University in 2008-2013, and worked as a visiting scholar at the University of North Carolina at Chapel Hill in 2011-2012. He is currently a professor at School of Pharmacy in Lanzhou University. His research interests include the design and synthesis of bioactive compounds as potential anticancer or pesticide agents, development of novel methodologies in organic chemistry, as well as drug mechanism and pharmacokinetics evaluation. He has published more than 50 research articles, applied for more than 30 patents, and received several projects.
Xiao Guo received her B.S. degree in Bioscience from Northwest Normal University in 2010 and her M.S degree in Plant sciences from Northwest Normal University in 2013, and her Ph.D. degree in Veterinary Science from Chinese Academy of Agricultural Science in 2017. now she is currently an assistant professor of Tibetan Medicine College in Qinghai University. Her research interests include the isolation, structural elucidation, and bioactivity of natural products.
Jing-Wen Peng received her B.S. degree in Pharmacy from Weifang Medical University in 2016, and she is currently a M.S. student in School of Pharmacy of Lanzhou University. Her research interests include the design and synthesis of bioactive compounds and natural products, and research of novel methodologies in medicinal chemistry.
Masuo Goto received his B.S. degree in pharmacology in 1987, M.S. in pharmaceutical science in 1989, and Ph.D. in molecular biology in 1993 from Kanazawa University. He was a research fellow in cell and developmental biology at the National Institutes of Health from 1999 to 2003, followed by a postdoctoral fellow at the School of Medicine, UNC-CH from 2003-2009. Currently, he is Research Assistant Professor, UNC Eshelman School of Pharmacy, UNC-CH. He is interested in elucidating the molecular mechanisms of action of antiproliferative small molecules mainly discovered and developed from natural products. His research goals are to develop novel strategies to prevent and overcome multidrug-resistant cancers.
Ji-Yu Zhang received his B.S. in veterinary science from Beijing Agricultural University in 1991 and his Ph.D. in basic veterinary medicine from Jilin University in 2002. He is currently a Professor at Lanzhou Institute of Husbandry and Pharmaceutical Sciences, CAAS. His research interests include the design and synthesis of bioactive compounds as veterinary medicines, as well as drug resistance and safety evaluation. He has published more than 100 research articles, applied for more than 30 patents, and directed several projects.
Kuo-Hsiung Lee received his B.S. in pharmacy from Kaohsiung Medical University, Taiwan (1961), M.S. in pharmaceutical chemistry from Kyoto University, Japan (1965), and Ph.D. in medicinal chemistry from University of Minnesota, Minneapolis (1968). He joined the faculty of UNC Eshelman School of Pharmacy, University of North Carolina-Chapel Hill, in 1970 and is now Kenan Distinguished Professor of Medicinal Chemistry and Director of the Natural Products Research Laboratories. He has published over 942 research articles, been granted over 121 patents, and received numerous awards, including most recently, the Third Cheung On Tak International Award for Outstanding Achievement in Chinese Medicine from Hong Kong Baptist University in 2016.
Footnotes
All authors declare that they have no competing interests.
References
- 1.Bhadra K, Kumar GS. Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: binding aspects and implications for drug design. Med Res Rev. 2011; 31:821–862. [DOI] [PubMed] [Google Scholar]
- 2.Koleva II, Beek TAV, Soffers AEMF, Dusemund B, Rietjens IMCM. Alkaloids in the human food chain–Natural occurrence and possible adverse effects. Mol Nutr Food Res. 2012; 56:30–52. [DOI] [PubMed] [Google Scholar]
- 3.Santoro D, Bellinghieri G, Savica VJ. Development of the concept of pain in history. J. Nephrol 2011; 24, 133–136. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Li Y, Dai Y, Peng JY. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol Res. 2018; 130:451–465. [DOI] [PubMed] [Google Scholar]
- 5.Weber C, Opatz T. Bisbenzylisoquinoline alkaloids. The Alkaloids Chemistry and Biology. 2019; 81:1–114. [DOI] [PubMed] [Google Scholar]
- 6.Nair JJ, Staden JV. Antifungal constituents of the plant family Amaryllidaceae. Phytother Res. 2018; 32:976–984. [DOI] [PubMed] [Google Scholar]
- 7.Simoben CV, Fidele NK, Sergi HA, Sippl W. Compounds from African medicinal plants with activities against selected parasitic diseases: Schistosomiasis, Trypanosomiasis and Leishmaniasis. Nat Prod Bioprospect. 2018; 8:151–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert F. Narciclasin-an Amaryllidaceae alkaloid with potent antitumor and anti-inflammatory properties. Planta Med. 2016; 82:1389–1394. [DOI] [PubMed] [Google Scholar]
- 9.Ashok P, Ganguly S, Murug S. Manzamine alkaloids: isolation, cytotoxicity, antimalarial activity and SAR studies. Drug Discov Today. 2014, 19, 1781–1791. [DOI] [PubMed] [Google Scholar]
- 10.Iranshahy M, Quinn RJ, Iranshahi M. ChemInform abstract: biologically active isoquinoline alkaloids with drug-like properties from the Genus Corydalis. RSC Adv. 2014; 4:15900–15913. [Google Scholar]
- 11.Maria AN, Andrei M, Echeverría J, et al. Berberine: botanical occurrence, traditional uses, extraction methods and relevance in cardiovascular, metabolic, hepatic and renal disorders. Front Pharmacol. 2018; 9:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu ZF, Feng W, Shen Q, et al. Rhizoma coptidis and berberine as a natural drug to combat aging and aging-related diseases via anti-oxidation and AMPK activation. Aging Dis. 2017, 8, 760–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016; 79:629–661. [DOI] [PubMed] [Google Scholar]
- 14.Freestone C, Eccles RJP. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J Pharm Pharmacol. 2011; 49:1045–1049. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med. 2008; 74:1423–1429. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich M, Teoh HL. Galanthamine from snowdrop--the development of a modern drug against Alzheimer's disease from local Caucasian knowledge. J Ethnopharmacol. 2004; 92:147–162. [DOI] [PubMed] [Google Scholar]
- 17.Iranshahy M, Quinn RJ and Iranshahi M. Biologically active isoquinoline alkaloids with drug-like properties from the genus Corydalis. RSC Adv., 2014, 4, 15900–15913 [Google Scholar]
- 18.Ibrahim Sabrin R.M., Mohamed Gamal A.. Naphthylisoquinoline alkaloids potential drug leads. Fitoterapia 106 (2015) 194–225 [DOI] [PubMed] [Google Scholar]
- 19.Bentley K, Robinson RS. The isoquinoline alkaloids: a course in organic chemistry. Pergamon Press; 1965:41–59. [Google Scholar]
- 20.Chen J, Gao K, Liu T, Zhao H, Wang J, Liu B, Wang W. Aporphine alkaloids: a kind of alkaloids’ extract source, chemical constitution and pharmacological actions in different botany: a review. Asian J Chem. 2013; 25:10015–10027. [Google Scholar]
- 21.Bringmann G, Pokorny F. Chapter 4: The naphthylisoquinoline alkaloids. Academic Press, New York, 1995; 46:127–271. [Google Scholar]
- 22.Ding D, Guo YR, Wu RL, Qi WY, Xu HM. Two new isoquinoline alkaloids from Scolopendra subspinipes mutilans induce cell cycle arrest and apoptosis in human glioma cancer U87 cells. Fitoterapia. 2016; 110:103–109. [DOI] [PubMed] [Google Scholar]
- 23.Guo YR, Wu PX, Xu HM, Qi WY. A new 1,5-dihydroxy-4-methoxyisoquinoline from Scolopendra subspinipes mutilans. Chem Biodivers. 2017;14: e1600478. [DOI] [PubMed] [Google Scholar]
- 24.Bouaziz A, Mhalla D, Zouari I, et al. Antibacterial and antioxidant activities of Hammada scoparia extracts and its major purified alkaloids. S Afr J Bot. 2016; 105:89–96. [Google Scholar]
- 25.Chlebek J, Novák Z, Kassemová D, et al. Isoquinoline alkaloids from Fumaria officinalis L. and their biological activities related to Alzheimer's disease. Chem Biodivers. 2016; 13:91–99. [DOI] [PubMed] [Google Scholar]
- 26.Kumar P, Rawat A, Keshari AK, et al. Antiproliferative effect of isolated isoquinoline alkaloid from Mucuna pruriens seeds in hepatic carcinoma cells. Nat Prod Res. 2016; 30:460–463. [DOI] [PubMed] [Google Scholar]
- 27.Lin CL, Kao CL, Li WJ, Li HT, Chen CY. Chemical constituents of the roots of Michelia champaca. Nat Compd. 2018; 54:324. [Google Scholar]
- 28.Wang Y, Wang D, Zhang J, Liu D, Wang Z, Meng D. Isoquinolines from Corydalis tomentella from Tibet, China, possess hepatoprotective activities. Phytochem. 2018; 155:93–99. [DOI] [PubMed] [Google Scholar]
- 29.Yin X, Zhao F, Feng X, et al. Four new spirobenzylisoquinoline N-oxide alkaloids from the whole plant of Corydalis hendersonii. Fitoterapia. 2018; 128:31–35. [DOI] [PubMed] [Google Scholar]
- 30.Tian W, Zhi H, Yang C, et al. Chemical composition of alkaloids of Plumula nelumbinis and their antioxidant activity from different habitats in China. Ind Crop Prod. 2018; 125:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin TY, Li SQ, Jin CR, et al. Catecholic isoquinolines from Portulaca oleracea and their anti-inflammatory and β2-adrenergic receptor agonist activity. J Nat Prod. 2018; 81:768–777. [DOI] [PubMed] [Google Scholar]
- 32.Tshitenge DT, Feineis D, Mudogo V, et al. Mbandakamine-type naphthylisoquinoline dimers and related alkaloids from the central African liana Ancistrocladus ealaensis with antiparasitic and antileukemic activities. J Nat Prod. 2018; 81:918–933. [DOI] [PubMed] [Google Scholar]
- 33.Akihisa T, Yokokawa S, Ogihara E, et al. Melanogenesis-inhibitory and cytotoxic activities of limonoids, alkaloids, and phenolic compounds from Phellodendron amurense Bark. Chem Biodivers. 2017;14. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Gao X, Cao Y, et al. Anti-inflammatory effects of boldine and reticuline isolated from Litsea cubeba through JAK2/STAT3 and NF-κB signaling pathways. Planta Med. 2018; 84:20–25. [DOI] [PubMed] [Google Scholar]
- 35.da Silva FMA, da Silva Filho FA, de Souza CAS, et al. Morphinadienone and other isoquinoline-derived alkaloids from the trunk bark of Unonopsis floribunda Diels (Annonaceae). Biochem Syst Ecol. 2018; 79:12–14. [Google Scholar]
- 36.da Silva FMA, da Silva Filho FA, de Souza CAS, et al. Isoquinoline-derived alkaloids from leaves of Unonopsis stipitata Diels (Annonaceae). Biochem Syst Ecol. 2018; 79:69–71. [Google Scholar]
- 37.Wan Othman WNN, Liew SY, Khaw KY, Murugaiyah V, et al. Cholinesterase inhibitory activity of isoquinoline alkaloids from three Cryptocarya species (Lauraceae). Bioorg Med Chem. 2016; 24:4464–4469. [DOI] [PubMed] [Google Scholar]
- 38.Zahari A, Cheah FK, Mohamad J, et al. Antiplasmodial and antioxidant isoquinoline alkaloids from Dehaasia longipedicellata. Planta Med. 2014; 80:599–603. [DOI] [PubMed] [Google Scholar]
- 39.Soares ER, da Silva FMA, de Almeida RA, et al. Direct infusion ESI-IT-MSn alkaloid profile and isolation of tetrahydroharman and other alkaloids from Bocageopsis pleiosperma maas (Annonaceae). Phytochem Anal. 2014; 26:339–345. [DOI] [PubMed] [Google Scholar]
- 40.Li YH, Li HM, Li Y, et al. New alkaloids sinomacutines A–E, and cephalonine-2-O-β-d-glucopyranoside from rhizomes of Sinomenium acutum. Tetrahedron. 2014; 70:8893–8899. [Google Scholar]
- 41.Wang L, Zhang SY, Chen L, et al. New enantiomeric isoquinoline alkaloids from Coptis chinensis. Phytochem Lett. 2014; 7:89–92. [Google Scholar]
- 42.Liu J, Peng C, Zhou QM, Guo L, Liu ZH, Xiong L. Alkaloids and flavonoid glycosides from the aerial parts of Leonurus japonicus and their opposite effects on uterine smooth muscle. Phytochemistry. 2017; 145:128–136. [DOI] [PubMed] [Google Scholar]
- 43.Yan ZR, Wang ZY, Wang B, et al. Immune-inhibitive phenyl-C1 substituent aporphine alkaloids from Thalictrum cirrhosum. Fitoterapia. 2018; 128:247–252. [DOI] [PubMed] [Google Scholar]
- 44.Kumar A, Chowdhury SR, Sarkar T, et al. A new bisbenzylisoquinoline alkaloid isolated from Thalictrum foliolosum, as a potent inhibitor of DNA topoisomerase IB of Leishmania donovani. Fitoterapia. 2016; 109:25–30. [DOI] [PubMed] [Google Scholar]
- 45.Hsiao SH, Lu YJ, Yang CC, et al. Hernandezine, a bisbenzylisoquinoline alkaloid with selective inhibitory activity against multidrug-resistance-linked ATP-binding cassette drug transporter ABCB1. J Nat Prod. 2016; 79:2135. [DOI] [PubMed] [Google Scholar]
- 46.Jin Q, Yang D, Dai Z, et al. Antitumor aporphine alkaloids from Thalictrum wangii. Fitoterapia. 2018; 128:204–212. [DOI] [PubMed] [Google Scholar]
- 47.Darvin SS, Toppo E, Esakkimuthu S, et al. Hepatoprotective effect of bisbenzylisoquinoline alkaloid tiliamosine from Tiliacora racemosa in high-fat diet/diethylnitrosamine-induced non-alcoholic steatohepatitis. Biomed Pharmacother. 2018; 108:963–973. [DOI] [PubMed] [Google Scholar]
- 48.Omole RA, Gathirwa J, Akala H, et al. Bisbenzylisoquinoline and hasubanane alkaloids from Stephania abyssinica (Dillon & Rich A) (Menispermeceae). Phytochem. 2014; 103:123–128. [DOI] [PubMed] [Google Scholar]
- 49.Su YF, Wink M. Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomed. 2014; 21:1110–1119. [DOI] [PubMed] [Google Scholar]
- 50.Gođevac D, Damjanović A, Stanojković TP, Anđelković B, Zdunić G. Identification of cytotoxic metabolites from Mahonia aquifolium using 1H NMR-based metabolomics approach. J Pharmaceut Biomed. 2018;150: 9–14. [DOI] [PubMed] [Google Scholar]
- 51.Gu DF, Li XL, Qi ZP, et al. Blockade of HERG K+ channel by isoquinoline alkaloid neferine in the stable transfected HEK293 cells. N S Arch Pharmacol. 2009; 380:143–151. [DOI] [PubMed] [Google Scholar]
- 52.Qian JQ. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol Sin. 2002; 23:1086–1092. [PubMed] [Google Scholar]
- 53.Chen J, Liu JH, Wang T, Xiao HJ, Yin CP, Yang J. Effects of plant extract neferine on cyclic adenosine monophosphate and cyclic guanosine monophosphate levels in rabbit corpus cavernosum in vitro. Asian J Androl. 2008; 10:307–312. [DOI] [PubMed] [Google Scholar]
- 54.Pan Y, Cai BC, Wang KL, et al. Neferine enhances insulin sensitivity in insulin resistant rats. Asian J Androl. 2009; 124:98–102. [DOI] [PubMed] [Google Scholar]
- 55.Xiong YQ, Zeng FD. Neferine enhances insulin sensitivity in insulin resistant rats. Acta Pharmacol Sin. 2003; 24:332–336. [PubMed] [Google Scholar]
- 56.Jung HA, Jin SE, Choi RJ, et al. Anti-amnesic activity of neferine with antioxidant and anti-inflammatory capacities, as well as inhibition of ChEs and BACE1. Life Sci. 2010; 87:420–430. [DOI] [PubMed] [Google Scholar]
- 57.Sugimoto Y, Furutani S, Itoh A, et al. Effects of extracts and neferine from the embryo of Nelumbo nucifera seeds on the central nervous system. Phytomed. 2008; 15:1117–1124. [DOI] [PubMed] [Google Scholar]
- 58.Zhao LB, Wang XM, Chang Q, et al. Neferine, a bisbenzylisoquinline alkaloid attenuates bleomycin-induced pulmonary fibrosis. Eur J Pharmacol. 2010; 627:304–312. [DOI] [PubMed] [Google Scholar]
- 59.Baskaran R, Poornima P, Huang CY, Padma VV. Neferine prevents NF-κB translocation and protects muscle cells from oxidative stress and apoptosis induced by hypoxia. BioFactors. 2016; 42:407–417. [DOI] [PubMed] [Google Scholar]
- 60.Zahari A, Ablat A, Omer N, et al. Ultraviolet-visible study on acid-base equilibria of aporphine alkaloids with antiplasmodial and antioxidant activities from Alseodaphne corneri and Dehaasia longipedicellata. Sci Rep. 2016; 6:21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian P, Yang XW. Five new alkaloids from Coptidis rhizoma-Euodiae Fructus couple and their cytotoxic activities against gastrointestinal cancer cells. Fitoterapia. 2014; 93:74–80. [DOI] [PubMed] [Google Scholar]
- 62.Perecim GP, Rodrigues A, Raminelli C. A convenient formation of aporphine core via benzyne chemistry: conformational analysis and synthesis of (R)-aporphine. Tetrahedron Lett. 2015; 56:6848–6851. [Google Scholar]
- 63.Guinaudeau H, Leboeuf M, Cave A. Aporphine alkaloids II. J Nat Prod. 1979; 42:325–360. [DOI] [PubMed] [Google Scholar]
- 64.Guinaudeau H, Leboeuf M, Cave A. Aporphinoid Alkaloids V. J Nat Prod. 1994; 57:1033–1135. [Google Scholar]
- 65.Guinaudeau H, Leboeuf M, Cave A. Dimeric aporphine-benzylisoquinoline and aporphinepavine alkaloids. J Nat Prod. 1979; 42:133–149. [DOI] [PubMed] [Google Scholar]
- 66.Guinaudeau H, Shamma M, Tantisewie B, Pharadai K. Aporphine alkaloids oxygenated at C-7. J Nat.Prod. 1982;45:355–357. [Google Scholar]
- 67.Yang X, Gao X, Cao Y, et al. Anti-inflammatory effects of boldine and reticuline isolated from Litsea cubeba through JAK2/STAT3 and NF-κB signaling pathways. Planta Med. 2018; 84:20–25. [DOI] [PubMed] [Google Scholar]
- 68.Zahari A, Ablat A, Sivasothy Y, Mohamad J, Choudhary MI, Awang K. In vitro antiplasmodial and antioxidant activities of bisbenzylisoquinoline alkaloids from Alseodaphne corneri Kosterm. Asian Pac J Trop Med. 2016; 9:328–332. [DOI] [PubMed] [Google Scholar]
- 69.Ravanelli N, Santos KP, Motta LB, Lago JHG, Furlan CM. Alkaloids from Croton echinocarpus Baill.: Anti-HIV potential. S Afr J Bot. 2016; 102:153–156. [Google Scholar]
- 70.Pereira MN, Justino AB, Martins MM, et al. Stephalagine, an alkaloid with pancreatic lipase inhibitory activity isolated from the fruit peel of Annona crassiflora Mart. Ind Crop Prod. 2017; 97:324–329. [Google Scholar]
- 71.Zeng YB, Wei DJ, Dong WH, et al. Antimicrobial glycoalkaloids from the tubers of Stephania succifera. Arch Pharm Res. 2017; 40:429–434. [DOI] [PubMed] [Google Scholar]
- 72.Rinaldi MVN, Díaz IEC, Suffredini IB, Moreno PRH. Alkaloids and biological activity of beribá (Annona hypoglauca). Rev Bras Farmacogn. 2017; 27:77–83. [Google Scholar]
- 73.Lee H, Tuong LT, Jeong JH, Lee SJ, Bae GU, Ryu JH. Isoquinoline alkaloids from Coptis japonica stimulate the myoblast differentiation via p38 MAP-kinase and Akt signaling pathway. Bioorg Med Chem Lett. 2017; 27:1401–1404. [DOI] [PubMed] [Google Scholar]
- 74.Sánchez G, Estrada O, Acha G, et al. The norpurpureine alkaloid from Annona purpurea inhibits human platelet activation in vitro. Cell Mol Bio Lett. 2018; 23:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.da Silva FMA, de Souza ADL, Koolen HF, et al. Phytochemical study of the alkaloidal fractions of Unonopsis duckei RE Fr. guided by electrospray ionisation ion-trap tandem mass spectrometry. Phytochem Anal. 2014: 25: 45–49. [DOI] [PubMed] [Google Scholar]
- 76.da Silva FMA, da Silva Filho FA, de Lima BR, et al. (+)-N-Formylnorglaucine Rotamers from Unonopsis stipitata Diels. Helv Chim Acta. 2016; 99:494–498. [Google Scholar]
- 77.Kim JH, Ryu YB, Lee WS, Kim YH. Neuraminidase inhibitory activities of quaternary isoquinoline alkaloids from Corydalis turtschaninovii rhizome. Bioorg Med Chem. 2014; 22:6047–6052. [DOI] [PubMed] [Google Scholar]
- 78.Wang B, Yang ZF, Zhao YL, et al. Anti-inflammatory isoquinoline with bis-seco-aporphine skeleton from Dactylicapnos scandens. Org Lett. 2018; 20:1647–1650. [DOI] [PubMed] [Google Scholar]
- 79.Mathouet H, Elomri A, Lameiras P, Daïch A, Verite P. An alkaloid, two conjugate sesquiterpenes and a phenylpropanoid from Pachypodanthium confine Engl. and Diels. Phytochem. 2007; 68:1813–1818. [DOI] [PubMed] [Google Scholar]
- 80.Del RCM, Kirby GC, Warhurst DC, Croft SL, Phillipson JD. Oxoaporphine alkaloids and quinones from Stephania dinklagei and evaluation of their antiprotozoal activities. Planta Med. 2000; 66:478–480. [DOI] [PubMed] [Google Scholar]
- 81.Lee JY, Kim KJ, Kim J, Choi SU, Kim SH, Ryu SY. Anti-osteoclastogenic effects of isoquinoline alkaloids from the rhizome extract of Sinomenium acutum. Archiv Pharm Res. 2016; 39:713–720. [DOI] [PubMed] [Google Scholar]
- 82.Costa EV, Soares LN, Pinheiro MLB, et al. Guaianolide sesquiterpene lactones and aporphine alkaloids from the stem bark of Guatteria friesiana. Phytochemistry. 2018; 145:18–25. [DOI] [PubMed] [Google Scholar]
- 83.Wu YC. New research and development on the Formosan Annonaceous plants. Studies in Natural Products. 2006; 33:957–1023. [Google Scholar]
- 84.Lúcio ASSC, Da Silva Almeida JRG, Da-Cunha EVL, Tavares JF, Filho JMB. Alkaloids of the Annonaceae: occurrence and a compilation of their biological activities. The Alkaloids: Chemistry and Biology. 2015; 74:233–409. [DOI] [PubMed] [Google Scholar]
- 85.Le PM, Vandana S, Thanh TN, Bruno P, Marylin M, Joel M, Thi TT, Hoyun L. Stephanine from Stephania venosa (Blume) Spreng showed effective antiplasmodial and anticancer activities, the latter by inducing apoptosis through the reverse of mitotic exit. Phytother Res. 2017; 31:1357–1368. [DOI] [PubMed] [Google Scholar]
- 86.Qin XD, Yang S, Zhao Y, Gao Y, Ren FC, Zhang DY, Wang F. A new aporphine alkaloid from Aconitum carmichaelii. Chem. Nat Compds 2017; 53:501–503. [Google Scholar]
- 87.Wu X, Li X, Dang Z, Jia Y. Berberine demonstrates anti-inflammatory properties in Helicobacter pylori-infected mice with chronic gastritis by attenuating the Th17 response triggered by the B cell-activating factor. J Cell Biochem. 2018; 119:5373–5381. [DOI] [PubMed] [Google Scholar]
- 88.Chen DL, Yang KY. Berberine alleviates oxidative stress in islets of diabetic mice by inhibiting miR-106b expression and up-regulating SIRT1. J Cell Biochem. 2017; 118:4349–4357. [DOI] [PubMed] [Google Scholar]
- 89.Yang TC, Chao HF, Shi LS, Chang TC, Lin HC, Chang WL. Alkaloids from Coptis chinensis root promote glucose uptake in C2C12 myotubes. Fitoterapia. 2014; 93:239–244. [DOI] [PubMed] [Google Scholar]
- 90.Han AR, Kim HR, Kil YS, Kang U, Jang DS, Seo EK. Isoquinoline alkaloids from Corydalis pallida. Chem Nat Compds. 2018; 54:1020–1022. [Google Scholar]
- 91.Naz I, Abdulkafi S, Munir I, et al. Cis- and trans-protopinium, a novel nematicide, for the eco-friendly management of root-knot nematodes. Crop Prot. 2016; 81:138–144. [Google Scholar]
- 92.Niu XF, Zhang HL, Li WF, Mu QL, Yao H, Wang Y. Anti-inflammatory effects of cavidine in vitro and in vivo, a selective COX-2 inhibitor in LPS-induced peritoneal macrophages of mouse. Inflammation. 2015; 38:923–933. [DOI] [PubMed] [Google Scholar]
- 93.Li L, Huang T, Tian C, et al. Thedefensive effect of phellodendrine against AAPH-induced oxidative stress throughregulating the AKT/NF-κB pathway in zebrafish embryos. Life Sci. 2016; 157:97–106. [DOI] [PubMed] [Google Scholar]
- 94.Costa RS, Lins MO, Hyaric ML, Barros TF, Velozo ES. In vitro antibacterial effects of Zanthoxylum tingoassuiba root bark extracts and two of its alkaloids against multiresistant Staphylococcus aureus. Rev Bras Farmacogn. 2016; 27:195–198. [Google Scholar]
- 95.Onozawa T, Kitajima M, Kogure N, Peerakam N, Santiarworn D, Takayama H. A cyclopeptide and a tetrahydroisoquinoline alkaloid from Ophiorrhiza nutans. J Nat Prod. 2017; 80: 2156–2160. [DOI] [PubMed] [Google Scholar]
- 96.Habartová K, Havelek R, Seifrtova M, et al. Scoulerine affects microtubule structure, inhibits proliferation, arrests cell cycle and thus culminates in the apoptotic death of cancer cells. Sci Rep. 2018; 8:4829–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takeuchi M, Saito Y, Goto M, et al. Novel sesquiterpene lactone analogues as potent anti-breast cancer agents. J Nat Prod. 2018; 81:1884–1891.30106296 [Google Scholar]
- 98.Naseri M, Emami SA, Asili J, Tayarani-Najaran Z, Dehghan G, Schneider B, Iranshahi M. Rupestrines A-D, alkaloids from the aerial parts of Corydalis rupestris. Bioorg Chem. 2018; 77:651–659. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J, Zhang QY, Tu PF, Xu FC, Liang H. Mucroniferanines A–G, Isoquinoline alkaloids from Corydalis mucronifera. J Nat Prod. 2018; 81:364–370. [DOI] [PubMed] [Google Scholar]
- 100.Lombe BK, Feineis D, Bringmann G. Dimerica naphthylisoquinoline alkaloids: polyketide-derived axially chiral bioactive quateraryls. Nat. Prod. Rep. 2019; 36:1513–1545. [DOI] [PubMed] [Google Scholar]
- 101.Bringmann G, Schneider C, Aké Assi L. Ancistrobarterine A: a new “mixed” Ancistrocladaceae Dioncophyllaceae-type alkaloid from Ancistrocladus barteri. Planta Med. 1993; 59:620–621. [Google Scholar]
- 102.Bringmann G, François G, Aké Assi L, Schlauer J. The alkaloids of Triphyophyllum peltatum (Dioncophyllaceae). Chimia Int J Chem. 1998; 52:18–28. [Google Scholar]
- 103.Bringmann G, Günther C, Ochse M, Schupp O, Tasler S. Biaryls in nature: a multi-facetted class of stereochemically, biosynthetically, and pharmacologically intriguing secondary metabolites. Prog. Chem. Org. Nat. Prod 2001; 82: 1–249. [DOI] [PubMed] [Google Scholar]
- 104.Bringmann G, Mühlbacher J, Repges C, Fleiscggauer J. MD-based CD calculations for the assignment of the absolute axial configuration of the naphthylisoquinoline alkaloid dioncophylline A. J Computat Chem. 2001; 22: 1–249. [Google Scholar]
- 105.Bringmann G, Seupel R, Feineis D, Zhang G, Xu M, Kaiser M, Brun R, Seo EJ, Efferth T. Ancistectorine D, a naphthylisoquinoline alkaloid with antiprotozoal and antileukemic activities, and further 5,8'- and 7,1'-linked metabolites from the Chinese liana Ancistrocladus tectorius. Fitoterapia. 2016; 115:1–8. [DOI] [PubMed] [Google Scholar]
- 106.Bringmann G, Seupel R, Feineis D, Xu M, Zhang G, Kaiser M, Brun R, Seo EJ, Efferth T. Antileukemic ancistrobenomine B and related 5,1′-coupled naphthylisoquinoline alkaloids from the Chinese liana Ancistrocladus tectorius. Fitoterapia. 2017; 121:76–85. [DOI] [PubMed] [Google Scholar]
- 107.Seupel R, Hemberger Y, Feineis D, Xu M, Seo EJ, Efferth T, Bringmann G. Ancistrocyclinones A and B, unprecedented pentacyclic N,C-coupled naphthylisoquinoline alkaloids, from the Chinese liana Ancistrocladus tectorius. Org Biomol Chem. 2018;16: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 108.Li J, Seupel R, Feineis D, Mudogo V, Kaier M, Brun R, Brünnert D, Chatterjee M, Seo EJ, Efferth T, Bringmann G. Dioncophyllines C2, D2, and F and related naphthylisoquinoline alkaloids from the Congolese liana Ancistrocladus ileboensis with potent activities against Plasmodium falciparum and against multiple myeloma and leukemia cell lines. J Nat Prod. 2017; 80:443–458. [DOI] [PubMed] [Google Scholar]
- 109.Kavatsurwa SM, Lombe BK, Feineis D, Dibwe DF, Maharaj V, Awale S, Bringmann G. Ancistroyafungines A-D, 5,8′- and 5,1′-coupled naphthylisoquinoline alkaloids from a Congolese Ancistrocladus species, with antiausterity activities against human PANC-1 pancreatic cancer cells. Fitoterapia. 2018; 130:6–16. [DOI] [PubMed] [Google Scholar]
- 110.Bringmann G, Steinert C, Feineis D, Mudogo V, Betzin J, Scheller C. HIV-inhibitory michellamine-type dimeric naphthylisoquinoline alkaloids from the Central African liana Ancistrocladus congolensis. Phytochemistry. 2016; 128:71–81. [DOI] [PubMed] [Google Scholar]
- 111.Lombe BK, Feineis D, Mudogo V, Brun R, Awale S, Bringmann G. Michellamines A6 and A7, and further mono- and dimeric naphthylisoquinoline alkaloids from a Congolese Ancistrocladus liana and their antiausterity activities against pancreatic cancer cells. RSC Adv. 2018; 8:5243–5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tshitenge DT, Feineis D, Mudogo V, Kaiser M, Brun R, Bringmann G. Antiplasmodial ealapasamines A-C, 'mixed' naphthylisoquinoline dimers from the central African liana Ancistrocladus ealaensis. Sci Rep. 2017; 7:5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bringmann G, Lombe BK, Steinert C, Ioset KN, Brun R, Turini F, Heubl G, Mudogo V. Mbandakamines A and B, unsymmetrically coupled dimeric naphthylisoquinoline alkaloids, from a Congolese Ancistrocladus species. Org Lett. 2013; 15:2590–2593. [DOI] [PubMed] [Google Scholar]
- 114.Kosina K, Gregorova P, Gruz J, et al. Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia. 2010; 81:1006–1012. [DOI] [PubMed] [Google Scholar]
- 115.Schmeller T, Latz-Bruning B, Wink M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochem.1997;44:257–266. [DOI] [PubMed] [Google Scholar]
- 116.Feng G, Zhang J, Liu Q. Inhibitory activity of dihydrosanguinarine and dihydrochelerythrine against phytopathogenic fungi. Nat Prod Res. 2011; 25:1082–1089. [DOI] [PubMed] [Google Scholar]
- 117.Niu X, Fan T, Li W, Zing W, Huang H. The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages. Eur J Pharmacol. 2012; 689:262–269. [DOI] [PubMed] [Google Scholar]
- 118.Walterova D, Ulrichova J, Valka I. Benzo[c]phenanthridine alkaloids sanguinarine and chelerythrine: biological activities and dental care applications. Acta Univ Palacki Olomuc. Fac Med 1995; 139:7–16. [PubMed] [Google Scholar]
- 119.Colombo ML, Bosisio E. Pharmacological activities of Chelidonium majusl L. (Papavercaeae). Pharmacol Res. 1996; 33:127–134. [DOI] [PubMed] [Google Scholar]
- 120.Wang K, Luo CH, Liu H, Xu J, Sun W, Zhou L. Nematicidal activity of the alkaloids from Macleaya cordata against certain nematodes. Afr J Agric Res. 2012;7:5925–5929. [Google Scholar]
- 121.Hammerová J, Uldrian S, Táborská E, Slaninová I. Benzo[c]phenanthridine alkaloids exhibit strong anti-proliferative activity in malignant melanoma cells regardless of their p53 status. J Dermatol Sci. 2011; 62:22–35. [DOI] [PubMed] [Google Scholar]
- 122.Juśkiewicz J, Gruzauskas R, Zduñczyk Z, et al. Effects of dietary addition of Macleaya cordata alkaloid extract on growth performance, caecal indices and breast meat fatty acids profile in male broilers. J Anim Physiol Anim Nutr. 2015; 95:171–178. [DOI] [PubMed] [Google Scholar]
- 123.Zou CS, Wang YJ, Zou H, et al. Sanguinarine in Chelidonium majus induced antifeeding and larval lethality by suppressing food intake and digestive enzymes in Lymantria dispar. Pestic Biochem Phys. 2019; 153:9–16. [DOI] [PubMed] [Google Scholar]
- 124.Zou C, Lv C, Wang Y, Cao C, Zhang G. Larvicidal activity and insecticidal mechanism of Chelidonium majus on Lymantria dispar. Pestic Biochem Phys. 2017; 142:123–132. [DOI] [PubMed] [Google Scholar]
- 125.Havelek R, Seifrtova M, Kralovec K, et al. Comparative cytotoxicity of chelidonine and homochelidonine, the dimethoxy analogues isolated from Chelidonium majus L. (Papaveraceae), against human leukemic and lung carcinoma cells. Phytomed. 2016; 23:253–266. [DOI] [PubMed] [Google Scholar]
- 126.Deng AJ, Zhang HJ, Li Q, et al. Six scalemic mixtures of 6-monosubstituted dihydrobenzophenanthridine alkaloids from Chelidonium majus and optically active structures of enantiomers. Phytochem. 2017; 144:159–170. [DOI] [PubMed] [Google Scholar]
- 127.Wangensteen H, Ho GTT, Tadesse M, et al. A new benzophenanthridine alkaloid and other bioactive constituents from the stem bark of Zanthoxylum heitzii. Fitoterapia. 2016; 109:196–200. [DOI] [PubMed] [Google Scholar]
- 128.Zhang HL, Gan XQ, Fan QF, et al. Chemical constituents and anti-inflammatory activities of Maqian (Zanthoxylum myriacanthum var. pubescens) bark extracts. Sci. Rep 2017; 7: 45805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dong ZB, Zhang YH, Zhao BJ, et al. Screening for anti-inflammatory components from Corydalis bungeana Turcz. based on macrophage binding combined with HPLC. BMC Complem Altern Med. 2015; 15:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang S, Yang Z, Han N, Liu Z, Yin J. The antithrombotic, anticoagulant activity and toxicity research of ambinine, an alkaloid from the tuber of Corydalis ambigua var. amurensis. Regul Toxicol Pharm. 2018; 95:175–181. [DOI] [PubMed] [Google Scholar]
- 131.Cahlíková L, Hrabinová M, Kulhánková A, et al. Alkaloids from Chlidanthus fragrans and their acetylcholinesterase, butyrylcholinesterase and prolyl oligopeptidase activities. Nat Prod Commun. 2013; 8:1541–1544. [PubMed] [Google Scholar]
- 132.Královec K, Chlebek J, CahlíkováL. Cytotoxic activities of Amaryllidaceae alkaloids against gastrointestinal cancer cells. Phytochem Lett. 2015; 13:394–398. [Google Scholar]
- 133.Havlasová J, Safratová M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014; 9:1151–1155. [PubMed] [Google Scholar]
- 134.Šafratová M, Novák Z, Kulhánková A, et al. Revised NMR data for 9-O-demethylgalanthine: an alkaloid from Zephyranthes robusta (Amaryllidaceae) and its biological activity. Nat Prod Commun. 2014; 9:787–788. [PubMed] [Google Scholar]
- 135.Vanĕčková N, Hoštoálková A, Šafratová M, et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities. Rsc Adv. 2016; 6:80114–80120. [Google Scholar]
- 136.Havelek R, Muthna D, Tomsik P, et al. Anticancer potential of Amaryllidaceae alkaloids evaluated by screening with a panel of human cells, real-time cellular analysis and Ehrlich tumor-bearing mice. Chem-Biol Interact. 2017; 275:121–132. [DOI] [PubMed] [Google Scholar]
- 137.Aldhaher AHS, Langat MK, Knirsch W, Andriantiana JL, Mulholl DA. Isoquinoline alkaloids from three Madagascan Crinum (Amaryllidaceae) species. Biochem Syst Ecol. 2018; 77:7–9. [Google Scholar]
- 138.Šafratová M, Hoštálková A, Hulcová D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018; 41:208–218. [DOI] [PubMed] [Google Scholar]
- 139.Ashok P, Lathiya H, Murugesan S. Manzamine alkaloids as antileishmanial agents: A review. Eur J Med Chem. 2015; 46:928–936. [DOI] [PubMed] [Google Scholar]
- 140.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep. 2016; 33:382–431. [DOI] [PubMed] [Google Scholar]
- 141.Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2008; 25:35–94. [DOI] [PubMed] [Google Scholar]
- 142.Nakamura H, Deng S, Kobayashi J, Ohizumi Y. Keramamine-A and -B, novel antimicrobial alkaloids from the Okinawan marine sponge Pellina sp. Tetrahedron Lett. 1987;28:621–624. [Google Scholar]
- 143.Sakai R, Higa T, Jefford CW, Manzamine A. Manzamine A, a novel antitumor alkaloid from a sponge. J Am Chem Soc.1986; 108:6404–6405. [Google Scholar]
- 144.Rao KV, Donia MS, Peng J, et al. Manzamine B and E and ircinal A related alkaloids from an Indonesian Acanthostrongylophora sponge and their activity against infectious, tropical parasitic, and Alzheimer's diseases. J Nat Prod. 2006; 69:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peng J, Rao KV, Choo YM, Hamann MT. Modern Alkaloids: Structure, Isolation, Synthesis and Biology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2008; 189. [Google Scholar]
- 146.Kim CK, Riswanto R, Won TH, et al. Manzamine alkaloids from an Acanthostrongylophora sp. Sponge. Nat Prod Rep. 2017; 80:1575–1583. [DOI] [PubMed] [Google Scholar]
- 147.Grollman AP. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci USA. 1966; 56:1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khandelwal N, Chander Y, Rawat KD, et al. Emetine inhibits replication of RNA and DNA viruses without generating drug-resistant virus variants. Antivir Res. 2017; 144:196–204. [DOI] [PubMed] [Google Scholar]
- 149.Krstin S, Mohamed T, Wang X, Wink M. How do the alkaloids emetine and homoharringtonine kill trypanosomes? An insight into their molecular modes of action. Phytomedicine. 2016; 23:1771–1777. [DOI] [PubMed] [Google Scholar]
- 150.Chen QB, Aisa HA. Alkaloid constituents from Viola tianschanica. Phytochem. 2017; 144:233–242. [DOI] [PubMed] [Google Scholar]
- 151.Shepherd C, Giacomin P, Navarro S, Miller C, Loukas A, Wangchuk P. A medicinal plant compound, capnoidine, prevents the onset of inflammation in a mouse model of colitis. J Ethnopharmacol. 2018; 211:17–28. [DOI] [PubMed] [Google Scholar]
- 152.Khdhairawi AAQ, Krishnan P, Mai CW, et al. A Bis-benzopyrroloisoquinoline alkaloid incorporating a cyclobutane core and a chlorophenanthroindolizidine alkaloid with cytotoxic activity from Ficus fistulosa var. tengerensis. J Nat Prod. 2017; 80:2734–2740. [DOI] [PubMed] [Google Scholar]
- 153.Koolen HHF, Pral EMF, Alfieri SC, et al. Antiprotozoal and antioxidant alkaloids from Alternanthera littoralis. Phytochem. 2017; 134:106–113. [DOI] [PubMed] [Google Scholar]
- 154.Sugisawa N, Ohnuma S, Ueda H, et al. Novel potent ABCB1 modulator, phenethylisoquinoline alkaloid, reverses multidrug resistance in cancer cell. Mol Pharm. 2018; 15:4021–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xia MY, Yang J, Zhang PH, et al. Amides, isoquinoline alkaloids and dipeptides from the aerial parts of Piper mullesua. Nat Prod Bioprosp. 2018. 10.1007/s13659-018-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Z, Yang F, Fu JJ, Shen YH, He W, Zhang WD. Delavatine A, a structurally unusual cyclopenta[de] isoquinoline alkaloid from Incarvillea delavayi. Rsc Adv. 2016; 6:65885–65888. [Google Scholar]
- 157.Xie Q, Wu ZG, Yang N, Shen YH, Tang J, Zhang WD. Delavatine A, an unusual isoquinoline alkaloid exerts anti-inflammation on LPS-induced proinflammatory cytokines production by suppressing NF-κB activation in BV-2 microglia. Biochem Biophys Res Commun. 2018; 502:202–208. [DOI] [PubMed] [Google Scholar]
- 158.Li J, Li ZX, Zhao JP, et al. A Novel tropoloisoquinoline tlkaloid, neotatarine, from Acorus calamus L. Chem Biodivers. 2017;14: e1700201. [DOI] [PubMed] [Google Scholar]
- 159.Pan X, Matsumoto M, Nishimoto Y, et al. Cytotoxic and nitric oxide production-inhibitory activities of limonoids and other compounds from the leaves and bark of Melia azedarach. Chem. Biodivers. 2014; 11:1121–1139. [DOI] [PubMed] [Google Scholar]
- 160.Hesari A, Ghasemi F, Cicero AFG, et al. Berberine: A potential adjunct for the treatment of gastrointestinal cancers? J Cell Biochem. 2018; 119:9655–9663. [DOI] [PubMed] [Google Scholar]
- 161.Liu B, Wang G, Yang J, Pan X, Yang Z, Zang L. Berberine inhibits human hepatoma cell invasion without cytotoxicity in healthy hepatocytes. PLoS One. 2011;6: e21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.He W, Wang B, Zhuang Y, Shao D, Sun K, Chen JP. Berberine inhibits growth and induces G1 arrest and apoptosis in human cholangiocarcinoma QBC939 cells. J Pharmacol Sci. 2012; 119:341–348. [DOI] [PubMed] [Google Scholar]
- 163.Ho YT, Yang JS, Li TC, et al. Berberine suppresses in vitro migration and invasion of human SCC-4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF-κB, u-PA and MMP-2 and -9. Cancer Lett. 2009; 279:155–162. [DOI] [PubMed] [Google Scholar]
- 164.Zhang L, Miao XJ, Wang X, et al. Antiproliferation of berberine is mediated by epigenetic modification of constitutive androstane receptor (CAR) metabolic pathway in hepatoma cells. Sci Rep. 2016; 6:28116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li J, Li O, Kan M, et al. Berberine induces apoptosis by suppressing the arachidonic acid metabolic pathway in hepatocellular carcinoma. Mol Med Rep. 2015; 12:4572–4577. [DOI] [PubMed] [Google Scholar]
- 166.Shukla S, Sharma A, Pandey VK, Raisuddin S, Kakkar P. Concurrent acetylation of FoxO1/3a and p53 due to sirtuins inhibition elicit Bim/PUMA mediated mitochondrial dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol Appl Pharm. 2016; 291:70–83. [DOI] [PubMed] [Google Scholar]
- 167.Zhao Y, Cui L, Pan Y, et al. Berberine inhibits the chemotherapy-induced repopulation by suppressing the arachidonic acid metabolic pathway and phosphorylation of FAK in ovarian cancer. Cell Proliferat. 2017;50: e12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Karnam KC, Ellutla M, Bodduluru LN, et al. Preventive effect of berberine against DMBA-induced breast cancer in female Sprague Dawley rats. Biomed Pharmacother. 2017; 92:207–214. [DOI] [PubMed] [Google Scholar]
- 169.Zhao Y, Jing Z, Lv J, et al. Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed Pharmacother. 2017; 95:18–24. [DOI] [PubMed] [Google Scholar]
- 170.Ma W, Zhu M, Zhang D, et al. Berberine inhibits the proliferation and migration of breast cancer ZR-75–30 cells by targeting Ephrin-B2. Phytomedicine. 2017; 25:45–51. [DOI] [PubMed] [Google Scholar]
- 171.Kalaiarasi A, Anusha C, Sankar R, et al. Plant isoquinoline alkaloid berberine exhibits chromatin remodeling by modulation of histone deacetylase to induce growth arrest and apoptosis in the A549 cell line. J Agric Food Chem. 2016; 64:9542–9550. [DOI] [PubMed] [Google Scholar]
- 172.Ruan H, Zhan YY, Hou J, et al. Berberine binds RXRα to suppress Î2-catenin signaling in colon cancer cells. Oncogene. 2017; 36:6906–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Q, Xu X, Zhao M, et al. Berberine induces senescence of human glioblastoma cells by downregulating the EGFR-MEK-ERK signaling pathway. Mol Cancer Ther. 2015; 14:355–363. [DOI] [PubMed] [Google Scholar]
- 174.Wang Z, Liu Y, Xue Y, et al. Berberine acts as a putative epigenetic modulator by affecting the histone code. Toxicol In Vitro. 2016; 36:10–17. [DOI] [PubMed] [Google Scholar]
- 175.Li L, Wang X, Sharvan R, Gao J, Qu S. Berberine could inhibit thyroid carcinoma cells by inducing mitochondrial apoptosis, G0/G1 cell cycle arrest and suppressing migration via PI3K-AKT and MAPK signaling pathways. Biomed Pharmacother. 2017; 95:1225–1231. [DOI] [PubMed] [Google Scholar]
- 176.Andreazza NL, Vevert-Bizet C, Bourg-Heckly G, et al. Berberine as a photosensitizing agent for antitumoral photodynamic therapy: Insights into its association to low density lipoproteins. Int J Pharm. 2016; 510:240–249. [DOI] [PubMed] [Google Scholar]
- 177.Huang T, Xiao Y, Yi L, et al. Coptisine from rhizoma Coptidis suppresses HCT-116 cells-related tumor growth in vitro and in vivo. Sci Rep. 2017; 7:38524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Han B, Jiang P, Li Z, Yu Y, Huang T, Ye X, Li X. Coptisine-induced apoptosis in human colon cancer cells (HCT-116) is mediated by PI3K/Akt and mitochondrial-associated apoptotic pathway. Phytomed. 2018; 48:152–160. [DOI] [PubMed] [Google Scholar]
- 179.Wu J, Xiao Q, Zhang N, et al. Palmatine hydrochloride mediated photodynamic inactivation of breast cancer MCF-7 cells: Effectiveness and mechanism of action. Photodiagn Photodyn Ther. 2016; 15:133–138. [DOI] [PubMed] [Google Scholar]
- 180.Skarydova L, Hofman J, Chlebek J, et al. Isoquinoline alkaloids as a novel type of AKR1C3 inhibitors J Steroid Biochem. 2014; 143:250–258. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y, Li YJ, Huang XH, et al. Liensinine perchlorate inhibits colorectal cancer tumorigenesis by inducing mitochondrial dysfunction and apoptosis. Food Funct. 2018; 14: 5536–5546. [DOI] [PubMed] [Google Scholar]
- 182.Poornima P, Kumar VB, Weng CF, Padma VV. Doxorubicin induced apoptosis was potentiated by neferine in human lung adenocarcima A549 cells. Food Chem Toxicol. 2014; 68:87–98. [DOI] [PubMed] [Google Scholar]
- 183.Poornima P, Weng CF, Padma VV. Neferine, an alkaloid from lotus seed embryo, inhibits human lung cancer cell growth by MAPK activation and cell cycle arrest. Biofactors. 2014; 40:121–131. [DOI] [PubMed] [Google Scholar]
- 184.Kumar P, Singh AK, Raj V, et al. 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid attenuates heptatocellular carcinoma in rats with NMR-based metabolic perturbations. Future Sci OA. 2017; 3:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mishra P, Raj V, Bhadauria AS, et al. 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid attenuates colon carcinogenesis via blockade of IL-6 mediated signals. Biomed Pharmacoth. 2018; 100:282–295. [DOI] [PubMed] [Google Scholar]
- 186.Shen J, Zhang T, Cheng Z, et al. Lycorine inhibits glioblastoma multiforme growth through EGFR suppression. J Exp Clin Cancer Res. 2018; 37:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moise NS, Reimers TJ. Insulin therapy in cats with diabetes mellitus. J Am Vet Med Assoc. 1983; 182:158–164. [PubMed] [Google Scholar]
- 188.Hakim ZS, Patel BK, Goya RK. Effects of chronic ramipril treatment in streptozotocin-induced diabetic rats. Indian J Physiol Pharmacol. 1997; 41:353–360. [PubMed] [Google Scholar]
- 189.American Diabetes Association. Executive summary: standards of medical care in diabetes-2014. Diabetes Care. 2014; 37: s5–13. [DOI] [PubMed] [Google Scholar]
- 190.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3: e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pang B, Zhou Q, Li JL, Zhao LH, Tong XL. Treatment of refractory diabetic gastroparesis: Western medicine and traditional Chinese medicine therapies. World J Gastroenterol. 2014; 20:6504–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xie W, Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diab Obes Metab. 2011; 13:289–301. [DOI] [PubMed] [Google Scholar]
- 193.Chandirasegaran G, Elanchezhiyan C, Ghosh K, Sethupathy S. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of Streptozotocin induced diabetic rats. Biomed Pharm. 2017; 95:175–185. [DOI] [PubMed] [Google Scholar]
- 194.Mokhber-Dezfuli N, Saeidnia S, Gohari AR, Kurepaz-Mahmoodabadi M. Phytochemistry and pharmacology of berberis species. Pharm Rev. 2014; 8:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang Q, Xiao X, Feng K, et al. Berberine moderate glucose and lipid metabolism through multipathway mechanism. Evid Based Complement Altern Med. 2011; 2011: 924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dong Y, Chen YT, Yang YX, et al. Metabolomics study of type 2 diabetes mellitus and the antiDiabetic effect of berberine in zucker diabetic fatty rats using Uplc-ESI-Hdms. Phytother Res. 2016; 30:823–828. [DOI] [PubMed] [Google Scholar]
- 197.Liu C, Wang Z, Song Y, et al. Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo. Biomed Res Int. 2015; 2015: 313808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adil M, Mansoori MN, Singh D, Kandhare AD, Sharma M. Pioglitazone-induced bone loss in diabetic rats and its amelioration by berberine: A portrait of molecular crosstalk. Biomed Pharmacother. 2017; 94:1010–1019. [DOI] [PubMed] [Google Scholar]
- 199.Wu YY, Zha Y, Liu J, et al. Effect of berberine on the ratio of high-molecular weight adiponectin to total adiponectin and adiponectin receptors expressions in high-fat diet fed rats. Chin J Nat Med. 2016;1–9. [DOI] [PubMed] [Google Scholar]
- 200.Adil M, Kandhare AD, Dalvi G, et al. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Renal Failure. 2016; 38:1. [DOI] [PubMed] [Google Scholar]
- 201.Li F, Zhao YB, Wang DK, et al. Berberine relieves insulin resistance via the cholinergic anti-inflammatory pathway in HepG2 Cells. J Huazhong U Sci-Med. 2016; 36: 64–69. [DOI] [PubMed] [Google Scholar]
- 202.Moghaddam HK, Baluchnejadmojarad T, Roghani M, et al. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induced diabetic rats. Mol Neurobiol. 2014; 49:820–826. [DOI] [PubMed] [Google Scholar]
- 203.Fetterman L, Holbrook M, Westbrook DG, et al. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc Diabetol. 2016; 15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sena CM, Pereira AM, Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013; 1832:2216–2231. [DOI] [PubMed] [Google Scholar]
- 205.Huang Z, Han Z, Ye B, et al. Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. Eur J Pharmacol. 2015; 762:1–10. [DOI] [PubMed] [Google Scholar]
- 206.Yu L, Li F, Zhao G, et al. Protective effect of berberine against myocardial ischemia reperfusion injury: role of Notch1/Hes1-PTEN/Akt signaling. Apoptosis. 2015; 20:796–810. [DOI] [PubMed] [Google Scholar]
- 207.Ma YG, Zhang YB, Bai YG, et al. Berberine alleviates the cerebrovascular contractility in streptozotocin-induced diabetic rats through modulation of intracellular Ca2+ handling in smooth muscle cells. Cardiovasc Diabetol. 2016; 15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Peng J, Li X, Zhang D, et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 2015; 87:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ni WJ, Tang LQ, Zhou H, Ding HH, Qiu YY. Renoprotective effect of berberine via regulating the PGE2-EP1-Gαq-Ca2+ signalling pathway in glomerular mesangial cells of diabetic rats. J Cell Mol Med. 2016; 20:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhu LP, Han JK, Yuan RR, Pang WY. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol Res. 2018; 51:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang Z, Li J, Xiong F, et al. Berberine attenuates high glucose-induced fibrosis by activating the G protein-coupled bile acid receptor TGR5 and repressing the S1P2/MAPK signaling pathway in glomerular mesangial cells. Exp Cell Res. 2016; 346:241–247. [DOI] [PubMed] [Google Scholar]
- 212.Tang LQ, Ni WJ, Cai M, et al. Renoprotective effects of berberine and its potential effect on the expression of β-arrestins and intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in streptozocin-diabetic nephropathy rats. J Diabetes. 2016; 8: 693–700. [DOI] [PubMed] [Google Scholar]
- 213.Zhao L, Jiang SJ, Lu FE, et al. Effects of berberine and cinnamic acid on palmitic acid-induced intracellular triglyceride accumulation in NIT-1 pancreatic β cells. Chin J Integr Med. 2016; 22:496–502. [DOI] [PubMed] [Google Scholar]
- 214.Xie X, Chang X, Chen L, et al. Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling. Mol Cell Endocrinol. 2013; 381:56–65. [DOI] [PubMed] [Google Scholar]
- 215.Komers R, Oyama TT, Beard DR, Anderson S. Effects of systemic inhibition of Rho kinase on blood pressure and renal haemodynamics in diabetic rats. Brit J Pharmacol. 2011; 162: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Awad AS, Rouse MD, Khutsishvili K, et al. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int. 2011; 79:1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li X, Liu W, Wang Q, et al. Emodin suppresses cell proliferation and fibronectin expression via p38MAPK pathway in rat mesangial cells cultured under high glucose. Mol Cell Endocrinol. 2009; 307:157–162. [DOI] [PubMed] [Google Scholar]
- 218.Lan T, Liu W, Xie X, et al. Berberine suppresses high glucose-induced TGF-β1 and fibronectin synthesis in mesangial cells through inhibition of sphingosine kinase 1/AP-1 pathway. Eur J Pharmacol. 2012; 697:165–172. [DOI] [PubMed] [Google Scholar]
- 219.Zhou J, Du X, Long M, et al. Neuroprotective effect of berberine is mediated by MAPK signaling pathway in experimental diabetic neuropathy in rats. Eur J Pharmacol. 2016; 774: 87–94. [DOI] [PubMed] [Google Scholar]
- 220.Qiu YY, Tang LQ, Wei W. Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Mol Cell Endocrinol. 2017; 443:89–105. [DOI] [PubMed] [Google Scholar]
- 221.Kopelman PG. Obesity as a medical problem. Nature. 2000; 404:635–643. [DOI] [PubMed] [Google Scholar]
- 222.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008; 8:923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhou L, Wang X, Yang Y, et al. Berberine attenuates cAMP-induced lipolysis via reducing the inhibition of phosphodiesterase in 3T3-L1 adipocytes. Biochem Biophys Acta. 2011; 1812: 527–535. [DOI] [PubMed] [Google Scholar]
- 224.Jiang D, Wang D, Zhuang X, et al. Berberine increases adipose triglyceride lipase in 3T3-L1 adipocytes through the AMPK pathway. Lipids Health Dis. 2016; 15:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zou Z, Hu Y, Ma H, et al. Coptisine attenuates obesity-related inflammation through LPS/TLR-4-mediated signaling pathway in Syrian golden hamsters. Fitoterapia. 2015; 105:139–146. [DOI] [PubMed] [Google Scholar]
- 226.Ma H, Hu Y, Zou Z, Feng M, Ye X, Li X. Antihyperglycemia and antihyperlipidemia effect of protoberberine alkaloids from Rhizoma coptidis in HepG2 cell and diabetic KK-Ay mice. Drug Develop Res. 2016; 77:163–170. [DOI] [PubMed] [Google Scholar]
- 227.Kou S, Han B, Wang Y, et al. Synergetic cholesterol-lowering effects of main alkaloids from Rhizoma Coptidis in HepG2 cells and hypercholesterolemia hamsters. Life Sci. 2016; 151:50–60. [DOI] [PubMed] [Google Scholar]
- 228.Chang W, Chen L, Hatch GM. Berberine treatment attenuates the palmitate-mediated inhibition of glucose uptake and consumption through increased 1,2,3-triacyl-sn-glycerol synthesis and accumulation in H9c2 cardiomyocytes. BBA-Mol Cell Biol L. 2016; 1861:352–362. [DOI] [PubMed] [Google Scholar]
- 229.Zhou Y, Cao SJ, Wang Y, et al. Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia. 2014; 92:230–237. [DOI] [PubMed] [Google Scholar]
- 230.Ju JQ, Li JG, Lin Q, Xu H. Efficacy and safety of berberine for dyslipidaemias: A systematic review and meta-analysis of randomized clinical trials. Phytomedicine. 2018; 50:25–34. [DOI] [PubMed] [Google Scholar]
- 231.He K, Ye X, Wu H, et al. The safety and anti-hypercholesterolemic effect of coptisine in Syrian Golden Hamsters. Lipids. 2015; 50:185–194 [DOI] [PubMed] [Google Scholar]
- 232.Li H, He C, Wang J, et al. Berberine activates peroxisome proliferator-activated receptor gamma to increase atherosclerotic plaque stability in Apoe−/− mice with hyperhomocysteinemia. J Diabetes Invest. 2016; 7: 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen H, Ji YS, Yan X, Su GF, Chen L, Xiao J. Berberine attenuates apoptosis in rat retinal Müller cells stimulated with high glucose via enhancing autophagy and the AMPK/mTOR signaling. Biomed Pharmacother. 2018; 108:1201–1207. [DOI] [PubMed] [Google Scholar]
- 234.Wang YY, Tang LQ, Wei W. Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFβ1-PI3K/AKT pathway. Eur J Pharmacol. 2018; 824:185–192. [DOI] [PubMed] [Google Scholar]
- 235.Xie HG, Wang QQ, Zhang XY, et al. Possible therapeutic potential of berberine in the treatment of STZ plus HFD-induced diabetic osteoporosis. Biomed Pharmacother. 2018; 108:280–287. [DOI] [PubMed] [Google Scholar]
- 236.Zhao ZM, Shang XF, Lawoe RK, et al. Anti-phytopathogenic activity and the possible mechanisms of action of isoquinoline alkaloid sanguinarine. Pest Biochem Physiol. 2019, in press. [DOI] [PubMed] [Google Scholar]
- 237.Andreazza NL, De Lourenço CC, Hernandez-Tasco ÁJ, et al. Antimicrobial photodynamic effect of extracts and oxoaporphine alkaloid isomoschatoline from Guatteria blepharophylla. J Photoch Photobio B. 2016;160: 154–162. [DOI] [PubMed] [Google Scholar]
- 238.Zorić N, Kosalec I, Tomić S, et al. Membrane of Candida albicans as a target of berberine. BMC Complem Altern Med. 2017; 17:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chu M, Xiao R, Yin Y, et al. Berberine: a medicinal compound for the treatment of bacterial infections. Clin Microbial. 2014; 3:3. [Google Scholar]
- 240.Tan L, Li C, Chen H, et al. Epiberberine, a natural protoberberine alkaloid, inhibits urease of Helicobacter pylori and jack bean: Susceptibility and mechanism. Eur J Pharm Sci. 2017; 110:77–86. [DOI] [PubMed] [Google Scholar]
- 241.Mihalyi A, Jamshidi S, Slikas J, Bugg TDH. Identification of novel inhibitors of phospho-MurNAc-pentapeptide translocase MraY from library screening: Isoquinoline alkaloid michellamine B and xanthene dye phloxine B. Bioorg Med Chem. 2014; 22:4566–4571. [DOI] [PubMed] [Google Scholar]
- 242.Centers for Disease Control and Prevention. U.S. Department of Health & Human Services, 2018. https://www.cdc.gov/. [Google Scholar]
- 243.Yu H, Kim K, Cha J, et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005; 8:454–461. [DOI] [PubMed] [Google Scholar]
- 244.Jin L, Hua GQ, Meng Z. Antibacterial mechanisms of berberine and reasons for little resistance of bacteria. C H M. 2011; 03:27–35. [Google Scholar]
- 245.Bavarsadi K, Mahdavi AH, Ansari-Mahyari S, Jahanian E. Effects of different levels of sanguinarine on antioxidant indices, immunological responses, ileal microbial counts and jejunal morphology of laying hens fed diets with different levels of crude protein. J Anim Physiol Anim Nutr. 2017; 101:936–948. [DOI] [PubMed] [Google Scholar]
- 246.Song S, Qiu M, Chu Y, et al. Downregulation of cellular c-Jun N-terminal protein kinase and NF-B activation by berberine may result in inhibition of herpes simplex virus replication. Antimicrob Agents Chemother. 2014; 58:5068–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fan XD, Wang J, Hou JC, et al. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J Transl Med. 2015; 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hayashi K, Minoda K, Nagaoka Y, Hayashi T, Uesato S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007; 17:1562–1564. [DOI] [PubMed] [Google Scholar]
- 249.Mahata S, Bharti AC, Shukla S, Tyagi A, Husain SA, Das BC. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol Cancer. 2011; 10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shin HB, Choi MS, Yi CM, Lee J, Kim NJ, Inn KS. Inhibition of respiratory syncytial virus replication and virus-induced p38 kinase activity by berberine. Int Immunopharmacol. 2015; 27:65–68. [DOI] [PubMed] [Google Scholar]
- 251.Song D, Song J, Wang C, Li Y, Li J. Berberine protects against light-induced photoreceptor degeneration in the mouse retina. Exp Eye Res. 2016; 145:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yan YQ, Fu YJ, Wu S, et al. Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phytother Res. 2018; 32: 2560–2567. [DOI] [PubMed] [Google Scholar]
- 253.Wang H, Li K, Ma L, et al. Berberine inhibits enterovirus 71 replication by downregulating the MEK/ERK signaling pathway and autophagy. Virol J. 2017; 14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Han Y, Park S, Kinyua AW, Andera L, Kim KW, Kim I. Emetine enhances the tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of pancreatic cancer cells by downregulation of myeloid cell leukemia sequence-1 protein. Oncol Rep. 2014; 31:456–462. [DOI] [PubMed] [Google Scholar]
- 255.Gupta RS, Siminovitch L. The isolation and preliminary characterization of somatic cell mutants resistant to the protein synthesis inhibitor-Emetine. Cell. 1976;9: 213–219. [DOI] [PubMed] [Google Scholar]
- 256.Jimenez A, Carrasco L, Vazquez D. Enzymic and nonenzymic translocation by yeast polysomes. Site of action of a number of inhibitors. Biochem. 1977; 16:4727–4730. [DOI] [PubMed] [Google Scholar]
- 257.Schweighoffer T, Schweighoffer E, Apati A, Antoni F. Cytometric analysis of DNA replication inhibited by emetine and cyclosporin A. Histochemistry.1991;96:93–97. [DOI] [PubMed] [Google Scholar]
- 258.Huang L, Chen J, Hu S, Yuan FH, Tu J. Neuroprotective effect of (−)-tetrahydropalmatine in Japanese encephalitis virus strain GP-78 infected mouse model. Microb Pathogenesis, 2018, 114, 197–203. [DOI] [PubMed] [Google Scholar]
- 259.Mo CF, Wang L, Zhang J, et al. β-Lapachone protects against doxorubicin-induced nephrotoxicity via NAD+/AMPK/NF-kB in mice. Redox Sign. 2014; 20:574–588. [Google Scholar]
- 260.Xiao HB, Sun ZL, Zhang HB, Zhang DS. Berberine inhibits dyslipidemia in C57BL/6 mice with lipopolysaccharide induced inflammation. Pharmacol Rep. 2012; 64:889–895. [DOI] [PubMed] [Google Scholar]
- 261.Maleki SN, Aboutaleb N, Souri F. Berberine confers neuroprotection in coping with focal cerebral ischemia by targeting inflammatory cytokines. J Chem Neuroanat. 2018; 87:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Choi SB, Bae GS, Jo IJ, Wang S, Song HJ, Park S. Berberine inhibits inflammatory mediators and attenuates acute pancreatitis through deactivation of JNK signaling pathways. Mol Immunol. 2016; 74:27–38. [DOI] [PubMed] [Google Scholar]
- 263.Li P, Liao S, Wang J, et al. Secondary Metabolites with chemical diversity from the marine-derived fungus pseudallescheria boydii F19–1 and their cytotoxic activity. RSC Adv. 2016; 6:47474. [Google Scholar]
- 264.Niu XF, Zhang HL, et al. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Chem-Bio Interact. 2015; 239:34–45. [DOI] [PubMed] [Google Scholar]
- 265.Li YJ, Xiao HT, Hua DD, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res. 2016; 110:227–239. [DOI] [PubMed] [Google Scholar]
- 266.Liu YX, Liu X, Hua WW, et al. Berberine inhibits macrophage M1 polarization via AKT1/SOCS1/NF-κB signaling pathway to protect against DSS-induced colitis. Int Immunopharmacol. 2018; 57:121–131. [DOI] [PubMed] [Google Scholar]
- 267.Zhang H, Shan Y, Wu Y, et al. Berberine suppresses LPS-induced inflammation through modulating Sirt1/NF-κB signaling pathway in RAW264.7 cells. Int Immunopharmacol. 2017; 52:93–100. [DOI] [PubMed] [Google Scholar]
- 268.Yue M, Xia Y, Shi C, et al. Berberine ameliorates collagen-induced arthritis in rats by suppressing Th17 cell responses via inducing cortistatin in the gut J Cell Sci. 2017; 284:2876–2801. [DOI] [PubMed] [Google Scholar]
- 269.Dinesh P, Rasool M. Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int Immunopharmacol. 2017; 44:26–37. [DOI] [PubMed] [Google Scholar]
- 270.Dinesh P, Rasool M. Berberine inhibits IL-21/IL-21R mediated inflammatory proliferation of fibroblast-like synoviocytes through the attenuation of PI3K/Akt signaling pathway and ameliorates IL-21 mediated osteoclastogenesis. Cytokine. 2018; 106:54–66. [DOI] [PubMed] [Google Scholar]
- 271.Lv Q, Wang K, Qiao SM, Dai Y, Wei ZF. Norisoboldine, a natural aryl hydrocarbon receptor agonist, alleviates TNBS-induced colitis in mice, by inhibiting the activation of NLRP3 inflammasome. Chin J Nat Med. 2018; 16:0161–0174. [DOI] [PubMed] [Google Scholar]
- 272.Luo YB, Liu M, Xia YF, Dai Y, Chou G, Wang Z. Therapeutic effect of norisoboldine, an alkaloid isolated from Radix Linderae, on collagen-induced arthritis in mice. Phytomed. 2010; 17:726–731. [DOI] [PubMed] [Google Scholar]
- 273.Luo YB, Liu M, Dai Y, et al. Norisoboldine inhibits the production of pro-inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 Cells by down-regulating the activation of MAPKs but not NF-κB. Inflammation. 2010; 33:389–397. [DOI] [PubMed] [Google Scholar]
- 274.Luo YB, Wei ZF, Chou GX, et al. Norisoboldine induces apoptosis of fibroblast-like synoviocytes from adjuvant-induced arthritis rats. Int Immunopharmacol. 2014; 20:110–116. [DOI] [PubMed] [Google Scholar]
- 275.Lu Q, Lu S, Gao XH, et al. Norisoboldine, an alkaloid compound isolated from Radix Linderae, inhibits synovial angiogenesis in adjuvant-induced arthritis rats by moderating Notch1 pathway-related endothelial tip cell phenotype. Exp Biol Med. 2012; 237:919–932. [DOI] [PubMed] [Google Scholar]
- 276.Lu Q, Tong B, Luo YB, et al. Norisoboldine suppresses VEGF-induced endothelial cell migration via the cAMP-PKA-NF-κB/Notch1 pathway. PLoS One. 2013; 8: e81220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tong B, Yuan X, Dou Y, et al. Norisoboldine, an isoquinoline alkaloid, acts as an aryl hydrocarbon receptor ligand to induce intestinal Treg cells and thereby attenuate arthritis. Int J Biochem Cell B. 2016; 75:63–73. [DOI] [PubMed] [Google Scholar]
- 278.Lv Q, Wang K, Qiao S, Yang L, Xin Y, Dai Y, Wei ZF. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD+/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 2018; 9:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tong B, Dou YN, Wang ZT, Kong LY, Dai Y, Xia YF. Norisoboldine ameliorates collagen-induced arthritis through regulating the balance between Th17 and regulatory T cells in gut-associated lymphoid tissues. Toxicol Appl Pharm. 2015, 282, 90–99. [DOI] [PubMed] [Google Scholar]
- 280.Di Donato JA, Mercurio F, Karin M. Analysis of LPS-induced, NFκB-dependent Interleukin-8 transcription in kidney embryonic cell line expressing TLR4 using luciferase Assay. Immunol Rev. 2012; 246:379–400.22435567 [Google Scholar]
- 281.Chen YY, Li RY, Shi MJ, et al. Demethyleneberberine alleviates inflammatory bowel disease in mice through regulating NF-κB signaling and T-helper cell homeostasis. Inflamm Res. 2016; 66:1–10. [DOI] [PubMed] [Google Scholar]
- 282.Liao W, He X, Yi Z, Xiang W, Ding Y. Chelidonine suppresses LPS-Induced production of inflammatory mediators through the inhibitory of the TLR4/NF-κB signaling pathway in RAW264.7 macrophages. Biomed Pharmacother. 2018; 107: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 283.Zhang ZH, Mi C, Wang KS, et al. Chelidonine inhibits TNF-α-induced inflammation by suppressing the NF-κB pathways in HCT116 cells. Phytother Res. 2017; 32:1–11. [DOI] [PubMed] [Google Scholar]
- 284.Yan B, Wang D, Dong S, et al. Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cells. Int Immunopharmacol. 2017; 45:194–200. [DOI] [PubMed] [Google Scholar]
- 285.Gao LN, Feng QS, Zhang XF, Wang QS, Cui YL. Tetrandrine suppresses articular inflammatory response by inhibiting pro-inflammatory factors via NF-κB inactivation. J Orthop Res. 2016; 34:1557–1568. [DOI] [PubMed] [Google Scholar]
- 286.Zhou K, Hu L, Liao W, Yin D, Rui F. Coptisine Prevented IL-β-induced expression of inflammatory mediators in chondrocytes. Inflammation. 2016; 39:1558–1565. [DOI] [PubMed] [Google Scholar]
- 287.Chen H, Luo C, Liang J, et al. Anti-inflammatory activity of coptisine free base in mice through inhibition of NF-κB and MAPK signaling pathways. Eur J Pharmacol. 2017; 811:222–231. [DOI] [PubMed] [Google Scholar]
- 288.Küpeli E, Koşar M, Yeşilada E, HüsnüK, Başer C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002; 72:645–657. [DOI] [PubMed] [Google Scholar]
- 289.Fang Q, Li HY. Effect of palmatine sequential combination of exercise therapy on serum inflammatory markers in pelvic inflammation and its clinical effect. Chin J Biochem Pharm. 2016; 6: 164–166. [Google Scholar]
- 290.Zhao W Reserach progress on Fibrauretin. China Anim Hus Vet Med. 2014; 5: 267–270. [Google Scholar]
- 291.Zhou X, Lin X, Xiong Y, Jiang L, Li W, Li J, Wu L. Chondroprotective effects of palmatine on osteoarthritis in vivo and in vitro: A possible mechanism of inhibiting the Wnt/β-catenin and Hedgehog signaling pathways. Int Immunopharmacol. 2016; 34:129–138. [DOI] [PubMed] [Google Scholar]
- 292.Zhang XJ, Yuan ZW, Qu C, et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacol Res. 2018; 137:34–46. [DOI] [PubMed] [Google Scholar]
- 293.Yang C, Zhang C, Wang Z, Tang Z, Kuang H, Kong AN. Corynoline isolated from Corydalis bungeana Turcz. exhibits anti-inflammatory effects via modulation of Nfr2 and MAPKs. Molecules. 2016; 21:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Liu Y, Song M, Zhu G, Xi X, Li K, Wu C, Huang L. Corynoline attenuates LPS-induced acute lung injury in mice by activating Nrf2. Int Immunopharmacol. 2017; 48:96–101. [DOI] [PubMed] [Google Scholar]
- 295.Feng AW, Yu C, Mao Q, Li N, Li QR, Li JS. Berberine hydrochloride attenuates cyclooxygenase-2 expression in rat small intestinal mucosa during acute endotoxemia. Fitoterapia. 2011; 82:976–982. [DOI] [PubMed] [Google Scholar]
- 296.Fu K, Lv X, Li W, et al. Berberine hydrochloride attenuates lipopolysaccharide-induced endometritis in mice by suppressing activation of NF-κB signal pathway. Int Immunopharmacol. 2015; 24:128–132. [DOI] [PubMed] [Google Scholar]
- 297.Choi YH. Berberine hydrochloride protects C2C12 myoblast cells against oxidative stress-induced damage via induction of Nrf-2-mediated HO-1 expression. Drug Develop Res. 2016; 77:310–318. [DOI] [PubMed] [Google Scholar]
- 298.Dkhil MA, Metwaly MS, Al-Quraishy S. Indigofera oblongifolia leaf extract regulates spleen macrophage response during Plasmodium chabaudi infection. Saudi J Biol Sci. 2017; 24:1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Hu YR, Ma H, Zou ZY, et al. Activation of Akt and JNK/Nrf2/NQO1 pathway contributes to the protective effect of coptisine against AAPH-induced oxidative stress. Biomed Pharmacother. 2017; 85:313–322. [DOI] [PubMed] [Google Scholar]
- 300.Baskaran R, Kalaiselvi P, Huang CY, Padma VV. Neferine, a bisbenzylisoquinoline alkaloid, offers protection against cobalt chloride-mediated hypoxia-induced oxidative stress in muscle cells. Integr Med Res. 2015; 4: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kawai N, Matsuda M, Uenishi J. Stereoselective synthesis of tetrahydroisoquinoline alkaloids: (−)-trolline, (+)-crispin A, (+)-oleracein E. Tetrahedron. 2011; 67:8648–8653. [Google Scholar]
- 302.Wang PP, Sun HX, Liu CJ, et al. Racemic oleracein E increases the survival rate and attenuates memory impairment in D-galactose/NaNO2-induced senescent mice. Phytomed. 2016; 23:460–467. [DOI] [PubMed] [Google Scholar]
- 303.Li L, Jiao Y, Jin T, et al. Phenolic alkaloid oleracein E attenuates oxidative stress and neurotoxicity in AlCl3-treated mice. Life Sci. 2017; 191:211–218. [DOI] [PubMed] [Google Scholar]
- 304.Mansueto P, Seidita A, Vitale G, Cascio A. Transfusion transmitted leishmaniasis. What to do with blood donors from endemic areas? Travel Med Infect Dis. 2014; 12:563–581. [DOI] [PubMed] [Google Scholar]
- 305.Ntie-Kang F, Onguene PA, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants, part II: a pharmacological evaluation of non-alkaloids and non-terpenoids. Malar J. 2014; 13:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.De Vries H, Wagelmans AP, Hasker E, Lumbala C, Lutumba P, et al. Forecasting human African trypanosomiasis prevalences from population screening data using continuous time models. PLoS Comput Biol. 2016;12: e1005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Copeland NK, Aronson NE. Leishmaniasis: treatment updates and clinical practice guidelines review. Curr Opin Infect Dis. 2015; 28:426–437. [DOI] [PubMed] [Google Scholar]
- 308.Deng YJ, Xu J, Zhang XY, et al. Berberine attenuates autophagy in adipocytes by targeting BECN1. Autophagy. 2014; 10:1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014; 383:2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Shang XF, Morris-Natschke SL, Liu YQ, et al. Biologically active quinoline and quinazoline alkaloids part I. Med Res Rev. 2018;38:775–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Nonaka M, Murata Y, Takano R, Han Y, Kabir MHB, Kato K. Screening of a library of traditional Chinese medicines to identify anti-malarial compounds and extracts. Malaria J. 2018; 17:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Malik TA, Kamili AN, Chishti MZ, Tanveer S, Ahad S, Johri RK. In vivo anticoccidial activity of berberine [18,5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a) quinolizinium] -an isoquinoline alkaloid present in the root bark of Berberis lycium. Phytomed. 2014; 21:663–669. [DOI] [PubMed] [Google Scholar]
- 313.Li MH, Zhang YJ, Yu YH, et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol. 2014; 728:67–76. [DOI] [PubMed] [Google Scholar]
- 314.Jiang W, Wei W, Gaertig MA, Li S, Li XJ. Therapeutic effect of berberine on huntington’s disease transgenic mouse model. PLoS One. 2015;10: e0134142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Kim M, Cho KH, Shin MS, et al. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int J Mol Med. 2014; 33:870–878. [DOI] [PubMed] [Google Scholar]
- 316.Zhang H, Zhao C, Cao G, et al. Berberine modulates amyloid-Î2 peptide generation by activating AMP-activated protein kinase. Neuropharmacol. 2017; 125:408–417. [DOI] [PubMed] [Google Scholar]
- 317.Liang Y, Huang M, Jiang X, Liu Q, Chang X, Guo Y. The neuroprotective effects of Berberine against amyloid β-protein-induced apoptosis in primary cultured hippocampal neurons via mitochondria-related caspase pathway. Neurosci Lett. 2017; 655:46–53. [DOI] [PubMed] [Google Scholar]
- 318.Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer's disease. Exp Gerontol. 2017; 91:25–33. [DOI] [PubMed] [Google Scholar]
- 319.Hussien HM, Abd Elmegied A, Ghareeb DA, et al. Neuroprotective effect of berberine against environmental heavy metals-induced neurotixicity and Alzheimer's-like disease in rats. Food Chem Toxicol. 2017; 111:432–444. [DOI] [PubMed] [Google Scholar]
- 320.Zhang Z, Li X, Li F, An L. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int Immunopharmacol. 2016; 38:426–433. [DOI] [PubMed] [Google Scholar]
- 321.Yu Y, Zhao Y, Teng F, et al. Berberine improves cognitive deficiency and muscular dysfunction via activation of the AMPK/SIRT1/PGC-1a pathway in skeletal muscle from naturally aging rats. J Nutr Health Aging. 2018; 22:710–717. [DOI] [PubMed] [Google Scholar]
- 322.Chang CF, Lee YC, Lee KH, et al. Therapeutic effect of berberine on TDP-43-related pathogenesis in FTLD and ALS. J Biomed Sci. 2016; 23:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Shen JD, Ma LG, Hu CY, et al. Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci Lett. 2016; 614:77–82. [DOI] [PubMed] [Google Scholar]
- 324.Lee B, Shim I, Lee H, Hahm DH. Berberine alleviates symptoms of anxiety by enhancing dopamine expression in rats with post-traumatic stress disorder. Korean J Physiol Pha. 2018; 22:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Fan J, Li B, Ge T, et al. Berberine produces antidepressant-like effects in ovariectomized mice. Sci Rep. 2017; 7: 1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.He W, Wang C, Chen Y, He Y, Cai Z. Berberine attenuates cognitive impairment and ameliorates tau hyperphosphorylation by limiting the self-perpetuating pathogenic cycle between NF-κB signaling, oxidative stress and neuroinflammation. Pharmacol Rep. 2017; 69:1341–1348. [DOI] [PubMed] [Google Scholar]
- 327.Baek SC, Ryu HW, Kang MG, et al. Selective inhibition of monoamine oxidase A by chelerythrine, an isoquinoline alkaloid. Bioorg Med Chem Lett. 2018; 28:2403–2407. [DOI] [PubMed] [Google Scholar]
- 328.Chang XX, Yan HM, Fei J, et al. Berberine reduces methylation of the MTTP promoter and alleviates fatty liver induced by a high-fat diet in rats. J Lipid Res. 2010; 51:2504–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Zhang ZG, Zhang HZ, Li B, et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2013; 5:5493. [DOI] [PubMed] [Google Scholar]
- 330.Chen W, Miao YQ, Fan DJ, et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS Pharm Sci Tech. 2011; 12:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hua W, Ding L, Chen Y, Gong B, He J, Xu GJ. Determination of berberine in human plasma by liquid chromatography-electrospray ionization-mass spectrometry. Pharm Biomed Anal. 2007; 44:931–937. [DOI] [PubMed] [Google Scholar]
- 332.Liu YT, Hao HP, Xie HG, et al. Extensive intestinal first-pass elimination and predominant hepatic distribution ofb erberine explain its low plasma levels in rats. Drug Metab Dispos. 2010; 38:1779–1784. [DOI] [PubMed] [Google Scholar]
- 333.Zhao L, Cang Z, Sun H, Nie X, Wang N, Lu Y. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. Endocr Disord. 2017; 17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Deng J, Sitou K, Zhang Y, Yan R, Hu Y. Analyzing the Chinese landscape in anti-diabetic drug research: leading knowledge production institutions and thematic communities. Chin Med. 2016; 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sun H, Liu Q, Hu H, et al. Berberine ameliorates blockade of autophagic flux in the liver by regulating cholesterol metabolism and inhibiting COX2-prostaglandin synthesis. Cell Death Dis. 2018; 9:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhang ZG, Li B, Meng XJ, et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci Rep. 2016; 6:20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mahmoud AM, Hozayen WG, Ramadan SM. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats. Biomed Pharmacother. 2017; 94:280–291. [DOI] [PubMed] [Google Scholar]
- 338.Du JY, Ding XH, Zhang XQ, Zhao XY, Shan HD, Wang FP. Berberine attenuate staphylococcal enterotoxin B-mediated acute liver injury via regulating HDAC expression. AMB Expr. 2018; 8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wang D, Wang K, Sui D, Zhen OY, Xu HY, Wei Y. Effects of tetrahydroberberine and tetrahydropalmatine on hepatic cytochrome P450 expression and their toxicity in mice. Chem-Bio Interact. 2017; 268:47–52. [DOI] [PubMed] [Google Scholar]
- 340.Liu X, Zhang X, Ye L, Yuan H. Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed Pharmacother. 2016; 79:222–230. [DOI] [PubMed] [Google Scholar]
- 341.Zhao G, Yu L, Gao W, et al. Berberine protects rat heart from ischemia/ reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol Sin. 2016; 37:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yu L, Li Q, Yu B, et al. Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: role of silent information regulator 1. Oxid Med Cell Longev. 2016; 6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Qing Y, Xu D, Li HL, Liu YH. Berberine promoted myocardial protection of postoperative patients through regulating myocardial autophagy. Biomed Pharmcother. 2018; 105: 1050–1053. [DOI] [PubMed] [Google Scholar]
- 344.Zhao GL, Yu LM, Gao WL, et al. Berberine protects rat heart from ischemia/ reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol Sin. 2016; 37:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Yang J, Yan H, Li SM, Zhang M. Berberine Ameliorates MCAO Induced Cerebral Ischemia/Reperfusion Injury via Activation of the BDNF–TrkB–PI3K/Akt Signaling Pathway. Neurochem Res. 2018; 43:702–710. [DOI] [PubMed] [Google Scholar]
- 346.Guo J, Wang S, Yuan T, et al. Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis. 2013; 231:384–391. [DOI] [PubMed] [Google Scholar]
- 347.Guan GY, Han H, Yang YL, Jin YM, Wang XT, Liu XM. Neferine prevented hyperglycemia-induced endothelial cell apoptosis through suppressing ROS/Akt/NF-κB signal. Endocrine. 2014; 47:764–771. [DOI] [PubMed] [Google Scholar]
- 348.Wang C, Wang H, Xiao JH, Wang JL, Xiang JZ, Tang Q. Inhibitory effects of neferine on Nav1.5 channels expressed in HEK293 cells. J Huazhong Univ Sci Technol [Med. Sci.] 2016; 36:487–493. [DOI] [PubMed] [Google Scholar]
- 349.Wang C, Chen YF, Quan XQ, et al. Effects of neferine on Kv4.3 channels expressed in HEK 293 cells and ex vivo electrophysiology of rabbit hearts. Acta Pharmacol Sin. 2015; 36:1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Luo C, Chen H, Wang Y, et al. Protective effect of coptisine free base on indomethacin-induced gastric ulcers in rats: Characterization of potential molecular mechanisms. Life Sci. 2018; 193:47–56. [DOI] [PubMed] [Google Scholar]
- 351.Wang L, Wang X, Zhang SL, et al. Gastroprotective effect of palmatine against acetic acid-induced gastric ulcers in rats. J Nat Med. 2017; 71:1–8. [DOI] [PubMed] [Google Scholar]
- 352.Li W, Wang X, Zhang H, et al. Anti-ulcerogenic effect of cavidine against ethanol-induced acute gastric ulcer in mice and possible underlying mechanism. Int Immunopharmacol. 2016;38:450–459. [DOI] [PubMed] [Google Scholar]
- 353.Hasanein P, Riahi H. Preventive use of berberine in inhibition of lead-induced renal injury in rats. Environ Sci Pollut Res. 2018, 25, 4896–4903. [DOI] [PubMed] [Google Scholar]
- 354.Lin YB, Sheng MW, Ding YJ, et al. Berberine protects renal tubular cells against hypoxia/reoxygenation injury via the Sirt1/p53 pathway. J Nat Med. 2018; 72:715–723. [DOI] [PubMed] [Google Scholar]
- 355.Yang S, Yu Z, Sun W, et al. The antiviral alkaloid berberine ameliorates neuropathic pain in rats with peripheral nerve injury. Acta Neurol. Belg 2018; doi: 10.1007/s13760-018-1006-9. [DOI] [PubMed] [Google Scholar]
- 356.Aicher WK, Rolauffs B. The spatial organisation of joint surface chondrocytes: review of its potential roles in tissue functioning, disease and early, preclinical diagnosis of osteoarthritis. Ann Rheum Dis. 2014; 73:645–653. [DOI] [PubMed] [Google Scholar]
- 357.Zhao H, Zhang T, Xia C, et al. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J Cell Mol Med. 2014; 18:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zhou Y, Liu SQ, Yu L, et al. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis. 2015; 20:1187–1199. [DOI] [PubMed] [Google Scholar]
- 359.Zhou Y, Tao H, Li Y, et al. Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via Wnt/β-catenin pathway. Eur J Pharmacol. 2016; 789:109–118. [DOI] [PubMed] [Google Scholar]
- 360.Tao K, Xiao D, Weng J, Xiong A, Kang B, Zeng H. Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway. Toxicol Lett. 2016; 240:68–80. [DOI] [PubMed] [Google Scholar]
- 361.Liu L, Luo N, Guo J, Xie Y, Chen L, Cheng ZP. Berberine inhibits growth and inflammatory invasive phenotypes of ectopic stromal cells: Imply the possible treatment of adenomyosis. J Pharmacol Sci. 2018; 137:5–11. [DOI] [PubMed] [Google Scholar]
- 362.Huang LN, Zhang XH, Ma XH, et al. Berberine alleviates endothelial glycocalyx degradation and promotes glycocalyx restoration in LPS-induced ARDS. Int Immunopharmacol. 2018; 65:96–107. [DOI] [PubMed] [Google Scholar]


