Abstract
Glomus tumors (GT), together with myofibroma (MF), myopericytoma (MP) and angioleiomyoma (AL) are classified as members of the perivascular myoid family of tumors. The reported genetic abnormalities across these neoplasms is dissimilar, arguing against a pathogenetically unified family; half of the GT showing NOTCH-gene fusions and a smaller subset BRAF V600E mutations, while PDGFRB mutations are noted in a subset of MF and MP. The aim of this study was to investigate the prevalence and specificity of NOTCH gene fusions in a large group of GT and correlate with clinical features. BRAF-VE1 and PDGFRB immunoexpression was also investigated in this cohort. 93 GT and 43 other pericytic lesions (11 MP, 13 MF, 19 AL) were selected. All cases were tested by fluorescence in situ hybridization for NOTCH1-4 and MIR143 gene abnormalities and 6 cases were investigated by targeted RNA sequencing. FISH revealed NOTCH gene rearrangements in 50 (54%) GT, 2 MP (18%) and 2 AL (11%). NOTCH-rearrangements were present in 34 (68%) benign and 16 (32%) malignant GT. Fusion-positive benign GT were overwhelmingly seen in males with a predilection for extremities, while the malignant GT occurred mostly in viscera. Among the fusion-negative GT, 88% were benign, 9% uncertain malignant potential and 2% malignant. Half of the fusion negative-GTs occurred in the finger/subungual region. In summary, rearrangements of NOTCH genes are seen in over half of GT, with NOTCH2-MIR143 being the most common fusion (73%), while only a small subset of AL and MP share these abnormalities. The common subungual GT subset lack NOTCH gene fusions suggesting an alternative pathogenesis. BRAF-VE1 was negative in all 37 cases studied while strong PDGFRB staining was seen in 14 (21%) cases. Additional studies are needed to investigate the genetic alterations in the fusion-negative cases.
Keywords: NOTCH2, MIR143, glomus tumor
INTRODUCTION
Glomus tumors (GT) are rare mesenchymal neoplasms composed of cells resembling the perivascular modified smooth muscle cells of the normal glomus body. They share variable histologic overlap with other tumors in the pericytic family including myofibroma (MF), myopericytoma (MP) and angioleiomyoma (AL). Studies investigating the genetic alterations of sporadic glomus tumors or other pericytic tumors, as well as the potential pathogenetic relationship between these neoplasms have been relatively sparse. Mosquera et al.1 first reported NOTCH1-3 gene rearrangements, with the most common alteration being NOTCH2-MIR143 gene fusion, in a group of 33 GT, while two other studies by Chakrapani et al.2 and Karamzadeh Dashti et al.3 described BRAF mutations in a small subset of GT. More recent studies have reported PDGFRB gene mutations in a small group of MF and MP.4, 5 Despite the morphologic similarities encountered among the entities of the pericytic tumor family, the emerging genetic data suggest a dichotomy of the perivascular myoid tumor spectrum into 2 categories: PDGFRB-mutant MF and MP and NOTCH-fusion positive GT. In this study, we investigate the prevalence of NOTCH gene abnormalities in a large group of GT and related pericytic lesions, and correlate with their clinicopathologic features. Additionally, BRAF-VE1 and PDGFRB immunohistochemistry is analyzed as surrogate alterations for alternative molecular events.
METHODS
Patient Selection
Archival material and personal consult files of the senior author (CRA) were searched for cases diagnosed morphologically as glomus tumor (GT), myofibromas (MF), myopericytoma (MP) and angioleiomyomas (AL) with available tissue for molecular analysis. In addition, a small number of cases (n=6) were initially unclassified or misclassified, and subsequently reclassified as malignant glomus tumor based on the molecular findings. Hematoxylin and eosin stained slides were reviewed. The study group was analyzed for demographic information, anatomic site, tumor size, and morphologic features, including nuclear features, mitotic activity and presence of necrosis. Available immunohistochemical stains were reviewed and reported immunohistochemical results were noted. A subset of cases was previously included in our initial study (Mosquera et al., 2013)1, including 33 GT, 18 AL, 6 MP and 9MF. GT were classified based on current WHO criteria6 as benign, of uncertain malignant potential, or malignant. According to WHO criteria, malignant glomus tumors are defined as tumors showing: (i) marked nuclear atypia and any level of mitotic activity; or (ii) atypical mitotic figures. Glomus tumors showing only a single atypical feature (deep location, tumor size ≥ 2 cm or mitotic activity ≥ 5/ 50 HPF) were designated GT-UMP. Cases with available FFPE material for ancillary testing were selected for the study. The clinical follow-up information was obtained for patients with malignant GT by review of the electronic medical records and from contacting referring pathologists and clinicians. The study was approved by the Institutional IRB.
Fluorescence in situ hybridization (FISH)
Formalin-fixed paraffin-embedded tissues were available in each case for FISH analysis. FISH for NOTCH2 was performed on all cases and, if negative, was followed by subsequent FISH for NOTCH1, NOTCH3 and NOTCH4. FISH for the gene fusion partner MIR143 was also performed on all cases positive for any of the NOTCH genes. FISH was performed on 4 μm-thick formalin-fixed paraffin-embedded (FFPE) tissue sections. Custom probes were made by bacterial artificial chromosomes (BAC) clones (Supplementary Table 1) flanking the target genes, according to UCSC genome browser (http://genome.ucsc.edu) and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (Oakland, CA; https://bacpacresources.org/). DNA from each BAC was isolated according to the manufacturer’s instructions. The BAC clones were labeled with fluorochromes by nick translation and validated on normal metaphase chromosomes. The slides were deparaffinized, pretreated, and hybridized with denatured probes. After overnight incubation, the slides were washed, stained with DAPI and mounted with an antifade solution. Two hundred successive nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Newton, MA). A positive score was interpreted when at least 20% of the nuclei showed a break-apart signal. Nuclei with incomplete set of signals were omitted from the score.
Targeted RNA sequencing and analysis
Six cases were investigated using two different targeted RNA sequencing platforms, including 3 with the TruSight RNA Fusion Panel and 3 with the Archer FusionPlex™. RNA was extracted from FFPE tissue using Amsbio’s ExpressArt FFPE Clear RNA Ready kit (Amsbio LLC, Cambridge, MA). Fragment length was assessed with an RNA 6000 chip on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA-seq libraries were prepared using 20-100 ng total RNA with the Trusight RNA Fusion Panel (Illumina, San Diego, CA). Each sample was subjected to targeted RNA sequencing on an Illumina MiSeq at 8 samples per flow cell (approximately 3 million reads per sample). All reads were independently aligned with STAR (ver 2.3) and BowTie2 against the human reference genome (hg19) for Manta-Fusion and TopHat-Fusion analysis, respectively.
Archer FusionPlex™-targeted RNA sequencing utilizes the Archer Anchored Multiplex PCR (AMP™) technology and next generation sequencing to detect gene fusions in solid tumor and sarcoma samples. The Archer™ custom solid panel targets 62 genes known to be recurrently involved in rearrangements associated with these tumors. RNA was extracted from formalin-fixed paraffin-embedded (FFPE) tumor material followed by cDNA synthesis. cDNA undergoes end repair, dA tailing, and ligation with Illumina molecular barcode adapters, using standard protocols.7 Sequencing was performed on an Illumina Miseq instrument (2x150bp) and analyzed on the Archer™ software V5.0.
Immunohistochemistry
Immunohistochemistry for BRAF V600E was performed using the VE1 mouse monoclonal antibody (Ventana, Tucson, AZ, USA; #790-4855) at a 1:3 dilution after antigen retrieval by citrate buffer pressure cook. The results were evaluated as positive or negative. Immunohistochemistry for PDGFRB (PDGFR beta) was performed using the antibody clone Y92 from Abcam (order # ab32570) at a dilution of 1:500 [0.32 ug/mL]. The staining was evaluated as negative and positive, with the positive cases further characterized on a scale of 1+ to 3+ as follows: 1+, weak staining in 0-25% of the tumor cells; 2+, weak to moderate staining in 25-50% of the tumor cells; and 3+, strong staining in >50% of the tumor cells.
RESULTS
NOTCH gene rearrangements occur in more than half of GT and correlate with risk of malignancy and clinical presentation.
Ninety-three patients with GT were selected, occurring in 55 males and 38 females, with an age range of 14 to 87 years (mean 48 years). Seventy-two cases were benign, 17 cases were malignant and 4 were of uncertain malignant potential. The tumors were located in the lower extremity (31 cases), upper extremity (12), fingers (22), visceral locations (21), bone (3), chest wall (2) and other sites (2). (Table 1)
Table 1:
Clinico-pathologic and molecular features.
| NOTCH rearranged benign GT | NOTCH rearranged malignant GT | Glomus tumors with no rearrangements | |
|---|---|---|---|
| Number of cases | 34 | 16 | 43 |
| Sex (M:F) | 29:5 | 8:8 | 19:24 |
| Age range (median age) | 22 – 87 years (52 years) | 14 – 80 years (48 yrs) | 14 – 84 yrs (47.5 yrs) |
| Location | Lower extremity – 17 Upper extremity – 9 Visceral – 5 Other - 3 |
Visceral – 10 (GI tract - 6, lung - 4) Lower extremity – 4 Chest wall - 2 |
Fingers – 22 (nailbed – 8) Lower extremity – 10 Upper extremity – 3 Visceral – 6 Bone - 3 |
| Size | 0.4 to 3.5 cm (median – 1.0 cm) | 0.8 to 10.7 cm (median – 4.2 cm) | 0.2 to 3.8 cm (median – 0.5 cm) |
| Gene rearrangements | 88% - NOTCH2 9% - NOTCH3 3% - NOTCH1 76% - MIR143 |
88% - NOTCH2 12% -NOTCH1 69% - MIR143 |
No rearrangements |
FISH studies showed rearrangements in one of the NOTCH genes in 50 cases (54%), including NOTCH2 in 44 (88%), NOTCH1 in 3 cases (3%) and NOTCH3 in 3 cases (3%). MIR143 was the most common partner gene identified in 37 cases. (74%) Among the 50 NOTCH-rearranged GT, 34 (68%) cases were benign and 16 (32%) were malignant. The NOTCH-rearranged tumors occurred in 36 males and 14 females, with an age range of 14 – 87 years (mean – 52 years). Twenty-one cases occurred in the lower extremity, 9 in the upper extremity, 15 in visceral locations, 2 in chest wall and 3 in other locations.
Targeted RNA-sequencing was performed in 6 cases, 3 cases each with the TruSight RNA Fusion Panel and Archer FusionPlex assay platforms. Overall, four cases showed NOTCH2-MIR143 gene fusion and 1 case showed NOTCH1-MIR143 gene fusion. One case did not reveal any candidate gene fusions.
Among the 34 cases of benign NOTCH-rearranged GT, most occurred in males (n=28, 82%), with a wide age range of 22-86 years (median – 52 years) at diagnosis. The lesions showed a variable anatomic distribution, with most occurring in the soft tissue of the extremities (82%, 17 lower extremity, 9 cases upper extremity), followed by visceral location (n=5, 15%). Tumors ranged in size from 0.4 to 3.3 cm with a mean of 1.3 cm. Morphologically, the cases showed typical features of monomorphic epithelioid to cuboidal cells arranged in solid pattern and encuffing the thin-walled blood vessels. Rare cases showed large cavernous vascular spaces surrounded by uniform lesional cells (glomangioma). One case showed symplastic changes with cytologic atypia. (Figure 1) Most cases (n=30; 88%) showed NOTCH2 gene rearrangement, and less commonly NOTCH3 (n=3, 9%) or NOTCH1 (n=1, 3%), while MIR143 gene partner also being rearrangement in 26 (76%) of the cases.
Figure 1: Morphologic spectrum of benign GT positive for NOTCH gene fusions.
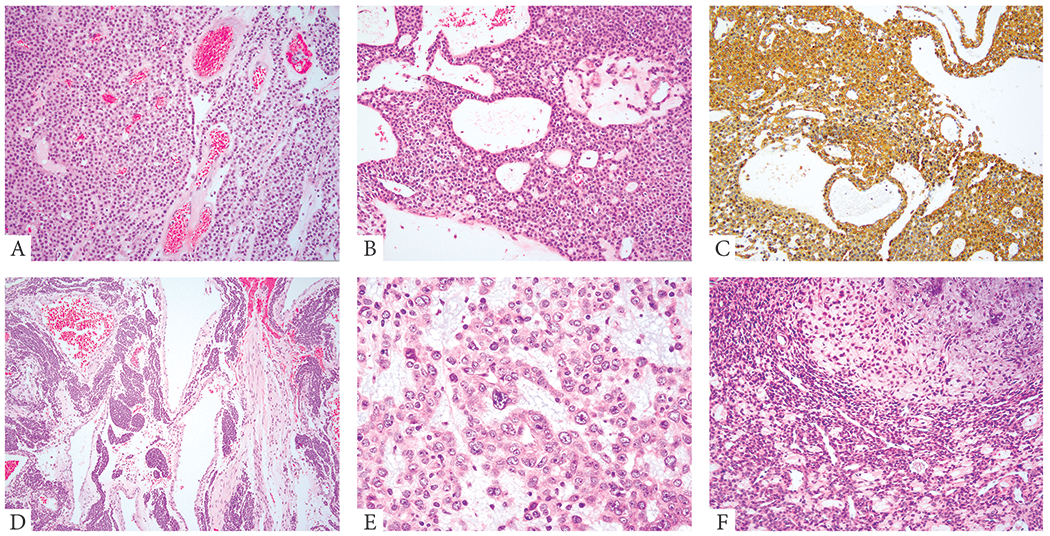
A. (68/M, thigh, NOTCH2-MIR143) Monomorphic round to ovoid cells in solid sheets and perivascular distribution. B, C. (48/M, thigh, NOTCH2-MIR143) Uniform cuboidal cells arranged in sheets and sieve-like, microcystic growth. Immunohistochemical stain for SMA (C) showing diffuse positivity. D. (31/M, knee, NOTCH3 rearranged) Large cavernous vascular spaces associated with solid nests of epithelioid cells (glomangioma). E. (36/M, kidney, NOTCH2 rearranged) Renal GT showing epithelioid cells with eosinophilic cytoplasm and enlarged nuclei with vesicular chromatin, conspicuous nucleoli and variable cytologic atypia (symplastic change). F. (74/M, foot, NOTCH2 rearranged) GT with focal myxoid stromal changes.
All except one malignant GT were positive for NOTCH gene rearrangements. The 16 patients with NOTCH-rearranged malignant GT had an equal gender distribution and a wide age range of 16 – 80 years (median – 48 years). Most cases (10, 63%) occurred in the viscera (GI tract, 6, lung, 4), while the remaining occurred in the soft tissues (lower extremity, 4; chest wall, 2). Tumors ranged in size from 0.8 to 10.7 cm with a mean of 5.0 cm. Morphologically, 5 cases showed evidence of conventional glomus tumor in focal areas. The malignant component of these cases ranged from cellular spindle cell areas (leiomyosarcoma-like) to undifferentiated spindle and round cell morphology. Some of the tumors showed a clear cell morphology. (Figure 2) Mitotic activity ranged from 2 to 30 per 10 high power fields (mean 11/ 10 high power fields).
Figure 2: Morphologic spectrum of malignant GT with NOTCH gene fusions.
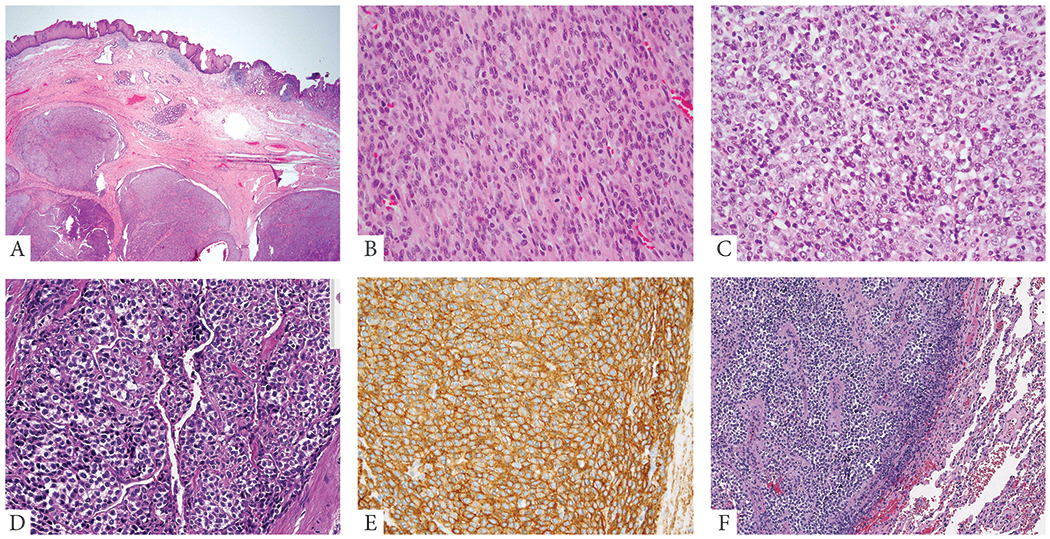
A. (67/M, esophageal, NOTCH2-MIR143) Low power image showing a multinodular lesion involving the submucosa of the gastro-esophageal junction. B. (77/F, small bowel, NOTCH2-MIR143) Malignant change in a GT in the form of sarcomatoid spindle cell areas with increased mitotic activity. C. (49/F, GE junction, NOTCH2 rearranged) Malignant GT in the form of undifferentiated round cell areas. D, E. (19/F, esophagus, NOTCH2-MIR143) Tumor shows a vague nested growth pattern, with epithelioid cells having sharply demarcated cell borders and variable clear cell change. Immunohistochemical stain for SMA (E) showing diffuse positivity in the tumor cells. F. (54/F, lung met, NOTCH2-MIR143) Metastatic GT to the lung with undifferentiated round cell morphology.
FISH analysis showed 14 cases (88%) being positive for NOTCH2 gene rearrangements, while remaining 2 cases had NOTCH1 rearrangements. MIR143 gene rearrangement was identified in 11 (76%) of the cases.
Follow-up was available in 7 patients with malignant GT (range 1-67 months), showing that all developed metastatic disease, most to the lung (n= 4), and one each to the brain, kidney and lymph node. At last follow-up, one patient, a 47 year-old male, with a primary thigh tumor and lung metastasis, had died of disease, 46 months following diagnosis.
The 43 NOTCH-negative GT occurred in 19 males and 24 females, with a wide age range at diagnosis of 14 – 84 years (median – 48 years). Notably, most cases in this negative-molecular subset were benign (n= 38, 88%), with a small number being defined as uncertain malignant potential (4 cases, 9%) and only one malignant GT (2%). About half of the cases (n=22) occurred in the fingers, including 8 involving the nailbed. The remaining were distributed in the soft tissue of the extremities (10 lower, 3 upper), 6 visceral and 3 in the bone. (Figure 3) Tumors ranged in size from 0.2 to 3.8 cm with a mean of 1.1 cm.
Figure 3: Morphologic spectrum of GT lacking NOTCH gene rearrangements.
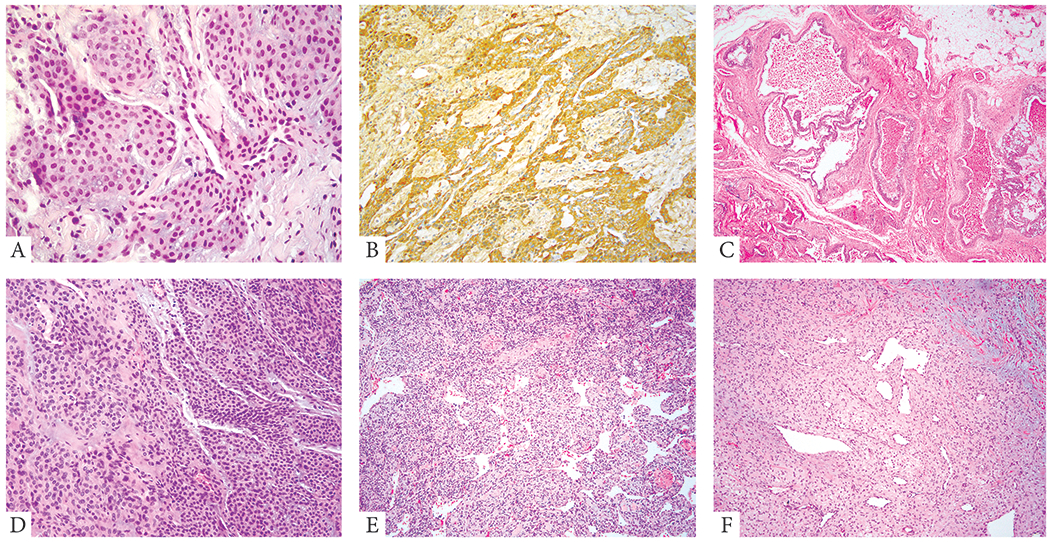
A, B. (51/F, finger) Characteristic pattern of GT with round to ovoid, monomorphic cells, arranged around blood vessels. Immunohistochemical stain for SMA (B) highlights the perivascular arrangement of the tumor cells. C. (17/F, extremity) GT showing large cavernous vascular spaces lined by a layer of monomorphic epithelioid cells (glomangioma) D. (43/M, subungual) GT with predominantly solid pattern of the epithelioid tumor cells. E. (69/F, forearm) and F. (39/F, ankle) GTs showing branching HPC-like vasculature in the background (glomangiopericytoma).
NOTCH-gene rearrangements occur in rare cases of angioleiomyoma and myopericytoma.
Among the 43 cases of other pericytic lesions, including 19 angioleiomyomas (AL), 11 myopericytomas (MP) and 13 myofibromas (MF), FISH analysis showed evidence of NOTCH2 gene rearrangements in 2 (11%) AL and one case of MP, while no MIR143 gene abnormalities were noted. One additional MP case showed evidence of NOTCH4 and MIR143 gene rearrangements by FISH analysis. The AL showed the characteristic morphology of vascular channels encuffed concentrically by cells with a well-differentiated smooth muscle appearance, as shown by the diffuse immunopositivity for desmin. (Supplementary Figure 1). The NOTCH-rearranged MP were composed of spindle cells with short ovoid nuclei, arranged concentrically around vascular spaces. (Supplementary Figure 2) None of the 13 MF showed NOTCH gene alterations by FISH or Archer FusionPlex (1 case).
PDGFRB Immunoreactivity does not correlate with NOTCH gene rearrangements
Immunohistochemistry for PDGFRB was performed on 67 cases, including 28 benign NOTCH-rearranged GT, 7 malignant NOTCH-rearranged GT and 32 NOTCH-negative GT. Overall, 14 cases (21%) showed a 3+ staining pattern (Supplementary Figure 3), 33 cases (49%) showed a 1+ - 2+ staining pattern and 19 (30%) were negative. Of the 14 cases with 3+ staining pattern, 9 cases were NOTCH-negative GT, 3 were benign NOTCH-rearranged GT and 2 were malignant NOTCH-rearranged GT.
Immunohistochemistry for BRAF (VE1) was performed on 37 of the 43 NOTCH-negative GT cases. None of the 37 cases showed BRAF expression in the tumor cells.
DISCUSSION
Novel NOTCH-gene fusions were first described in glomus tumors (GT) by Mosquera et al.1 using whole-transcriptome RNA-sequencing. In this initial study, about half of the 33 cases investigated showed NOTCH gene fusions, with NOTCH2 being the most common abnormality (81%), resulting in overexpression of NOTCH2 mRNA. Only rare cases of GT harbored NOTCH3 and NOTCH1 gene rearrangements. In two-thirds of the cases MIR143 was detected as the gene fusion partner. Interestingly, in that study, MIR143 miRNA cluster was found to be overexpressed not only in GT but also in a wide range of smooth muscle neoplasms, irrespective of the MIR143 fusions. These results suggested that the MIR143 strong promoter in these fusions lead to oncogenic overexpression of NOTCH2 expression. Moreover, all 5 malignant examples of GT included in that study showed the presence of NOTCH gene rearrangements.
There have been additional genetic abnormalities reported in small subsets of sporadic GT including BRAF and KRAS gene mutations. 2, 3 Chakrapani et al. studied 28 GT and identified 3 cases with BRAF V600E mutation and one case with KRAS G12A mutation. In the study by Dashti et al., 6 of the 95 glomus tumors tested had BRAF V600E gene mutation, with 3 of them occurring in malignant GT and 3 in uncertain malignant potential GT. In familial GT, inactivating mutations of the glomulin (GLMN) gene, located in 1p22.1, have been reported. 8–10
The current study investigated the largest cohort to date of GT and other pericytic lesions for the presence of NOTCH gene rearrangements and related abnormalities, using a combined methodology including FISH and targeted RNA sequencing. The results showed that 54% of GT harbor NOTCH gene fusions, with NOTCH2 being the most common abnormality (88%), with only 3 cases each with NOTCH3 and NOTCH1 rearrangements. MIR143 was the most common gene partner, detected in 76% of the cases, being fused to NOTCH2 in 67% and NOTCH1/3 in 4%. Among the NOTCH-fusion positive GT, two-thirds of the cases were benign and one third were malignant. However, most of the GT lacking rearrangements were benign (88%), while all except one malignant GT was positive for NOTCH fusions. NOTCH fusion positive benign GT occurred predominantly in males, while the fusion negative GT occurred slightly more common in females. Although the age range and the median age at diagnosis were similar among these molecular subgroups, there were striking correlations with tumor location. Most benign NOTCH fusion-positive GT were located in the soft tissues of the extremities (76%), while malignant NOTCH-fusion-positive tumors were frequently located in the viscera (63%), commonly in the gastrointestinal tract and lung. Half of the fusion-negative GT were located in the fingers, including all of the sub-ungual lesions. Based on our study findings, one could broadly sub-classify GT into 2 clinicopathologic / genetic categories, such as the fusion-negative benign acral GT and the NOTCH-rearranged GT occurring in the soft tissues of the extremities and viscera. Moreover, most, if not all, GT with malignant histologic features were positive for NOTCH fusions, which can be used as a powerful molecular diagnostic tool in challenging cases. In our series, one third (6 of 16) of malignant GTs could not be correctly subclassified based on morphology and immunohistochemical studies, being diagnosed descriptively (‘spindle and epithelioid neoplasm’, ‘undifferentiated sarcoma with small round cell features’, ‘unusual epithelioid neoplasm’, ‘malignant mesenchymal neoplasm’). Only after the molecular results became available, showing either NOTCH gene rearrangements or NOTCH-MIR143 gene fusion, the diagnosis of malignant GT was rendered.
Visceral GT are rare and diagnostically challenging in malignant examples, which often lack a benign counterpart.6 The gastrointestinal (GI) tract is one of the common visceral sites, with the gastric locations being the most common site of occurrence.11 In a study by Miettinen et al., 32 patients with GI tract GT, 31 occuring in the stomach, followed a good prognosis overall, with only one patient dying of metastatic disease to the liver. In our study, 23% of the entire cohort occurred in the viscera, with the GI tract being the most common location, followed by lung. Two-thirds of our visceral GT were classified as malignant or of uncertain malignant potential. Half of the malignant NOTCH-rearranged GTs arose in visceral locations, with the stomach being the most common site. A number of the malignant examples of the visceral GT were misdiagnosed. One of the gastric tumors showed diffuse synaptophysin positivity suggestive of neuroendocrine differentiation. Some of the initial diagnoses rendered in our cohort of malignant GTs included undifferentiated sarcoma with small blue round cell morphology, unclassified spindle and epithelioid neoplasm, unusual malignant mesenchymal neoplasm, etc.
The other members of the perivascular smooth muscle family, including myofibromas (MF), myopericytomas (MP) and angioleiomyomas (AL), appear to have distinct genetic abnormalities from GT, although most of these entities have not been thoroughly investigated by next generation sequencing. One potential exception is the presence of BRAF V600E mutations reported not only in a small proportion of GT but also in a small subset (3/20, 15%) of MP.12 However, in a subsequent study, none of the 20 MP tested showed immunopositivity for BRAF VE1 antibody (known to be highly concordant with BRAF mutation status).13 These results suggest that BRAF V600E mutations occur at a low frequency in both GT and MP, and its relationship with the more common genetic alterations seen in these tumors remains uncertain. Our findings of negative immunostaining with VE1 antibody in all fusion-negative GT tested further support the overall low impact of this abnormality in the pathogenesis of these neoplasms.
Agaimy et al.4 investigated a group of 8 infantile and 16 adult sporadic MF and found PDGFRB mutations in 6 of 8 infantile and 11 of 16 adult MF, while none of the 6 MP and 8 AL analyzed showed this abnormality. In the study by Hung et al.5, targeted next-generation DNA sequencing identified PDGFRB gene mutations in 4 of 5 myopericytomatosis (a phenomenon of diffuse dermal / subcutaneous involvement by myopericytomatous nodules) and 3 of 5 conventional MP. In addition, low-level amplification at the 5q32 locus (containing PDGFRB and CSF1R) were detected in 2 of the 5 myopericytomatosis and 4 of 5 MP, which were not mutually exclusive from the PDGFRB mutations, were of uncertain significance. No BRAF or NOTCH abnormalities were noted. In our study, 11% of AL and 18% of MP showed evidence of NOTCH gene rearrangements, suggesting some limited overlap with GT. PDGFRB immunohistochemistry was used in our study as a surrogate marker for PDGFRB alterations. In analyzing 67 cases of GTs from both NOTCH-rearranged and NOTCH-negative groups, we identified 14 cases (21%) with strong PDGFRB staining and 33 cases (49%) with mild to moderate staining pattern. Additional studies are required to investigate the significance of this finding, which does not appear to correlate with the presence NOTCH gene rearrangements. Based on the findings, the perivascular smooth muscle family can be subdivided into three genetic groups, based on NOTCH fusions, PDGFRB gene abnormalities and undetermined (lacking genetic alterations).
In conclusion, this is the largest study to date investigating the prevalence and specificity of NOTCH gene abnormalities among a group of GT and other members of the perivascular smooth muscle family. Our results highlight some important correlations between the molecular signature and clinico-pathologic features. Benign NOTCH fusion positive GT occur with predilection in males and in the soft tissues of the extremities, while malignant NOTCH-rearranged GT occur preferentially in visceral locations. In fact, all except one malignant GT were positive for NOTCH gene abnormalities. Thus, FISH or targeted RNA sequencing for detecting NOTCH gene rearrangement can be used as an ancillary test in challenging cases, especially when lacking a benign component. Acral and subungual GT lack NOTCH gene fusions and might be pathogenetically unrelated from other soft tissue and visceral GT. Although a small subset of AL and MP showed NOTCH gene rearrangements, most members of the perivascular smooth muscle tumor family appear genetically distinct from soft tissue and visceral GT.
Supplementary Material
Supplementary Figure 1: Angioleiomyoma with NOTCH2 gene fusion. (67/F, thigh) A. Low power image showing a well-circumscribed lesion with increased vascularity. B. High power image showing well-differentiated smooth muscle concentrically arranged around small and large vessels. C. FISH for NOTCH2 showing break-apart red (c-) and green (t-) signals (arrows). D. Immunohistochemical stain for Desmin showing diffuse positivity in the lesional cells.
Supplementary Figure 2: Myopericytomas with NOTCH2 rearrangement . A. (47/M, ankle) Low power image showing cellular spindle cell lesion with spindle cells arranges in whorled pattern around intermediate sized vessels. and NOTCH4-MIR143 gene fusion B - D. (62yM, thumb) B. Low power image showing cellular lesion with spindle cells en-cuffing the thin walled vasculature. FISH images for C. NOTCH4 and D. MIR143 gene probes showing break-apart red (c-) and green (t-) signals (arrows).
Supplementary Figure 3: Immunohistochemical stain for PDGFRB showing 3+ positivity in GT, including benign NOTCH-rearranged GTs (A, 41yM, mainstem bronchus, NOTCH2-MIR143; B, 38yF, kidney, NOTCH2 rearranged), malignant NOTCH-rearranged GTs (C, 47yM, thigh, NOTCH2 rearranged; D, 67yM, esophagus, NOTCH2-MIR143) and in NOTCH-negative GTs (E, 69yF, forearm; F, 55yM, calf).
ACKNOWLEDGEMENTS
The authors would like to thank Bruce Crilly for preparation of composite figures. The authors would also like to thank Denise Frosina, Jerica A. Geronimo and Emily Hernandez from the Developmental Immunohistochemistry Laboratory for assistance with the PDGFRB stains.
Supported in part by: P50 CA140146 (CRA); P30-CA008748 (NA, CRA); P50 CA217694 (NA, CRA), Kristen Ann Carr Foundation (CRA); Cycle for Survival (CRA)
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Mosquera JM, Sboner A, Zhang L, et al. , Novel MIR143-NOTCH fusions in benign and malignant glomus tumors. Genes Chromosomes Cancer, 2013. 52(11): p. 1075–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrapani A, Warrick A, Nelson D, et al. , BRAF and KRAS mutations in sporadic glomus tumors. Am J Dermatopathol, 2012. 34(5): p. 533–5. [DOI] [PubMed] [Google Scholar]
- 3.Karamzadeh Dashti N, Bahrami A, Lee SJ, et al. , BRAF V600E Mutations Occur in a Subset of Glomus Tumors, and Are Associated With Malignant Histologic Characteristics. Am J Surg Pathol, 2017. 41(11): p. 1532–1541. [DOI] [PubMed] [Google Scholar]
- 4.Agaimy A, Bieg M, Michal M, et al. , Recurrent Somatic PDGFRB Mutations in Sporadic Infantile/Solitary Adult Myofibromas But Not in Angioleiomyomas and Myopericytomas. Am J Surg Pathol, 2017. 41(2): p. 195–203. [DOI] [PubMed] [Google Scholar]
- 5.Hung YP and Fletcher CDM, Myopericytomatosis: Clinicopathologic Analysis of 11 Cases With Molecular Identification of Recurrent PDGFRB Alterations in Myopericytomatosis and Myopericytoma. Am J Surg Pathol, 2017. 41(8): p. 1034–1044. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher C, Bridge JA, Hogendoorn PC, et al. , WHO Classification of Tumours of Soft Tissue and Bone. 2013: IARC: Lyon. [Google Scholar]
- 7.Zhu G, Benayed R, Ho C, et al. , Diagnosis of known sarcoma fusions and novel fusion partners by targeted RNA sequencing with identification of a recurrent ACTB-FOSB fusion in pseudomyogenic hemangioendothelioma. Mod Pathol, 2019. 32(5): p. 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boon LM, Brouillard P, Irrthum A, et al. , A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet, 1999. 65(1): p. 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouillard P, Olsen BR, and Vikkula M, High-resolution physical and transcript map of the locus for venous malformations with glomus cells (VMGLOM) on chromosome 1p21-p22. Genomics, 2000. 67(1): p. 96–101. [DOI] [PubMed] [Google Scholar]
- 10.Amyere M, Aerts V, Brouillard P, et al. , Somatic uniparental isodisomy explains multifocality of glomuvenous malformations. Am J Hum Genet, 2013. 92(2): p. 188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miettinen M, Paal E, Lasota J, et al. , Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol, 2002. 26(3): p. 301–11. [DOI] [PubMed] [Google Scholar]
- 12.Sadow PM, Priolo C, Nanni S, et al. , Role of BRAFV600E in the first preclinical model of multifocal infiltrating myopericytoma development and microenvironment. J Natl Cancer Inst, 2014. 106(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mito JK and Jo VY, BRAF V600E is not a consistent feature of myopericytoma. J Cutan Pathol, 2016. 43(12): p. 1248–1249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Angioleiomyoma with NOTCH2 gene fusion. (67/F, thigh) A. Low power image showing a well-circumscribed lesion with increased vascularity. B. High power image showing well-differentiated smooth muscle concentrically arranged around small and large vessels. C. FISH for NOTCH2 showing break-apart red (c-) and green (t-) signals (arrows). D. Immunohistochemical stain for Desmin showing diffuse positivity in the lesional cells.
Supplementary Figure 2: Myopericytomas with NOTCH2 rearrangement . A. (47/M, ankle) Low power image showing cellular spindle cell lesion with spindle cells arranges in whorled pattern around intermediate sized vessels. and NOTCH4-MIR143 gene fusion B - D. (62yM, thumb) B. Low power image showing cellular lesion with spindle cells en-cuffing the thin walled vasculature. FISH images for C. NOTCH4 and D. MIR143 gene probes showing break-apart red (c-) and green (t-) signals (arrows).
Supplementary Figure 3: Immunohistochemical stain for PDGFRB showing 3+ positivity in GT, including benign NOTCH-rearranged GTs (A, 41yM, mainstem bronchus, NOTCH2-MIR143; B, 38yF, kidney, NOTCH2 rearranged), malignant NOTCH-rearranged GTs (C, 47yM, thigh, NOTCH2 rearranged; D, 67yM, esophagus, NOTCH2-MIR143) and in NOTCH-negative GTs (E, 69yF, forearm; F, 55yM, calf).


