Abstract
Background
To achieve goals, organisms are often faced with complex tasks that require an enhanced control of cognitive faculties for optimal performance. However, the neural circuit mechanisms underlying this ability are unclear. The claustrum is proposed to mediate a variety of functions ranging from sensory binding to cognitive control of action, but direct functional assessments of this telencephalic nucleus are lacking.
Methods
Here, we employ the guanine nucleotide-binding subunit beta-4 cre driver line in mice to selectively monitor and manipulate claustrum projection neurons during 1-choice versus 5-choice serial reaction time task performance.
Results
Using fiber photometry, we find elevated claustrum activity prior to an expected cue during correct performance on a cognitively demanding five-choice response assay relative to the less-demanding one-choice version of the task. Claustrum activity during reward acquisition is also enhanced when task demand is higher. Furthermore, optogenetically inhibiting claustrum prior to the onset of the cue reduces choice accuracy on the five-choice but not the one-choice task.
Discussion
These results suggest the claustrum supports a cognitive control function necessary for optimal behavioral performance under cognitively demanding conditions.
Keywords: Photometry, Networks, cognition, attention, GNB4, glutamate
Introduction
The allocation of cognitive resources to meet task demands is critical for survival and impaired in several neuropsychiatric disorders (1). This cognitive control function recruits constellations of cortical regions for optimal behavioral performance (2,3), but the role of subcortical nuclei in gating or orchestrating cortical responses are poorly defined. Nuclei with widespread innervation of cortical regions are proposed to mediate this function, and some evidence exists that monoaminergic nuclei may contribute (4–6). However, a role for the claustrum (CL), which is also widely connected with neocortex (7,8) and significantly negatively modulates its activity (9) has not been previously considered.
The connectivity of the claustrum with cortex has instead motivated a number of other functional hypotheses (8,10–13), including that the CL binds sensory information to generate conscious percepts (7). Direct analysis of CL function is historically intractable, particularly in the rodent. A recent human study using a method to accurately detect CL BOLD signal using functional magnetic resonance imaging showed the CL is activated with the switch to a difficult version of the multi-source interference task (14). While this suggests that the CL may be involved in controlling cognition to meet task demand, a causal assessment of this role is lacking.
In this study, we use the guanine nucleotide binding protein beta 4 cre driver line (GNB4-cre) for monitoring and manipulation of CL projection neurons in awake, freely moving mice (15). This line exhibits specific expression in claustrum relative to neighboring striatum and insular cortex and labels projection neurons (15), making heretofore intractable examinations of claustrum function now possible. The importance of anterior cingulate cortex (ACC) input to CL for five-choice response task performance (16) and claustrum processing/propagation of ACC signals to posterior cortices (17), suggest the CL is a critical nucleus for top-down coordination of distal cortical areas. Models of higher order brain functions propose the necessity of such coordination, for example in models of attention (18) and consciousness (19,20). As such, examination of the role of CL in cognitively demanding conditions, stands to provide important insight into mechanisms of higher order brain function.
Materials and methods
Animals.
34 GNB4-cre mice bred from a C57BL/6J background of both sexes were used (15). Mice used for electrophysiology were 10–20 weeks of age at the time of experiments and group-housed with food and water available ad libitum. In contrast, mice used for behavioral experiments were 12–28 weeks of age at the time of experiments. These mice were singly-housed, weighed daily, and fed daily to maintain 90% of ad libitum weigh. All mice were on a 12h light-dark cycle beginning at 0700 and behavior experiments were performed during the light cycle. For optogenetic experiments, control and experimental groups were comprised of mice from the same litters to minimize any litter effects. This study was performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the University of Maryland, School of Medicine, Animal Care and Use Committee.
Viral vectors and stereotaxic procedures.
For optogenetic inhibition or in vivo fiber photometry monitoring of CL 80–110 nL of AAV vectors expressing loxP-flanked double inverted open reading frames (DIO) of halorhodopsin (AAV5-eF1a-DIO-eNPhR3.0-eYFP; University of Pennsylvania Vector Core) or GCaMP6f (AAV9-EF1a-DIO-GCaMP6f; University of Pennsylvania Vector Core), respectively, were injected bilaterally at two rostrocaudal levels of the CL (4 injections) in GNB4-cre mice. In control mice and mice used for whole-cell electrophysiology, the same approach was used but with a vector expressing eYFP (AAV5-eF1a-DIO-eYFP; University of Pennsylvania Vector Core). Relative to bregma, CL coordinates were 1) anterior-posterior: +1.34 mm, medial-lateral ± 2.3 mm, dorsal-ventral (from the brain surface): −2.35 mm; and 2) anterior-posterior: +0.86 mm, medial-lateral ± 2.75 mm, dorsal-ventral (from the brain surface): −2.55 mm. Viral incubation was no fewer than 3 weeks.
Mice used for in vivo optogenetics experiments were implanted bilaterally with chronic indwelling fiber optic implants into CL. Fiber optic implants were custom-made using high NA (0.66) fiber (Prizmatix Ltd; Giv’at Shmuel, Israel) epoxied into ceramic ferrules (ThorLabs Inc; Newton, NJ) and affixed to the skull with dental cement. Mice used for in vivo fiber photometry received a unilateral implant into CL, which was custom-made from low (0.22) NA fiber and ceramic ferrules (ThorLabs Inc; Newton, NJ). Fiber implant placement was confirmed post-hoc using immunohistochemistry. The light path angle (θ) was approximated to ensure accurate implantation using the known refractive index (n) of cortical tissue (21) and the respective fiber NA according to the following formula: NA = nsinθ. The distance of light penetration was approximated using previous estimates for each type of fiber (22,23).
Ex vivo brain slice preparation for electrophysiology.
Following anesthetization, mice were decapitated, the brains were extracted, and 250 μm coronal sections were sliced using a vibrating microtome in a high-sucrose artificial cerebrospinal fluid (aCSF). The aCSF was ice-cold, carbogen (95% O2, 5% CO2)-bubbled, and consisted of 194 mM sucrose, 30 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 26 mM NaHCO3, 1.2 mM NaH2PO4, and 10 mM D-glucose. Sections were incubated after slicing for 30 min at 33°C in carbogen-bubbled aCSF (315–320 mOsm) that contained 124 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 26 mM NaHCO3, 1.2 mM NaH2PO4, and 10 mM D-glucose. Sections were incubated at room temperature until whole-cell patch-clamp recordings that were performed in the same aCSF formulation used for incubation.
Whole-cell current-clamp recordings.
Whole-cell recordings were performed at 29°C – 31°C using borosilicate glass recording pipettes of 3–7 MΩ resistance. Recording pipettes were filled with a potassium-based solution (290–295 mOsm; pH 7.3) composed of 126 mM potassium gluconate, 4 mM KCl, 10 mM HEPES, 4 mM ATP-Mg, 0.3 mM GTP-Na and 10 mM phosphocreatine. Clampex software (version 10.4; Molecular Devices; Sunnyvale, CA) was used for electrophysiological recordings, which were filtered at 2 kHz and digitized at 10 kHz. Internal pipette solutions also contained hydrazide dye conjugated with AlexaFluor®−488 (40 µM) for visualization of dendritic spines. GNB4+ neurons expressing eYFP or halorhodopsin were identified using epifluorescence and 470 nm light was delivered with an external LED.
Five-choice serial reaction-time task (5CSRTT).
Mice were trained to perform the 5CSRTT (24–27) in operant chambers (Med Associates; St. Albans, VT) housed within sound-attenuating cabinets as previously described (16). Briefly, mice are trained to nose poke into one of five pseudo-randomly illuminated apertures (cue). Trials are preceded by a 5 s inter-trial interval (ITI) and responses are allowed during the cue and up to 5 s after the cue. Correct nose pokes resulted in a sucrose pellet dispensed into a receptacle on the wall opposite to the five apertures. A new trial ITI did not begin until 5 s after the reinforcement was collected. Nose pokes into the incorrect aperture, no response (omission), and nose pokes during the ITI resulted in a 5 s time out period, during which the house light was extinguished. Any nose poke during the time out period restarted the 5 s time out.
For optogenetic experiments, 470 nm light was delivered bilaterally during experimental sessions using an LED system (Plexon Inc; Dallas, TX); light delivery occurred pseudo-randomly on 33% of trials during the ITI period continuously for 4 s prior to the onset of the cue. A session ended after 100 trials or 30 min, whichever occurred first, and data was averaged across five sessions.
Mice were habituated, shaped, and trained before experiments as previously described (16). Mice were habituated to the operant chamber for two 15 min sessions with sucrose pellets available in the receptacle. Shaping occurred in two phases. First, all apertures were illuminated and any nose poke was reinforced. Apertures were initially loaded with sucrose pellets to facilitate initial poking. In the second phase, only one aperture was illuminated and only a nose poke into the illuminated aperture was reinforced. Mice progressed through each shaping phase after meeting criterion, which was defined as 30 correct nose pokes in a 30 min session. No time outs were given during shaping.
Mice were then progressed through two 5CSRTT training phases that were identical to the final 5CSRTT except for the cue duration. For the first training phase, the cue duration was 10 s and for the second training phase, the cue duration was 5 s. For training phases, criterion was defined as 60% accuracy and 30 responses within a session. Mice were over-trained on the final task (1 s cue) until choice accuracy reached a stable baseline before manipulations. Subsequent to 5CSRTT experimental sessions, mice were trained to perform a one-choice modification of the 5CSRTT (1CSRTT). All aspects of the task remained the same, except the middle aperture was illuminated and active on every trial. Mice were over-trained until performance stabilized before beginning experimental sessions. In 1CSRTT experimental sessions, 470 nm light was delivered as described above for the 5CSRTT; continuously for 4 s prior to the onset of the cue.
Real-time place preference (RTPP) and open field assay.
The RTPP apparatus consisted of a two-sided chamber connected with a narrow corridor. The RTPP assay consisted of habituation and test sessions. In the habituation session, mice were placed initially in the narrow corridor of the chamber and allowed free exploration for 20 min. On the following day, mice performed the test session, which paired one side of the chamber with 4 s of continuous 470 nm light bouts. These bouts were repeated at most every 20 s in order to approximate the amount of stimulation that occurred during the 5CSRTT sessions. The amount of time spent in each side of the chamber during sessions and ambulatory speed was recorded with EthoVision XT v 11.5 (Noldus, Wageningen, The Netherlands). The speed during the 0.5 s of light delivery and the subsequent 2 s was compared to the 2.5 s preceding light delivery to determine any effect of light delivery on movement. The same apparatus was used as an open field to measure calcium-dependent activity of CL during movement using in vivo fiber photometry.
In vivo fiber photometry.
Photometry data were collected using a customized in vivo fiber photometry system. Two single wavelength laser modules were used (Opto Engine LLC; Midvale, Utah), a 473 nm laser for optimal GCamP6f excitation, and a 405 nm laser to excite GCaMP6f at its isosbestic wavelength (28). As such, emission from 405 nm excitation was used to control for image artifacts due to photometry cable motion, background fluorescence, and other sources of noise (28). The two lasers were multiplexed at 10 Hz, resulting in a continuous 20 Hz pulse train. Both laser beams were two-bounced nto a dichroic filter cube optimized for 473 and 405 nm excitation, as well as for 510 nm emission (Chroma Technology Corporation; Bellow Falls, VT). The two excitation wavelengths were focused through a 4X fluorite objective (Olympus; Tokyo, Japan) onto a multimode fiber bundle that consisted of seven individual multimode fibers. One fiber was connected to the mouse via chronic multimode fiber implant, while another fiber was placed inside a tube of AlexaFluor®−488 (40 µM) to control for variability in laser energy. Emissions from GCaMP6f and AlexaFluor®−488 through the multimode fibers were detected as an image of the fiber bundle using the ORCA Flash 4.0LT high-resolution CMOS camera (Hamamatsu Photonics KK; Hamamatsu City, Japan). Laser multiplexing and image acquisition were synchronized using an Arduino Leonardo microcontroller and time-locked to trials when necessary. Camera image acquisition parameters were controlled through the HCImage Software for Hamamatsu cameras.
Data analysis and statistics.
Photometry data were analyzed using a combination of custom MATLAB code (Mathworks; Natick, Massachusetts) and Prism v 6.0.1 (GraphPad Software; La Jolla, CA). Pixel intensities imaged from the fibers implanted in CL and the fluorophore control fiber were first averaged. The background signal in the absence of laser transmission was then subtracted from the averaged signals. Two separate regressions were performed to minimize any noise sources. First, 473 and 405 nm photometry signals were regressed with the corresponding control fluorophore signal, and the residuals of the regression were then used for further processing. The 473 nm signal was then regressed with the 405 nm signal as a covariate, and the residuals of the regression were extracted as the fully processed photometry signal from the CL. The processed signal was then sorted based on trial type (correct, incorrect or omission), and averaged across trials for each specific trial type, and then across all five 5CRSTT runs for each animal. Further statistical analysis was performed in GraphPAD Prism. All statistics are displayed as mean ± standard error unless otherwise noted.
Results
GNB4-cre transgenic mouse line allows access to CL projection neurons
To confirm that CL projection neurons express cre recombinase in the GNB4-cre mouse, we injected a virus expressing eYFP in a cre-dependent manner into the CL of GNB4-cre mice (Figure 1A). We used parvalbumin (PV) immunostaining to delineate CL borders (Figure 1B) (16,29) and observed that virus expression and PV immunostaining were isomorphic (Figure 1C). We next examined the morphological and electrophysiological identity of GNB4-positive (+) neurons by performing whole-cell recordings from labeled neurons using recording pipettes filled with AlexaFluor®−594. We found that GNB4+ neurons were spiny (Figure 1D), consistent with a CL projection neuron identity (30–32). Basic membrane properties of these neurons are shown in Figure 1E and representative responses to current injection steps are shown in Figure 1F. Membrane capacitance delineates two types of CL projection neurons (33). In GNB4-positive neurons, we find a wide range of membrane capacitance values (89 to 176 pF), which is consistent with sampling from both CL projection neuron subtypes (type I = 118 ± 16 pF; type II = 158 ± 9 pF [mean ± SD]; (33)).
Figure 1: The GNB4-cre transgenic mouse line provides genetic access to claustrum (CL) projection neurons and allows for recording CL activity.
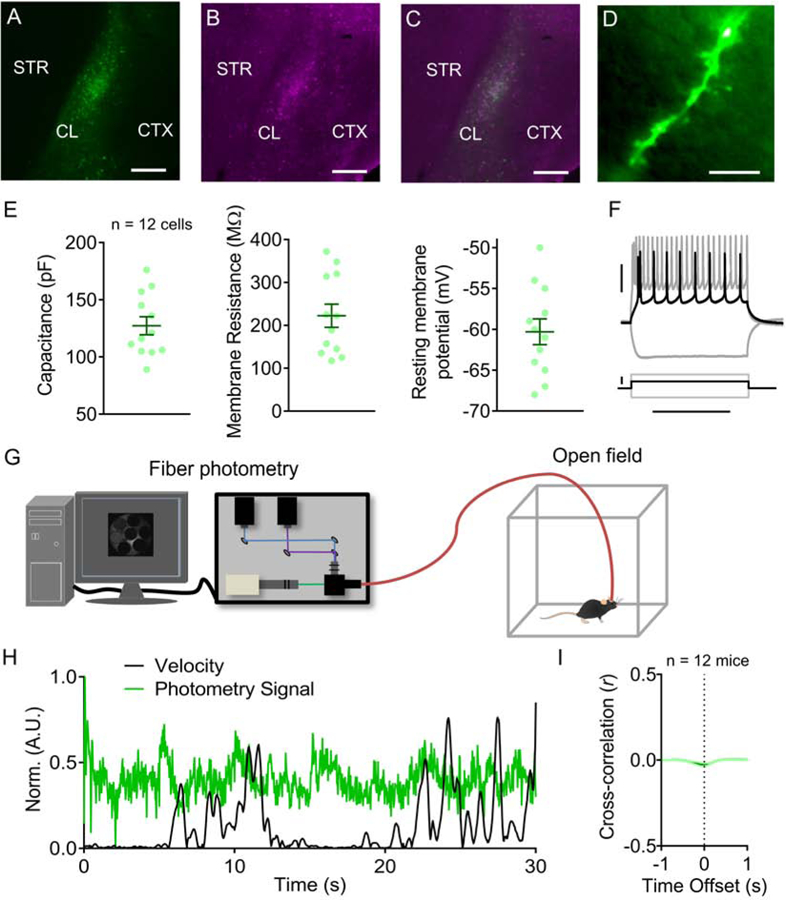
(A) Cre-dependent viral expression of eYFP in mouse CL. (B) Immunostaining of CL parvalbumin (PV) expression. (C) Labeling of virally-expressed eYFP and immunolabeled PV were isomorphic. (D) Dendritic spines were evident upon whole-cell patch clamp records of GNB4-positive CL neurons using an internal solution including AlexFluor®−594. (E) Left: Mean membrane capacitance from labeled neurons was 127 ± 9 (mean ± SD) pF. Middle: Mean membrane resistance was 222 ± 34 MΩ. Right: Mean resting membrane potential was −60 ± 2 mV. (F) Representative traces from labeled neurons in response to current injection steps. (G) Experimental schematic illustrating that CL activity was monitored during freely moving behavior in GNB4-cre mice injected with AAV-FLEX-GCaMP6f into the CL. (H) Representative traces of normalized CL activity (green) and speed (black) during movement from GNB4-cre mice. (I) Cross-correlation between the photometry signal and movement speed. Horizontal scale bars = 200 µm (A-C), 10 µm (D), 400 ms (F). Vertical scale bars = 30 mV (F [top]), 200 pA (F [bottom]).
To monitor calcium-dependent activity of these identified CL neurons in GNB4-cre mice, we employed in vivo fiber photometric detection of a neuronal activity-dependent calcium indicator. To do this, GNB4-cre mice were injected with a virus expressing GCaMP6f into the CL (AAV-FLEX-GCaMP6f; Figure 1G). To first assess if CL activity relates to movement, or if our photometry signal was itself influenced by movement, we monitored CL activity during freely moving behavior (Figure 1H). CL activity was weakly negatively correlated with movement speed (Average r = −0.0062 Figure 1I).
CL activity is sensitive to task demand
Next, we monitored CL activity using the same viral strategy in GNB4-cre mice (Figure 2A). The photometry system and data processing were designed to minimize sources of noise, such as fluctuations in excitation laser intensity, motion-related artifacts, and bleaching artifacts. Fluctuations in excitation laser intensity were controlled for by exciting a stable fluorophore, AlexaFluor®−488 (AF®−488; Figure S1A–S1C). Motion-related artifacts were controlled for by multiplexing excitation of CL with 405 nm light, the isosbestic wavelength for GCaMP6 (28), and 473 nm light (Figure S1A and S1B). Regressing out the signals from 405 nm excitation and AF®−488 excitation attenuated noise (Figure S1). To control for photo-bleaching during 30 min behavior sessions, signals were examined in 10 min trial bins.
Figure 2: CL activity during 5-choice serial reaction time task (5CSRTT) and one-choice serial reaction time task (1CSRTT) performance.

(A) Experimental schematic illustrating that CL activity was monitored during 5CSRTT performance in GNB4-cre mice injected with AAV-FLEX-GCaMP6f (n = 12). (B) Left: Average calcium-dependent activity of CL aligned to 5CSRTT cue onset is shown for correct (green) and incorrect (gray) trials. Right: During the ITI period (5 to 0s prior to cue onset), CL activity was greater for correct trials relative to incorrect trials. Paired t test, t(23) = 3.183, P = 0.004. (C) Left: Average CL activity aligned to correct or incorrect nose pokes during 5CSRTT performance is shown. Right: Average CL activity (1 s prior to 1 s after poke) was greater for correct nose pokes compared to incorrect nose pokes. Paired t test, t(11) = 3.14, P = 0.0095. (D) Average CL activity during the ITI for correct 5CSRTT trials (green) and correct 1CSRTT trials (blue). (E) CL activity during the ITI was greater for correct 5CSRTT trials compared to 1CSRTT trials. Paired t test, t(23) = 2.464, P = 0.0144. (F) Left: Average CL activity aligned to correct nose pokes during 5CSRTT performance and right: during 1CSRTT performance. (G) Left: CL activity prior to nose poke (average of 0.5 s prior, gray box) was not different between 5CSRTT and 1CSRTT. Paired t test, t(11) = 0.88, P = 0.40. Right: activity immediately subsequent to nose poke (average of 0.5 s subsequent, brown box) was not different between 5CSRTT and 1CSRTT. Paired t test, t(11) = 1.85, P = 0.09. (H) Left: Average CL activity aligned to collection of the reinforcement (sucrose pellet) on correct 5CSRTT trials and right: on correct 1CSRTT trials. (I) Left: CL activity prior to collection of reward pellet (average of 0.5 s prior) was greater for the 5CSRTT relative to the 1CSRTT. Paired t test, t(11) = 3.11, P = 0.01. Right: activity immediately subsequent to collection (average of 0.5 s subsequent) was greater for the 5CSRTT compared to the 1CSRTT. Paired t test, t(11) = 4.41, P = 0.0010.
During five-choice serial reaction time task (5CSRTT) performance, CL activity was elevated on correct trials in the inter-trial interval (ITI) relative to incorrect trials (Figure 2B). However, on both trial types activity of claustrum neurons exhibits an initial rise at trial onset. To control for the larger proportion of correct trials relative to incorrect trials, we matched the numbers of correct and incorrect trials for each mouse and found a similar difference in CL activity between the two trial types (Figure S2). We next aligned CL activity to correct and incorrect nose pokes during 5CSRTT performance. Average CL activity around the time of the nose poke was significantly greater for correct nose pokes compared to incorrect nose pokes (Figure 2C). We next examined if CL activity during correct 5CSRTT performance reflects task complexity. To do this, we first compared CL activity during the ITI between correctly performed 5CSRTT versus the less-demanding one-choice serial reaction time task (1CSRTT) trials (Figure 2D) where mice solely need to attend to a single hole that illuminates repeatedly to signal a nose poke for reward. As such, the 1CSRTT retains the basic sensory and motor components of the 5CSRTT while requiring less cognitive demand. We found increased CL activity during the 5CSRTT ITI compared to the 1CSRTT ITI (Figure 2E). We next compared the two tasks by aligning activity to correct nose pokes (Figure 2F). We did not observe any activity differences between the two tasks immediately prior to or subsequent to nose pokes (Figure 2G). However, when CL activity was aligned to the acquisition of sucrose pellets (Figure 2H), activity was greater for the 5CSRTT relative to the 1CSRTT immediately before and after acquisition of the sucrose pellet (Figure 2I).
CL contributes to complex, but not simple, task performance
To determine if CL is critical for 5CSRTT task performance, we injected a cre-dependent halorhodopsin virus (AAV-DIO-eNPhR3.0) or eYFP-expressing virus control (AAV-DIO-eYFP) into the CL of GNB4-cre mice and implanted optical fibers to expose the CL to 470 nm light (Figure 3A). In acute brain slices, 470 nm light faithfully blocked action potential spike generation in CL neurons expressing eNPhR3.0 during a depolarizing current injection (Figure 3B). To rule out the possibility that eNPhR3.0 stimulation alters claustrum neuron firing subsequent to 470 nm exposure, we quantified action potential frequency after 470 nm exposure in these neurons and compared it to the same epoch on current injections without any light exposure. The frequency of action potentials was not different following 470 nm exposure (7.46 ± 3.15 Hz [mean ± SD]) compared to control current injections (7.42 ± 2.89 [mean ± SD]; Wilcoxon rank-sum test, n = 8, P = 1). Experimental and control mice were trained to perform the 5CSRTT and 1CSRTT. The CL was exposed to 470 nm light for 4s during the inter-trial interval (ITI) pseudo-randomly on 33% of 5CSRTT and 1CSRTT trials (Figure 3C). This protocol was derived from our previous work showing ACC input to CL prior to cue onset is critical for optimal task performance (16). On 5CSRTT trials paired with 470 nm stimulation, AAV-DIO-eNPhR3.0 mice were less accurate compared to control trials; whereas no difference in accuracy was observed in AAV-DIO-eYFP mice (Figure 3D). Light delivery did not change the number of omissions or the response latency on correctly performed 5CSRTT trials in AAV-DIO-eNPhR3.0 or AAV-DIO-eYFP mice (Figure 3E and 3F). For AAV-DIO-eNPhR3.0 mice, we found that accuracy deficits on inactivation trials during the 1CSRTT were less than those on the 5CSRTT (Figure 3G). For AAV-DIO-eYFP mice, no differences in accuracy deficits were observed between the two tasks (Figure 3G). Overall, choice accuracy of AAV-DIO-eNPhR3.0 mice was lower in the absence of stimulation (Figure S3A), which suggests that eNPhR3.0 activation might lead to long-term synaptic changes that impair task performance.
Figure 3: Inactivation of CL prior to cue presentation disrupts response accuracy on 5CSRTT, but not 1CSRTT.
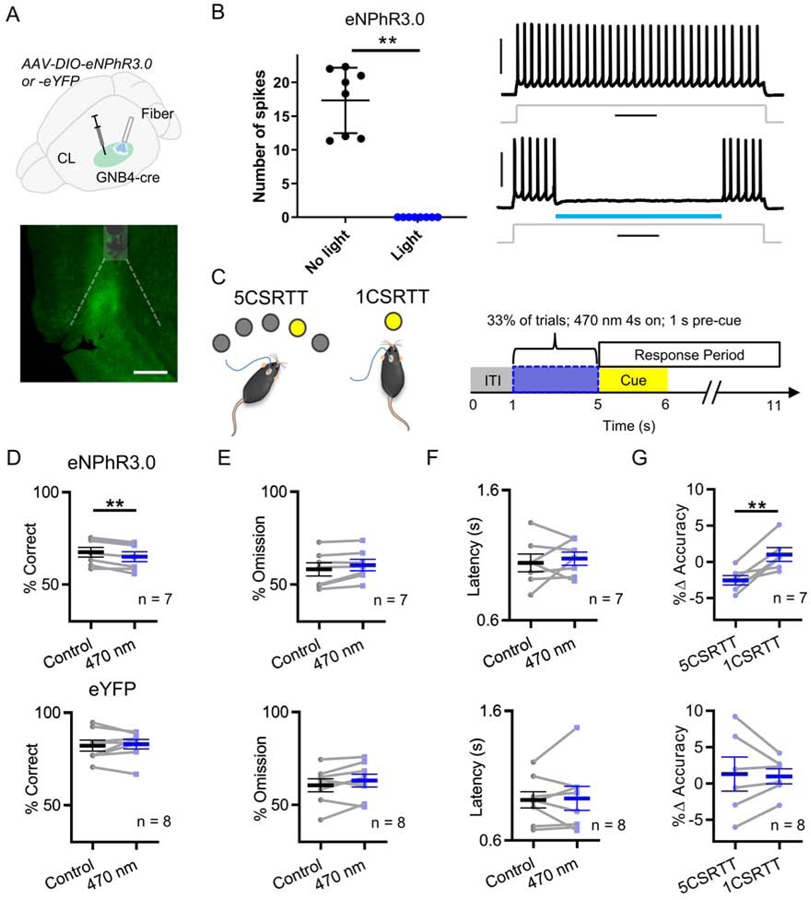
(A) Top: Schematic illustrating that a virus expressing either halorhodopsin (n = 7) or eYFP (n = 8) in a cre-dependent manner (AAV-DIO-eNPhR3.0 or -eYFP, respectively) was injected bilaterally into the CL of GNB4-cre mice. Optical fibers were chronically implanted bilaterally in the CL. Bottom: Photomicrograph illustrating fiber optic implant (white box) position above CL, estimated light path (dotted lines), and viral expression. (B) Left: CL neurons expressing halorhodopsin (eNPhR3.0) show loss of action potential firing in the presence of 470 nm light (4 s) compared to absence of 470 nm light during a depolarizing current injection (Wilcoxon rank-sum test, n = 8, P = 0.0078). Right: Top trace shows representative example of claustrum neuron expressing eNPhR3.0 generating action potentials in response to depolarizing current injection in the absence of 470 nm light. Bottom trace shows representative example of the same claustrum neuron, for which 470 nm light abolishes action potential generation. (C) Left: Mice were trained to perform the 5CSRTT and subsequently a one-choice control task (1CSRTT). Right: Experimental schematic illustrating that 470 nm light was delivered to the CL during the inter-trial interval (ITI) 4 s prior to the onset of the cue on 33% of 5CSRTT or 1CSRTT trials. (D) Top: For eNPhR3.0 mice performing the 5CSRTT, choice accuracy was reduced on trials paired with 470 nm light delivery pre-cue compared to control trials. Paired t test, t(7) = 4.41, P = 0.005. Bottom: No changes in choice accuracy were found on 5CSRTT trials paired with light delivery in eYFP mice. Paired t test, t(7) = 0.42, P = 0.691. (E) Top: The omission rate was not different on 5CSRTT trials paired with 470 nm light delivery compared to control trials for eNPhR3.0 mice. Paired t test, t(6) = 2.35, P = 0.057. Bottom: Light delivery did not alter omission rate in eYFP mice. Paired t test, t(7) = 1.57, P = 0.161. (F) Top: The latency to correctly respond was not different on 5CSRTT trials paired with 470 nm light delivery compared to control trials for eNPhR3.0 mice. Paired t test, t(6) = 0.55, P = 0.6029. Bottom: Light delivery did not alter latency to correctly respond in eYFP mice. Paired t test, t(7) = 0.26, P = 0.806. (G) Top: For eNPhR3.0 mice, the reduction in choice accuracy on the 5CSRTT resulting from 470 nm light delivery was significantly larger than the reduction during 1CSRTT performance. Paired t test, t(6) = 4.46, P = 0.0067. Bottom: For eYFP mice, there was no difference in the change in choice accuracy on the 5CSRTT resulting from 470 nm light delivery compared to the change during 1CSRTT performance. Paired t test, t(7) = 0.24, P = 0.823. Horizontal scale bars = 200 µm (A), 500 ms (B). Vertical scale bar = 30 mV.
To exclude reward-related effects elicited by CL inactivation, we performed a real-time place preference (RTPP) assay. We found that both AAV-DIO-eNPhR3.0 and AAV-DIO-eYFP mice did not exhibit a preference for either the control side of the chamber where no 470 nm light was delivered to CL or the side of the chamber paired with 470 nm light stimulation (Figure S3B and S3C). Assessing whether CL inactivation affects movement, we found no difference in the velocity of AAV-DIO-eNPhR3.0 and AAV-DIO-eYFP mice before 470 nm light exposure compared to the 4 s of inactivation + the 1.5 s following inactivation (Figure S3D). The two groups did not differ in the amount of time spent in the middle transition zone, suggesting exploration did not differ between the two groups (Figure S3E).
Discussion
Our results show that the CL is required for optimal performance on the cognitively-demanding 5CSRTT but not the simpler 1CSRTT version of the task. CL activity on the 5CSRTT is higher on accurately performed compared to inaccurately performed trials on this task. In addition, relative to the 1CSRTT, CL activity on the 5CSRTT is higher prior to the cue and at reward acquisition. These findings are in line with the previous finding that the activity of ACC inputs to CL are greater during 5CSRTT than 1CSRTT (16) and that the human CL activates at the switch to a difficult version of the multi-source interference task (14). Notably, in some mice we see an increase in CL activity when task demand is low, which may reflect experimenter inability to fully govern cognitive loading in performing mice. Nevertheless, these data taken together suggest that the claustrum may be recruited to some extent under a variety of cognitive conditions but may only serve a critical role for performance when task demand, and cognitive load geared toward that task, is high.
It is important to note that our photometry analysis detects population level CL projection neuron activity. Therefore, it is difficult to determine, for example, whether increased activity-dependent calcium signals reflect recruitment of more CL neurons, an increase in firing of a subset of CL neurons, synchrony of CL neurons, or some combination of these possibilities. As such, future studies will need to assess if selective activation of functionally distinct subpopulations may explain our finding of increased activity on the 5CSRTT versus the 1CSRTT. This is of particular interest as electrophysiologically distinct claustrum subtypes show differential cortical targeting and may serve different roles in cognition (33). Another caveat of the present study is that we did not attempt to improve behavioral importance using stimulation of claustrum neurons. Our prior work suggests that at optimal performance exogenous activation of critical circuits, such as ACC input to claustrum, may not improve behavioral performance (16). However, it is possible that earlier in training claustrum neuron stimulation may improve task acquisition, which warrants future investigation.
The CL exhibits widespread bidirectional connectivity with many areas of cerebral cortex (15,34,35). Amongst the strongest connections are with frontal cortices, including the anterior cingulate cortex (12). Anterior cingulate cortex input to CL and the human CL both respond to task demand (14,16). The CL activates along with, and is also functionally connected to, task-positive networks when cognitive demand increases on an attention task (14). Taken together with the present results, this suggests a role for the CL in supporting cortical networks underlying cognitive control of goal-directed behavior under cognitively demanding conditions.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | ||||
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | Gnb4-IRES2-Cre-D knock-in | Allen Institute for Brain Science | Jax Stock No:029587 | |
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | ||||
| Transfected Construct | ||||
| Other |
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants K22AA021414 and R01AA024845 (B.N.M.), Whitehall Foundation grant 2014–12-68 (B.N.M.), National Institute of General Medical Sciences grant T32GM008181 (M.G.W.), National Institute of Neurological Disorders and Stroke grant T32NS063391 (M.G.W.), and National Institute of Mental Health grant F31MH112350 (M.G.W.). The authors also are grateful for the assistance of Dr. Christof Koch and the Allen Institute for Brain Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure
We report no biomedical financial interests or potential conflicts of interest.
References
- 1.Heinrichs RW, Zakzanis KK (1998): Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 12: 426–445. [DOI] [PubMed] [Google Scholar]
- 2.Bressler SL, Menon V (2010, June): Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences, vol. 14 pp 277–290. [DOI] [PubMed] [Google Scholar]
- 3.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012): Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12: 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins TW, Arnsten AFT (2009): The Neuropsychopharmacology of Fronto-Executive Function: Monoaminergic Modulation. Annu Rev Neurosci 32: 267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayraghavan S, Major AJ, Everling S (2017, December 5): Neuromodulation of prefrontal cortex in non-human primates by dopaminergic receptors during rule-guided flexible behavior and cognitive control Frontiers in Neural Circuits, vol. 11 Frontiers Media S.A., p 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mäki-Marttunen V, Hagen T, Espeseth T (2019): Task context load induces reactive cognitive control: An fMRI study on cortical and brain stem activity. Cogn Affect Behav Neurosci 19: 945–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crick FC, Koch C (2005): What is the function of the claustrum? Philos Trans R Soc B Biol Sci 360: 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur BN (2014): The claustrum in review. Front Syst Neurosci 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson J, Karnani MM, Zemelman BV, Burdakov D, Lee AK (2018): Inhibitory Control of Prefrontal Cortex by the Claustrum. Neuron 99: 1029–1039.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smythies J, Edelstein L, Ramachandran V (2012): Hypotheses relating to the function of the claustrum. Front Integr Neurosci 6: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remedios R, Logothetis NK, Kayser C (2010): Unimodal Responses Prevail within the Multisensory Claustrum. J Neurosci 30: 12902–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JB, Alloway KD (2010): Functional Specificity of Claustrum Connections in the Rat: Interhemispheric Communication between Specific Parts of Motor Cortex. J Neurosci 30: 16832–16844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patru MC, Reser DH (2015): A New Perspective on Delusional States - Evidence for Claustrum Involvement. Front psychiatry 6: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krimmel SR, White MG, Panicker MH, Barrett FS, Mathur BN, Seminowicz DA (2019): Resting state functional connectivity and cognitive task-related activation of the human claustrum. Neuroimage 196: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Ng L, Harris JA, Feng D, Li Y, Royall JJ, et al. (2017): Organization of the connections between claustrum and cortex in the mouse. J Comp Neurol 525: 1317–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White MG, Panicker M, Mu C, Carter AM, Roberts BM, Dharmasri PA, Mathur BN (2018): Anterior Cingulate Cortex Input to the Claustrum Is Required for Top-Down Action Control. Cell Rep 22: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White MG, Mathur BN (2018): Frontal cortical control of posterior sensory and association cortices through the claustrum. Brain Struct Funct 1–8. [DOI] [PMC free article] [PubMed]
- 18.Miller EK, Buschman TJ (2013): Cortical circuits for the control of attention. Curr Opin Neurobiol 23: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch C, Massimini M, Boly M, Tononi G (2016): Neural correlates of consciousness: progress and problems. Nat Rev Neurosci 17: 307–21. [DOI] [PubMed] [Google Scholar]
- 20.Tononi G, Boly M, Massimini M, Koch C (2016): Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci 17: 450–61. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Lee SJ, Wu L, Sarntinoranont M, Xie H (2012): Refractive index measurement of acute rat brain tissue slices using optical coherence tomography. Opt Express 20: 1084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM (2013): Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Juboori SI, Dondzillo A, Stubblefield EA, Felsen G, Lei TC, Klug A (2013): Light Scattering Properties Vary across Different Regions of the Adult Mouse Brain ((Aegerter CM, editor)). PLoS One 8: e67626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muir JL, Everitt BJ, Robbins TW (1996): The Cerebral Cortex of the Rat and Visual Attentional Function: Dissociable Effects of Mediofrontal, Cingulate, Anterior Dorsolateral, and Parietal Cortex Lesions on a Five-Choice Serial Reaction Time Task. Cereb Cortex 6: 470–481. [DOI] [PubMed] [Google Scholar]
- 25.Passetti F, Chudasama Y, Robbins TW (2002): The Frontal Cortex of the Rat and Visual Attentional Performance: Dissociable Functions of Distinct Medial Prefrontal Subregions. Cereb Cortex 12: 1254–1268. [DOI] [PubMed] [Google Scholar]
- 26.Dalley JW, Cardinal RN, Robbins TW (2004): Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28: 771–784. [DOI] [PubMed] [Google Scholar]
- 27.Robinson ESJ, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, et al. (2008): Similar Effects of the Selective Noradrenaline Reuptake Inhibitor Atomoxetine on Three Distinct Forms of Impulsivity in the Rat. Neuropsychopharmacology 33: 1028–1037. [DOI] [PubMed] [Google Scholar]
- 28.Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, et al. (2016): Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat Methods 13: 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathur BN, Caprioli RM, Deutch AY (2009): Proteomic analysis illuminates a novel structural definition of the claustrum and insula. Cereb Cortex 19: 2372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braak H, Braak E (1982): Neuronal types in the claustrum of man. Anat Embryol (Berl) 163: 447–60. [DOI] [PubMed] [Google Scholar]
- 31.Hur EE, Zaborszky L (2005): Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected]. J Comp Neurol 483: 351–73. [DOI] [PubMed] [Google Scholar]
- 32.Watakabe A, Ohsawa S, Ichinohe N, Rockland KS, Yamamori T (2014): Characterization of claustral neurons by comparative gene expression profiling and dye-injection analyses. Front Syst Neurosci 8: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White MG, Mathur BN (2018): Claustrum circuit components for top–down input processing and cortical broadcast. Brain Struct Funct 223: 3945–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White MG, Cody PA, Bubser M, Wang H-D, Deutch AY, Mathur BN (2017): Cortical hierarchy governs rat claustrocortical circuit organization. J Comp Neurol 525: 1347–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zingg B, Dong HW, Tao HW, Zhang LI (2018): Input–output organization of the mouse claustrum. J Comp Neurol 526: 2428–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


