Rare hypereosinophilic syndromes (HES) are a heterogeneous group of disorders characterized by peripheral and tissue eosinophilia resulting in a wide variety of clinical manifestations and distinct subtypes. Not only is HES heterogeneous from an etiologic perspective (i.e., comprised of myeloid, secondary and idiopathic disorders), but organ-system involvement is highly variable from patient to patient(1,2). Novel treatments including biologic therapies, may have differential effects on tissue depletion or eosinophil activation. Despite the recognition of HES and its pathologic presentations, few studies have assessed the patient experience or interviewed patients with the disease to date. A patient-reported outcomes (PRO) instrument measuring symptoms of HES would provide valuable information for use in clinical practice as well as inform endpoint selection and assessment of treatment effects in clinical trials (3,4). Although PRO’s have been developed in a wide variety of disorders, including both organ-restricted eosinophilic disorders (5) and disorders with heterogeneous, multisystem involvement, such as systemic lupus erythematosus (6), no PRO questionnaires developed specifically to measure the symptoms, disease impacts, or health-related quality-of-life (HRQoL) of patients with HES has been created due to the rarity of HES, the heterogeneity of clinical presentations, and confounding by the wide array of medications used to treat HES(1,2,7). Understanding the patient experience of disease is essential to support qualitative research and the development of PROs specific to HES. Accordingly, the main objective of the current study was to understand the HES patient’s symptoms, and disease experience for endpoint selection and PRO development for use in clinical care and clinical trials of novel therapies in accordance with the FDA PRO Guidance (3,4).
Literature review and concept elicitation interviews of 26 HES patients (Figure E4, Table E1–3) were used to understand important symptom concepts and impacts on HRQoL. A conceptual model with the goal of developing an HES-specific PRO instrument was constructed (Figure E3). Patients recruited from an IRB approved study for evaluation of patients with eosinophilic disease had standard assessments and were interviewed (see Online Repository for assessments, demographics and interview methods).
Patients reported a wide variety of symptoms (Figure 1), and impacts (Figure E2), and most patients experienced multiple HES symptoms over the course of their disease (median 12, SD 8.3). However, not all symptoms were present at the time of interview or were equally bothersome and physician assessed symptoms and patient reported symptoms differed (Figure E1). The most commonly reported symptom was fatigue (n=17, 65%), followed by itching (n=13, 50%). and shortness of breath (n=10, 38%) (Figure 1). Other common symptoms (present in >20% of patients) included abdominal pain, diarrhea, muscle pain, sinus congestion or rhinorrhea, chills or sweats, cough and wheezing. The most bothersome symptoms to patients varied considerably and depended on the organs affected; for example, most patients with dermatological involvement (n=17) reported itching as their most bothersome symptom, whereas those with GI involvement (n=12) more frequently reported abdominal pain. A variety of constitutional symptoms were reported including fatigue (n=17) which was described frequently as a “full body” sensation akin to “having the flu” or being “run over by a truck” accompanied by lack of desire to be active (n=6). Four patients described this as the most bothersome symptom of their HES. Physicians noted GI, cardiac, and sinus/nasal symptoms more than patients complained of symptoms referable to those organ systems. Conversely, patients often reported pulmonary, constitutional, musculoskeletal and neurologic/cognitive symptoms in the absence of documented objective evidence of eosinophilic organ involvement.
Figure 1.
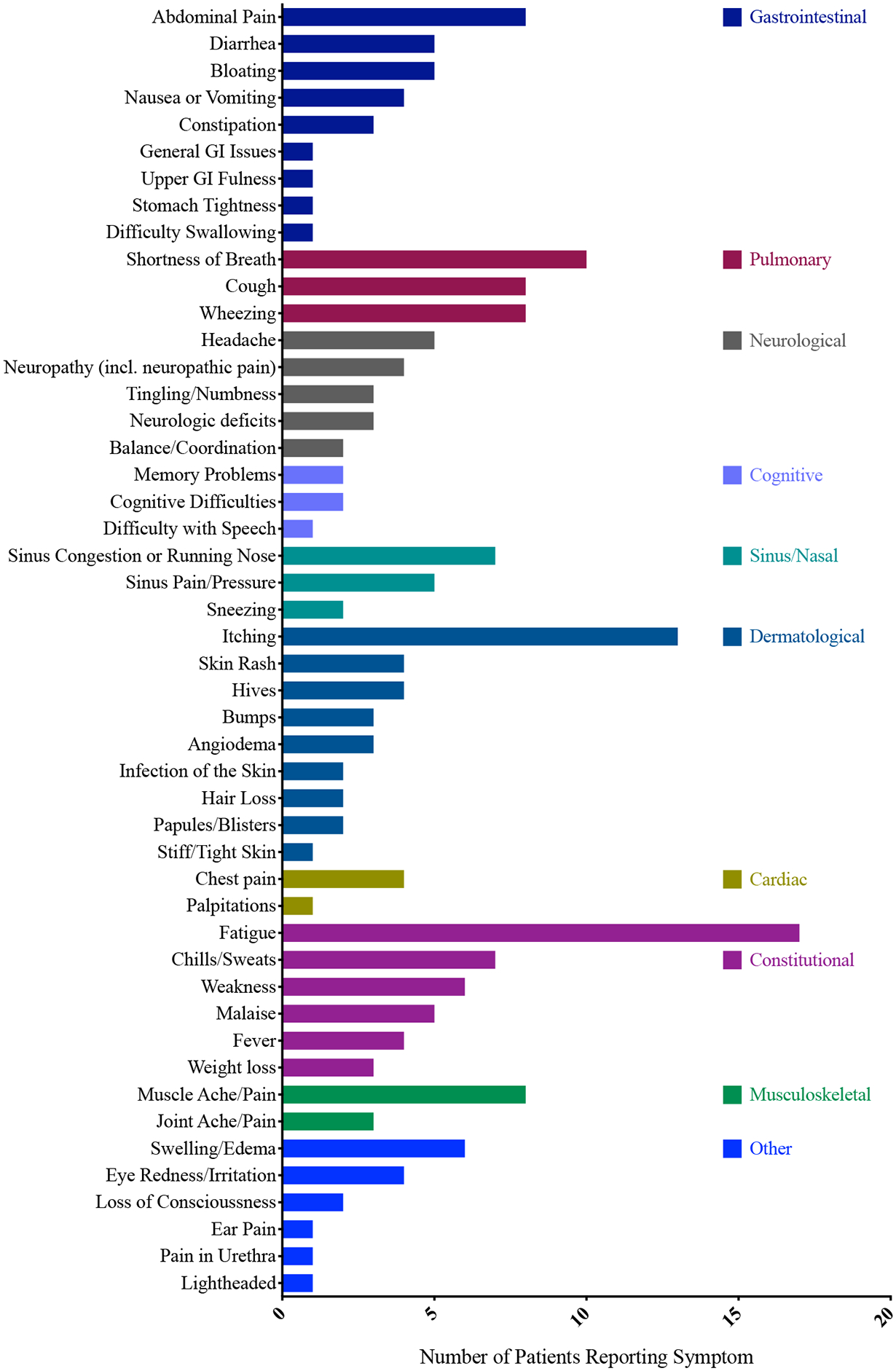
Symptoms reported by HES patients
Nineteen patients (73%) reported that HES symptoms impacted their activities of daily living (ADLs), including their ability to get out of bed (n=6), perform chores (n=8), leave the house (n=7) or care for themselves (n=5) (Figure E2). Nine patients had to stop working temporarily or permanently due to their symptoms. HES affected all areas of patient functioning, with >80% of patients reporting an effect on their physical, emotional and social functioning (Figure 2). Some symptoms were associated with an emotional burden including “annoyance”, “frustration,” and associated feelings of depression and isolation. The most common functional impacts were on social activities (n=18, 69%), sleep (n=17, 65%) and walking (n=15, 58%). A majority of patients had been treated with glucocorticoids sometime during the course of their disease and most reported some side effects from their use.
Figure 2.
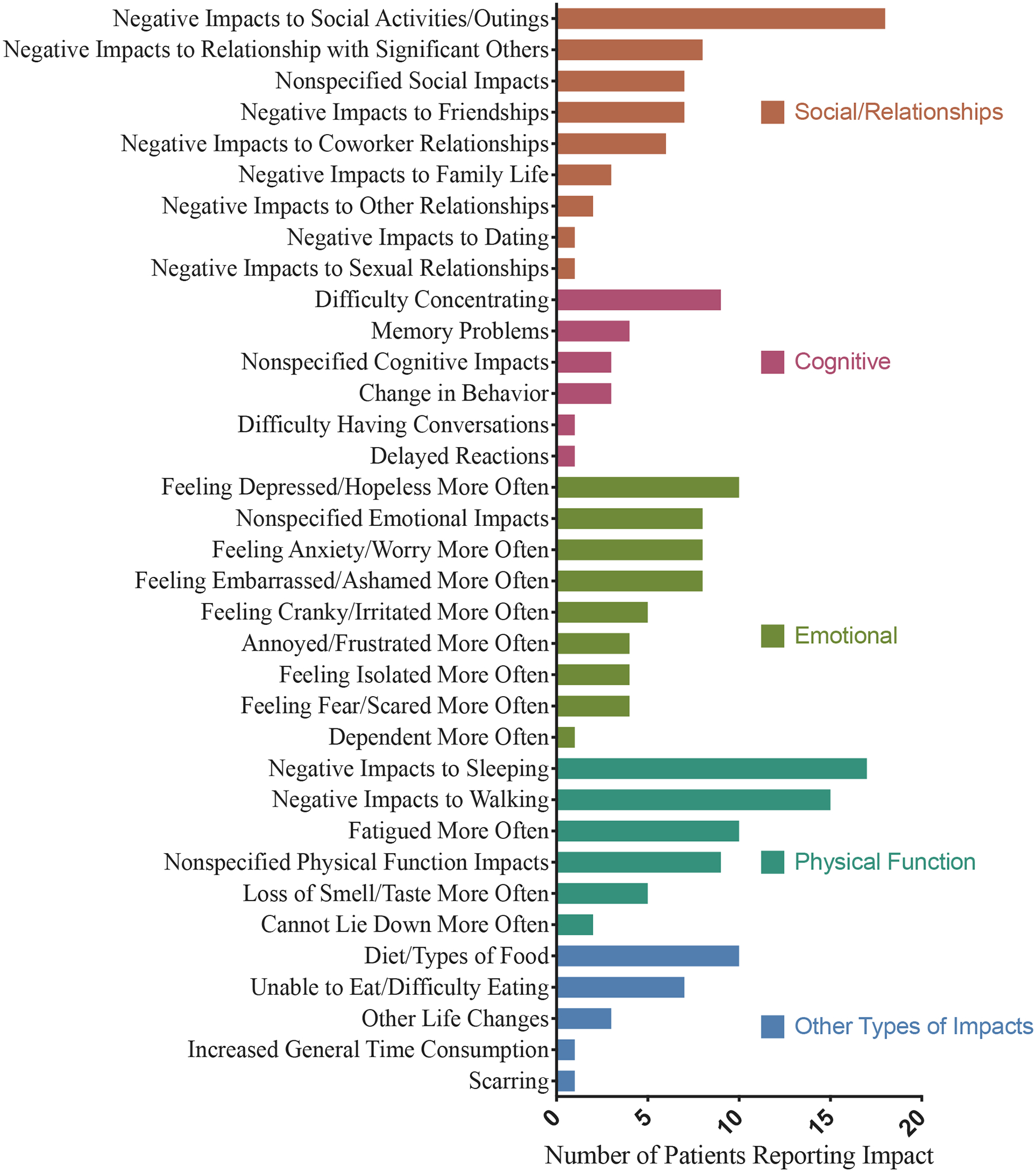
Patient-reported impact of HES on Social, Cognitive, Emotional and Physical functioning.
A draft conceptual model (Figure E3) depicts the impacts of the disease, the relationships among the impacts and compiles the effects of symptoms on patient functioning and well-being. The number of concepts and symptom domains identified precluded clustering of any one symptom or combination of symptoms for creation of a de-novo PRO with response options that reflect the totality of patient experience, whether between patients or for the same patient over time.
With the development of novel therapeutic agents that reduce or deplete blood eosinophils, it will be increasingly important to identify meaningful changes in disease activity and improvement in HES. The FDA PRO guidance issued in 2009(4) emphasizes use of metrics that would show improvement in how a patient “feels, functions, or survives.” Consistent with a prior multicenter study of 188 patients with HES (1), dermatologic, pulmonary and GI involvement were most common, although considerable inter-individual variability of reported symptoms, characterization of symptom experience, and recognition of the large burden of impact to ADLs and IADLs were identified supporting the need for additional efforts to develop safe and effective treatments for HES.
In summary, the present study highlights the difficulties in designing a measure sensitive enough to capture changes in PROs over time using a standard approach. Future efforts using other modalities, such as composite scoring systems utilizing validated instruments (e.g. PROMIS scales or HAQ subscales) or a most-bothersome symptom approach, that take into consideration the heterogeneity of HES and individual experiences of the disease are needed to advance efforts to create an HES-specific PRO.
Supplementary Material
Clinical Implications.
HES is a heterogenous disease with distinct subtypes, pathogenesis and myriad organ involvement and symptomatology making development of a patient reported outcome measure difficult. We describe the range of patient reported symptoms in a diverse group of HES patients.
Funding:
This study was funded by the Division of Intramural Research, NIAID, NIH, and by GlaxoSmithKline (HO-14-15311). Linda Nelsen, Jonathan Steinfeld are employees of GlaxoSmithKline. Hector Ortega and Suyong Yun Kirby were employees of GlaxoSmithKline when this research was conducted. ICON received funding from GlaxoSmithKline to conduct research on this study. Olga Moshkovich and Ethan J. Schwartz are employees of ICON. Katy Benjamin was an employee of ICON at the time this research was conducted. Paneez Khoury, Amy Klion, Nick Kovacs, and Nicole Holland-Thomas have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
Paneez Khoury, Amy Klion, Nick Kovacs, and Nicole Holland-Thomas have nothing to disclose.
Linda Nelsen, Jonathan Steinfeld are employees of and hold stock in GlaxoSmithKline. Hector Ortega and Suyong Yun Kirby were employees of GlaxoSmithKline when this research was conducted.
Bibliography
- 1.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009. December;124(6):1319–25.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helbig G, Moskwa A, Hus M, Woźniczka K, Wieczorkiewicz A, Dziaczkowska-Suszek J, et al. Heterogeneity among characteristics of hypereosinophilic syndromes. Journal of Allergy and Clinical Immunology. 2010. June;125(6):1399–1401.e2. [DOI] [PubMed] [Google Scholar]
- 3.Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1--eliciting concepts for a new PRO instrument. Value Health. 2011. December;14(8):967–77. [DOI] [PubMed] [Google Scholar]
- 4.Guidance for IndustryPatient-Reported Outcome Measures:Use in Medical Product Developmentto Support Labeling Claims [Internet]. FDA; 2009. [cited 2009 Dec 31]. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf [Google Scholar]
- 5.Schoepfer AM, Straumann A, Panczak R, Coslovsky M, Kuehni CE, Maurer E, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014. December;147(6):1255–66.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahieu M, Yount S, Ramsey-Goldman R. Patient-Reported Outcomes in Systemic Lupus Erythematosus. Rheum Dis Clin North Am. 2016. May;42(2):253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine. 1975. January;54(1):1–27. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


