Abstract
This paper reports a feasibility study designed to evaluate the behavioral and neurological effects of using transcranial direct current stimulation (tDCS) in conjunction with speech motor learning treatment for individuals with acquired speech impairment subsequent to stroke. Most of the research using tDCS to enhance treatment outcomes in stroke recovery has focused on either limb motor control or aphasia treatment. Using a multiple-baseline multiple-probe crossover design, we compared both behavioral and brain connectivity-based outcomes following speech motor learning treatment with both Active tDCS and Sham tDCS. We observed that both treatment phases led to improvement in short-term maintenance, but that Active tDCS was associated with greater long-term maintenance improvement. Active tDCS was also associated with an increase in functional connectivity in the left hemisphere and interhemispherically in an ROI-based network analysis examining correlations among areas associated with speech production and acquired speech impairment. This report supports the possibility that tDCS may enhance both behavioral and neurological outcomes and indicates the importance of additional work in this area, although replication is required to confirm the extent and consistency of tDCS benefits on speech motor learning treatment outcomes.
Keywords: tDCS, speech, motor learning, motor control, apraxia of speech
1. Introduction
Chronic stroke is typically associated with limited recovery of premorbid ability. Over the past decade, there has been great interest in combining non-invasive neuromodulation techniques such as transcranial direct current stimulation (tDCS) with traditional behavioral intervention to enhance stroke recovery outcomes. While the precise biological mechanism of action remains an active area of inquiry, tDCS modulates cortical excitability with subthreshold stimulation that can promote (or inhibit) neural activity in the stimulated region in the presence of potentiating neural activity (Chhatbar et al., 2018; Kronberg, Bridi, Abel, Bikson, & Parra, 2017; Ziemann, Muellbacher, Hallett, & Cohen, 2001). In stroke rehabilitation, the aim has primarily been to use tDCS to enhance performance by increasing activation in specific brain regions that are engaged via task performance. At present, the preponderance of research has focused on using tDCS to enhance motor recovery, with a particular focus on upper extremity recovery with stimulation that targets motor cortex (Figlewski et al., 2017; Hummel et al., 2005; Kim et al., 2010). Language impairment has also received attention, with some strong preliminary evidence suggesting that tDCS may enhance the effects of anomia treatment (Fiori, Nitsche, Cucuzza, Caltagirone, & Marangolo, 2019; Fridriksson et al., 2018; Marangolo et al., 2016). In this paper, we present a study that incorporates elements of these domains, focused on enhancing motor learning in the speech domain.
Intervention focused on speech motor learning has been reported to be effective in treating apraxia of speech, a motor planning/programming disorder that frequently co-occurs with aphasia subsequent to stroke (Ballard et al., 2015). Motor learning is typically associated with practice-dependent neuroplasticity through long-term potentiation. This mechanism is suspected to underlie both novel skill acquisition and recovery of impaired motor skills subsequent to acquired brain injury. Recent in vitro studies have suggested that tDCS can enhance synaptic plasticity in the presence of potentiating neural activity (Kronberg et al., 2017; Kronberg, Rahman, Lafon, Bikson, & Parra, 2019), and this has been recently demonstrated in an in vivo study involving the auditory neurophysiological pathway (Boroda, Sponheim, Fiecas, & Lim, 2020). Thus, given that motor learning involves associative learning and long-term potentiation, and that tDCS may enhance this type of plasticity in the presence of potentiating neural activity, tDCS may be particularly well-suited to enhancing motor learning. Most of the literature on tDCS and human performance has focused on limb-based motor learning in both unimpaired and impaired individuals. Recent work by Buchwald and colleagues (2019) demonstrated that tDCS can enhance novel consonant cluster acquisition in healthy adults in a single-session speech learning task, demonstrating a parallel between motor learning in speech and non-speech domains.
With respect to stroke recovery, tDCS may be particularly useful for engaging brain regions that had not been central in spontaneous recovery but may facilitate improvement. For example, Marangolo et al. (2016) performed a crossover study with active and sham phases in conjunction with aphasia treatment and a bilateral electrode montage (anode: LH inferior frontal gyrus (IFG); cathode: RH IFG). They reported that the active phase was associated with an improvement in speech and language measures as well as an increase in functional connectivity within the left hemisphere. This is consistent with the idea that anodal tDCS over the left hemisphere increases its engagement when there is potentiating neural activity (in this case, performing the treatment task). This can be particularly important given research indicating weaker functional connectivity in individuals with stroke-based impairment, including both aphasia (Sandberg, 2017) and apraxia of speech (New et al., 2015).
In this paper, we report on a crossover treatment study performed to address whether tDCS can enhance speech motor learning in an individual with acquired apraxia of speech by targeting left ventral premotor and motor cortices. Given the considerable inter- and intra-individual variability in this population, we used a multiple-baseline, multiple-probe crossover design in which we combined speech motor learning treatment with active tDCS in one phase and sham tDCS in the other phase. To evaluate possible changes in functional connectivity, we obtained 12 minutes of fMRI during rest at the beginning of the study and after each treatment phase, and we used region of interest (ROI)-based network analyses to examine intra- and interhemispheric functional connectivity at each time point. The preliminary findings presented here suggest that tDCS targeted to deliver maximal current to perilesional left ventral premotor and motor cortices can enhance speech motor learning treatment effects, and that this benefit is associated with greater functional connectivity in a bilateral cortical speech network defined based on lesion and neuroimaging research. Our findings indicate promise for tDCS to improve the long-term outcomes of speech motor learning treatment in individuals with AOS and suggest that tDCS in conjunction with this treatment can strengthen the cortical network associated with speech production.
2. Method
2.1. Participant
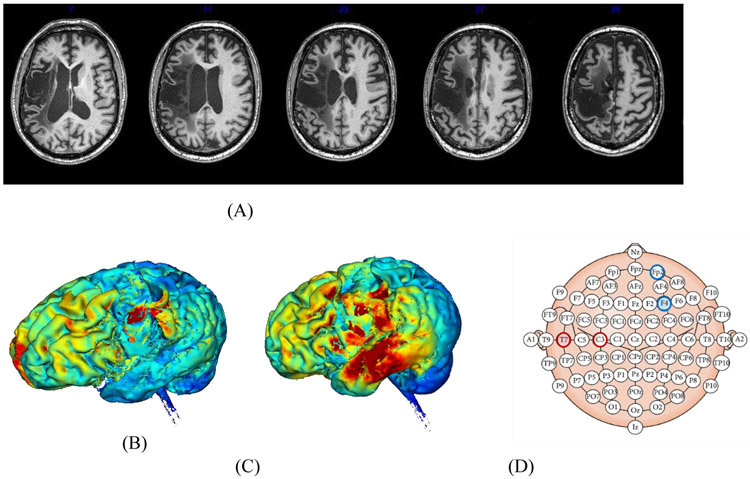
The participant in this study, P1, was a right-handed male who was 60 years old at the onset of this investigation. He had suffered a left middle cerebral artery ischemic stroke six years prior to the study leading to infarction of left fronto-parietal and temporal regions (see Fig 1A). P1 was administered the Western Aphasia Battery – Revised (WAB-R; (Kertesz, 2006)) and his Aphasia Quotient was 44.1, with classification as Broca’s aphasia. P1 exhibited non-fluent output with limited spontaneous speech primarily restricted to formulaic phrases (e.g., “thank you very much”). He correctly named 1/15 pictures as part of a single-word confrontation naming task from the short version of the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983). His auditory perception was intact as indicated by 100% accuracy (72/72) on a minimal pair discrimination test from the Psycholinguistic Assessment of Language Processing in Aphasia (PALPA 2; Kay, Lesser, & Coltheart, 1992).
Figure 1:
Aspects of P1’s lesion and approach to identifying an electrode montage. (A) Axial slices from T1-weighted structural scan arranged from inferior to superior. (B) Simulation of current flow for P1 based on placing electrodes in typical positions for motor learning studies (Anode: C3; Cathode: Fp2). (C) Simulation of current flow for P1 based on electrode placement used in this study (Anode: T7; Cathode: F4). (D) Schematic of 10-20 EEG system, with coordinates from (B) represented with unfilled red (anode) and blue (cathode) circles, and coordinates from (C) represented with filled circles.
Assessment of AOS was based on the informed clinical judgment of two certified speech-language pathologists (SLPs) with experience diagnosing motor speech disorders who reviewed a variety of speech samples, including those collected as part of standardized tests (e.g., WAB) as well as descriptions of other pictures and a spontaneous speech sample. Both SLPs independently rated P1 on a 3-point scale (following Maas et al., 2015): 1 = no AOS; 2 = possible AOS; 3 = AOS. The characteristics indicative of AOS diagnosis included slowed speech with segmental and intersegmental prolongations, dysprosody, speech sound distortions, and distorted substitutions, with dysprosody as a necessary feature. This approach to AOS diagnosis follows the literature on the differential diagnosis of AOS and other impairments, particularly aphasia subtypes involving phonological impairment (Ballard et al., 2015; McNeil, 2009). In addition to the clinician ratings, the first author independently rated the participant based on over ten years of experience as an AOS researcher. Each assessor assigned P1 a rating of 3, indicating perfect agreement on the presence of AOS.
2.2. Speech motor learning intervention
P1 was determined to have difficulty with the production of word-initial consonant clusters, as has previously been reported for others with AOS (Buchwald, Gagnon, & Miozzo, 2017; Buchwald & Miozzo, 2011, 2012). We created two lists of words employing different consonant clusters for the two treatment phases. One list consisted of words with initial clusters that began with stop consonants (e.g., /pr/, /bl/, /gr/) and the other list had initial clusters that began with fricative consonants (e.g., /fr/, /sl/, /st/). These two lists were generated based on previous findings that training within one of these categories can generalize to other sounds in that same category but does not transfer to sounds in the other category (Ballard, Maas, & Robin, 2007). Thus, the items trained in the first phase of the crossover should not lead to improvement in the items from the second phase. Each list had 84 cluster-initial monosyllabic words, with 48 items designated for training and testing. An additional 36 items were included for generalization testing containing both trained and untrained clusters. We report on the trained items in this paper due to the small number of items per cluster among the untrained items.
All testing was performed by a licensed and ASHA-certified SLP, and all sessions were audio-recorded. The single-subject intervention research design we used included obtaining 5 baseline sessions prior to Phase 11. Baseline sessions involved probing each stimulus from both lists (168 total words). Stimuli were elicited in a repetition format with no feedback provided. Each treatment phase then consisted of 9 treatment sessions; the list being trained was probed immediately prior to every third session. The sessions began with pre-practice in which the participant was guided through the production of 1-2 treatment items. During the treatment task, each treated word was elicited 3 times through repetition in randomized order. For 25% of items (randomly pre-selected before the session), P1 was provided with feedback giving knowledge of results (i.e., correct, incorrect, or ‘close’ for a distortion). The overall structure of the practice and feedback was developed in accordance with principles of motor learning (Maas et al., 2008) with a large amount of random and variable practice and low-frequency knowledge of results feedback. Each treatment session lasted approximately 40 minutes after the application of tDCS.
2.3. Behavioral data processing
Analysis of the speech tokens occurred after all data were collected by two independent and blinded coders. The speech data from the baseline, maintenance, and probe sessions were extracted for analysis from the full recorded session by a trained research assistant using Praat software (Boersma & Weenink, 2020). Groupings of 24-36 items were spliced into separate files and randomized so that coders were blind to the session they came from, as well as which items were trained in the Active phase vs. the Sham phase. The coders then independently transcribed each item produced by P1, and the dependent variable was the accuracy of the segments in the onset consonant cluster. Items with disagreement (N=156; 8.9%) were resolved by a third rater. We coded the first complete response for each item, and did not code for other aspects of accuracy throughout the rest of the word.
2.4. Neuromodulation
A 1x1 Soterix battery-driven current stimulator delivered current using rubber-carbon electrodes encased in 5cm x 7cm (35cm2) saline-soaked sponges. The electrode placement was selected based on individual neuroanatomy to maximize the electrical current targeting the left ventral premotor and motor cortices. A high-resolution T1 scan was used to draw a lesion map and create a model of P1’s head including the lesion. This head model was constructed using MRI signal intensities to segment P1’s head into various tissue types and cerebrospinal fluid, then used his individualized anatomy and the known conductivities of different tissues to determine the current flow and density in target regions using a finite elements model (Datta, Baker, Bikson, & Fridriksson, 2011). Using tDCS targeting software (Datta et al., 2011), we then compared montages to determine the appropriate electrode placement. Figures 1B and 1C depict current flow for the commonly used motor cortex montage (anode: C3; cathode: Fp2) and the montage we ultimately used (anode: T7; cathode: F4) respectively, and Figure 1D shows those montages on a figure representing 10-20 EEG space. As can be seen in Figure 1B, current modeling indicated ventral premotor and motor cortices would not be targeted using the more traditional montage. Although this montage also provides notable temporal stimulation, we selected the montage that maximized current flow to the intact left ventral premotor and motor cortices, independent of other regions receiving stimulation. As tDCS provides subthreshold stimulation and its effects are known to be activity-selective (Bikson & Rahman, 2013), significant plasticity would be unlikely in cortices that were not critically engaged by our speech motor treatment, such as the temporal lobe.
During the Active tDCS phase, the device delivered 1mA of current for 20 minutes (current density = 0.029 mA/cm2). For Sham tDCS, the electrode montage was identical and current ramped up to 1mA over 30 seconds to simulate the sensation of stimulation, but then was immediately decreased and turned off (Ambrus et al., 2012). Stimulation intensity and duration were selected based on the most commonly used parameters in the literature, as these have previously demonstrated behavioral effects in healthy participants and those with stroke. Both the participant and the testing clinician were blind to the order of conditions. During the maintenance sessions after Phase 2, they were each asked which phase they thought had Active tDCS. They each stated they were guessing; the clinician guessed correctly and the participant guessed incorrectly.
2.5. Neuroimaging
2.5.1. Data acquisition and preprocessing.
Neuroimaging data was collected at three time points on a Siemens Prisma 3T MRI scanner at the NYU Center for Brain Imaging. Scan 1 was acquired three weeks prior to initiating treatment and included both structural and functional neuroimaging. Anatomical images were acquired during the first session only with a T1-MPRAGE sequence, with TR=2250 ms, TE = 4.11ms, TI = 925ms, flip angle=9°, FOV = 256x256 mm, and 1mm isotropic voxel size. These high-resolution images were used to delineate the lesion extent as described below. Resting-state fMRI (rs-fMRI) images were acquired with an EPI sequence with TR = 1650ms, TE = 35ms, flip angle = 72°, FOV = 216x216mm, matrix size = 90x90, 50 axial slices with 2mm thickness (no gap), inplane voxel size of 2.4x2.4mm, and multi-band acceleration factor of 2. During 12 minutes of scanning, 427 volumes were collected. Scans 2 and 3 occurred within 72 hours of the ends of Phase 1 and 2 (respectively).
The lesion was manually drawn on the T1 scans using MRICron (Rorden & Brett, 2000) by the second author under training and supervision by the last author. Using the T1 scan, we filled the left-hemisphere lesion with mirrored tissue from the intact right hemisphere using the Virtual Brain Transplant approach (Solodkin et al., 2010). This was done to facilitate precise nonlinear registration with a structural template (MNI152) in standard space. We used symmetric diffeomorphic image registration (Advanced Normalization Tools (ANTs); Avants, Epstein, Grossman, & Gee, 2008) to register the transplanted brain to the template, and then used the inverse transform to return the template (and corresponding atlases) to native space. We used the Brainnetome Atlas (Fan et al., 2016; Jiang, 2013) to segment the whole brain into 246 regions (105 cortical and 18 subcortical regions in each hemisphere) to permit identification of our ROIs. Analyses of the functional data were conducted in native space, i.e., the space in which the functional data were originally acquired.
2.5.2. Functional MRI processing.
Our preprocessing primarily used the Analysis of Functional Neuroimages (AFNI; Cox, 1996) and FMRIB (Functional Magnetic Resonance Imaging of the Brain) Software Library (FSL; Smith et al., 2004) software packages, and consisted of despiking (outlier removal), head motion correction, spatial smoothing (6mm FWHM), and co-registration with the T1 structural scans. Following preprocessing, we applied a General Linear Model for artifact removal by including motion, ventricle, and white matter time series as nuisance regressors and excluding time points exhibiting excessive head motion (>3° rotation or 3 mm translation) from further analysis. We retained a minimum of 93% of the volumes recorded at each time point (Scan 1: 423 volumes; Scan 2: 398 volumes; Scan 3: 415 volumes). The signal was then bandpass-filtered (0.01Hz-0.1Hz) to identify the low frequency fluctuations of interest in rs-fMRI, and we used an independent component analysis approach (using FSL’s MELODIC) to detect and remove potential lesion-driven artifacts using the procedure outlined by Yourganov et al. (Yourganov, Fridriksson, Stark, & Rorden, 2018), following their demonstration of the superiority of this approach in revealing expected activation patterns highly correlated with behavioral change.
2.6. Statistical analysis
The dependent variable of the treatment study is cluster production accuracy during the non-treatment sessions (baselines, probes, and retentions). There are multiple approaches for determining the size of an effect given single-subject multiple-baseline treatment data (Olive & Smith, 2005). One class of approaches involves determining the extent of overlapping data between baseline sessions and the practice and retention sessions and comparing treatment conditions on this metric. A second approach which has been common in speech and language treatment studies involves the computation of Cohen’s d for single subject participant design as outlined by Beeson and Robey (2006). This statistic is calculated in a similar fashion to Cohen’s d but tends to be higher and has different standards for small, medium, and large effects for different research areas. For a new treatment domain, many cases are required to establish benchmarks to identify small, medium, and large effect sizes. We consider our preliminary data using benchmarks from the related area of phonological treatment where Cohen’s d of 1.4, 3.5, and 10.1 have been identified as small, medium, and large effects respectively based on 135 participants across multiple treatment sites (Gierut, Morrisette, & Dickinson, 2015). We calculated d according to this formula using the baseline sessions from the first arm to calculate the standard deviation.
2.7. Neuroimaging data analysis
To examine changes in functional connectivity in the rs-fMRI data, we extracted the average time series from the preprocessed data for the preserved (non-lesioned) portions of each of 12 ROIs. These ROIs were composed of six bilateral areas, defined by the Brainnetome Atlas (Fan et al., 2016), that have been identified as integral to speech production (New et al., 2015). The ROIs corresponded to pars opercularis of the inferior frontal gyrus, premotor and motor cortices, somatosensory cortex, and the anterior insula (see Table 1 for area labels and corresponding MNI coordinates). For each of the three rs-fMRI scans acquired, we computed correlations between these time series for each pair of ROIs, followed by z-transformation (inverse hyperbolic tangent) of these values to permit comparison across time points. These pairwise scores were then compared using paired t-tests on normalized connectivity values (z-scores) before and after each treatment phase.
Table 1:
List of the corresponding region numbers, labels, and MNI coordinates of our ROIs from the Brainnetome Atlas (Fan et al., 2016). Region abbreviations: IFG (inferior frontal gyrus); PreCG (Precentral gyrus); INS (insula). Numbers correspond to Brodmann areas.
| Left Hemisphere | Right Hemisphere | ||||
|---|---|---|---|---|---|
| # | Label | MNI (x, y, z) | # | Label | MNI (x, y, z) |
| 37 | IFG, A44op (opercular) | −39, 23, 4 | 38 | IFG, A44op (opercular) | 42, 22, 3 |
| 39 | IFG, A44v (ventral) | −52, 13, 6 | 40 | IFG, A44v (ventral) | 54, 14, 11 |
| 53 | PreCG, A4hf (head/face) | −49, −8, 39 | 54 | PreCG, A4hf (head/face) | 55, −2, 33 |
| 61 | PreCG A4tl (tongue/larynx) | −52, 0, 8 | 62 | PreCG A4tl (tongue/larynx) | 54, 4, 9 |
| 157 | A1/2/3uhlf (upper limb/head/face) | −56, −14, 16 | 158 | A1/2/3uhlf (upper limb/head/face) | 56, −10, 15 |
| 165 | INS, via (ventral agranular insula) | −32, 14, −13 | 166 | INS, via (ventral agranular insula) | 33, 14, −13 |
3. Results
3.1. Treatment outcomes
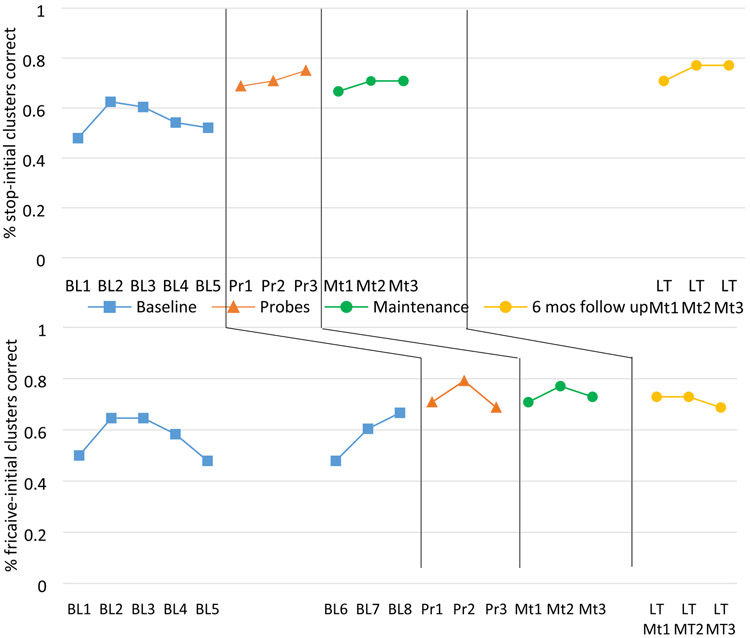
Accuracy by session is presented in Figure 2, with the top panel presenting data on the targets treated in Phase 1 and the bottom panel presenting the targets treated in Phase 2. As can be seen from the figure, there were no overlapping data between baseline sessions and probe or maintenance sessions for either treatment condition, verifying that the treatment enhanced the speech production performance when combined with both Sham tDCS and Active tDCS. For the short-term maintenance retention sessions (two weeks after the treatment phase had ended), the Cohen’s d for each condition was associated with a small treatment effect (Active: 2.46; Sham: 2.41). At the long-term maintenance session, Cohen’s d for the targets treated with Active tDCS indicated a medium effect size (3.69) whereas it remained a small effect size for the Sham tDCS targets (2.27). These findings indicate that both phases led to an improvement in P1’s productions, with the only difference between the phases occurring at long-term retention when there was a benefit for the targets treated during the Active condition.
Figure 2:
Speech production accuracy data from Phase 1 (Active) and Phase 2 (Sham) for P1. Treatment phases coincide with Probes which were collected every third treatment session.
3.2. Neuroimaging data
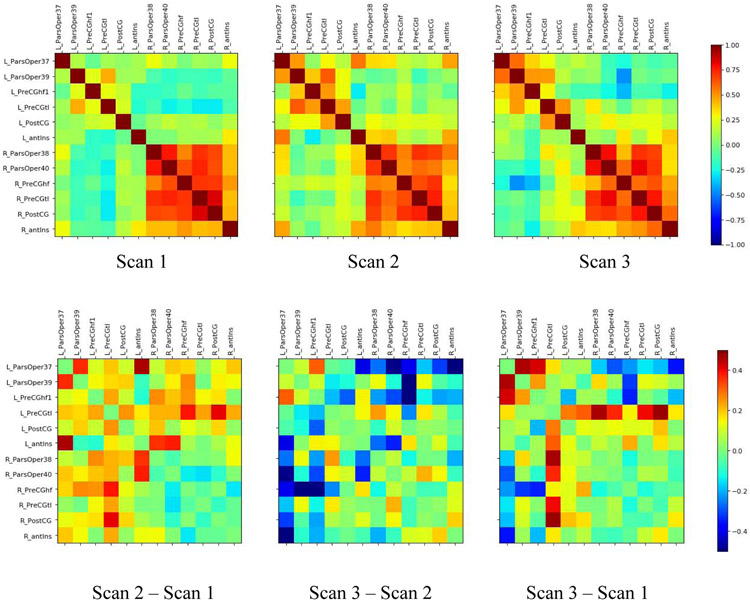
Correlation matrices representing the ROI-based network analyses for the three time points are presented in Figure 3A, with matrices indicating the differences between these conditions presented in Figure 3B. Paired t-tests examined changes in connectivity among LH regions, RH regions, and interhemispherically (i.e., all 36 pairwise connections between the 6 LH and 6 RH ROIs). There was a significant increase in connectivity from Timepoint 1 (baseline) to Timepoint 2 (after the Active phase) among LH regions [mean difference = 0.169, 95% Confidence Interval (CI; 0.101, 0.237), t(14) = 5.33, p = 0.0001] and interhemispheric regions [difference = 0.149, CI (0.105, 0.194), t(35) = 6.86, p < 0.0001], with no difference among the RH regions [difference = −0.054, CI (−0.127, 0.02), t(14) =−1.56, p = 0.14). This indicates that there was greater functional connectivity within the left hemisphere, as well as across hemispheres, after the Active tDCS treatment phase. When comparing Timepoint 2 to Timepoint 3 (after the Sham phase), there was a significant decrease in connectivity among interhemispheric regions [difference = −0.165, CI (−0.237, −0.093), t(35) = −4.64, p < 0.0004], and no significant change in connectivity among the LH [difference = −0.060, CI (−0.142, 0.022), t(14) =−1.58, p = .14] or RH [difference = 0.028, CI (−0.068, 0.124), t(14) =0.62, p = .54] regions, indicating that the interhemispheric connectivity returned to approximate baseline levels after the Sham Phase. When comparing Timepoint 3 to the Timepoint 1 baseline, we confirmed that the only significant change was an increase in LH connectivity [difference = .109, CI (0.21, 0.199), t(14) = 2.64, p = 0.019]. This increase in LH connectivity had been observed at Timepoint 2 and was durable enough to remain following the Sham phase. The RH connectivity and interhemispheric connectivity did not differ between Timepoint 3 and Timepoint 1.
Figure 3:
Correlation matrices representing the correlation across the entire time series for each neuroimaging scan. Regions are listed in the order they are presented in Table 1, with left hemisphere regions listed first followed by homologous right hemisphere regions. The top row presents data from each time point, and the bottom row shows difference matrices from consecutive time points. Regions (from left-right) correspond to Fan et al. regions 37, 39, 53, 61, 157, 165, 38, 40, 54, 62, 158, 166 (see Table 1).
4. Discussion
This single-subject intervention study in an individual with apraxia of speech provides the first evidence that non-invasive neuromodulation using tDCS can enhance long-term treatment outcomes following speech motor learning treatment. Additionally, consistent with findings from Marangolo et al. (2016), our neurological findings suggest that Active tDCS in conjunction with speech therapy enhances functional connectivity involving the left hemisphere. In our study, we examined a bilateral network that was defined based on the literature on apraxia of speech (AOS) and related speech motor control research. Following the treatment phase with Active tDCS, we observed an increase in connectivity within the left hemisphere as well as interhemispherically. In this individual, the sham phase came second and was associated with a decrease in interhemispheric connectivity (i.e., a return to baseline), but no decrease within the left hemisphere. Thus, the enhanced left hemisphere connectivity from the Active Phase was durable enough to be maintained throughout the following treatment phase with Sham tDCS.
The behavioral outcomes revealed that the speech motor learning treatment led to an increase in speech production accuracy for both treatment phases. At short-term maintenance (two weeks after the treatment phase ended), the improvement for each phase was associated with a small effect size. However, at long-term follow up, the effect size for items trained during the Active phase had increased to a medium effect while the Sham phase was still associated with a small effect. This may indicate that the immediate difference between treatment with and without adjunctive tDCS is not significant and examining more cases will be critical to evaluating whether the difference between the conditions is typically restricted to the long-term follow-up. At this point, we also cannot rule out the possibility that the specific order of treatment phases (Active then Sham) or the order of, or inherent differences between, treated items (Stops then Fricatives) can explain this overall pattern. One change we believe will be crucial moving forward is to increase the number of treatment sessions, and this has been done for our future cases. We also note that we did not control what the participant did between the end of the second phase and the long-term maintenance session. It remains possible, although unlikely, that when the participant engaged in treatment again outside of the study, this resulted in further improvement that specifically targeted the stop-initial items compared to the fricative-initial items.
We also note that there was a rising baseline immediately prior to treatment for the Sham Phase targets. A rising baseline could suggest that the participant was improving at the production of those items prior to the onset of treatment. If this were the case, it would call into question whether the behavioral treatment effects that were observed in the Sham Phase truly came from the treatment in that phase. The expectation around a rising baseline is that we should expect that condition to show a larger benefit because it was already improving. Thus, this rising baseline makes it unclear whether the small effect sizes in the Sham group truly come from the treatment in that phase. We hasten to add that we can only speculate on this point at this juncture, but it reinforces the importance of continuing to examine these issue with a larger number of cases permitting appropriate controls.
The neurological outcomes revealed a significant change in connectivity following the treatment phase with Active stimulation in which the left-hemisphere portion of the bilateral network exhibits an increase in connectivity, and that increase is maintained following the Sham phase as well. The Active phase is also associated with an increase in interhemispheric connectivity which is not maintained following the Sham phase. In this context, these findings are compelling but may raise more questions than they answer. For example, the durability of the LH increase coupled with the transitory interhemispheric connectivity increase may reflect some aspect of how recovery works when attempting to re-engage the LH in the speech task, which may have been performed by the RH more following the brain injury (although we did not observe intrahemispheric RH changes following either condition). It is possible that the interhemispheric connectivity increase is a temporary effect required to successfully transition to the LH being more active in the task, and following up on this finding will be of crucial importance to testing such hypotheses.
In total, the neurobiological changes are suggestive of an effect of tDCS condition but highlight the need to acquire data from additional participants to determine how and whether the order of the treatment phases (Active-Sham vs. Sham-Active) affects these changes. In particular, we hypothesized that tDCS could enhance treatment effects by specifically and consistently engaging an appropriate speech network in performing the treatment task, thereby strengthening the network through associative learning. These findings are consistent with that hypothesis, but there remains the distinct possibility that the pattern of effects we observed come solely from the behavioral treatment. Thus, we must compare multiple cases receiving both treatment phase orders to sufficiently evaluate this hypothesis. We also cannot speak to the specificity of the findings with respect to speech vs. other motor skills in the absence of a control task.
Despite the preliminary nature of our findings, we believe that they suggest that tDCS may be effective for enhancing motor learning-based rehabilitation in the speech domain and justify a call for further research. In particular, we believe that this study demonstrates that the single-subject intervention research approach and our methods are appropriate for addressing this issue in this population. Further cases will elucidate the effects of tDCS on behavior and speech motor network connectivity when we compare individuals who receive both orders of treatment phases and across levels of impairment severity.
Highlights.
Speech motor learning treatment enhances speech outcomes following stroke
Long-term outcomes are enhanced when treatment is paired with targeted Active tDCS
Resting-state functional connectivity in cortical speech network is increased when treatment is paired with Active tDCS
Acknowledgments
The authors thank Alexandra Gordon, Chiara Repetti-Ludlow, and Christine Laganella for assistance with data analysis.
Funding
This work was supported by an award from the National Institute on Deafness and other Communication Disorders (NIDCD) to AB (K01DC014298). ESD’s time was supported by a grant from the Louisiana Board of Regents (Research Competitiveness Subprogram; LEQSF(2017-20)-RD-A-03).
Footnotes
Conflict of interest: The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This case is the first of a planned series of cases which will vary the number of baseline sessions to establish experimental control across participants. We will also balance the order of which manner of articulation is trained first (e.g., stops vs. fricatives) and the order of the treatment phases (active-sham vs. sham-active). In anticipation of the full series, we generated random experimental conditions, and P1 was assigned to the condition described above (5 baselines, stops first, active first).
REFERENCES
- Ambrus GG, Al-Moyed H, Chaieb L, Sarp L, Antal A, & Paulus W (2012). The fade-in – Short stimulation – Fade out approach to sham tDCS – Reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimulation, 5(4), 499–504. doi: 10.1016/j.brs.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, & Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal, 12(1), 26–41. doi: 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard K, Maas E, & Robin D (2007). Treating control of voicing in apraxia of speech with variable practice. Aphasiology, 21(12), 1195–1217. doi: 10.1080/02687030601047858 [DOI] [Google Scholar]
- Ballard K, Wambaugh JL, Duffy JR, Layfield C, Maas E, Mauszycki S, & McNeil MR (2015). Treatment for Acquired Apraxia of Speech: A Systematic Review of Intervention Research Between 2004 and 2012. American Journal of Speech-Language Pathology, 24(2), 316–337. doi: 10.1044/2015_AJSLP-14-0118 [DOI] [PubMed] [Google Scholar]
- Beeson PM, & Robey RR (2006). Evaluating Single-Subject Treatment Research: Lessons Learned from the Aphasia Literature. Neuropsychology Review, 16(4), 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, & Rahman A (2013). Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Frontiers in Human Neuroscience, 7, 688–688. doi: 10.3389/fnhum.2013.00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P, & Weenink D (2020). Praat: doing phonetics by computer (Version 6.1.12) [Computer program]. Retrieved from http://www.praat.org/. [Google Scholar]
- Boroda E, Sponheim SR, Fiecas M, & Lim KO (2020). Transcranial direct current stimulation (tDCS) elicits stimulus-specific enhancement of cortical plasticity. Neuroimage, 211, 116598. doi: 10.1016/j.neuroimage.2020.116598 [DOI] [PubMed] [Google Scholar]
- Buchwald A, Calhoun H, Rimikis S, Lowe MS, Wellner R, & Edwards DJ (2019). Using tDCS to facilitate motor learning in speech production: The role of timing. Cortex, 111, 274–285. doi: 10.1016/j.cortex.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald A, Gagnon B, & Miozzo M (2017). Identification and Remediation of Phonological and Motor Errors in Acquired Sound Production Impairment. Journal of Speech, Language, and Hearing Research, 60(6S), 1726–1738. doi: 10.1044/2017_JSLHR-S-16-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald A, & Miozzo M (2011). Finding abstract structure in word production: Evidence from aphasia. Psychol Sci, 22(9), 1113–1119. [DOI] [PubMed] [Google Scholar]
- Buchwald A, & Miozzo M (2012). Phonological and motor errors in individuals with acquired impairment. Journal of Speech, Language and Hearing Research, 55(5), 1573–1586. [DOI] [PubMed] [Google Scholar]
- Chhatbar PY, Kautz SA, Takacs I, Rowland NC, Revuelta GJ, George MS,… Feng W (2018). Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimulation, 11(4), 727–733. doi: 10.1016/j.brs.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Datta A, Baker JM, Bikson M, & Fridriksson J (2011). Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimulation, 4(3), 169–174. doi: 10.1016/j.brs.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L,… Jiang T (2016). The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex, 26(8), 3508–3526. doi: 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewski K, Blicher JU, Mortensen J, Severinsen KE, Nielsen JF, & Andersen H (2017). Transcranial direct current stimulation potentiates improvements in functional ability in patients with chronic stroke receiving constraint-induced movement therapy. Stroke, 48(1), 229–232. doi: 10.1161/STROKEAHA.116.014988 [DOI] [PubMed] [Google Scholar]
- Fiori V, Nitsche MA, Cucuzza G, Caltagirone C, & Marangolo P (2019). High Definition Transcranial Direct Current Stimulation (HD-tDCS) Improves Verb Recovery in Aphasic Patients Depending on Current Intensity. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Rorden C, Elm J, Sen S, George MS, & Bonilha L (2018). Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: A randomized clinical trial. JAMA Neurology. doi: 10.1001/jamaneurol.2018.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierut JA, Morrisette ML, & Dickinson SL (2015). Effect Size for Single-Subject Design in Phonological Treatment. J Speech Lang Hear Res, 58(5), 1464–1481. doi: 10.1044/2015_JSLHR-S-14-0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, & Cohen LG (2005). Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain, 128(Pt 3), 490–499. doi: 10.1093/brain/awh369 [DOI] [PubMed] [Google Scholar]
- Jiang T (2013). Brainnetome: a new -ome to understand the brain and its disorders. NeuroImage, 80, 263–272. doi: 10.1016/j.neuroimage.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). Boston naming test. Philadelphia: Lea & Febiger. [Google Scholar]
- Kay J, Lesser R, & Coltheart M (1992). PALPA: Psycholinguistic Assessments of Language Processing in Aphasia. Hove: Lawrence Erlbaum Associates. [Google Scholar]
- Kertesz A (2006). Western Aphasia Examination -- Revised. . San Antonio, TX: Pearson. [Google Scholar]
- Kim D-YMDP, Lim J-YMD, Kang EKMDP, You DSMD, Oh M-KMD, Oh B-MMD, & Paik N-JMDP (2010). Effect of Transcranial Direct Current Stimulation on Motor Recovery in Patients with Subacute Stroke. American Journal of Physical Medicine & Rehabilitation, 89(11), 879–886. [DOI] [PubMed] [Google Scholar]
- Kronberg G, Bridi M, Abel T, Bikson M, & Parra LC (2017). Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul, 10(1), 51–58. doi: 10.1016/j.brs.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronberg G, Rahman A, Lafon B, Bikson M, & Parra LC (2019). Direct current stimulation boosts associative Hebbian synaptic plasticity and maintains its pathway specificity. bioRxiv, 562322. doi: 10.1101/562322 [DOI] [Google Scholar]
- Maas E, Robin DA, Hula SNA, Freedman SE, Wulf G, Ballard KJ, & Schmidt RA (2008). Principles of motor learning in treatment of motor speech disorders. American Journal of Speech-Language Pathology, 17(3), 277–298. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=2009991377&site=ehost-live [DOI] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Sabatini U, De Pasquale G, Razzano C, Caltagirone C, & Gili T (2016). Bilateral transcranial direct current stimulation language treatment enhances functional connectivity in the left hemisphere: preliminary data from aphasia. Journal of Cognitive Neuroscience, 28(5), 724–738. [DOI] [PubMed] [Google Scholar]
- McNeil MR (Ed.) (2009). Clinical Management of Sensorimotor Speech Disorders (Second ed.). New York: Thieme. [Google Scholar]
- New AB, Robin DA, Parkinson AL, Duffy JR, McNeil MR, Piguet O,… Ballard KJ (2015). Altered resting-state network connectivity in stroke patients with and without apraxia of speech. NeuroImage. Clinical, 8, 429–439. doi: 10.1016/j.nicl.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive ML, & Smith BW (2005). Effect size calculations and single subject designs. Educational Psychology, 25(2-3), 313–324. doi: 10.1080/0144341042000301238 [DOI] [Google Scholar]
- Rorden C, & Brett M (2000). Stereotaxic display of brain lesions. Behav Neurol, 12(4), 191–200. [DOI] [PubMed] [Google Scholar]
- Sandberg CW (2017). Hypoconnectivity of Resting-State Networks in Persons with Aphasia Compared with Healthy Age-Matched Adults. Front Hum Neurosci, 11, 91. doi: 10.3389/fnhum.2017.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hasson U, Siugzdaite R, Schiel M, Chen EE, Kotter R, & Small SL (2010). Virtual brain transplantation (VBT): a method for accurate image registration and parcellation in large cortical stroke. Arch Ital Biol, 148(3), 219–241. [PubMed] [Google Scholar]
- Yourganov G, Fridriksson J, Stark B, & Rorden C (2018). Removal of artifacts from resting-state fMRI data in stroke. NeuroImage: Clinical, 17, 297–305. doi: 10.1016/j.nicl.2017.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, & Cohen LG (2001). Modulation of practice-dependent plasticity in human motor cortex. Brain, 124(6), 1171–1181. doi: 10.1093/brain/124.6.1171 [DOI] [PubMed] [Google Scholar]