Abstract
Introduction
Post-partum women are at increased risk for lower limb musculoskeletal disorders. Foot arch collapse following pregnancy has been reported as a mechanism for this increased risk. However, dynamic changes during gait in post-partum women have not been reported. Therefore, we assessed the association between parity and dynamic foot pronation during gait.
Objective
To determine: (1) if there is an association between parity and dynamic foot pronation (center of pressure excursion index, CPEI) during gait, and (2) the extent to which there is a dose-effect of parity on foot pronation.
Design
The Multicenter Osteoarthritis Study (MOST) Study is a longitudinal cohort study of adults with or at risk for knee osteoarthritis (OA).
Setting
Two communities in the US, Birmingham, Alabama and Iowa City, Iowa.
Interventions
Not applicable
Participants
A population-based sample of 1177 MOST participants who were female, had complete CPEI and parity data and completed the baseline, 30- and 60-month visits.
Main Outcome Measure(s)
Odds of a one quintile decrease in CPEI by parity group and mean CPEI by parity group.
Results
In 1177 women, mean age was 67.7 years and mean BMI was 30.6 kg/m2. As parity increased, there was significantly greater foot pronation, lower mean CPEI: 19.1 (18.2–20.1), 18.9 (18.4–19.4), 18 (17.5–18.6) to 17.5 (16.4–18.6) in the 0, 1–2, 3–4 and ≥ 5 children groups, respectively; p= 0.0021), which remained significant after adjusting for race and clinic site (p=0.0052). There was a positive linear trend (β= 1.08, 1.03–1.14) in odds ratios of a one quintile decrease in CPEI (greater pronation) with increasing parity level (p=0.0037), which remained significant after adjusting for race and clinic site (p=0.0099). After adjusting for age and BMI, these two associations were no longer statistically significant.
Conclusion(s)
This study indicates a positive correlation between parity and greater dynamic pronation of the feet.
Keywords: Parity, pronation, osteoarthritis
Introduction
Women report musculoskeletal pain and functional limitations due to foot, back and hip problems more often than men and are at greater risk for musculoskeletal problems than men.[1,2] In addition, knee osteoarthritis (OA), one of the most common causes of pain and disability,[3] is disproportionately seen in women,[4] occurring 1.8 times more often in women than men.[5] Understanding and preventing musculoskeletal disorders in women is key to minimizing risk for disablement and maximizing quality of life.
The increased risk that women face may be associated with biochemical or biomechanical changes that occur during pregnancy. Increased body mass, an anteriorly displaced center of mass and hormonal changes that occur during pregnancy alter the musculoskeletal structure of women and may lead to functional changes in their lower limbs during pregnancy.[6,7,8] Multiple researchers reported an increase in lower-limb musculoskeletal discomfort, such as increased back,[9] hip,[10] knee,[10] and foot pain[8,10,11] during pregnancy. While musculoskeletal symptoms increase during pregnancy, Vullo and colleagues found that the risk for musculoskeletal disorders persists postpartum and parous women are almost twice as likely as nulliparous women to develop new lower limb musculoskeletal disorders later in life.[10] Furthermore, Wise et al reported that parity is associated with an increase in the incidence of both radiographic osteoarthritis and knee replacements.[12]
Malalignment at the feet, such as over-pronation or supination, differences in pressure distribution and planus foot morphology may play an important role in determining knee joint alignment and loading through altering lower limb kinematics and kinetics.[13,14,15,16] Proper functional alignment of the feet is important in absorbing mechanical stress from ground contact[17] in order to prevent injury.[13,15] Therefore, changes in foot structure associated with pregnancy may explain why parous women are at greater risk for musculoskeletal disorders than nulliparous women.
Anthropometric changes in the feet, such as increased foot length, width and volume are commonly reported during pregnancy.[18] In a study of 111 women, 13.2% of nulliparous women, 31.2% of primiparous women, 58.3% of women who had carried 2 pregnancies and 66.7% of women who carried 3 or more pregnancies reported a permanent increase in shoe size since age 18 years. [19] More importantly, some changes in the feet persist following pregnancy.[7] Segal et al found that arch collapse is persists following pregnancy to at least 19 weeks post-partum in 40–60% of women and in a follow-up study, 11 of the women from the original study with 19-week follow-up had persistent foot length/width and arch changes greater than 1 year after the original study.[20] Planus foot posture has been associated with more eversion excursion than rectus foot posture in runners[16] and may lead to excessive pronation of the foot during both standing and walking activities.[20] A recent study showed that foot pronation in women may cause kinematic changes that are transmitted to more proximal structures, resulting in low back pain.[21] Therefore, persistent changes in dynamic foot function and foot biomechanics due to pregnancy may affect loading and motion patterns of more proximal structures in the kinetic chain.
Although collapse of the arch in a static standing posture during pregnancy and its persistence following pregnancy have been reported, to our knowledge, changes in the dynamic function of the foot during gait have not been reported. Detecting an effect would provide additional information regarding a potential mechanism for pregnancy altering lower limb loading. Considering the number of gait cycles completed in the decades following reproduction, this may elucidate a reason for increased risk for musculoskeletal disorders in post-partum women and older women and guide preventative strategies. Therefore, our aim was to assess for an association between the number of children a woman gave birth (parity) and dynamic foot pronation during gait (center of pressure excursion index: CPEI)[7], and the extent to which there is a dose-effect of parity on pronatory foot function.
Methods
Participants
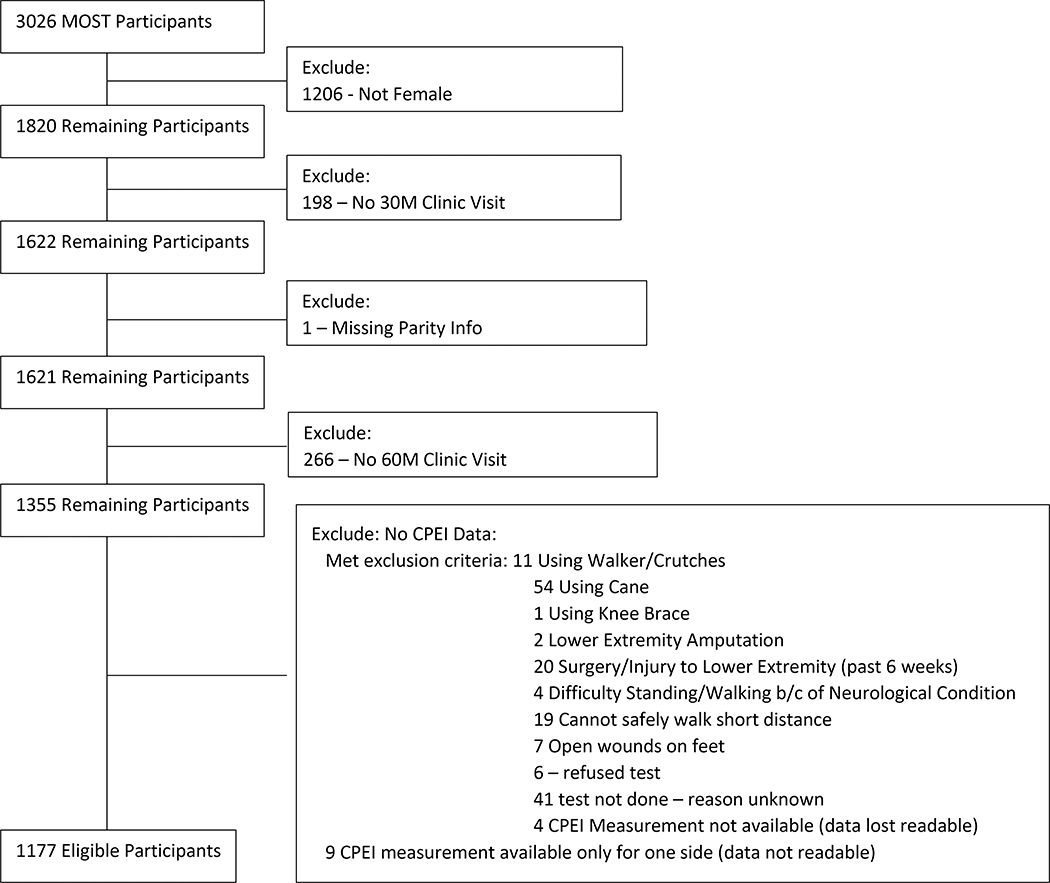
The Multicenter Osteoarthritis Study (MOST) is a longitudinal cohort study of adults, age 50–79 years at baseline, with or at an increased risk for knee OA.[22] Participants were recruited based on characteristics known to confer elevated risk for knee OA— frequent knee pain, history of injury or surgery, or being overweight or obese. Distribution of race was kept in proportion to that of the US population. Our study included MOST participants who were female, had complete CPEI and parity data and completed the baseline, 30- and 60-month measurement visits (Figure 1). The study was approved by local institutional review boards and study participants underwent an informed consent process.
Figure 1.
Association between Parity and Dynamic Foot Function Inclusion/Exclusion Flow Chart
Assessments
Data was collected at baseline and at the 30-month and the 60-month clinic visits. Participants’ responses to the question, “How many children did you give birth to?” at the 30-month clinic visit were used as surrogate for parity number. Age, sex, height, self-reported weight and height at age 25 years old and other demographic characteristics were assessed by questionnaire at the 30-month visit. At the 60-month clinic visit, participant body mass index (BMI) and dynamic foot function were measured. BMI was calculated as body mass (kilograms) divided by height (meters) squared. Height and weight were measured per protocol by certified staff members, as previously described (appendix 1).[23] Dynamic foot function (greater foot pronation during gait) was measured using an emed X digital pedobarograph (Novel Electronics Inc., St. Paul, MN) to quantify CPEI. CPEI is side-specific and averaged over 5 trials for each foot, computed from a composite “ensemble averaged” footprint for all 5 trials.[7]
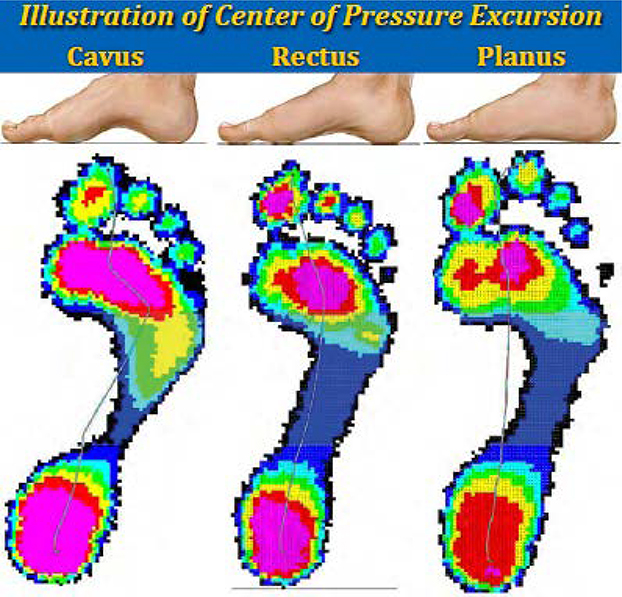
Data were collected at 50 Hz with a 15-kPa threshold. A customized Matlab (Version 7.8.0 R2009a; Natick, MA) program was used to calculate CPEI. CPEI was defined as the lateral displacement of the center of pressure curve from a reference line drawn from the initial to the final centers of pressure during the stance phase of gait. This measurement was standardized to the width of the anterior third of the foot. [7] Example CPEI measurements are represented by blue lines for cavus, rectus and planus feet in Figure 2. [24]
Figure 2.
Illustration of Center of Pressure Excursion Measurement
Statistical Analysis
Parity was divided into four categories: 0 births, referent group in all analyses; 1–2 births; 3–4 births; and 5 or more births. We compared the demographic and general characteristics of the participants on a participant level and individual knee level based on parity category using the chi-square test for categorical variables and analysis of variance for continuous variables. CPEI quintiles were established based on knee level samples in women using the mean CPEI percentage of five trials on the left and five trials on the right.[25] Bivariate association was performed between CPEI quintiles and continuous measurements using analysis of variance with p-values calculated for linear trends. The odds of a one quintile decrease in CPEI (i.e. more foot pronation) by parity group was modeled using a proportional odds, generalized estimation equation (GEE), to account for correlation between knees within a person, adjusted for race and clinic site, age, BMI at the 60 month visit, or BMI at 25 years of age. Since CPEI quintiles and parity groups varied by clinic site, we performed a clinic-specific analysis of the data (see supplemental material). Alpha level for statistical significance was set at 0.05.
Results
The MOST study included 1820 women. 1815 reported being post-menopausal and 5 stated that they did not know. The following numbers of participants were excluded from this analysis due to missing the 30-month visit (n=198), missing the 60-month visit (n=266), missing parity data (n=1) or missing CPEI data (n=178). A total of 1177 women were included in this analysis. Participants’ mean ± SD age was 67.7 ± 7.5 years and BMI was 30.6 ± 6.2 kg/m2. As presented in Table 1, 154 participants (13%) were nulliparous, and parity was 1–2 for 511 (43%), 3–4 for 407 (35%) and ≥ 5 for 105 (9%). There were significant differences in clinic site, age, marital status and type of work between the parity groups (p<.0001) as well as BMI at age 25 years old (p= 0.0252) (Table 1). However, there were no significant differences in BMI at the 60-month visit or physical activity scale for the elderly (PASE) score when comparing the parity groups. The mean CPEI for each CPEI quintile (Severe over-pronation, moderate over-pronation, rectus and over-supinating were 9.07 ± 3.12, 14.92 ± 1.19, 18.56 ± 0.95, 22.17 ± 1.18 and 27.83 ± 3.03, respectively (Table 1b).
Table 1.
Descriptive Statistics by Parity Group.
| Characteristic | Total | 0 Children | 1–2 Children | 3–4 Children | ≥5 Children | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Number of Participants | 1177 | 154 | 511 | 407 | 105 | -- | ||
| N (%) Clinic site | UAB | 526 (44.7%) | 73 (47.4%) | 263 (51.5%) | 161 (39.6%) | 29 (27.6%) | <.0001 | |
| UIowa | 651 (55.3%) | 81 (52.6%) | 248 (48.5%) | 246 (60.4%) | 76 (72.4%) | |||
| N (%) Race | White or Caucasian | 1023 (86.9%) | 139 (90.3%) | 429 (84.0%) | 362 (88.9%) | 93 (88.6%) | 0.1807 | |
| Black or African American | 141 (12.0%) | 13 (8.4%) | 74 (14.5%) | 42 (10.3%) | 12 (11.4%) | |||
| Other | 13 (1.1%) | 2 (1.3%) | 8 (1.6%) | 3 (0.7%) | 0 (0.0%) | |||
| Mean ± SD Age at 60m | 67.7 ± 7.5 | 65.5 ± 7.7 | 66.1 ± 7.2 | 69.3 ± 7.3 | 72.6 ± 6.7 | <.0001 | ||
| Mean ± SD BMI at 60m | 30.6 ± 6.1 | 31.6 ± 6.6 | 30.3 ± 6.4 | 30.6 ± 5.9 | 30.0 ± 4.6 | .0950 | ||
| Mean ± SD BMI at BL (enrollment visit) | 30.1 ± 5.9 | 31.0 ± 6.1 | 29.9 ± 6.1 | 30.2 ± 5.8 | 29.8 ± 4.9 | .2552 | ||
| Mean ± SD BMI at 25 yo (self-reported weight and height) | 22.2 ± 3.5 | 22.9 ± 4.2 | 22.0 ± 3.5 | 22.3 ± 3.5 | 22.2 ± 2.5 | .0252 | ||
| Mean ± SD PASE at BL (enrollment visit) | 162.8 ± 76.5 | 173.9 ± 72.9 | 164.9 ± 76.5 | 158.5 ± 78.4 | 153.4 ± 73.3 | .0916 | ||
| N (%) Marital Status | Married | 809 (68.7%) | 78 (50.6%) | 357 (69.9%) | 297 (73.0%) | 77 (73.3%) | <.0001 | |
| Widowed | 140 (11.9%) | 12 (7.8%) | 56 (11.0%) | 54 (13.3%) | 18 (17.1%) | |||
| Separated | 6 (0.5%) | 1 (0.6%) | 3 (0.6%) | 2 (0.5%) | 0 (0.0%) | |||
| Divorced | 161 (13.7%) | 12 (7.8%) | 88 (17.2%) | 51 (12.5%) | 10 (9.5%) | |||
| Single, never married | 54 (4.6%) | 46 (29.9%) | 6 (1.2%) | 2 (0.5%) | 0 (0.0%) | |||
| Other | 3 (0.3%) | 3 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||
| No answer | 4 (0.3%) | 2 (1.3%) | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | |||
| N (%) Type of work – Most adult life | Sales, Office Work | 628 (53.4%) | 99 (64.3%) | 310 (60.7%) | 181 (44.5%) | 38 (36.2%) | <.0001 | |
| Skilled, Unskilled, Farming, Technician, Housework | 280 (23.8%) | 24 (15.6%) | 82 (16.0%) | 133 (32.7%) | 41 (39.0%) | |||
| Other | 269 (22.9%) | 31 (20.1%) | 119 (23.3%) | 93 (22.9%) | 26 (24.8%) | |||
| Mean ± SD CPEI (average right and left) | 18.5 ± 5.7 | 19.1 ± 6.0 | 18.9 ± 5.6 | 18.0 ± 5.7 | 17.5 ± 5.7 | .0172 | ||
| Mean ± SD CPEI (left side only) | 17.9 ± 6.6 | 18.6 ± 6.9 | 18.3 ± 6.5 | 17.6 ± 6.6 | 16.5 ± 6.7 | .0236 | ||
| Mean ± SD CPEI (right side only) | 19.1 ± 6.7 | 19.6 ± 6.6 | 19.5 ± 6.6 | 18.5 ± 7.0 | 18.5 ± 6.2 | .0826 | ||
Abbreviations: BMI, body mass index; CPEI, center of pressure excursion index; PASE, physical activity scale for the elderly
Table 1B.
Knee level Descriptive Statistics by CPEI quintiles.
| Characteristic | Q1 | Q2 | Q3 | Q4 | Q5 | p-value | |
|---|---|---|---|---|---|---|---|
| Number of Knees | 467 | 483 | 460 | 475 | 469 | -- | |
| Mean ± SD CPEI | 9.07 ± 3.12 | 14.92 ± 1.19 | 18.56 ± 0.95 | 22.17 ± 1.18 | 27.83 ± 3.03 | ||
| Min, P25, P50, P75, max CPEI | −7.7, 7.5, 9.7, 11.5, 12.7 | 12.8, 13.9, 15, 16, 16.8 | 16.9, 17.8, 18.6, 19.3, 20.2 | 20.3, 21.2, 22.1, 23.2, 24.3 | 24.4, 25.3, 27, 29.4, 40.2 | ||
| Mean ± SD Number of children (self-reported at 30m) | 2.61 ± 1.65 | 2.51 ± 1.71 | 2.44 ± 1.71 | 2.34 ± 1.48 | 2.27 ± 1.55 | .0131 | |
| Min, P25, P50, P75, max Number of children | 0, 2, 2, 3, 12 | 0, 2, 2, 3, 12 | 0, 2, 2, 3, 12 | 0, 1, 2, 3, 8 | 0, 1, 2, 3, 9 | ||
| N (%) Parity | 0 | 50 (10.7%) | 64 (13.3%) | 64 (13.9%) | 61 (12.8%) | 69 (14.7%) | 0.1526 |
| 1 child | 50 (10.7%) | 46 (9.5%) | 47 (10.2%) | 64 (13.5%) | 57 (12.2%) | ||
| 2 children | 137 (29.3%) | 152 (31.5%) | 153 (33.3%) | 149 (31.4%) | 167 (35.6%) | ||
| 3–4 children | 180 (38.5%) | 174 (36.0%) | 152 (33.0%) | 168 (35.4%) | 140 (29.9%) | ||
| 5–12 children | 50 (10.7%) | 47 (9.7%) | 44 (9.6%) | 33 (6.9%) | 36 (7.7%) | ||
| N (%) Clinic site | UAB | 184 (39.4%) | 219 (45.3%) | 196 (42.6%) | 217 (45.7%) | 236 (50.3%) | 0.0147 |
| UIowa | 283 (60.6%) | 264 (54.7%) | 264 (57.4%) | 258 (54.3%) | 233 (49.7%) | ||
| N (%) Race | White or Caucasian | 428 (91.6%) | 423 (87.6%) | 405 (88.0%) | 403 (84.8%) | 387 (82.5%) | 0.0026 |
| Black or African American | 37 (7.9%) | 54 (11.2%) | 47 (10.2%) | 67 (14.1%) | 77 (16.4%) | ||
| Other | 2 (0.4%) | 6 (1.2%) | 8 (1.7%) | 5 (1.1%) | 5 (1.1%) | ||
| N (%) Type of work – Most adult life | Sales, Office Work | 236 (50.5%) | 244 (50.5%) | 254 (55.2%) | 260 (54.7%) | 262 (55.9%) | 0.5006 |
| Skilled, Unskilled, Farming, Technician, Housework | 122 (26.1%) | 129 (26.7%) | 102 (22.2%) | 104 (21.9%) | 103 (22.0%) | ||
| Other | 109 (23.3%) | 110 (22.8%) | 104 (22.6%) | 111 (23.4%) | 104 (22.2%) | ||
| Mean ± SD Age at 60m | 68.9 ± 7.4 | 68.3 ± 7.7 | 67.3 ± 7.4 | 67.3 ± 7.5 | 66.6 ± 7.4 | <.0001 | |
| Mean ± SD BMI at 60m | 29.9 ± 5.4 | 29.6 ± 5.7 | 30.7 ± 6.5 | 31.4 ± 6.5 | 31.1 ± 6.2 | <.0001 | |
| Mean ± SD BMI at BL (enrollment visit) | 29.4 ± 5.2 | 29.3 ± 5.3 | 30.4 ± 6.4 | 31.0 ± 6.4 | 30.5 ± 5.9 | <.0001 | |
| Mean ± SD BMI at 25 yo (self-reported weight and height) | 21.9 ± 3.3 | 21.9 ± 3.3 | 22.4 ± 4.0 | 22.4 ± 3.4 | 22.7 ± 3.4 | .0020 | |
| Mean ± SD PASE at BL (enrollment visit) | 159.6 ± 77.1 | 161.8 ± 76.9 | 162.4 ± 72.3 | 161.9 ± 76.0 | 168.4 ± 80.0 | 0.4761 | |
| ROA status at baseline | No | 248 (53.1%) | 276 (57.1%) | 273 (59.3%) | 276 (58.1%) | 275 (58.6%) | .9627 |
| Yes | 209 (44.8%) | 199 (41.2%) | 179 (38.9%) | 190 (40.0%) | 185 (39.4%) | ||
| KR or exclusion | 9 (1.9%) | 7 (1.4%) | 7 (1.5%) | 8 (1.7%) | 8 (1.7%) | ||
| no TF/PF unknown | 1 (0.2%) | 1 (0.2%) | 1 (0.2%) | 1 (0.2%) | 1 (0.2%) | ||
| ROA status at 60m | No | 185 (39.6%) | 224 (46.4%) | 208 (45.2%) | 203 (42.7%) | 207 (44.1%) | .5108 |
| Yes | 224 (48.0%) | 208 (43.1%) | 197 (42.8%) | 217 (45.7%) | 216 (46.1%) | ||
| KR or exclusion | 53 (11.3%) | 50 (10.4%) | 53 (11.5%) | 52 (10.9%) | 41 (8.7%) | ||
| no TF/PF unknown | 5 (1.1%) | 1 (0.2%) | 2 (0.4%) | 3 (0.6%) | 5 (1.1%) | ||
Abbreviations: BMI, body mass index; CPEI, center of pressure excursion index; KR, Knee replacement; PASE, physical activity scale for the elderly; ROA, radiographic osteoarthritis; TF/PF, Tibiofemoral/Patellofemoral
There were significant differences in mean number of children, BMI at 60 month, baseline visit and BMI at age 25 years old and average age, with a trend towards lower mean number of children (p=0.0131), higher BMI at 60 month visit (p<0.0001), higher BMI at baseline visit (p<0.0001), BMI at age 25 years old (p=0.0020) and younger women (p<0.0001) as CPEI quintile increased (Table 1b). There were also significant differences in clinic site and race with a trend towards more patients at the University of University of Alabama Birmingham (UAB) clinic site (p=0.0147) and more non-white or Caucasian patients as CPEI quintile increased (Table 1b). There were no differences in radiographic osteoarthritis or type of work (Table 1b).
Bivariate analyses of the associations BMI at the 60-month visit, baseline visit and at 25 years old and quintiles of CPEI revealed correlations between higher values of all BMI parameters (p<.0002) and higher CPEI (less pronation) (Table 2). The same analyses of age and number of children also showed correlations between higher age (p<0.0001) and parity (reported number of children) (p= 0.0027) and lower CPEI (greater pronation) (Table 2).
Table 2.
Bivariate association between CPEI quintiles and continuous measurements: BMI, Age and Number of children.
| CPEI quintile description | Range of CPEI in Quintile | 60m BMI (kg/m2) Mean (95% CI) |
BL BMI (kg/m2) Mean (95% CI) |
25yo BMI (kg/m2) Mean (95% CI) |
60m Age Mean (95% CI) |
Reported N of children Mean (95% CI) |
|
|---|---|---|---|---|---|---|---|
| Lowest Quintile | Q1 | [−7.7 – 12.7] (N=467) | 29.9 (29.3 – 30.5) | 29.4 (28.8 – 30) | 21.9 (21.5– 22.3) | 68.9 (68.1 – 69.8) | 2.61 (2.43 – 2.78) |
| 2nd Quintile | Q2 | [12.8 – 16.8] (N=483) | 29.6 (29 – 30.2) | 29.3 (28.8 – 29.9) | 21.9 (21.6 – 22.3) | 68.3 (67.5 – 69) | 2.51 (2.34 – 2.68) |
| 3rd Quintile | Q3 | [16.9 – 20.2] (N=460) | 30.7 (30 – 31.4) | 30.4 (29.8 – 31.1) | 22.4 (21.9 – 22.8) | 67.3 (66.6 – 68.1) | 2.44 (2.25 – 2.62) |
| 4th Quintile | Q4 | [20.3 – 24.3] (N=475) | 31.4 (30.7 – 32.1) | 31 (30.3 – 31.6) | 22.4 (22 – 22.7) | 67.3 (66.6 – 68.1) | 2.34 (2.19 – 2.5) |
| Highest Quintile | Q5 | [24.4 – 40.2] (N=469) | 31.1 (30.4 – 31.8) | 30.5 (29.9 – 31.2) | 22.7 (22.3 – 23) | 66.6 (65.8 – 67.4) | 2.27 (2.1 – 2.44) |
| p-value for linear trend | 0.0002 | 0.0005 | 0.0004 | <.0001 | 0.0027 | ||
Abbreviations: CPEI, center of pressure excursion index; BMI, body mass index
As presented in (Table 3), there was significantly greater foot pronation (lower mean CPEI), with higher parity, 19.1 (18.2–20.1), 18.9 (18.4–19.4), 18 (17.5–18.6) to 17.5 (16.4–18.6) in the 0, 1–2, 3–4 and ≥ 5 children groups, respectively(p= 0.0021), which remained significant after adjusting for race and clinic site (p=0.0052). Also, there was a positive linear trend (β= 1.08, 1.03–1.14) in odds ratios of a one quintile decrease in CPEI (greater pronation) with increasing parity level (p=.0037), which remained significant after adjusting for race and clinic site (p=0.0099) (Table 4). The distribution of CPEI values for each parity group are summarized in Table 3.
Table 3.
Association between Parity and CPEI – reported CPEI mean (95% CI) and p-value for linear trend
| Unadjusted | Model 1† | Model 2‡ | Model 3A§ | Model 3B¶ | |
|---|---|---|---|---|---|
| 0 Children | 19.1 (18.2 – 20.1) | 19.8 (18.6 – 21) | 19.5 (18.3 – 20.7) | 19.4 (18.2 – 20.5) | 19.4 (18.2 – 20.5) |
| 1–2 Children | 18.9 (18.4 – 19.4) | 19.5 (18.6 – 20.3) | 19.2 (18.3 – 20.1) | 19.2 (18.3 – 20) | 19.2 (18.4 – 20) |
| 3–4 Children | 18 (17.5 – 18.6) | 18.7 (17.8 – 19.7) | 18.7 (17.8 – 19.6) | 18.6 (17.7 – 19.5) | 18.7 (17.7 – 19.6) |
| ≥5 Children | 17.5 (16.4 – 18.6) | 18.2 (16.9 – 19.6) | 18.5 (17.1 – 19.8) | 18.4 (17.1 – 19.7) | 18.5 (17.2 – 19.8) |
| p-value for linear trend: | 0.0021 | 0.0052 | 0.0919 | 0.0976 | 0.1112 |
Model 1: adjusted for race and clinic site
Model 2: model 1 plus additionally adjusted for age
Model 3A: model 2 plus additionally adjusted for BMI at 60m (current)
Model 3B (sensitivity): model 2 plus additionally adjusted for BMI at 25 years-old (self-reported)
Abbreviations: CPEI, center of pressure excursion index; CI, confidence interval
Table 4.
Odds Ratio of a one quintile decrease in CPEI (lower CPEI) by parity group and by covariate††.
| Unadjusted Odds Ratio (95% CI) p-value | Model 1† | Model 2‡ | Model 3A§ | Model 3B¶ | |
|---|---|---|---|---|---|
| Parity 1–2 vs. 0 | 1.03 (0.78 – 1.358) p= 0.8222 |
1.06 (0.8 – 1.393) p= 0.6902 |
1.05 (0.79 – 1.381) p= 0.7422 |
1.02 (0.78 – 1.346) p= 0.8778 |
1.01 (0.77 – 1.337) p= 0.9341 |
| Parity 3–4 vs. 0 | 1.31 (0.99 – 1.744) p= 0.0582 |
1.3 (0.98 – 1.736) p= 0.0679 |
1.21 (0.91 – 1.623) p= 0.1903 |
1.2 (0.9 – 1.6) p= 0.2215 |
1.19 (0.89 – 1.594) p= 0.2359 |
| Parity ≥5 vs. 0 | 1.48 (1.01 – 2.17) p= 0.0442 |
1.46 (1 – 2.147) p= 0.0525 |
1.27 (0.85 – 1.881) p= 0.2393 |
1.25 (0.84 – 1.848) p= 0.2705 |
1.23 (0.83 – 1.832) p= 0.2985 |
| Parity ≥3 vs. 0 | 1.95 (1.08 – 3.499) p= 0.0261 |
1.91 (1.06 – 3.448) p= 0.0325 |
1.54 (0.84 – 2.829) p= 0.1653 |
1.49 (0.81 – 2.742) p= 0.1945 |
1.47 (0.8 – 2.705) p= 0.215 |
| Parity ≥1 vs. 0 | 2.01 (0.89 – 4.513) p= 0.0913 |
2.02 (0.89 – 4.562) p= 0.0918 |
1.61 (0.7 – 3.696) p= 0.2596 |
1.53 (0.67 – 3.492) p= 0.3158 |
1.49 (0.65 – 3.418) p= 0.3492 |
| Linear trend (1 child increment) | 1.08 (1.03 – 1.14) p= 0.0037 |
1.07 (1.02 – 1.133) p= 0.0099 |
1.04 (0.99 – 1.106) p= 0.129 |
1.04 (0.99 – 1.105) p= 0.1349 |
1.04 (0.99 – 1.105) p= 0.1415 |
| Additional covariates | |||||
| ††Race (AA vs. W) | 0.66 (0.49 – 0.89) p= 0.006 |
0.72 (0.53 – 0.97) p= 0.0332 |
0.79 (0.58 – 1.08) p= 0.1343 |
0.77 (0.57 – 1.04) p= 0.0883 |
|
| ††Race (other vs. W) | 0.81 (0.4 – 1.66) p= 0.5712 |
0.87 (0.43 – 1.73) p= 0.6847 |
0.86 (0.44 – 1.67) p= 0.6513 |
0.85 (0.43 – 1.69) p= 0.646 |
|
| ††Site (UIowa vs UAB) | 1.09 (0.9 – 1.31) p= 0.3919 |
1.12 (0.93 – 1.36) p= 0.2283 |
1.16 (0.96 – 1.4) p= 0.126 |
1.19 (0.98 – 1.45) p= 0.0775 |
|
| ††Age (1 year) | 1.02 (1.01 – 1.03) p= 0.0016 |
1.02 (1 – 1.03) p= 0.0096 |
1.02 (1 – 1.03) p= 0.0086 |
||
| ††BMI (1 kg/m2) | 0.98 (0.97 – 0.99) p= 0.0056 |
0.97 (0.94 – 0.99) p= 0.0154 |
Model 1: adjusted for race and clinic site
Model 2: model 1 plus additionally adjusted for age
Model 3A: model 2 plus additionally adjusted for BMI at 60m (current)
Model 3B (sensitivity): model 2 plus additionally adjusted for BMI at 25 years-old (self-reported)
Abbreviations: AA, African American; BMI, body mass index; CPEI, center of pressure excursion index; CI, confidence interval; W, White
After additionally adjusting for age (p=0.0942), age and BMI at 60 month visit (p=0.1001), and age and BMI at age 25 years (p=0.1134), the mean CPEI remained lower, but to a lesser extent, and this was no longer statistically significant (Table 3). The positive linear trend in odds ratio for a one quintile decrease in CPEI (more pronation) with increasing parity level persisted after adjusting for age (p=0.129), age and BMI at the 60-month visit (p=0.1349), and age and BMI at age 25 years old (p=0.1415) but did not reach statistical significance (Table 4).
Discussion
The purpose of this study was to determine the relationship between dynamic foot arch function and parity status. We hypothesized that women with higher parity have greater functional foot pronation (lower CPEI) than those with lower parity. The results of this study show a statistically significant linear trend in odds ratios, indicating increased functional foot pronation (lower CPEI) with greater parity, that persisted after controlling for BMI and age but not to a degree that reached statistical significance. Nulliparous women were chosen as the referent group and men were excluded. No men would be included in the parity groups and participant sex may have been a confounder if men were included in the referent group. There are sex-based differences in the dynamic function of the foot and women have different hormonal milieu, life experiences, undergo menopause and differ from men in a variety of other physiological and biomechanical parameters. Since CPEI differed by clinic, we ran the models with clinic-specific CPEI quintiles and found the same trend toward increased functional foot pronation (lower CPEI) with greater parity. These findings suggest that higher parity may be a risk factor for increasing functional foot pronation, which could contribute to the higher incidence of lower limb musculoskeletal disorders in women compared to in men. [4]
Srikanth et al previously reported that women are at increased risk of knee OA[4] and planus feet have been associated with medial tibiofemoral cartilage damage.[15] Greater pronation of the foot causes increased rotation of the tibia,[26] which can be transferred through the knee to the femur and is a characteristic of women who have lost arch height with pregnancy.[20] Salsich et al found that subjects with patellofemoral pain syndrome with a medial collapse movement fault, had higher tibiofemoral rotation[27] and Kalichman et al found that patellar malalignment was associated with patellofemoral OA[28]. Thus, increased pronation of the foot during walking (lower CPEI) in those with higher parity potentially could lead to increased articular contact stress at the knee and more proximal joints, which could partially explain the increased risk for knee OA in women. There were no differences in radiographic knee OA between CPEI groups, but the MOST study recruited participants with preexisting knee OA or at elevated risk for knee OA, so this study was not designed to assess the relationship between lower CPEI and knee OA.
Hagedorn et al found no association between BMI and CPEI.[29] However, Tománková et al found that BMI was significantly positively correlated with increased relative pressure impulse in the midfoot and lateral part of the forefoot.[30] The results of our study indicate that higher BMI is associated with decreased functional foot pronation (higher CPEI), consistent with the findings of Tománková et al but discordant from Hagedorn et al. Our results may have differed from Hagedorn et al for several reasons. We treated BMI as a continuous variable whereas their study examined BMI categorically as greater than or less than 30 kg/m2 and our population had a higher BMI (mean BMI 30.6 kg/m2 vs 27.9 kg/m2).[29] The mean CPEI for women in their study was 12.29 compared to 18.5 in our study.[29] Also, we calculated CPEI based on an average of 5 trials per foot compared to 1 trial per foot which may have allowed us to decrease our measurement error and elicit more subtle associations. This association may reflect changes in foot function due to chronically higher BMI and increased loads. To better support the chronically increased load, the foot may adopt a more rectus position. However, pregnant women only experience a temporary increase in BMI, reducing the amount of time for this adaptation to occur. In the presence of estrogen and relaxin during pregnancy, arch height is lost in some women,[7] which could result in increased pronation (lower CPEI). If the increased BMI in pregnancy becomes permanent, then boney remodeling could occur, which may reduce the over-pronation seen in relation to pregnancy. Our dataset did not include changes in BMI in relation to parity. However, when the odds ratio for increased pronation was adjusted for BMI at the 60-month visit and age, the positive linear trend for increased parity being associated with a one quintile decrease in CPEI was no longer statistically significant. Multiple studies have examined the effect of childbearing on weight gain and increasing BMI, with results ranging from a postpartum weight gain of none to 2.0 kg per live birth [31,32,33]. If participant weight, and BMI, are in part dependent on parity, then increased BMI during pregnancy may be a mediator of the effect of pregnancy on CPEI therefore we would expect that adjusting for BMI would weaken the association between number of pregnancies and CPEI. We also found that BMI at 25 years old, an age closer to time of pregnancies, also made the effect between higher parity and increased risk for pronation non-significant which further supports the argument that increased BMI due to pregnancy is a mediator of the effect of pregnancy on CPEI. Also, the positive linear trend in odds ratio of a one quintile decrease in CPEI with increasing parity level was not significant when adjusted for age and BMI at age 25 years.
When the odds ratio for increased pronation was adjusted only for age, the positive linear trend for increased parity being associated with a one quintile decrease in CPEI was no longer statistically significant. The association between older age in women and lower CPEI has been previously reported. [29] There was a significant difference in mean age based on parity groups and CPEI quintiles therefore our findings prior to adjusting for age may have been confounded by age. Further studies evaluating changes in BMI and CPEI during pregnancy and over long-term follow-up as well as studies examining age matched controls or using years removed from first and last pregnancy as variables in addition to age would be necessary to further elucidate these associations.
Kim et al reported that brief foot exercises improved medial longitudinal arch height in patients with functional flat feet, and thereby improved the ability to distribute weight during gait[34]. They were not able to examine the use of foot exercises for prevention of flat feet because their study population already had the diagnosis. The use of short foot exercises or arch-supportive insoles [24] may be useful for women during the pre-natal period, given our findings of increased functional foot pronation with increasing parity. These preventative measures could potentially decrease the risk of knee OA and chronic musculoskeletal disorders later in life; however longitudinal studies are necessary to determine if such interventions may prevent incident decreases in medial longitudinal arch height that persist following pregnancy.[7]
Type of work was associated with parity groups with higher parity groups tending to work less in office work and more in manual labor jobs. However, type of work was not associated with CPEI quintiles therefore jobs requiring more standing and walking may not predispose women to increased foot pronation. This study wasn’t designed to address why women with more children tended to work more manual labor jobs but may be due to socioeconomic differences in the groups.
Study Limitations
Strengths of this study included a large sample size and the use of a validated measurement of dynamic foot function, CPEI. The study was limited by quantifying the number of children women had given birth to rather than the number of pregnancies they had carried. Therefore, we may have underestimated parity. Since it is most likely the hormonal and anthropometric changes that occur during pregnancy that lead to increased pronation of the foot,[35] more specific predictor measurements could improve estimates of association. BMI at age 25 was calculated based on self-reported weight and height data which may be less accurate than measured values. Participants were far removed from actual pregnancies as their mean age was 67.7 years therefore the differences in dynamic foot function that we found are more persistent changes rather than acute changes occurring during pregnancy or early post-partum.
Alternatively, our findings could be explained by the mechanistic differences in childcare strategies, such as the work involved with raising infants and toddlers rather than to parity itself. However, there is an association between carrying pregnancies and raising more children, so it is difficult to definitively identify the mechanism for this epidemiological association. Women included in this analysis were born between 1924–1953, a time when over 90% of women carried pregnancies, which is substantially higher than the percentage of women who carry pregnancies in more recent years.[36] Therefore, the pregnancy history and childcare patterns of our study participants may differ from women who are currently carrying pregnancies.
Future studies examining the correlation between different childcare activities and dynamic foot function with comparisons between men and women whose main physical involvement with number of children is childcare could be helpful as results in men would not be confounded by pregnancy.
Conclusions
Results from this study indicate a positive correlation between carrying more children and greater dynamic pronation of the feet that persisted after adjusting for age and BMI but not at a statistically significant level. These changes in dynamic foot function could potentially contribute to the increased risk for musculoskeletal disorders in women compared to men. If longitudinal studies confirm parity as a potential risk factor for knee osteoarthritis and other lower-limb musculoskeletal disorders, it would suggest that implementation of preventative measures, such as orthoses or exercises during pregnancy, could potentially reduce risk for these disorders later in life.
Supplementary Material
References
- 1.Bingefors K, Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain--a gender perspective. Eur J Pain 2004; 8(5):435–450. [DOI] [PubMed] [Google Scholar]
- 2.Wijnhoven HA, de Vet HC, Picavet HS. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain 2006; 22(8):717–724. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 2005; 13(9):769–781. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum 1997; 40(4):728–733. [DOI] [PubMed] [Google Scholar]
- 6.Nyska M, Sofer D, Porat A, Howard CB, Levi A, Meizner I. Planter foot pressures in pregnant women. Isr J Med Sci 1997; 33(2):139–146. [PubMed] [Google Scholar]
- 7.Segal NA, Boyer ER, Teran-Yengle P, Glass NA, Hillstrom HJ, Yack HJ. Pregnancy leads to lasting changes in foot structure. Am J Phys Med Rehabil 2013; 92(3):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponnapula P, Boberg JS. Lower extremity changes experienced during pregnancy. J Foot Ankle Surg 2010; 49(5):452–458. [DOI] [PubMed] [Google Scholar]
- 9.Wang SM, Dezinno P, Maranets I, Berman MR, Caldwell-Andrews AA, Kain ZN. Low back pain during pregnancy: prevalence, risk factors, and outcomes. Obstet Gynecol 2004; 104(1):65–70. [DOI] [PubMed] [Google Scholar]
- 10.Vullo VJ, Richardson JK, Hurvitz EA. Hip, knee, and foot pain during pregnancy and the postpartum period. J Fam Pract 1996; 43(1):63–68. [PubMed] [Google Scholar]
- 11.Block RA, Hess LA, Timpano EV, Serlo C. Physiologic changes in the foot during pregnancy. J Am Podiatr Med Assoc 1985; 75(6):297–299. [DOI] [PubMed] [Google Scholar]
- 12.Wise BL, Niu J, Zhang Y, et al. The association of parity with osteoarthritis and knee replacement in the multicenter osteoarthritis study. Osteoarthritis Cartilage 2013; 21(12):1849–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cote KP, Brunet ME, Gansneder BM, Shultz SJ. Effects of Pronated and Supinated Foot Postures on Static and Dynamic Postural Stability. J Athl Train 2005; 40(1):41–46. [PMC free article] [PubMed] [Google Scholar]
- 14.Erhart JC, Mundermann A, Mundermann L, Andriacchi TP. Predicting changes in knee adduction moment due to load-altering interventions from pressure distribution at the foot in healthy subjects. J Biomech 2008; 41(14):2989–2994. [DOI] [PubMed] [Google Scholar]
- 15.Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken) 2011; 63(7):937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.III DSW, McClay IS, Hamill J, Buchanan TS. Lower Extremity Kinematic and Kinetic Differences in Runners with High and Low Arches. Journal of Applied Biomechanics 2001; 17(2):153–163. [Google Scholar]
- 17.Williams Iii DS, McClay IS, Hamill J. Arch structure and injury patterns in runners. Clinical Biomechanics 2001; 16(4):341–347. [DOI] [PubMed] [Google Scholar]
- 18.Wetz HH, Hentschel J, Drerup B, Kiesel L, Osada N, Veltmann U. [Changes in shape and size of the foot during pregnancy]. Orthopade 2006; 35(11):1124, 1126–1130. [DOI] [PubMed] [Google Scholar]
- 19.Segal N, Eagles MS. The effects of pregnancy on shoe size and knee laxity. Am J Phys Med Rehabil 2010; 89:S41. [Google Scholar]
- 20.Rabe KG, Segal NA, Waheed S, Anderson DD. The Effect of Arch Drop on Tibial Rotation and Tibiofemoral Contact Stress in Postpartum Women. PM R 2018; 10(11):1137–1144. [DOI] [PubMed] [Google Scholar]
- 21.Menz HB, Dufour AB, Riskowski JL, Hillstrom HJ, Hannan MT. Association of planus foot posture and pronated foot function with foot pain: the Framingham foot study. Arthritis Care Res (Hoboken) 2013; 65(12):1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal NA, Nevitt MC, Gross KD, et al. The Multicenter Osteoarthritis Study: opportunities for rehabilitation research. PM R 2013; 5(8):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal NA, Felson DT, Torner JC, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil 2007; 88(8):988–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segal NA, Neuman LN, Hochstedler MC, Hillstrom HL. Static and dynamic effects of customized insoles on attenuating arch collapse with pregnancy: A randomized controlled trial. Foot (Edinb) 2018; 37:16–22. [DOI] [PubMed] [Google Scholar]
- 25.Hillstrom HJ, Song J, Kraszewski AP, et al. Foot type biomechanics part 1: structure and function of the asymptomatic foot. Gait & posture 2013; 37(3):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coplan JA. Rotational motion of the knee: a comparison of normal and pronating subjects. J Orthop Sports Phys Ther 1989; 10(9):366–369. [DOI] [PubMed] [Google Scholar]
- 27.Salsich GB, Perman WH. Tibiofemoral and Patellofemoral Mechanics are Altered at Small Knee Flexion Angles in People with Patellofemoral Pain. Journal of science and medicine in sport / Sports Medicine Australia 2013; 16(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalichman L, Zhu Y, Zhang Y, et al. The association between patella alignment and knee pain and function: an MRI study in persons with symptomatic knee osteoarthritis. Osteoarthritis Cartilage 2007; 15(11):1235–1240. [DOI] [PubMed] [Google Scholar]
- 29.Hagedorn TJ, Dufour AB, Golightly YM, et al. Factors affecting center of pressure in older adults: the Framingham Foot Study. J Foot Ankle Res 2013; 6(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tománková K, Přidalová M, Svoboda Z, Cuberek R. Evaluation of Plantar Pressure Distribution in Relationship to Body Mass Index in Czech Women During Walking. Journal of the American Podiatric Medical Association 2017; 107(3):208–214. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe WS, Sobal J, Olson CM, Frongillo EA Jr., Williamson DF. Parity-associated weight gain and its modification by sociodemographic and behavioral factors: a prospective analysis in US women. Int J Obes Relat Metab Disord 1997; 21(9):802–810. [DOI] [PubMed] [Google Scholar]
- 32.Brown JE, Kaye SA, Folsom AR. Parity-related weight change in women. Int J Obes Relat Metab Disord 1992; 16(9):627–631. [PubMed] [Google Scholar]
- 33.Rookus MA, Rokebrand P, Burema J, Deurenberg P. The effect of pregnancy on the body mass index 9 months postpartum in 49 women. Int J Obes 1987; 11(6):609–618. [PubMed] [Google Scholar]
- 34.Kim EK, Kim JS. The effects of short foot exercises and arch support insoles on improvement in the medial longitudinal arch and dynamic balance of flexible flatfoot patients. J Phys Ther Sci 2016; 28(11):3136–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marnach ML, Ramin KD, Ramsey PS, Song SW, Stensland JJ, An KN. Characterization of the relationship between joint laxity and maternal hormones in pregnancy. Obstet Gynecol 2003; 101(2):331–335. [DOI] [PubMed] [Google Scholar]
- 36.Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U.S. women continue to drop. NCHS Data Brief 2013; (136):1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.