Abstract
The mortality effects and risk-benefit profile of low dose rivaroxaban (2.5 mg twice daily) in patients with coronary heart disease are not completely understood. Five randomized controlled trials (26,110 patients) were selected using PubMed and Cochrane library till April 2019. The background antiplatelet therapy was aspirin in 3 trials, P2Y12 inhibitor in 1 trial, and in 1 trial 65% patients received aspirin and 35% were on dual antiplatelet therapy (DAPT). The outcomes of interest were cardiovascular mortality, all-cause mortality, myocardial infarction (MI), stroke and major bleeding events. Random effects hazard ratios (HR) with 95% confidence intervals (CI) were calculated. Low dose rivaroxaban did not reduce the risk of cardiovascular mortality (HR 0.90, 95% CI 0.73–1.11, P = 0.34) or all-cause mortality (HR 0.91, 95% CI 0.74–1.12, P = 0.38) compared with control. However, low dose rivaroxaban was associated with reduction in MI (HR 0.85, 95% CI 0.73–0.99, P = 0.04), and stroke (HR 0.59, 95%CI 0.48–0.73, P < 0.001) at the expense of major bleeding (HR 1.64, 95% CI 1.39–1.94, P < 0.001) compared with control. These effects did not vary according to acute coronary syndrome or stable coronary heart disease (P-interaction > 0.05). The use of low dose rivaroxaban in patients with coronary heart disease predominantly receiving antiplatelet monotherapy did not reduce cardiovascular or all-cause mortality. The benefits of preventing MI and stroke were balanced by increased risk of major bleeding.
Keywords: Rivaroxaban, Mortality, Meta-analysis
Introduction
Despite the use of guideline directed optimal medical therapy, 12% of patients with stable coronary heart disease and 18% of patients with recent acute coronary syndrome experience recurrent major adverse cardiovascular events [1]. The risk of recurrent cardiovascular events may be related to persistent elevation of thrombin beyond the index event [2, 3] which leads to progression of cardiovascular disease by inducing inflammation, endothelial dysfunction and thrombosis [4]. In patients with coronary heart disease, vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs) have been explored as secondary prevention strategies and have shown cardiovascular benefits at the cost of higher bleeding events [5–8]. The European Society of Cardiology (ESC) recommends low dose rivaroxaban 2.5 mg twice daily for patients with Non-ST elevation myocardial infarction (NSTEMI) in addition to dual antiplatelet therapy (DAPT) if they have high ischemic burden and low bleeding risk (class II b) [9]. Based on the results of the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial [10–12] the US Food and Drug Administration (FDA) has recently approved rivaroxaban 2.5 mg twice daily for the prevention of recurrent adverse cardiovascular events in patients with stable coronary heart disease and peripheral arterial disease (PAD). However, the risk–benefit profile of low dose rivaroxaban when used with single antiplatelet therapy (aspirin or P2Y12 inhibitor monotherapy) in patients with coronary heart disease are not completely understood. Herein, we performed meta-analysis to fill this knowledge gap.
Methods
This trial level meta-analysis was conducted in accordance with the Cochrane Collaboration guidelines and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) [13, 14].
Data sources and searches
Two independent researchers (M.U.K and S.V) conducted the literature search using PubMed and Cochrane library databases till April 2019. A search strategy included key search terms: “rivaroxaban”, “mortality”, “cardiovascular outcomes”, “myocardial infarction”, and “stroke” (Supplementary Table 1). The search filters were applied on “Human and clinical trials”. All citations were downloaded into Endnote X9.1 (Clarivate Analytics). Duplicate records were removed electronically and manually. Two authors (M.U.K and S.V) screened the remaining articles at the title and abstract level followed by full text screening based on the pre-determined selection criteria.
Study selection
The pre-defined inclusion criteria were: (1) randomized controlled trials of low dose rivaroxaban (2.5 mg twice daily), (2) trials must have participants with recent acute coronary syndrome or stable coronary heart disease, (3) trials must report mortality and cardiovascular outcomes of interest in adult patients (≥ 18 years), and (4) trials must have follow-up of ≥ 6 months to provide more reliable estimates. There were no restrictions on language and sample size.
Quality assessment and data extraction
Data was collected on a standard data collection form by two investigators (M.U.K and M.S.K), who adjudicated the data and resolved any disagreements related to data with the opinion of third investigator (S.U.K). Following information was abstracted: baseline characteristics of the trials and participants, background antiplatelet therapy, control groups, events and sample sizes, crude point estimates and follow-up duration. While the included trials reported different doses and combination of rivaroxaban at different follow-ups, we extracted data on low dose rivaroxaban from each trial at maximum follow-up duration. The data extraction was performed according to intention to treat principle. The risk of bias assessment was performed at trial level according to the Cochrane Risk of Bias Tool by M.U.K and M.S.K (Supplementary Table 2) [15].
Outcome measures
The outcomes of interest were cardiovascular mortality, all-cause mortality, myocardial infarction (MI), stroke, and major bleeding. The definition of major bleeding varied across the trials. Trials grouped under recent acute coronary syndrome category defined major bleeding as per TIMI (Thrombolysis in Myocardial Infarction) criteria, and those grouped under stable coronary heart disease category defined major bleeding as per ISTH (International Society of Thrombosis and Hemostasis) criteria. For all other endpoints, we used definitions as used in the original trials.
Estimates were pooled using generic invariance random effects model and calculated as hazard ratios (HR) with 95% confidence intervals (CI). Heterogeneity was evaluated using the Cochrane Q statistics with I2 > 75% being consistent with a high degree of heterogeneity [16]. Publication bias was not assessed due to small number of studies (< 10). Subgroup analysis were performed according to acute coronary syndrome and stable coronary heart disease. For all-analyses, statistical significance was set at 5%. Meta-analyses were performed using the Comprehensive Meta-Analysis Software 3.0 (Biostat, Englewood, NJ).
Results
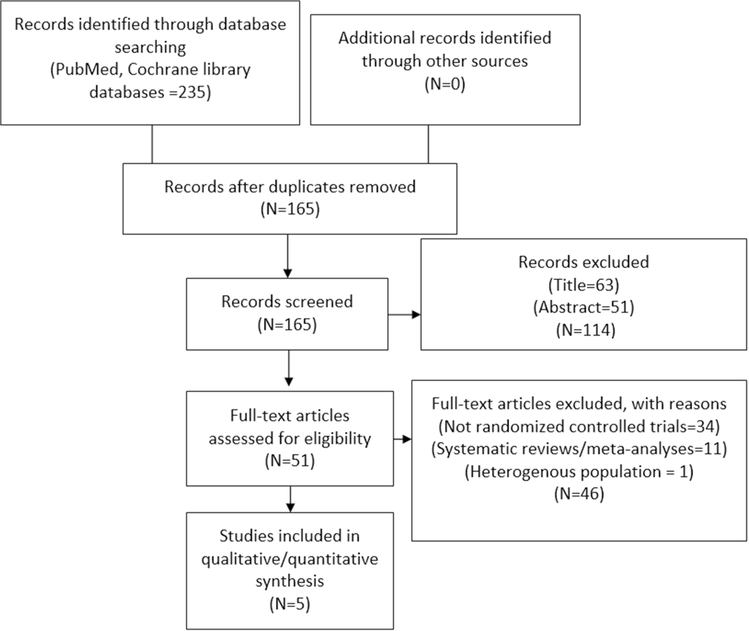
Of 235 records, 165 were screened after removal of duplicates, 114 articles were excluded at title and abstract level, 45 full text articles were removed based on a priori selection criteria, and one trial, PIONEER AF-PCI (Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose Adjusted Oral Vitamin K Antagonist [VKA] Treatment Strategy in Subjects with Atrial Fibrillation [AF] who Undergo Percutaneous Coronary Intervention [PCI]) was excluded because this study was conducted in unique population who had AF and PCI, and the comparator groups had higher doses of rivaroxaban 15 mg once daily plus P2Y12 inhibitor or VKA once daily plus DAPT [17].
Ultimately, five randomized controlled trials (27,814 patients) met inclusion criteria [11, 12, 18–22] (Fig. 1). Data for recent acute coronary syndrome patients were abstracted from three trials: GEMINI-ACS-1 (Clinically Significant Bleeding with Low-Dose Rivaroxaban vs Aspirin, in Addition to P2Y12 Inhibition, in Acute Coronary Syndrome) [18] which compared low dose rivaroxaban 2.5 mg twice daily with aspirin receiving background P2Y12 inhibitor. Data from ATLAS ACS 2-TIMI 51 (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome-Thrombolysis in MI 51) and ATLAS ACS- TIMI 46 (Rivaroxaban in Combination With Aspirin Alone or With Aspirin and a Thienopyridine in Patients With Acute Coronary Syndrome) were abstracted from report by Gibson et al., who pooled patients with acute coronary syndrome from both trials receiving aspirin monotherapy [20–22]. Two trials studied low dose rivaroxaban in patients with stable coronary heart disease: the COMPASS trial examined rivaroxaban 2.5 mg twice daily plus aspirin, rivaroxaban 5 mg twice and aspirin alone [11, 12], and the COMMANDER HF (A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, MI, or Stroke in Participants with Heart Failure [HF] and Coronary Artery Disease Following an Episode of Decompensated HF) [19] assessed rivaroxaban 2.5 mg twice daily vs placebo in patients with chronic systolic HF, stable coronary heart disease and sinus rhythm receiving aspirin monotherapy or DAPT. Table 1 reports the characteristics of the included trials. Table-2 reports the Key outcomes of primary and secondary endpoints of included trials.
Fig. 1.
Study selection process. Study flow chart according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)
Table 1.
Baseline characteristics of the trials and participants
| Study (year) | Age (years) | Females | Active drug (dose) | Control (dose) | Participants | Background antiplatelet therapy | Primary composite outcome | Bleeding criteria | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| ATLAS ACS-TIMI 46 (2009) [20, 21] | 60.3 | 294 (69%) | Rivaroxaban (2.5 or 5 mg BID) | Placebo | 427 | Aspirin (low dose) | Cardiovascular death, MI, or stroke (ischemic, hemorrhagic, or of uncertain cause | TIMI | 6 |
| ATLAS ACS2-TIMI 51 (2012) [19, 20] | 61.6 | 573 (54%) | Rivaroxaban (2.5 or 5 mg BID) | Placebo | 1050 | Aspirin (low dose) | Cardiovascular death, MI, or stroke (ischemic, hemorrhagic, or of uncertain cause | TIMI | 13 |
| GEMINI ACS-1 (2017) [17] | 62.0 | 762 (25%) | Rivaroxaban (2.5 mg BID) | Aspirin (100 mg) | 3037 | P2Y12 inhibitor (clopidogrel or ticagrelor) | cardiovascular death, MI, stroke, or definite stent thrombosis | TIMI | 11 |
| COMPASS (2018) | 69 | 3382 (20%) | Rivaroxaban (2.5 mg BID) | Aspirin (100 mg) | 16,574 | Aspirin (100 mg) | Cardiovascular death, stroke, or MI | ISTH | 23 |
| COMMANDER HF (2018) [18] | 66.4 | 1150 (23%) | Rivaroxaban (2.5 mg BID) | Placebo | 5022 | Aspirin/DAPT | Death from any cause, MI, or stroke | ISTH | 21 |
ATLAS ACS- TIMI 46, Rivaroxaban in Combination With Aspirin Alone or With Aspirin and a Thienopyridine in Patients With Acute Coronary Syndromes; ATLAS ACS2-TIMI 51, Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome-Thrombolysis in Myocardial Infarction 5; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; COMMANDER HF, A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure; DAPT, Dual Antiplatelet Therapy, GEMINI ACS-1, Clinically Significant Bleeding with Low-Dose Rivaroxaban versus Aspirin, in Addition to P2Y12 Inhibition, in Acute Coronary Syndromes; ISTH, International Society of Thrombosis and Hemostasis; MI, Myocardial Infarction; TIMI, Thrombolysis in Myocardial Infarction
Table 2.
Key outcomes of primary and secondary endpoints of included trials
| Primary compositea | All-cause mortalityb | Myocardial infarctionb | Strokeb | Major bleedingc | |
|---|---|---|---|---|---|
| ATLAS ACS-TIMI 46 (2009) [20, 21] and ATLAS ACS2-TIMI 51 (2012) [19, 20] | |||||
| Active (%) | 62 (11.4) | 25 (5.6) | 33 (7.3) | 12 (1.9) | 8 (1.5) |
| Control (%) | 65 (16.3) | 16 (3.2) | 44 (11.7) | 9 (2.6) | 2 (0.3) |
| HR [95% CI] | 0.69 [0.45–1.07] | 1.25 (0.61–2.59) | 0.59 [0.34–1.03] | 0.42 [0.11–1.59] | 1.01 [0.14–7.23] |
| P value | 0.09 | 0.99 | 0.06 | 0.20 | 0.99 |
| GEMINI ACS-1 (2017) [17] | |||||
| Active (%) | 76 (5) | 22 (1) | 56 (4) | 7 (< 1) | 10 (1) |
| Control (%) | 72 (5) | 23 (1.5) | 49 (3) | 12 (1) | 8 (1) |
| HR [95% CI] | 1.06 [0.77–1.46] | 0.95 [0.53–1.71] | 1.15 [0.78–1.68] | 0.58 [0.23–1.48] | 1.25 [0.49–3.17] |
| P value | 0.73 | 0.87 | 0.48 | 0.25 | 0.63 |
| Compass (2018) | |||||
| Active (%) | 347 (4) | 262 (3) | 195 (2) | 74 (1) | 263 (3) |
| Control (%) | 460 (6) | 339 (4) | 98 (3.9) | 130 (2) | 158 (2) |
| HR [95% CI] | 0.74 [0.65–0.86] | 0.77 [0.65–0.90] | 0.86 [0.70–1.05] | 0.56 [0.42–0.75] | 1.66 [1.37–2.03] |
| P value | < 0.01 | 0.001 | 0.15 | < 0.01 | < 0.01 |
| Commander HF (2018) [18] | |||||
| Active (%) | 626 (25) | 546 (21.8) | 98 (3.9) | 51 (2) | 82 (3.3) |
| Control (%) | 658 (26.2) | 556 (22.1) | 118 (4.7) | 76 (3) | 50 (2) |
| HR [95% CI] | 0.94 [0.84–1.05] | 0.98 [0.87–1.10] | 0.83 [0.63–1.08] | 0.66 [0.47–0.95] | 1.68 [1.18–2.39] |
| P-interaction | 0.27 | – | – | – | < 0.01 |
ATLAS ACS- TIMI 46, Rivaroxaban in Combination With Aspirin Alone or With Aspirin and a Thienopyridine in Patients With Acute Coronary Syndromes; ATLAS ACS2-TIMI 51, Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome-Thrombolysis in Myocardial Infarction 5; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; COMMANDER HF, A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure; GEMINI ACS-1, Clinically Significant Bleeding with Low-Dose Rivaroxaban versus Aspirin, in Addition to P2Y12 Inhibition, in Acute Coronary Syndromes; HR hazard ratio
Primary efficacy outcome
Secondary efficacy outcome
Primary safety outcome
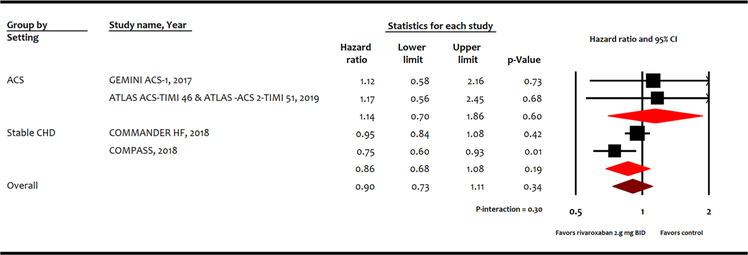
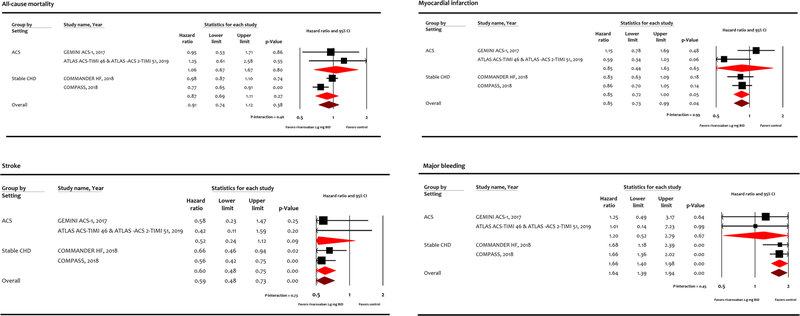
Low dose rivaroxaban did not reduce the risk of cardiovascular mortality (HR 0.90, 95% CI 0.73–1.11, P = 0.34; Fig. 2) or all-cause mortality (HR 0.91, 95% CI 0.74–1.12, P = 0.38; Fig. 3) compared with control. However, low dose rivaroxaban was associated with reduction in MI (HR 0.85, 95% CI 0.73–0.99, P = 0.04), and stroke (HR 0.59, 95%CI 0.48–0.73, P < 0.001) at the expense of major bleeding events (HR 1.64, 95% CI 1.39–1.94, P < 0.001) compared with control. While these benefits appear to be driven by results in stable coronary heart disease patients, subgroup interaction between acute coronary syndrome or stable coronary heart disease was not statistically significant (P-interaction > 0.05).
Fig. 2.
Meta-analysis for cardiovascular mortality. ATLAS ACS-TIMI 46, Rivaroxaban in Combination With Aspirin Alone or With Aspirin and a Thienopyridine in Patients With Acute Coronary Syndromes; ATLAS ACS2-TIMI 51, Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome-Thrombolysis in Myocardial Infarction 5; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; COMMANDER HF, A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure; GEMINI ACS-1, Clinically Significant Bleeding with Low-Dose Rivaroxaban versus Aspirin, in Addition to P2Y12 Inhibition, in Acute Coronary Syndromes
Fig. 3.
Meta-Analysis for secondary endpoints. ATLAS ACS- TIMI 46, Rivaroxaban in Combination With Aspirin Alone or With Aspirin and a Thienopyridine in Patients With Acute Coronary Syndromes; ATLAS ACS2-TIMI 51, Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome-Thrombolysis in Myocardial Infarction 5; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; COMMANDER HF, A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure; GEMINI ACS-1, Clinically Significant Bleeding with Low- Dose Rivaroxaban versus Aspirin, in Addition to P2Y12 Inhibition, in Acute Coronary Syndromes
Discussion
These analyses showed that use of low dose rivaroxaban with predominantly single antiplatelet therapy was not associated with reducing cardiovascular or all-cause mortality in patients with coronary heart disease. Low dose rivaroxaban reduced MI and stroke at the expense of higher bleeding rates. The lack of mortality benefit shown by low dose rivaroxaban could be because the low event rates and limited follow up of trials, which makes it difficult for any therapy to demonstrate a significant survival benefit [23, 24]; or the low dose rivaroxaban 2.5 twice daily may simply does not confer the desired therapeutic benefits and higher doses should be tried in future trials. It is also possible that the combined beneficial effects of antithrombotic and antiplatelet therapies may have plateaued, and addition of further similar therapies will merely increase the bleeding risk without reducing ischemic events. It is also noteworthy that 75% of the included trials used single antiplatelet therapy, with three trials using aspirin monotherapy [11, 12, 20–22], 1 trial using P2Y12 inhibitor [18], and in 1 trial ~ 35% patients were on DAPT [19]. Moreover, variation in PCI techniques including vascular access, complexity of coronary atherosclerosis, types and sizes of stents and procedural anticoagulation may have an impact on cardiovascular outcomes and could have confounded the results. Finally, the inherent risks of concurrent comorbidities could have influenced the mortality rates. For instance, the event rates for primary outcome in COMPASS were low (4.1% vs 5.4%) and majority of events were atherothrombotic (cardiovascular death, MI and stroke) vs higher event rates in COMMANDER-HF (25% vs 26.2%) where majority of deaths were caused by progression of HF rather than atherothrombotic events.
In this analysis the effects of low dose rivaroxaban appeared to be stronger in patients with stable coronary heart disease than recent acute coronary syndrome. The most likely explanations are that patients with recent acute coronary syndrome were assigned low dose rivaroxaban with single antiplatelet therapy, while only the COMMANDER-HF utilized 35% DAPT which can potentially bias the effect in favor of low dose rivaroxaban. In a meta-analysis of seven trials encompassing 31,574 patients with recent acute coronary syndrome, addition of a DOAC to single antiplatelet agent neither increased the risk of clinically significant bleeding nor reduced the major adverse cardiovascular events (MACE). Conversely, there was 14% risk reduction in MACE but more than twice the risk of bleeding with DOAC plus DAPT [8]. Second, the acute coronary syndrome data were somewhat influenced by phase II trials and only ATLAS ACS 2-TIMI 51 was phase III clinical trial [20]. The European Medicinal Agency approved the use of low dose rivaroxaban with DAPT in NSTEMI based on the findings of ATLAS ACS-2 TIMI 51 trial [9, 20]. However, this trial had issues related to incomplete follow-up, uncounted mortality and informative censoring, leading to decline in the drug approval by the FDA for acute coronary syndrome in 2012 [8]. Finally, since acute coronary syndrome is a higher risk condition compared with stable coronary heart disease [1], use of low dose rivaroxaban with single antiplatelet therapy might not provide enough therapeutic protection against recurrent MACE.
We compared our results with other meta-analyses. Khan and colleagues showed reduction in MACE by use of rivaroxaban at the expense of higher bleeding risk [8]. This meta-analysis did not investigate important cardiovascular endpoints such as mortality, MI or stroke and the analyses were not adjusted for different doses of DOACs [8]. More recently, Gibson and colleagues reported pooled data from the ATLAS ACS-2 TIMI-51 and COMMANDER-HF trial and showed all-cause mortality (HR 0.71, 95% CI 0.61–0.83) reduction with low dose rivaroxaban compared with placebo. There were higher rates of major bleeding with rivaroxaban vs. placebo (2.7 vs. 1.5 events per 100 patient years; P < 0.01) [25]. Since baseline risk varies substantially between both groups, we performed separate analyses to examine effects of low dose rivaroxaban in these distinct patient populations.
This meta-analysis has certain limitations. The baseline characteristics of the participants, concurrent medical therapy and follow up varied considerably among trials. The patient population had comorbidities with different inherent risks of mortality and cardiovascular outcomes. Due to limited available data, effects of low dose rivaroxaban with background DAPT could not be examined. These findings might not be universally applicable to all candidates because some trials recruited patients with low bleeding risk, concurrent use of proton pump inhibitors was limited, and presentation of acute coronary syndrome was variable. A participant level analysis will be ideal in exploring the such differences.
In summary, the use of low dose rivaroxaban with antiplatelet monotherapy did not reduce cardiovascular or all-cause mortality in patients with recent acute coronary syndrome or stable coronary heart disease. The benefits of MI and stroke reduction were achieved at the cost of major bleeding risk. Antiplatelet therapy is the standard approach for the secondary prevention of cardiovascular disease [26, 27]. Additional use of oral anticoagulant on top of antiplatelet monotherapy calls for a trade-off between higher bleeding risk and prevention of recurrent MACE [8]. Therefore, use of low dose rivaroxaban should be carefully prescribed only to subjects having ischemic risk exceeding their bleeding propensity.
Supplementary Material
Highlights.
The US Food and Drug Administration (FDA) has recently approved rivaroxaban 2.5 mg twice daily for the prevention of recurrent adverse cardiovascular events in patients with stable coronary heart disease.
There is still paucity of data related to effects of low dose rivaroxaban on mortality in patients with recent acute coronary syndrome and stable coronary heart disease.
This meta-analysis shows that low dose rivaroxaban with antiplatelet monotherapy did not reduce cardiovascular or all-cause mortality in patients with recent acute coronary syndrome or stable coronary heart disease.
The benefits of MI and stroke reduction were achieved at the cost of major bleeding risk.
Acknowledgments
Funding The authors have not received any funding for this project.
Footnotes
Conflict of interest No conflict of interest for authors Muhammad Zia Khan; Safi U. Khan; Zain Ul Abideen Asad; Shahul Valavoor; Muhammad Usman Khan, Muhammad Shahzeb Khan; Troy Krupica; and Mohamad Alkhouli. Dr. Kaluski is a speaker and consultant for Bristol-Myers Squibb, Pfizer, Janssen, and Daiichi-Saknyo.
Compliance with ethical standards
Ethical approval This article does not contain any studies with human participants performed by any of the authors. This article does not contain any studies with animals performed by any of the authors.
Informed consent None needed.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11239-020-02114-7) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D’Agostino R, Liau CS, Mas JL, Rother J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG, Investigators RR (2010) Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 304(12):1350–1357 [DOI] [PubMed] [Google Scholar]
- 2.Merlini PA, Bauer KA, Oltrona L, Ardissino D, Cattaneo M, Belli C, Mannucci PM, Rosenberg RD (1994) Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation 90(1):61–68 [DOI] [PubMed] [Google Scholar]
- 3.Ueda Y, Ogasawara N, Matsuo K, Hirotani S, Kashiwase K, Hirata A, Nishio M, Nemoto T, Wada M, Masumura Y, Kashiyama T, Konishi S, Nakanishi H, Kobayashi Y, Akazawa Y, Kodama K (2010) Acute coronary syndrome: insight from angioscopy. Circ J 74(3):411–417 [DOI] [PubMed] [Google Scholar]
- 4.Borissoff JI, Spronk HM, Heeneman S, ten Cate H (2009) Is thrombin a key player in the ‘coagulation-atherogenesis’ maze? Cardiovasc Res 82(3):392–403 [DOI] [PubMed] [Google Scholar]
- 5.van Es RF, Jonker JJ, Verheugt FW, Deckers JW, Grobbee DE, Antithrombotics in the Secondary Prevention of Events in Coronary Thrombosis-2 Research G (2002) Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet 360(9327):109–113 [DOI] [PubMed] [Google Scholar]
- 6.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H (2002) Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 347(13):969–974 [DOI] [PubMed] [Google Scholar]
- 7.Anand SS, Yusuf S (1999) Oral anticoagulant therapy in patients with coronary artery disease: a meta-analysis. JAMA 282(21):2058–2067 [DOI] [PubMed] [Google Scholar]
- 8.Khan SU, Arshad A, Riaz IB, Talluri S, Nasir F, Kaluski E (2018) Meta-analysis of the safety and efficacy of the oral anticoagulant agents (apixaban, rivaroxaban, dabigatran) in patients with acute coronary syndrome. Am J Cardiol 121(3):301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 37(3):267–315 [DOI] [PubMed] [Google Scholar]
- 10.News V Rivaroxaban gets FDA approval for treatment in CAD or PAD. https://vascularnews.com/rivaroxaban-fda-approval-cad-pad/
- 11.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Stork S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S (2017) Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 377(14):1319–1330 [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, Metsarinne K, O’Donnell M, Dans AL, Ha JW, Parkhomenko AN, Avezum AA, Lonn E, Lisheng L, Torp-Pedersen C, Widimsky P, Maggioni AP, Felix C, Keltai K, Hori M, Yusoff K, Guzik TJ, Bhatt DL, Branch KRH, Cook Bruns N, Berkowitz SD, Anand SS, Varigos JD, Fox KAA, Yusuf S, COMPASS investigators (2018) Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 391(10117):205–218 [DOI] [PubMed] [Google Scholar]
- 13.van Tulder M, Furlan A, Bombardier C, Bouter L (2003) Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine 28(12):1290–1299 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gptzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA (2016) Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med 375(25):2423–2434 [DOI] [PubMed] [Google Scholar]
- 18.Ohman EM, Roe MT, Steg PG, James SK, Povsic TJ, White J, Rockhold F, Plotnikov A, Mundl H, Strony J, Sun X, Husted S, Tendera M, Montalescot G, Bahit MC, Ardissino D, Bueno H, Claeys MJ, Nicolau JC, Cornel JH, Goto S, Kiss RG, Guray U, Park DW, Bode C, Welsh RC, Gibson CM (2017) Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEM-INI-ACS-1): a double-blind, multicentre, randomised trial. Lancet 389(10081):1799–1808 [DOI] [PubMed] [Google Scholar]
- 19.Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, van Veldhuisen DJ, Greenberg B (2018) Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med 379(14):1332–1342 [DOI] [PubMed] [Google Scholar]
- 20.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM (2012) Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 366(1):9–19 [DOI] [PubMed] [Google Scholar]
- 21.Gibson William J, Gibson CM, Yee Megan K, Korjian S, Daaboul Y, Plotnikov Alexei N, Burton P, Braunwald E (2019) Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post-acute coronary syndrome patients: a pooled analysis study of ATLAS ACS-TIMI 46 and ATLAS ACS 2-TIMI 51. J Am Heart Assoc 8(5):e009451 [Google Scholar]
- 22.Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, Hricak V, Barnathan ES, Bordes P, Witkowski A, Markov V, Oppenheimer L, Gibson CM (2009) Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet 374(9683):29–38 [DOI] [PubMed] [Google Scholar]
- 23.Betensky RA (2015) Measures of follow-up in time-to-event studies: Why provide them and what should they be? Clin Trials 12(4):403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SU, Rahman H, Talluri S, Kaluski E (2018) The clinical benefits and mortality reduction associated with catheter ablation in subjects with atrial fibrillation: a systematic review and meta-analysis. JACC Clin Electrophysiol 4(5):626–635 [DOI] [PubMed] [Google Scholar]
- 25.Gibson CM (2019) Rivaroxaban 2.5 mg BID combined with dual antiplatelet therapy for the prevention of death/MI/stroke: a patient level data meta-analysis of ATLAS-ACS-2 TIMI-51 and COMMANDER-HF. CRT, Washington, DC [Google Scholar]
- 26.Khan SU, Riaz IB, Rahman H, Lone AN, Raza M, Khan MS, Riaz A, Kaluski E (2019) Meta-analysis of duration of dual antiplatelet therapy in patients with acute coronary syndrome after percutaneous coronary intervention. Eur J Prev Cardiol 26(4):429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lettino M, Leonardi S, De Maria E, Halvorsen S (2017) Antiplatelet and antithrombotic treatment for secondary prevention in ischaemic heart disease. Eur J Prev Cardiol 24:61–70 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.