Abstract
In March 2019, a scientific meeting was held at the UCLA Luskin Center to discuss approaches to expedite the translation of neurobiological insights to advances in the treatment of alcohol use disorder (AUD). A guiding theme that emerged was that while translational research in AUD is clearly a challenge, it is also a field ripe with opportunities. Herein, we seek to summarize and disseminate the recommendations for the future of translational AUD research using four sections. First, we briefly review the current landscape of AUD treatment including the available evidence-based treatments and their uptake in clinical settings. Second, we discuss AUD treatment development efforts from a translational science viewpoint. We review current hurdles to treatment development as well as opportunities for mechanism-informed treatment. Third, we consider models of translational science and public health impact. Together, these critical insights serve as the bases for a series of recommendations and future directions. Towards the goal of improving clinical care and population health for AUD, scientists are tasked with bolstering the clinical applicability of their research findings so as to expedite the translation of knowledge into patient care.
Keywords: translational, AUD, research, treatment, treatment-gap, medications
Introduction
Alcohol use disorder (AUD) is highly prevalent and costly to individuals and society 1,2. It is a heterogeneous and progressive disorder, ranging from mild, time-limited, alcohol-related problems to severe, chronic and relapsing presentation often termed addiction. AUD is also a heritable disorder, with a host of promising advances in understanding genetic causation and genetic predictors of treatment response 3,4. While the past two decades have seen remarkable advances in our understanding of the neuroscience of addiction 5,6, the translation of that knowledge to addiction therapeutics has been slower to materialize 7,8. Efforts to improve this translation are timely in face of the BRAIN Initiative which seeks to catalyze neuroscience discoveries to improve the treatment of neuropsychiatric disorders 9. In order to discuss approaches to expediting the translation (and reverse translation) of neurobiological insights to advances in the treatment of AUD, the authors gathered for a one-day meeting at the University of California, Los Angeles (UCLA) with support from the UCLA Luskin Lecture for Thought Leadership. This meeting included an open session in the morning and a closed session in the afternoon among opinion leaders. The meeting consisted of brief individual presentations on perceived challenges and opportunities for translational research in AUD followed by an extended period of group discussions which sought to garner concurrence around ideas presented by the individual authors. By design, the authors included individuals trained in either or both basic and clinical science whose primary focus is on the study of AUD. Specifically, the authors were invited based on their expertise in the domains of animal models, genetics, human laboratory models, and clinical trials. This article seeks to disseminate the conclusions from the “UCLA-Luskin Translational Research in Alcoholism Meeting” and focuses on the key priorities and strategies that were identified to advance translational research for AUD.
While a host of concepts were presented and discussed, this review of the proceedings consists of the following key areas. First, we provide a summary of considerations regarding the current landscape of AUD identification and treatment. These include practical considerations such as treatment-seeking rates, patient and provider needs, and the overall accessibility of resources to consumers, particularly the accessibility of evidenced-based practices. Second, we consider the scope of translational science, and its specific implications for translational research in the field of AUD. Third, we discuss previously successful models, such as the transition of tobacco dependence and major depression to broad-scale clinical practice within the scope of primary care. While elements of AUD are quite unique, research in AUD can be informed by lessons from sister fields that have been successful in translation and bringing forward effective pharmacotherapies to improve public health. Finally, we conclude with recommendations and future directions for converting the neurobiological understanding of AUD into more precise and effective treatment and interventions.
Landscape of AUD Treatment in the United States
Millions of Americans suffer from AUD 10, while only a small subset of those seek treatment 11. The FDA-approved medications for AUD (i.e., disulfiram, acamprosate, oral and injectable naltrexone) are not widely used in medical practice 12, despite their documented benefits on healthcare utilization and costs 13. In fact, it is estimated that medications for AUD are prescribed to only 9% of patients who need AUD treatment 14. These finding are noted despite several rigorous meta-analyses showing effect sizes for naltrexone and acamprosate equivalent to that of selective serotonin reuptake inhibitors for depression 15,16. As with medications, behavioral treatments for AUD have received empirical support. Cognitive Behavioral Therapy (CBT) is arguably a first-line behavioral treatment for AUD, with consistent support for the treatment of substance use disorder more broadly 17. Despite its documented and durable effects, CBT for AUD has had limited diffusion in clinical practice in the US, except in the case of the Veterans Affairs system where CBT has been widely used 18. The accessibility of competent providers of CBT for AUD may pose an obstacle to consumers, an issue that has prompted the development of alternative CBT delivery methods, such as computer-based and mobile CBT platforms 19. Of note, CBT has been effectively deployed and integrated into routine AUD treatment care in other regions of the world, including Europe and Australia. In brief, for both medications and behavioral treatments, it appears that the evidence-based first-line treatments are seldom integrated into and utilized within standard clinical practice. While natural recovery or self-change (i.e., symptom recovery without treatment) has been documented across the lifespan 20, for most persons with moderate-to-severe AUD, behavioral and/or pharmacological interventions are needed to catalyze the beginning of the change process requisite in the reduction of excessive drinking and its consequences 21. Moreover, the large gap between AUD incidence, treatment-seeking rates, and treatment accessibility underscores that major changes in medical care and clinical practice are needed to lessen the morbidity associated with the disorder. Further, it is crucial to recognize that these gaps are largely being filled by non-scientifically supported treatments; it is estimated that the addiction residential “treatment” industry earns $35 billion annually. It is also critical to note that a “one size fits all” approach to treatment will not be effective for a disorder as heterogenous as AUD 22. Over the recent past, several efforts have been made to improve personalized medicine for AUD treatment to better tailor treatments to individuals with differing presentations of AUD. These studies have emphasized that patient characteristics, such as drinking phenotype or neural response to alcohol cues, can predict treatment response 23–26. Continued work in the realm of personalized medicine represents a goal for the field of AUD treatment.
An even broader discussion regarding the treatment of AUD has to do with the very definition of recovery itself. There is an active and ongoing discussion about what constitutes recovery from an alcohol use disorder 27,28. This debate extends to the clinical outcomes used to evaluate novel treatments, both pharmacological and psychosocial 29. Importantly, recent work indicates that harm reduction endpoints, such as reductions in the World Health Organization (WHO) drinking risk level reflect improvements in how patients feel and function 30–32, and represent a better match with the treatment goals of many patients. Currently, the FDA only accepts abstinence and no heavy drinking days as primary outcomes for phase 3 clinical trials; the inclusion of reduction in WHO drinking risk levels as an additional primary outcome may provide more efficiency in treatment development for AUD 29. Moreover, the current FDA accepted endpoints may not be appropriate for many early in their AUD trajectories, including adolescents and young adults. The resolution of such important scientific questions is likely to have large implications for the treatment of AUD. Insofar as translational research in AUD can inform the conceptualization of recovery, the field stands to benefit from an integrative perspective. To that end, biomarkers, defined as measurable and biologically-based phenotypes, that correlate with and predict clinically meaningful outcomes, represent a critical priority in the field of AUD 7. Notable medical examples from other fields include C-Reactive Protein (CRP) to assess the severity of treatment response in infectious and/or inflammatory diseases and pro b-type natriuretic peptide (proBNP) to assess the severity of heart failure.
In summary, consideration of the broader landscape of AUD treatment in the U.S. shaped the authors’ discussion about the priorities and strategies to expedite translational research in AUD. It was widely agreed upon that the current treatment landscape poses challenges and opportunities to advance neuroscience-informed treatments for AUD, many of which are discussed herein.
Treatment Development for AUD
Treatment development for AUD follows a stage model. For medications, this model spans from target discovery and development, to preclinical development, to efficacy studies of candidate pharmacotherapies, to formal regulatory approval, to post-market monitoring 33,34. Likewise, behavioral treatment development follows a prescribed format from preclinical concept validation through open-label feasibility studies to large scale trials 35. In the domain of medications development, there are several documented obstacles, from the uptake of preclinical findings to human testing (termed the “valley of death” in medications development 36 to challenges in patient recruitment, cost of clinical trials, and small effect sizes 33. Expediting the development of novel therapeutics and overcoming the “valley of death” has been the topic of considerable discussion 36,37. The recent interest by scientific funding agencies on the development of novel compounds for AUD treatment, as well as on repurposing of FDA-approved medications for the indication of AUD, brings a host of unique opportunities and challenges 38,39. Several issues related to optimizing medications development were discussed at the UCLA Luskin meeting and are summarized herein. Such issues include, neuroscience-based phenotypes and biomarkers of clinical utility, optimal methods for clinical trials design, the utilization of validated animal and human laboratory models for assessing pharmacotherapy effects, the selection of phenotypes for mechanism-based treatment, and the identification of compounds with novel targets. As efforts to develop novel medications for AUD have been challenging in the past two decades, the advantages of the proposed approach remain to be determined and evaluated by its outcomes.
The translation of preclinical findings to clinical samples is thought to be optimized through the use of human laboratory studies. In addition, the heterogeneity of patients with AUD suggests the need for comprehensive screening that is informed by neurobiological effects and mechanisms. As proposed by Koob, Lloyd, and Mason (2009), a “Rosetta Stone approach” entails using existing medications to validate and refine research paradigms across clinical and preclinical domains 40. Domains proposed include cue-reactivity, affective priming, stress-induced craving, alcohol self-administration, subjective and physiological responses to alcohol self-administration, and impulse control 41. These refined behavioral pharmacology models could be used to accelerate the screening and clinical testing of novel compounds and combinations of compounds in the spectrum of AUD neurobehavioral targets 40,41.
In the context of patient heterogeneity, however, it is plausible that medications may work through some, but not other, mechanisms. This suggests the need for a comprehensive screening that is informed by neurobiological effects and mechanisms. Consistent with an iterative model whereby preclinical and clinical findings converge to inform treatment development, recent preclinical studies have emphasized the clinical utility of compounds that affect animals mimicking the AUD phenotype 42. Other paradigm-shifting approaches in animal modeling of AUD include the comparison of alcohol seeking and drug-seeking versus another high-value rewards, such as social interaction or a sugar solution 43,44, which may provide more clinically-relevant insights into the underlying construct of alcohol seeking. Whether it be the feedback between preclinical and clinical assessments (i.e., Rosetta Stone approach to validating clinically meaningful paradigms), the focus on effects specific to the AUD condition in preclinical animal models (i.e., dependent versus non-dependent animals), or the refinement of preclinical models (i.e., via comparison to competing rewards), there was consensus in our discussion that substantial changes, and perhaps an entire paradigm-shift, may be required in order to expedite translational efforts in medication development. Likewise, medications may work for some patients and not others, thus personalized treatment and pharmacogenetics may be useful in identifying treatment responders. As the behavioral genetics of alcohol consumption and AUD progresses to account for more of its phenotypic expression 4, the opportunities for precision medicine and pharmacogenetics of AUD treatment also evolve 45,46.
Other avenues by which translational research can be expedited include methodological adjustments for clinical studies. For example, it has been documented that treatment-seekers with AUD differ from non-treatment seekers with AUD in a host of measures including patterns of alcohol use, AUD severity, age, impulsivity, and liver function 47–50, yet the majority of human laboratory models in medications development enroll non-treatment seeking individuals 37. Differences in sample characteristics and motivation for change may contribute to the lack of consilience between behavioral pharmacology and clinical trials and from preclinical to early efficacy human studies. A number of ethical issues arise in this arena including limitations around medications development and testing in younger samples, including those with AUD below the legal drinking age, who are also often non-treatment-seeking. A similar issue arises with the enrollment of individuals with AUD and comorbid liver disease, which poses additional ethical issues for inclusion in experimental medicine studies. However, this is a population for which abstinence is a crucial therapeutic goal, treatment for AUD is very much needed, and yet access to treatment has been limited, highlighting the need for multidisciplinary approaches, e.g. active team-driven treatments led by both addiction and hepatology specialists 51. Efforts to synergize team-science for alcoholic hepatitis are currently underway through the Alcohol Hepatitis Network project (www.alchepnet.org). While the ethical considerations for the administration of alcohol or alcohol cues have been reviewed elsewhere 52, it is notable that the standards for clinical studies in AUD differ markedly from other disciplines in which enrollment of treatment-seeking samples is the norm (e.g., oncology) 53,54. Notably, performing a challenge test is the norm in other biomedical fields, e.g. an oral glucose tolerance test for patients with suspected diabetes. Again, ethical considerations are key, nonetheless it is undoubtable that stigma still puts AUD, and addictions at large, far from other mental health and medical disorders. Experimental pharmacotherapy studies in treatment seeking populations, (e.g. studies where treatment-seekers receive alcohol), are feasible to carry-out under ethically acceptable conditions and do not place the patients at a disadvantage 55. Further, there is evidence that participation in alcohol administration studies results in reductions in alcohol use in study participants 56–58, indicating that participation may be beneficial for at least some participants. In the context of translational research in AUD, the use of clinical samples that represent the target population we wish to generalize is a key area of opportunity for enhancing translation.
Another important consideration in treatment development relates to clinical targets and the very definition of AUD. Without revisiting the long debate about what constitutes AUD, the study of mental disorders has moved toward a cross-diagnostic approach that focuses on specific domains that can help explain illness as a result of varying degrees of dysfunction in psychology/biological systems 59,60. AUD itself is heterogeneous, with every patient developing an AUD through an interaction of underlying neurobiological and environmental mechanisms 22. The field of addiction too has taken on the challenge of describing its phenomenology through several domains of functioning, such as negative emotionality, executive (dys)function, and incentive salience 61,62. Such efforts have recently received empirical support through the Addictions Neuroclinical Assessment (ANA), a heuristic framework for addictive disorders, designed to create deep-phenotyping profiles coupled with factor analytic methods 63. The neuroscience-based framework for the assessment and conceptualization of addiction, through the ANA, presents new opportunities whereby dysfunctions in these domains may serve as treatment targets. Recent efforts, such as the ANA development 61, addiction models involving competing active rewards 43,64, and human laboratory testing of neurobiological theories 65,66, provide a few emergent examples of translation and reverse translation in the field of AUD.
Another approach to improving our understanding of the heterogeneity of AUD has been suggested by an international Delphi Consensus study, in which experts in the field of addiction came to consensus on seven constructs that are primary to the understanding of addiction: reward valuation, expectancy, action selection, reward learning, habit, response selection/inhibition, and compulsivity 67. The suggested constructs constitute a range of valence (from positive to negative) and are implicated in different stages of addiction (from vulnerable to chronic use).
The dimensional approach offered by the ANA can be used to identify novel addiction biomarkers and to refine existing ones 68. To do so would entail filling the gaps between behavioral and biological phenotypes, an ongoing challenge in neuroscience and psychiatry. For instance, deep behavioral phenotyping derived from clinical, behavioral, and self-report measures, suggest that motor impulsivity, attentional impulsivity, and negative urgency load into the construct of executive dysfunction 63. What is known about the underlying neurobiology of these constructs? Can biomarkers be discerned from them? As one example, D1/D2 receptor availability is a putative biomarker associated with executive function domains in substance using populations 69,70 – might this finding be driven by association with one of these factors that make up the larger construct of executive dysfunction? A related question has to do with whether these phenotypes and targets are “druggable” (i.e. amendable as targets of pharmacological interventions). Which pharmacological agents can be used to selectively target such phenotypes? Perhaps neuromodulation and/or behavioral interventions may be more successful? Could precision medicine be achieved through behavioral and/or biomarkers derived from a dimensional approach? Mechanism-informed treatment represents a unique opportunity to translate neuroscientific findings into advancements in clinical care. These are some of the big questions facing translational scientists in AUD but also the field of neuropsychiatry more broadly 71. During the scientific meeting from which this report is based, there was ample recognition of the opportunities afforded by dimensional models of AUD. There was also consensus about the need to utilize a broader range of pharmacological interventions and the underutilized potential of resources drawn from the National Center for Advancing Translational Sciences (NCATS), including its pharmaceutical collection 72. Importantly, there was agreement around the need for reliable biomarkers that can facilitate translation and reverse translation of therapeutic discoveries 7,73,74. Facilitating biomarker development, refinement, and validation represents a critical research priority with potential to impact clinical care. Deep phenotyping efforts informed by dimensional models of AUD across multiple levels of analyses represent a unique opportunity to consolidate and advance discoveries in the field.
Models of Translational Research
The panel considered and discussed current models of translational research as a conduit to refining and implementing such approaches to AUD. As a starting point, the NIH has proposed a continuum of research on neurobiological variables in behavior change research, from the neurobiological substrate (level 1) all the way to a direct manipulation of the neurobiological variable to induce behavior change (level 5) 75,76. This approach is useful in outlining a clear progression from the identification of a neurobiological substrate to refining it and ultimately leveraging it to obtain a desired outcome, in this case, behavior change. Another important function of the model is to focus attention on mechanisms that represent malleable targets. As with our discussion of “druggable” or “modifiable” biomarkers (above), a mechanisms-focused experimental medicine approach holds great promise to inform intervention development pipelines. Consistent with this approach, the dimensional variables captured by the ANA model serve as candidate targets/mechanisms for experimental medicine manipulations leading to treatment development 68.
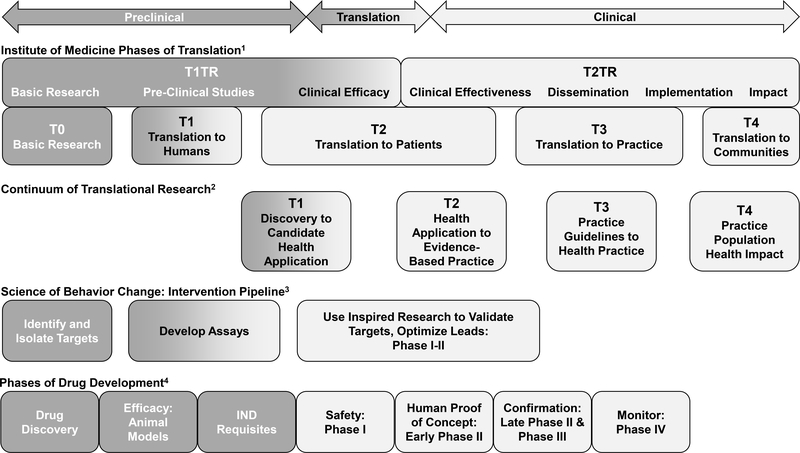
A related model to understanding translational research has been put forth by the Institute of Medicine which consists of classifying research from T0 to T4 77. Specifically, T0 consists of basic research, T1 represents a translation to humans, T2 addresses the translation to patients, T3 is a translation to practice, and T4 is a translation to communities. The model proposes a continuum that can engage both academicians and communities in knowledge exchange and partnership and has been applied to cancer epidemiology 78. A related model from Khoury and colleagues 79 describes phases of translation as being Discovery (T0), Characterization (T1), Evaluation (T2), Implementation and Health Services (T3), and Outcome Research (T4). The first half of the model (from T0 to T2) is termed Type 1 Translational Research (T1TR) and focuses on basic research to clinical efficacy. The second half (from T2 to T4) is termed Type 2 Translational Research (T2TR) and proceeds from clinical efficacy to dissemination, implementation, and impact. Relatedly, “Drivers” of translation are described as collaboration, knowledge-integration, technology, and multi-level analysis 80. These recommendations and models are informative insofar as they provide a vision for translating findings from discovery to population health impact, along a translational research continuum. And while most of the discussion at the UCLA Luskin meeting centered on expediting T0 to T2 translation (also described as T1TR), the authors clearly recognized gaps in dissemination, implementation and impact (T3 to T4), often recognized as a second “valley of death”, as critical to changing the landscape of clinical care for AUD. The models of translational research discussed above are summarized in Figure 1.
Figure 1.
Models of Translational Research
Four models of translational research reviewed in the manuscript are displayed to highlight their overlap and opportunities for translational and reverse translational research. The Institute of Medicine1 classifies research in two halves: Type 1 Translational Research (T1TR) which focuses on basic research to clinical efficacy, and Type 2 Translational Research (T2TR) emphasizes clinical efficacy, dissemination, implementation, and impact (Lam et al., 2013). The continuum of translational research2 includes four phases beginning with the identification of targets to candidate health application (T1) and ending with the evaluation of real-world health outcomes in the population (T4) (Khoury et al., 2007). The Science of Behavior Change proposed intervention pipeline3 highlights the identification of intervention targets, the development of appropriate assays, and the optimization of trial designs to measure target engagement (Nielsen et al., 2018). Finally, the Phases of Drug Development represents the pathway from drug discovery and pre-clinical animal research through translation to patient populations and safety monitoring of approved medications (Litten et al., 2012).
Phases of preclinical research are colored in dark gray, phases which overlap preclinical and clinical research, termed translation, are colored in a gradient from dark to light gray, and clinical research phases are colored in light gray.
The scientific panel at the meeting also discussed recent advances in health care as plausible models for translational research. Beyond informing the translational research process, this discussion focused on the “end result” of impacting health care, and mental disorders in particular. Two examples were discussed as exemplifying changes in the landscape of health care. First, as smoking cessation treatments were developed, stakeholders remained alert to the negative health consequences of cigarette smoking, and therefore, treatments had quick uptake by providers. Treatment guidelines for smoking cessation are firmly in place 81 and are regularly updated as new evidence on treatment efficacy from clinical research evolves 82. Furthermore, the transition from prescription to over-the-counter (OTC) use of nicotine replacement therapy (NRT) provides a unique example of balancing risks with public health benefits. Results of a literature review suggest that OTC NRT has been used safely and effectively with continued physician engagement, increasing access to an evidence-based treatment 83.
The treatment of depression follows a similar pattern of change over time with advances in pharmacological treatment becoming increasingly accessible to patients. There was a documented expansion in the treatment of depression with antidepressants in the U.S. from 1996 to 2005, jumping from 5.84% to 10.12% rate of antidepressant treatment 84, highlighting the acceptance of pharmacological treatment for depression within the United States. It is notable that primary care physicians often prescribe these medications for nicotine dependence and depression, further highlighting the successful integration of these treatments in the primary care setting, where individuals are much more likely to seek and receive treatment than within specialty care clinics where addiction is most routinely treated. While the examples of smoking cessation and antidepressant medication treatment are notable in health care, there are unique features to AUD that must be considered. Nevertheless, a mature field of study must move towards firm guidelines, dissemination, and accessibility of care with recurrent updates in guidelines and clinical practices that are informed by the science. For this to become a reality, addiction treatment must become a part of mainstream healthcare. The current landscape of addiction treatment is such that the majority of treatments are delivered outside of medical settings, unlike treatment of nicotine dependence and depression. The American Psychiatric Association offers clinical guidelines for pharmacotherapy for AUD 85; however a more expansive set of guidelines are required for treatment that include evidence-based psychosocial treatments. The discussion of these examples served to engage the authors in a discussion of a long-term vision for the field of AUD.
Considering the “big picture” of translational science while being mindful of the landscape of AUD treatment, there was a recognition that gaps in evidence-based clinical recommendations quickly get filled by market-driven services of unknown (or even questionable) efficacy. The authors shared anecdotes of ill-informed AUD treatments offered in the market place while access to evidence-based care was deemed harder to achieve. In order to fill the gaps in evidence-based clinical recommendations, several notable initiatives were discussed and opportunities for wider dissemination of these (and other) resources were considered. For example, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) has developed and launched the NIAAA Alcohol Treatment Navigator (https://alcoholtreatment.niaaa.nih.gov/) with the overarching goal of helping consumers find treatment options that are evidence-based. A related NIAAA online resource consists of “Rethinking Drinking” (https://www.rethinkingdrinking.niaaa.nih.gov/), a program designed to help individuals evaluate their drinking pattern, identify signs of a problem, and access tools to make changes in their drinking. Notably, elements from the Rethinking Drinking program have been evaluated as a computer-delivered behavioral platform for AUD clinical trials 86. These publicly available resources generated from NIAAA and informed by its federally-funded research portfolio were largely discussed as examples of needed efforts to address knowledge and treatment gaps in AUD (i.e., defined as the low rates of treatment seeking for the disorder). There was agreement among the authors for the need to widely disseminate these resources; however, it still remains unclear which agencies should be responsible for funding and supporting this effort. In summary, the larger discussion about models of translational research served to identify gaps and opportunities. A more detailed set of recommendations for translational research in AUD is provided next.
Recommendations for Translational Research in AUD
Owing to the issues and opportunities highlighted above, the authors identified a series of recommendations for advancing translational research in AUD (see summary in Table 1). While these recommendations are not exhaustive, they represent some of key ideas discussed during the scientific meeting and are meant to generate further discussion and broader consideration by alcohol and addiction researchers.
Table 1.
Summary of recommendations and associated objective
| Recommendation | Objective |
|---|---|
| 1. Refine language for basic and clinical phenotypes | Bridge key constructs in alcohol research to facilitate translational research. |
| 2. Biomarker Development | Develop translatable, scalable biomarkers for pharmacotherapy and psychotherapy AUD treatments. |
| 3. Conduct large scale longitudinal studies | Link neurobiological substrates to clinically relevant outcomes. |
| 4. Conduct generalizable clinical research | Develop and refine clinical methods that increase external validity for AUD treatment. |
| 5. Leverage resources | Increase awareness in resources available to improve medication development. |
| 6. Support translational and team-based training | Promote scientific exchange between scientists across the translational spectrum and encourage team-based science. |
| 7. Close the treatment gap | Use currently available resources to benefit individuals with AUD. |
| 8. Fill the evidence-based practice knowledge gap | Improve communication between scientists and the public regarding evidence-based prevention and treatment strategies. |
1. Refine language for basic and clinical phenotypes
Part of the obstacle to translational research in AUD has to do with the ability of translational teams to effectively communicate and share scientific findings. Collaboration and knowledge integration have been proposed as two of the “drivers” in translational science 80. To that end, the development of common language that is both precise and agreed upon by basic and clinical scientists, can facilitate translational research. For example, the construct of alcohol withdrawal is often described in terms of acute and protracted withdrawal. However, the behavioral and phenomenological definitions of alcohol withdrawal in preclinical and clinical samples are often disparate. Bridging key constructs in alcohol research with precision represents both a challenge and an outstanding opportunity to bolster translational efforts 87. Notably, the ANA and RDoC approaches are challenging the field to develop more precise and operationally defined constructs.
2. Biomarker development
At the level of Type 1 Translational Research (T1TR), the development, refinement, and application of biomarkers was a central theme of discussion. Biomarker development for AUD represents a high priority area with potential to accelerate the pace of therapeutic development. A recently proposed set of criteria for biomarkers illustrates the complexity of biomarker development as it describes robust psychometric and functional characteristics of translational biomarkers 71. The criteria also emphasize the need for the biomarker to be scalable so that it can be used in real-world clinical settings. Behavioral markers, including constructs such as cue reactivity, attention bias, and decision-making, are also needed to advance translation and should be viewed in the same light as traditional biomarkers. Of note, liver dysfunction, as assessed by widely available liver function tests (ALT, AST and GGT) or specialized laboratory analysis of carbohydrate-deficient transferrin, is an identified biomarker which is underutilized in clinical practice. For example, a variant in carbohydrate-deficient transferrin detection may lead to actionable early detection of liver disease in heavy drinkers 88. In cases of clinically significant liver dysfunction there is a need for behavioral medicine “swat” teams to intervene and provide treatment referrals. In essence, the need for AUD biomarkers and avenues for their development encapsulates a high priority area set forth by the authors.
3. Conduct large scale longitudinal studies to link neurobiological substrates to clinical outcomes
Consistent with the overarching goal of developing and refining translational biomarkers for AUD, large scale longitudinal studies are needed to demonstrate several of the key properties of biomarkers. Specifically, as proposed by Heilig, Sommer, and Spanagel (2016), a biomarker strategy may select biological substrates because of their mechanistic relationship to alcohol effects, strong psychometric properties, accessibility in animals and humans and importantly, responsivity to intervention 7. This approach requires both controlled experimental medicine studies and longitudinal clinical studies to fully capture these biomarker characteristics. Importantly, as science progresses from controlled experimental studies to large scale clinical and population studies, there will invariably be a change in the “signal-to-noise ratio” of the treatment effect on a given biomarker. In order to impact health care, the question is whether the signal is favorable enough to warrant dissemination with the end goal of improving population health. Longitudinal studies are key in following a promising “signal” into the noisy world of clinical practice. Of note, the ongoing open-access data resources obtained within the 12+ year longitudinal Adolescent Brain Cognitive Development study (ABCD) (www.abcdstudy.org), will offer deep phenotypic data (neuroimaging, genetic, behavioral, clinical measures) on school-age children throughout the United States; providing an invaluable resource to investigate current and future questions regarding genetic, neural, and clinical predictors of substance use disorders, particularly within adolescents and young adults 89.
4. Conduct clinical research in generalizable settings and formats
In the vein of detecting a clinically meaningful treatment “signal” that is scalable, there was considerable discussion about efforts to increase the external validity of clinical studies. In AUD treatment development, this involves the wider inclusion of treatment-seeking individuals in clinical trials 47,48, and the inclusion of patients with AUD of a severity comparable to that seen in the treatment-seeking clinical population. It is noteworthy that translation to patients (T2) in the IOM classification, represents a bridge between type 1 and type 2 translational research categories. Therefore, translation to patients (i.e., T2 research) may be bolstered by enhancing external and ecological validity of currently-used laboratory paradigm methods. In other words, laboratory paradigms that more effectively capture clinically-meaningful phenomenology will facilitate the screening of novel medications by providing a more robust (i.e., reliable) test of early efficacy. Finally, clinical research that is embedded in clinical care settings e.g., 90 has potential to generate findings that are more generalizable and scalable.
5. Leverage resources
The group discussion agreed on the need to utilize a broader range of pharmacological interventions. It was also noted that resources from the National Center for Advancing Translational Sciences (NCATS), including its pharmaceutical collection, were largely under-utilized. While academia-industry collaborations are complex, the NCATS resources are accessible to qualified scientists providing excellent opportunities for therapeutic development for AUD. Moreover, the increasing availability of datasets, including alcohol-informative datasets, provides unique opportunities to expedite discovery by leveraging existing data. Recent changes in the data-sharing policy for NIAAA are meant to enhance accessibility to the scientific community (https://grants.nih.gov/grants/guide/notice-files/NOT-AA-19-020.html).
6. Support scientific training that is translational and team-based
The authors discussed the training pipeline as an opportunity to nurture translational and team-based science. Promoting ongoing scientific exchange from scientists across the translational science continuum is critical to the long-term success of translational research in AUD. The practical aspects of team science were discussed, such as accepting the notion that one cannot be an expert in all levels of the translational research process and as a result, there are no “silly questions” as much as there are opportunities to more fully understand each other’s perspectives. The academic environment often incentivizes individualism and independence, therefore a shift towards team-based science is in many respects a culture shift. Nonetheless, such changes are within reach and they start with close attention to the training pipeline.
7. Close the treatment gap
Discussions about the treatment of AUD cannot ignore the abysmally low rates of treatment seeking for this disorder across all age groups 91,92. Closing the treatment gap represents a critical direction for the field and will likely engage efforts at multiple levels, from prevention, dissemination, implementation and overall public education about AUD and its treatment. Certainly, the development of novel and more effective treatments can enhance these efforts and instill even greater hopes of long-term recovery. Nevertheless, the individuals and families currently affected by AUD cannot afford to wait until new discoveries are available. We must instead use the resources currently available to us through scientific advances to benefit individuals who need them now.
8. Fill the knowledge gap with evidence-based practices
Related to the call for efforts to close the treatment gap in AUD, there was ample recognition of a knowledge gap whereby evidence-based prevention and treatment strategies are slow to reach the public. Along with NIAAA-led initiatives to educate the public about identifying alcohol problems (e.g., Rethinking Drinking) and finding high quality treatments (e.g., Treatment Navigator), scientists are tasked with disseminating the science of AUD. Furthermore, there is a substantial clinician knowledge gap 93, and perhaps more so for physician-scientists 94. Significant efforts have been made by both ASAM and AAAP, and progress in this domain is reflected in the Addiction Psychiatry specialization of the American Board of Psychiatry and Neurology, the American College of Academic Addiction Medicine (ACAAM) fellowship program (https://www.acaam.org), and the successful certification of addiction medicine as a sub-specialty of Preventive Medicine. Moreover, NIAAA is currently developing a core resource for clinicians to provide basic knowledge about alcohol to all clinicians, medical doctors, physician assistants, nurses, clinical psychologists, and pharmacists. Taking opportunities to share evidence-based resources and to provide the public with insights from years and years of alcohol research has become increasingly a priority for investigators in the field. Putting scientific knowledge to the greater good is the ultimate goal of science and as such, efforts to facilitate the uptake of scientific findings in AUD are incumbent on scientists as stakeholders. This realization is much more salient in today’s society in which the flow of information is more fluid and faster, and often involves social media outlets.
In conclusion, while translational research in AUD is clearly a challenge, it is also a field ripe with opportunities. Considering the current landscape of AUD treatment and with the end goal of improving clinical care for AUD, the field of alcohol research is tasked with moving our research questions further along the translational continuum. Although it will be challenging, the priorities articulated here – refining AUD phenotypes and biomarkers, embedding these concepts in observational and clinical longitudinal studies, leveraging existing resources, and supporting the career pipeline of translational scientists – provide a scientific blueprint to ultimately provide substantive improvements in the treatment of AUD.
Acknowledgments
Funding: This meeting was supported by a competitive award from the UCLA Meyer and Renee Luskin Lecture for Thought Leadership in the College of Letters and Sciences (PI: Lara Ray). This work was also supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to LAR (K24AA025704).
Footnotes
None of the authors have conflicts of interest to disclose.
REFERENCES
- 1.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–2233. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019;394(10200):781–792. [DOI] [PubMed] [Google Scholar]
- 3.Kranzler HR, Edenberg HJ. Pharmacogenetics of alcohol and alcohol dependence treatment. Curr Pharm Des. 2010;16(19):2141–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kranzler HR, Zhou H, Kember RL, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10(1):1499–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekhtiari H, Nasseri P, Yavari F, Mokri A, Monterosso J. Neuroscience of drug craving for addiction medicine: From circuits to therapies. Prog Brain Res. 2016;223:115–141. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiat. 2016;3(8):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilig M, Sommer WH, Spanagel R. The Need for Treatment Responsive Translational Biomarkers in Alcoholism Research. Current topics in behavioral neurosciences. 2016;28:151–171. [DOI] [PubMed] [Google Scholar]
- 8.Egli M Advancing Pharmacotherapy Development from Preclinical Animal Studies. Handb Exp Pharmacol. 2018. [DOI] [PubMed] [Google Scholar]
- 9.Jorgenson LA, Newsome WT, Anderson DJ, et al. The BRAIN Initiative: developing technology to catalyse neuroscience discovery. Philos Trans R Soc Lond B Biol Sci. 2015;370(1668). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco C, Iza M, Rodriguez-Fernandez JM, Baca-Garcia E, Wang S, Olfson M. Probability and predictors of treatment-seeking for substance use disorders in the U.S. Drug Alcohol Depend. 2015;149:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark TL, Kassed CA, Vandivort-Warren R, Levit KR, Kranzler HR. Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug Alcohol Depend. 2009;99(1–3):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mark TL, Montejano LB, Kranzler HR, Chalk M, Gastfriend DR. Comparison of healthcare utilization among patients treated with alcoholism medications. The American journal of managed care. 2010;16(12):879–888. [PMC free article] [PubMed] [Google Scholar]
- 14.Kranzler HR, Soyka M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. Jama. 2018;320(8):815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. Jama. 2014;311(18):1889–1900. [DOI] [PubMed] [Google Scholar]
- 17.Magill M, Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. J Stud Alcohol Drugs. 2009;70(4):516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll KM, Kiluk BD. Cognitive behavioral interventions for alcohol and drug use disorders: Through the stage model and back again. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2017;31(8):847–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiluk BD, Nich C, Buck MB, et al. Randomized Clinical Trial of Computerized and Clinician-Delivered CBT in Comparison With Standard Outpatient Treatment for Substance Use Disorders: Primary Within-Treatment and Follow-Up Outcomes. Am J Psychiatry. 2018;175(9):853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MR, Boness CL, McDowell YE, Verges A, Steinley DL, Sher KJ. Desistance and Severity of Alcohol Use Disorder: A Lifespan-Developmental Investigation. Clinical psychological science : a journal of the Association for Psychological Science. 2018;6(1):90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray LA, Bujarski S, Grodin E, et al. State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. Am J Drug Alcohol Ab. 2019;45(2):124–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39(4):579–584. [DOI] [PubMed] [Google Scholar]
- 23.Witkiewitz K, Roos CR, Mann K, Kranzler HR. Advancing Precision Medicine for Alcohol Use Disorder: Replication and Extension of Reward Drinking as a Predictor of Naltrexone Response. Alcoholism: Clinical and Experimental Research. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roos CR, Mann K, Witkiewitz K. Reward and relief dimensions of temptation to drink: construct validity and role in predicting differential benefit from acamprosate and naltrexone. Addiction biology. 2017;22(6):1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann K, Vollstädt-Klein S, Reinhard I, et al. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcoholism: Clinical and Experimental Research. 2014;38(11):2754–2762. [DOI] [PubMed] [Google Scholar]
- 26.Schacht JP, Randall PK, Latham PK, et al. Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status. Neuropsychopharmacology. 2017;42(13):2640–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witkiewitz K, Wilson AD, Pearson MR, et al. Profiles of recovery from alcohol use disorder at three years following treatment: can the definition of recovery be extended to include high functioning heavy drinkers? Addiction. 2019;114(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray LA, Lim AC, Shoptaw S. What defines a clinically meaningful outcome in the treatment of substance use disorders: ‘Getting your life back’. Addiction. 2019;114(1):18–20. [DOI] [PubMed] [Google Scholar]
- 29.Falk DE, O’Malley SS, Witkiewitz K, et al. Evaluation of Drinking Risk Levels as Outcomes in Alcohol Pharmacotherapy Trials: A Secondary Analysis of 3 Randomized Clinical Trials. JAMA Psychiatry. 2019;76(4):374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witkiewitz K, Kranzler HR, Hallgren KA, et al. Drinking Risk Level Reductions Associated with Improvements in Physical Health and Quality of Life Among Individuals with Alcohol Use Disorder. Alcoholism, clinical and experimental research. 2018;42(12):2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knox J, Wall M, Witkiewitz K, et al. Reduction in Nonabstinent WHO Drinking Risk Levels and Change in Risk for Liver Disease and Positive AUDIT-C Scores: Prospective 3-Year Follow-Up Results in the U.S. General Population. Alcoholism, clinical and experimental research. 2018;42(11):2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasin DS, Wall M, Witkiewitz K, et al. Change in non-abstinent WHO drinking risk levels and alcohol dependence: a 3 year follow-up study in the US general population. The lancet Psychiatry. 2017;4(6):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litten RZ, Wilford BB, Falk DE, Ryan ML, Fertig JB. Potential medications for the treatment of alcohol use disorder: An evaluation of clinical efficacy and safety. Subst Abus. 2016;37(2):286–298. [DOI] [PubMed] [Google Scholar]
- 34.Litten RZ, Egli M, Heilig M, et al. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17(3):513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162(8):1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray LA, Bujarski S, Roche DJO, Magill M. Overcoming the “Valley of Death” in Medications Development for Alcohol Use Disorder. Alcohol Clin Exp Res. 2018;42(9):1612–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yardley MM, Ray LA. Medications development for the treatment of alcohol use disorder: insights into the predictive value of animal and human laboratory models. Addict Biol. 2017;22(3):581–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mason BJ. Emerging pharmacotherapies for alcohol use disorder. Neuropharmacology. 2017;122:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litten RZ, Falk D, Ryan M, Fertig J. Research opportunities for medications to treat alcohol dependence: addressing stakeholders’ needs. Alcohol Clin Exp Res. 2014;38(1):27–32. [DOI] [PubMed] [Google Scholar]
- 40.Koob GF, Kenneth Lloyd G, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov. 2009;8(6):500–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason BJ, Higley AE. A translational approach to novel medication development for protracted abstinence. Current topics in behavioral neurosciences. 2013;13:647–670. [DOI] [PubMed] [Google Scholar]
- 42.Bell RL, Lopez MF, Cui C, et al. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 2015;20(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Augier E, Barbier E, Dulman RS, et al. A molecular mechanism for choosing alcohol over an alternative reward. Science. 2018;360(6395):1321–1326. [DOI] [PubMed] [Google Scholar]
- 44.Venniro M, Zhang M, Caprioli D, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21(11):1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kranzler HR, Smith RV, Schnoll R, Moustafa A, Greenstreet-Akman E. Precision medicine and pharmacogenetics: what does oncology have that addiction medicine does not? Addiction (Abingdon, England). 2017;112(12):2086–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartwell EE, Kranzler HR. Pharmacogenetics of alcohol use disorder treatments: an update. Expert Opin Drug Metab Toxicol. 2019;15(7):553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse. 2017;43(6):703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohn MC, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L. Differences Between Treatment-Seeking and Nontreatment-Seeking Alcohol-Dependent Research Participants: An Exploratory Analysis. Alcohol Clin Exp Res. 2017;41(2):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee MR, Sankar V, Hammer A, et al. Using Machine Learning to Classify Individuals With Alcohol Use Disorder Based on Treatment Seeking Status. EClinicalMedicine. 2019;12:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fein G, Landman B. Treated and treatment-naive alcoholics come from different populations. Alcohol. 2005;36(2):19–26. [PMC free article] [PubMed] [Google Scholar]
- 51.Leggio L, Lee MR. Treatment of Alcohol Use Disorder in Patients with Alcoholic Liver Disease. The American journal of medicine. 2017;130(2):124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enoch MA, Johnson K, George DT, et al. Ethical considerations for administering alcohol or alcohol cues to treatment-seeking alcoholics in a research setting: can the benefits to society outweigh the risks to the individual? A commentary in the context of the National Advisory Council on Alcohol Abuse and Alcoholism -- Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation (2005). Alcohol Clin Exp Res. 2009;33(9):1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(8):2109–2117. [DOI] [PubMed] [Google Scholar]
- 54.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283(20):2701–2711. [DOI] [PubMed] [Google Scholar]
- 55.Spagnolo PA, Ramchandani VA, Schwandt ML, et al. Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcohol-dependent patients. Alcohol Clin Exp Res. 2014;38(12):3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pratt WM, Davidson D. Does participation in an alcohol administration study increase risk for excessive drinking? Alcohol. 2005;37(3):135–141. [DOI] [PubMed] [Google Scholar]
- 57.Drobes DJ, Anton RF. Drinking in alcoholics following an alcohol challenge research protocol. J Stud Alcohol. 2000;61(2):220–224. [DOI] [PubMed] [Google Scholar]
- 58.Farokhnia M, Schwandt ML, Lee MR, et al. Biobehavioral effects of baclofen in anxious alcohol-dependent individuals: a randomized, double-blind, placebo-controlled, laboratory study. Transl Psychiat. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167(7):748–751. [DOI] [PubMed] [Google Scholar]
- 60.Clark LA, Cuthbert B, Lewis-Fernandez R, Narrow WE, Reed GM. Three Approaches to Understanding and Classifying Mental Disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychological science in the public interest : a journal of the American Psychological Society. 2017;18(2):72–145. [DOI] [PubMed] [Google Scholar]
- 61.Kwako LE, Momenan R, Grodin EN, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A reverse translational approach. Neuropharmacology. 2017;122:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biological psychiatry. 2016;80(3):179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwako LE, Schwandt ML, Ramchandani VA, et al. Neurofunctional Domains Derived From Deep Behavioral Phenotyping in Alcohol Use Disorder. Am J Psychiatry. 2019;176(9):744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Venniro M, Zhang M, Caprioli D, et al. Volitional social interaction prevents drug addiction in rat models. Nature neuroscience. 2018;21(11):1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D. A Prospective 5-Year Re-examination of Alcohol Response in Heavy Drinkers Progressing in Alcohol Use Disorder. Biol Psychiatry. 2016;79(6):489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bujarski S, Ray LA. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: an examination of Koob’s allostatic model in humans. Drug Alcohol Depend. 2014;140:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yücel M, Oldenhof E, Ahmed SH, et al. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. 2019;114(6):1095–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwako LE, Bickel WK, Goldman D. Addiction Biomarkers: Dimensional Approaches to Understanding Addiction. Trends in molecular medicine. 2018;24(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robertson CL, Ishibashi K, Mandelkern MA, et al. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci. 2015;35(15):5990–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.London ED. Impulsivity, Stimulant Abuse, and Dopamine Receptor Signaling. Advances in pharmacology (San Diego, Calif). 2016;76:67–84. [DOI] [PubMed] [Google Scholar]
- 71.Light GA, Swerdlow NR. Selection criteria for neurophysiologic biomarkers to accelerate the pace of CNS therapeutic development. Neuropsychopharmacology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colvis CM, Austin CP. Innovation in therapeutics development at the NCATS. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(1):230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heilig M, Leggio L. What the alcohol doctor ordered from the neuroscientist: Theragnostic biomarkers for personalized treatments. Prog Brain Res. 2016;224:401–418. [DOI] [PubMed] [Google Scholar]
- 74.Volkow ND, Koob G, Baler R. Biomarkers in substance use disorders. ACS chemical neuroscience. 2015;6(4):522–525. [DOI] [PubMed] [Google Scholar]
- 75.Nielsen L, Riddle M, King JW, et al. The NIH Science of Behavior Change Program: Transforming the science through a focus on mechanisms of change. Behav Res Ther. 2018;101:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sumner JA, Beauchaine TP, Nielsen L. A mechanism-focused approach to the science of behavior change: An introduction to the special issue. Behav Res Ther. 2018;101:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liverman CT, Schultz AM, Terry SF, Leshner AI. The CTSA program at NIH: Opportunities for advancing clinical and translational research. National Academies Press; 2013. [PubMed] [Google Scholar]
- 78.Lam TK, Chang CQ, Rogers SD, Khoury MJ, Schully SD. Evolution of the “drivers” of translational cancer epidemiology: analysis of funded grants and the literature. Am J Epidemiol. 2015;181(7):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in medicine : official journal of the American College of Medical Genetics. 2007;9(10):665–674. [DOI] [PubMed] [Google Scholar]
- 80.Lam TK, Spitz M, Schully SD, Khoury MJ. “Drivers” of translational cancer epidemiology in the 21st century: needs and opportunities. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(2):181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 82.Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. Journal of the American College of Cardiology. 2018;72(25):3332–3365. [DOI] [PubMed] [Google Scholar]
- 83.Shiffman S, Sweeney CT. Ten years after the Rx-to-OTC switch of nicotine replacement therapy: what have we learned about the benefits and risks of non-prescription availability? Health Policy. 2008;86(1):17–26. [DOI] [PubMed] [Google Scholar]
- 84.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. [DOI] [PubMed] [Google Scholar]
- 85.Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. American Journal of Psychiatry. 2017;175(1):86–90. [DOI] [PubMed] [Google Scholar]
- 86.Devine EG, Ryan ML, Falk DE, Fertig JB, Litten RZ. An exploratory evaluation of Take Control: A novel computer-delivered behavioral platform for placebo-controlled pharmacotherapy trials for alcohol use disorder. Contemporary clinical trials. 2016;50:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Becker HC. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology. 2017;122:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stewart SH, Reuben A, Anton RF. Relationship of Abnormal Chromatographic Pattern for Carbohydrate-Deficient Transferrin with Severe Liver Disease. Alcohol and Alcoholism. 2016;52(1):24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Volkow ND, Koob GF, Croyle RT, et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental cognitive neuroscience. 2018;32:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Costello MJ, Ropp C, Sousa S, Woo W, Vedelago H, Rush B. The development and implementation of an outcome monitoring system for addiction treatment. Canadian Journal of Addiction. 2016;7(3):15–24. [Google Scholar]
- 91.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of general psychiatry. 2007;64(7):830–842. [DOI] [PubMed] [Google Scholar]
- 93.Gottesman MM. The role of the NIH in nurturing clinician-scientists. The New England journal of medicine. 2013;368(24):2249–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Milewicz DM, Lorenz RG, Dermody TS, Brass LF, National Association of MDPPEC. Rescuing the physician-scientist workforce: the time for action is now. The Journal of clinical investigation. 2015;125(10):3742–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]