Abstract
The prevalence and disease burden of atopic dermatitis (AD) is substantial. AD causes significant impairment in quality of life. It is also associated with mental disorders as well as cardiovascular diseases. Many factors including race, environment, skin barrier dysfunction, immune regulatory abnormalities, and microbiome have been reported to affect the pathophysiology of AD. A variety of cell types including Th2, Th17, Th22, and type 2 innate lymphoid cells contribute to AD. Cytokines from these immune cells cause abnormal epidermal differentiation and skin barrier dysfunction. Moreover, microbial dysbiosis and deficiency of antimicrobial peptides result in Staphylococcus aureus infection. Recently, new drugs have been successfully launched to target polarized immune pathways that lead to moderate-to-severe AD.
Keywords: Atopic dermatitis, Epidermal barrier, Microbiome, Biologics
Introduction
Atopic dermatitis (AD) is characterized by immune dysregulation, epidermal barrier defects, and microbial dysbiosis [1**, 2*, 3, 4**]. The prevalence of AD has increased in both children and adults [5*,6]. AD is associated with mental problems, cardiovascular diseases, autoimmunity, and recurrent infections [6–8]. Additionally, AD causes marked impairment to quality of life for patients and their families [6,7]. Recently, researchers have advanced our understanding of the pathophysiology of AD. Many factors including race, onset of AD, environmental factors, altered epidermal lipid profiles, immune dysregulation, and microbial dysbiosis play critical roles in AD and modify the course of this common skin disease [3,9,10]. New strategies, including the correction of microbial dysbiosis and new biologics and small molecules, are being used to control disease activity in patients with moderate-to-severe AD.
Epidemiology
Pediatric AD is significantly associated with mental disorders including impairment of emotional behavior, peer relationships, and attention [11]. AD is also associated with depression and suicidal ideations in children and adults [6,7]. A systematic review and meta-analysis of cohort studies has demonstrated that AD is associated with increased risk of cardiovascular complications such as myocardial infarction, stroke, ischemic stroke, angina, and heart failure along with anaphylaxis to egg and milk [8,12]. More attention should therefore be paid to the comorbidities of AD.
Recently, there has been great interest on AD subgroups. Using data from 1,437 mother-child pairs of a prospective prebirth cohort in eastern Massachusetts, it was found that early childhood AD was more likely to persist in non-Hispanic blacks (aOR, 6.26; 95% CI, 2.32–16.88) and Hispanics (aOR, 6.42; 95% CI, 1.93–21.41) compared to non-Hispanic whites [9]. In another prospective cohort study of 4,898 women and their children in 20 large cities, female gender (aOR, 1.56; 95% CI, 1.02–2.37) and black race (aOR, 1.80; 95% CI, 1.07–3.01) were associated with persistent AD through ages 5, 9, and 15 years [13]. These findings indicate that AD persistence is higher in specific subgroups and would be important to consider in our understanding of AD phenotypes and endotypes.
Clinically, it is known that AD develops primarily in children and can resolve over time. However, there have been controversies about persistence of AD beyond childhood. In a systematic review and meta-analysis of population-based longitudinal studies of AD patients ranging from age 3 months to 26 years, the percentage decrease in prevalence after age 12 was only 1% [14*]. These investigators suggested that this is due to a combination of factors including disease persistence, decreased remission, and later-onset disease. In fact, the estimated prevalence of AD among US adults with a mean age of 51.25 years is 7.3%, indicating that a substantial number of people in this age group have AD [6]. In particular, 26.1% of AD in adults is an adult-onset disease which has a distinct clinical phenotype according to Lee et al [5*]. Increasing awareness of adult AD is needed for timely diagnosis and proper management.
Environmental factors
Our understanding of environmental factors that trigger AD is critical because they are modifiable. A recent cross-sectional study among South African toddlers aged 12–36 months reported that consumption of fermented milk products is strongly associated with reduced AD in an urban cohort. However, this effect was not found in the rural population, suggesting a role for urbanization and loss of gut microbial diversity in AD development [10]. A systematic review and meta-analysis revealed that there was a significant association between AD and fall birth (OR, 1.16; 95% CI, 1.06–1.28; P=0.0018) and winter birth (OR, 1.15; 95% CI, 1.04–1.27; P=0.0076) in the northern hemisphere when compared to spring birth. Although the exact mechanism remains unclear, the authors proposed reduced ultraviolet radiation (UVR) exposure, increased immune activity, and increased air pollution in specific seasons contribute to a higher prevalence of AD [15].
Air pollution is a growing concern with urbanization and industrialization. Rutter et al collected the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3 survey data of 546,348 children from 53 countries and assessed the individual- and school-level effects of environmental factors to exclude reverse causation. They found that current exposure to heavy traffic is significantly associated with eczema symptoms in 13–14-year-olds during the previous 12 months [16]. In a consortium of six birth cohorts from Europe and Canada, genetic risk scores from glutathione S-transferase P1, tumor necrosis factor, Toll-like receptor (TLR)-2, and TLR-4 single-nucleotide polymorphisms were associated with AD up to the age of 2 years [17]. Furthermore, oxidative stress and inflammation were associated with the prevalence of childhood AD and they may modify susceptibility to air pollution-induced AD [17]. However, traffic-related air pollution (TRAP) did not show an association with AD in the general population [17]. In elderly participants over age 50, exposure to TRAP was significantly associated with increased odds of incident eczema and this effect was more pronounced with nonatopic eczema [18]. Therefore, exposure to air pollution may involve the development or aggravation of AD through oxidative stress and inflammation, especially in susceptible children and elderly. However, further study is needed to clarify the association between TRAP and AD.
The concept of exposome has been introduced to improve our understanding of the pathophysiology of AD. Exposome is the sum of environmental influences throughout an individual’s lifetime. Exposomal domains are stratified into external nonspecific (e.g. climate, migration, urbanization), external specific (e.g. humidity, UVR, diet, pollution, allergens, water hardness), and internal (e.g. microbiome) exposures [19*]. Future research will focus on exposome characterization and whether its modification alters the disease course of AD [19*].
Epidermal barrier
Epidermal barrier dysfunction contributes to the development of AD and food allergy [2*]. Type 2 cytokines inhibit the expression of structural cornified barrier proteins such as filaggrin (FLG), loricrin, involucrin, antimicrobial peptides (AMPs), and tight junctions [2*,3]. IL-17-producing T helper (Th) 17 and Th22 subsets can also be highly upregulated in certain AD subtypes and are associated with abnormal keratinocyte differentiation and epidermal barrier dysfunction [3]. FLG is a key epidermal barrier protein required for formation of the stratum corneum (SC) and is influenced by environmental factors such as climate, pollution, and microbiome [20,21]. A recent study demonstrated that the epidermal mammalian target of rapamycin complex 2 activity orchestrated epidermal barrier formation through FLG processing and de novo epidermal lipogenesis [22*]. Additionally, siRNA-knockdown of EMSY, also characterized as a transcriptional regulator, increased the number of layers within the SC and the expression of corneodesmosomes and FLG [23]. These studies have focused on novel mechanistic insights into epidermal barrier formation, which may be used as a future therapeutic target to improve epidermal barrier conditions [22*,23]. Recently, human keratinocyte proline-rich proteins in the upper part of the granular layer have also been reported to play a crucial role in skin barrier function and percutaneous immune responses [24].
The corneocyte lipid envelope becomes a hydrophobic impermeable epidermal layer that prevents water loss and antigen penetration [3]. AD skin is associated with a reduced free fatty acid chain length and an increased proportion of ceramides with an unsaturated acyl-chain and sphingosine subclass. This correlates with an aberrant lipid organization and decreased skin barrier function [25]. It is also known that the ratio between ω-esterified fatty acid sphingosine (EOS) ceramides and nonhydroxy fatty acid sphingosine (NS) ceramides is higher in AD patients than those in normal controls [1**]. Interestingly, a recent study demonstrated that Th2 cytokines downregulated fatty acid elongases 3 and 6 in human keratinocytes in a signal transducer and activator of transcription (STAT)-6-dependent way, indicating the role of Th2 immune activation in epidermal lipid metabolism in AD patients [26**]. Staphylococcus aureus (S. aureus)-colonized AD patients reveal lower levels of long chain ceramides than those without S. aureus colonization. This suggests that S. aureus colonization affects lipid composition and enhances skin barrier impairment [27].
Immune dysregulation
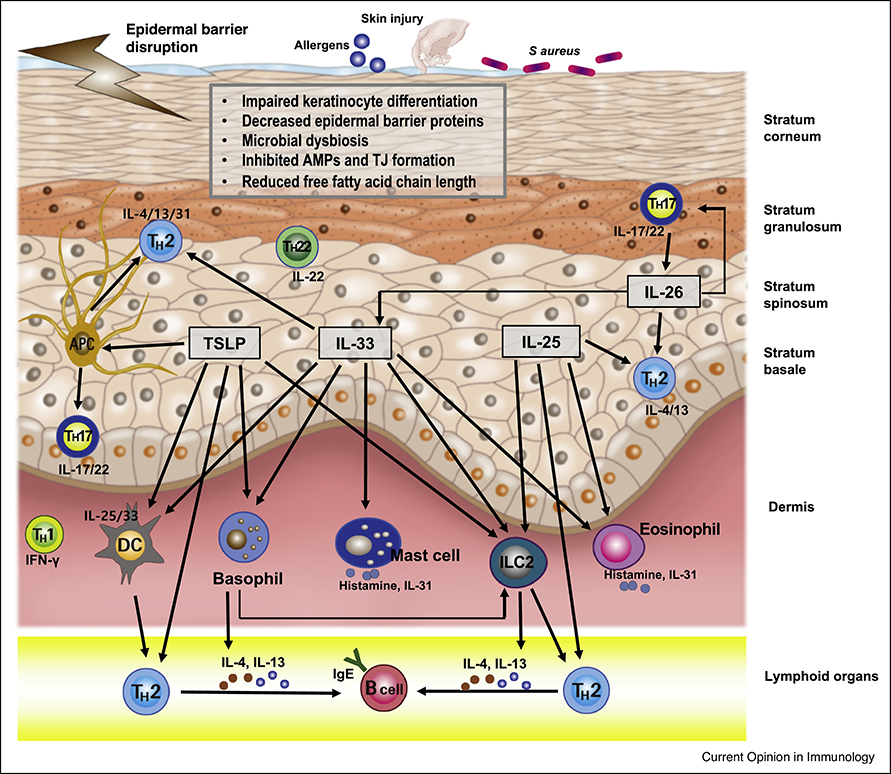
It is well known that Th2, Th17, Th22, and type 2 innate lymphoid cells (ILC2) play central roles in AD pathobiology [2*,3,20] (Figure 1). Researchers have recently found additional cytokines that are significant to the pathogenesis of AD. Kamijo et al have reported that IL-26, which is produced by Th17 cells, induces production of Th2 and Th17-associated cytokines such as IL-4, IL-13, IL-17A, IL-33, etc. in a mouse model of AD [28**]. Therefore, it has been suggested that IL-26 may exacerbate AD and act as an important bridge between Th2 and Th17 responses in AD skin. It has been known that regulatory B cells suppress inflammation by the secretion of IL-10. Moreover, it has recently been reported that IL-10-producing regulatory B cells are decreased in severe AD patients compared to mild AD patients and normal control subjects [29].
Figure 1. Pathophysiology of atopic dermatitis.
Epidermal barrier defects, cutaneous dysbiosis, and immune dysregulation play important roles in the pathophysiology of AD. TSLP, IL-25, IL-33, and ILC2 induce production of Th2, Th17, and Th22 cytokines directly or indirectly. TSLP activates antigen presenting cells, dendritic cells, basophil, and ILC2 to produce Th2 and Th17 cytokines. IL-26 from Th17 cells induces production of IL-4, IL-13, IL-17A, and IL-33.
Abbreviations: AMPs, antimicrobial peptides; ILC2, type 2 innate lymphoid cell; Th2, T helper type 2; Th17, T helper type 17; Th22, T helper type 22; TJ, tight junction; TSLP, thymic stromal lymphopoietin.
S. aureus infection correlates with type 2 responses in AD skin, but it has not been elucidated how S. aureus infection aggravates type 2 inflammation in AD skin. Brauweiler et al have demonstrated that S. aureus lipoteichoic acid inhibits expression of skin barrier proteins [30] and causes the expression of IL-4 from basophil by the production of thymic stromal lymphopoietin (TSLP) [31]. Ryffel et al have reported that basophil and ILC2 contribute to IL-33 mediated AD-like skin inflammation without adaptive immune cells [32]. TSLP upregulates Fc receptor γ receptors on antigen-presenting cells through STAT-5 and induces Th2/Th17 polarization through dectin-2 [33].
Cutaneous microbiome
The commensal microbiome communicates with host immune systems and plays a key role in maintaining cutaneous homeostasis [34,35*]. Commensal bacteria are able to produce AMPs and prevent invasion of pathogenic microorganisms such as S. aureus on the skin of healthy subjects [34,35]. Decreased microbial diversity and deficiency of AMPs, which lead to frequent S. aureus infection and microbial dysbiosis, are characteristic findings in AD skin [4**,35*]. S. aureus infection aggravates AD skin and is strongly associated with the severity of AD [4**,34].
Conversely, normalization of microbial signature by transplantation of cutaneous commensal bacteria reduces S. aureus colonization, skin inflammation, and promotes clinical improvement of AD [34,35*]. Callewaert et al have recently reported that an antibody to IL-4 receptor α (dupilumab) decreases S. aureus abundance and increases microbial diversity in AD skin [36]. It has been reported that aryl hydrocarbon receptor (AHR) plays a major role in cutaneous microbial-host interactions [37,38]. Yu et al have demonstrated that skin microbiome-derived tryptophan metabolites, which are decreased in AD skin, may attenuate skin inflammation through the AHR [37]. Additionally, topical treatment of coal tar upregulates the levels of AMPs in an AHR dependent manner and changes microbiota composition toward that of healthy subjects by decreasing Staphylococcal abundance and increasing Propionibacterium abundance [38].
Clinical application of new drug targets
As AD treatment has begun to move toward precision medicine, various biologic and small molecule agents have been developed to block specific cytokines, cytokine receptors, or transcription factors (Table 1). Dupilumab is a monoclonal antibody that reduces type 2 inflammtion by antagonizing IL-4 and IL-13 action and has been approved by the US Food and Drug Administration for patients with moderate-to-severe AD [39,40]. In a phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial, subcutaneous injections of dupilumab monotherapy in adolescents every 2 weeks or 4 weeks led to a significantly higher proportion of patients with EASI75 improvement at week 16 [39]. In this study, safety was acceptable [39]. Clinical trials of many new biologics are in progress to assess their efficacy and safety in both adults and pediatric populations [40–45, 46**, 47–53].
Table 1.
Summary of recently published clinical trials and retrospective review of new drugs in atopic dermatitis
| Biologic agents | Target | Phase | Region | Study population | Administration | Study duration | Efficacy | Safety | Reference |
|---|---|---|---|---|---|---|---|---|---|
| In adults and adolescents | |||||||||
| Dupilumab | IL4 receptor alpha chain | Real-life multicenter retrospective cohort study | France | 241 adults (>18 years) with moderate-to-severe AD | Subcutaneous injection | 3.8±3.7 months | Significant improvement in disease severity at 3 months of treatment | High frequency of conjunctivitis and eosinophilia | 40 |
| Dupilumab | IL4 receptor alpha chain | Phase 3, RCT (3-arm trial) | USA, Canada | 251 adolescents (12–17 years) with moderate-to-severe AD | Subcutaneous injections with dulipumab 200 mg (baseline weight <60 kg) or 300 mg (baseline weight ≥ 60 kg) every 2 weeks, 300 mg every 4 weeks, or placebo | 16 weeks | Significant improvement in AD signs, symptoms and quality of life; efficacy of the every-2-week regimen was generally superior to the every-4-week regimen | No significant difference between dupilumab and placebo groups; safety is acceptable | 39 |
| Nemolizumab | IL31 receptor alpha subunit | Phase 2b, RCT (4-arm trial) | North America (USA, Canada), Europe (France, Germany, Poland), Australia | 226 adults with moderate-to-severe AD | Subcutaneous injections with a loading dose of 20, 60, or 90 mg on day 1, followed by 10, 30, or 90 mg, respectively, every 4 weeks | 20-week treatment and 12 week follow-up | Rapid and sustained improvement with maximal efficacy observed at 30 mg | No significant difference between treatment and placebo groups; safe and well tolerated | 43 |
| Tezepelumab | TSLP | Phase 2a, RCT (2-arm trial) | Australia, Canada, Germany, Hungary, New Zealand, USA | 113 adults (18–75 years) with moderate-to-severe AD | Subcutaneous injections of 280 mg every 2 weeks plus class 3 topical corticosteroids | 12 weeks | No significant difference between treatment and placebo groups | No significant difference between treatment and placebo groups | 48 |
| Tralokinumab | IL13 | Phase 2b, RCT (4-arm trial) | Australia, Canada, Germany, Japan, Poland, USA | 299 adults (18–75 years) with moderate-to-severe AD | Subcutaneous injections with 45, 150, or 300 mg of tralokinumab or placebo every 4 weeks | 12 weeks | Early and sustained improvements in AD symptoms | No significant difference between treatment and placebo groups; safe and well tolerated | 51 |
| GBR 830 | OX40 | Phase 2a, RCT (2-arm trial) | USA, Canada | 64 adults with moderate-to-severe AD | Two intravenous administration of 10 mg/kg 4 weeks apart (day 1, day 29) | 4-week treatment and 14 week follow-up | Significant progressive tissue and clinical improvements until day 71 (42 days after the last dose) | Well tolerated with equal TEAE distribution | 45 |
| Fezakinumab | IL22 | Phase 2a, RCT (2-arm trial) | USA | 59 adults with moderate-to-severe AD | Intravenous administration with a loading dose of 600 mg followed by 300 mg every 2 weeks for 10 weeks | 10-week treatment and 10 week follow-up | A significant decline in disease severity for severe AD patients; a profound effect of IL22 blockade on multiple inflammatory pathways | No significant difference between treatment and placebo groups | 50,53 |
| Ruxolitinib | JAK1/JAK2 | Phase 2, RCT (6-arm trial) | USA, Canada | 252 adults (18–70 years) with history of AD > 2 years, IGA score of 2 or 3 and BSA involvement of 3%–20% | 0.15% RUX cream qd, 0.5% RUX cream qd, 1.5% RUX cream qd, 1.5% RUX cream bid, vehicle cream bid, 0.1% triamcinolone cream bid | 8-week of double-blind treatment, 4-week of open-label treatment and 4-week of follow-up | Rapid and sustained improvement in AD symptoms | Unremarkable safety profile with no notable systemic effects and good tolerability | 41 |
| Tapina rof | AHR | Phase 2, RCT (6-arm trial) | USA, Japan | 247 adolescents and adults (12–65 years) with moderate-to-severe AD | 1% tapinarof cream bid, 1% tapinarof cream qd, 0.5% tapinarof cream bid, 0.5% tapinarof cream qd, vehicle bid, vehicle qd | 12-week treatment and 4-week follow-up | Significantly higher success rate in the treatment grouop | More TEAEs with mild to moderate intensity in treatment group | 47 |
| Apremilast | PDE4 | Phase 2, RCT (3-arm trial) | North America, Japan | 185 adults (≥18 years) with moderate-to-severe AD | 30 mg tables bid (APR30), apremilast 40 mg tables bid (APR40), placebo | 12 weeks | Modest clinical efficacy for APR40 with decreased AD-related biomarkers | More frequent AEs including cellulitis in APR40-treated group, leading to discontinuation of treatment | 49 |
| Baricitinib | JAK1/JAK2 | Phase 2, RCT (3-arm trial) | USA, Japan | 124 adults (26–52 years) with moderate-to-severe AD | 2 mg tablet qd, 4 mg baricitinib tablet qd, placebo qd | 16 weeks | Significantly reduced inflammation and pruritus | More frequent headache, increased creatine phosphokinse level and nasopharyngitis in 4 mg baricitinib-treated group | 52 |
| In children | |||||||||
| Dupilumab | IL4 receptor alpha chain | Multi-center retrospective review of off-label use | USA | 111 children with the age of 13.0±3.9 years (range 3.1 to 18.0) with moderate-to-severe AD | Subcutaneous injections of a mean of 8.7 mg/kg (range, 4–15.5; SD 2.2) loading dose followed by a mean of 5.1 mg/kg (range, 2.0–15.3; SD 2.2) maintenance dose every other week | 9 weeks | 64.3% experienced ≥ 2-point IGA improvement; 22.1% reported a 1-point improvement, and 12.6% experienced no improvement. | AEs are comparable to previous adolescent and adult trials. | 42 |
| Delgocitinib | JAK (JAK1/JAK2/JAK3/tyrosine kinase) | Phase 2, RCT (3-arm trial) | Japan | 103 children (2–15 years) with AD (modified EASI ≥ 5, IGA ≥ 2 and BSA involvement of 5%–30%) | 0.25% or 0.5% delgocitinib ointment bid, placebo bid | 4 weeks | Significant improvement in clinical signs and symptoms | No significant difference between treatment and placebo groups; mild AEs with no serious events | 44 |
Indications are expanding from adults to children and formulations are becoming more diverse, ranging from injections to topical cream and oral forms [42,44,48]. These drugs are targeting various key molecules responsible for skin inflammation, i.e., IL-4 receptor α chain, IL-31 receptor α subunit, TSLP, IL-13, IL-22, OX40, AHR, phosphodiesterase 4 (PDE4), and Janus kinase (JAK). Most of the newly developed biologics and small molecule antagonists have been reported to be efficacious and well tolerated [47–49,52]. Further research in large populations of AD are needed to improve therapeutic outcomes of precision medicine and to guarantee drug safety.
Conclusions
AD is a heterogenous skin disease charcterized by skin barrier dysfunction, systemic immune dysregulation, systemic comorbidities, and microbial dysbiosis. In recent years, a better understanding of AD has been achieved by various research investigations. New treatments such as microbial skin transplantation, biologics, and small molecular antagonists targeting key immune pathways will improve our treatment strategies in AD.
Acknowledgements
This work was funded by NIH/NIAMS grant AR41256 and The Environmental Health Center Project of The Ministry of Environment, Republic of Korea. The authors would like to thank Sungkab Kim from Multimedia Services Part, Samsung Medical Center (Seoul, Republic of Korea) for the preparation of a figure for this manuscript. The authors acknowledge Nicole Meiklejohn for her editorial assistance in preparing this manuscript.
Conflict of interest
Donald YM Leung has consulted for Regeneron, Boehringer-Ingelheim, and Sanofi/Genzyme. He has also received grant support from MedImmune/Astra-Zeneca, Incyt,e and Pfizer. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- AD
atopic dermatitis
- AE
adverse event
- AHR
aryl hydrocarbon receptor
- AMP
antimicrobial peptide
- BSA
body surface area
- CI
confidence interval
- EASI
eczema area and severity index
- EOS ceramide
ω-esterified fatty acid sphingosine ceramide
- FLG
filaggrin
- IGA
investigator’s global assessment
- ILC2
type 2 innate lymphoid cell
- JAK
Janus kinase
- NS ceramide
nonhydroxy fatty acid sphingosine ceramide
- OR
odds ratio
- PDE4
phosphodiesterase 4
- S. aureus
Staphylococcus aureus
- SC
stratum corneum
- SCORAD
scoring of atopic dermatitis
- STAT
signal transducer and activator of transcription
- TEAE
treatment-emergent adverse event
- Th
T helper
- TJ
tight junction
- TLR
Toll-like receptor
- TRAP
traffic-related air pollution
- TSLP
thymic stromal lymphopoietin
- UVR
ultraviolet radiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References and recomended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Leung DYM, Calatroni A, Zaramela LS, LeBeau PK, Dyjack N, Brar K, David G, Johnson K, Leung S, Ramirez-Gama M, et al. : The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med 2019, 11:eaav2685.**This study demonstrates that children with AD and food allergy (AD FA+) have unique properites associated with an immature skin barrier and type 2 immune activation. Transepidermal water loss, abundance of S. aureus, type 2 immune pathways, and abnormal keratinocyte proliferation are increased in non-lesional skin of AD FA+ subjects compared to AD FA- or nonatopic controls.
- 2.Goleva E, Berdyshev E, Leung DY: Epithelial barrier repair and prevention of allergy. J Clin Invest 2019, 129:1463–1474.*This article describes the formation of the skin barrier and demonstrates the link between altered skin barrier formation and the pathogenesis of AD. Authors also suggest epidermal barrier repair strategies as an approach for AD prevention or intervention.
- 3.Kim J, Kim BE, Leung DYM: Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc 2019, 40:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung DYM: The microbiome and allergic diseases: a struggle between good and bad microbes. Ann Allergy Asthma Immunol 2019, 122:231–232.** This paper provides comprehensive knowledge of microbiomes in allergic diseases such as AD.
- 5.Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI: A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol 2019, 80:1526–1532.e7.*This paper describes adult-onset disease as a distinct clinical phenotype and shows that AD is not only a disease of childhood.
- 6.Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, Grayson MH, Margolis DJ, Mitchell L, Silverberg JI, et al. : Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol 2019, 139:583–590. [DOI] [PubMed] [Google Scholar]
- 7.Patel KR, Immaneni S, Singam V, Rastogi S, Silverberg JI: Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis. J Am Acad Dermatol 2019, 80:402–410. [DOI] [PubMed] [Google Scholar]
- 8.Ascott A, Mulick A, Yu AM, Prieto-Merino D, Schmidt M, Abuabara K, Smeeth L, Roberts A, Langan SM: Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol 2019, 143:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Blomberg M, Rifas-Shiman SL, Camargo CA Jr., Gold DR, Thyssen JP, Litonjua AA, Oken E, Asgari MM: Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol 2019, 139:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin ME, Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, Kiragu W, Ramjith J, Watkins A, Genuneit J: Environmental factors associated with allergy in urban and rural children from the South African Food Allergy (SAFFA) cohort. J Allergy Clin Immunol 2020, 145:415–426. [DOI] [PubMed] [Google Scholar]
- 11.Wan J, Takeshita J, Shin DB, Gelfand JM: Mental health impairment among children with atopic dermatitis: a U.S. population-based cross-sectional study of the 2013–2017 National Health Interview Survey. J Am Acad Dermatol 2019. 10.1016/j.jaad.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman BC, Garcia S, Everett DC, Leung DYM, Cho CB: Association of atopic dermatitis with increased risk of anaphylaxis to egg and milk. Ann Allergy Asthma Immunol 2019, 123:620–622. [DOI] [PubMed] [Google Scholar]
- 13.McKenzie C, Silverberg JI: The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol 2019, 123:173–178.e1. [DOI] [PubMed] [Google Scholar]
- 14.Abuabara K, Yu AM, Okhovat JP, Allen IE, Langan SM: The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy 2018, 73:696–704.*The authors demonstrate that the prevalence of AD is not decreased significantly beyond childhood and provide more information on the natural course of AD.
- 15.Calov M, Alinaghi F, Hamann CR, Silverberg J, Egeberg A, Thyssen JP: The association between season of birth and atopic dermatitis in the Northern hemisphere: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2020, 8:674–680.e5. [DOI] [PubMed] [Google Scholar]
- 16.Rutter CE, Silverwood RJ, Williams HC, Ellwood P, Asher I, Garcia-Marcos L, Strachan DP, Pearce N, Langan SM: Are environmental factors for atopic eczema in ISAAC Phase Three due to reverse causation? J Invest Dermatol 2019, 139:1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huls A, Klumper C, MacIntyre EA, Brauer M, Melen E, Bauer M, Berdel D, Bergstrom A, Brunekreef B, Chan-Yeung M, et al. : Atopic dermatitis: interaction between genetic variants of GSTP1, TNF, TLR2, and TLR4 and air pollution in early life. Pediatr Allergy Immunol 2018, 29:596–605. [DOI] [PubMed] [Google Scholar]
- 18.Huls A, Abramson MJ, Sugiri D, Fuks K, Kramer U, Krutmann J, Schikowski T: Nonatopic eczema in elderly women: effect of air pollution and genes. J Allergy Clin Immunol 2019, 143:378–385.e9. [DOI] [PubMed] [Google Scholar]
- 19.Stefanovic N, Flohr C, Irvine AD: The exposome in atopic dermatitis. Allergy 2020, 75:63–74.*This article emphasizes the role of environmental factors in the pathophysiology of AD. The authors of this paper highlight current approaches to exposome modification in AD and propose future directions for exposome characterization and modification using novel research techniques.
- 20.Kim BE, Leung DYM: Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res 2018, 10:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drislane C, Irvine AD: The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol 2020, 124:36–43. [DOI] [PubMed] [Google Scholar]
- 22.Ding X, Willenborg S, Bloch W, Wickstrom SA, Wagle P, Brodesser S, Roers A, Jais A, Bruning JC, Hall MN, et al. : Epidermal mammalian target of rapamycin complex 2 controls lipid synthesis and filaggrin processing in epidermal barrier formation. J Allergy Clin Immunol 2020, 145:283–300.e8.*This paper reveals a key role of epidermal mTORC2 activity in epidermal lipid metabolism and in assembly of a protective SC.
- 23.Elias MS, Wright SC, Remenyi J, Abbott JC, Bray SE, Cole C, Edwards S, Gierlinski M, Glok M, McGrath JA, et al. : EMSY expression affects multiple components of the skin barrier with relevance to atopic dermatitis. J Allergy Clin Immunol 2019, 144:470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suga H, Oka T, Sugaya M, Sato Y, Ishii T, Nishida H, Ishikawa S, Fukayama M, Sato S: Keratinocyte proline-rich protein deficiency in atopic dermatitis leads to barrier disruption. J Invest Dermatol 2019, 139:1867–1875.e7. [DOI] [PubMed] [Google Scholar]
- 25.Boiten W, van Smeden J, Bouwstra J: The cornified envelope-bound ceramide fraction is altered in patients with atopic dermatitis. J Invest Dermatol 2019. 10.1016/j.jid.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Berdyshev E, Goleva E, Bronova I, Dyjack N, Rios C, Jung J, Taylor P, Jeong M, Hall CF, Richers BN, et al. : Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018, 3:e98006.**This article demonstrates that Th2 immune activation increases the proportion of short chain molecular species in epidermal lipids and decreases the expression of elongases in a STAT-6 dependent way.
- 27.Baurecht H, Ruhlemann MC, Rodriguez E, Thielking F, Harder I, Erkens AS, Stolzl D, Ellinghaus E, Hotze M, Lieb W, et al. : Epidermal lipid compostion, barrier integrity, and eczematous inflammation are associated with skin microbiome configuration. J Allergy Clin Immunol 2018, 141:1668–1676.e16. [DOI] [PubMed] [Google Scholar]
- 28.Kamijo H, Miyagaki T, Hayashi Y, Akatsuka T, Watanabe-Otobe S, Oka T, Shishido-Takahashi N, Suga H, Sugaya M, Sato S: Increased IL-26 expression promotes T helper type 17- and T helper type 2-associated cytokine production by keratinocytes in atopic dermatitis. J Invest Dermatol 2019. 10.1016/j.jid.2019.07.713.**This paper reports that IL-26 from Th17 cells is increased in lesional skin of AD patients. Additionally, IL-26 promotes Th2 and/or Th17 immune responses by inducing production of Th2 and Th17 cytokines.
- 29.Yoshihara Y, Ishiuji Y, Yoshizaki A, Kurita M, Hayashi M, Ishiji T, Nakagawa H, Asahina A, Yanaba K: IL-10-producing regulatory B cells are decreased in patients with atopic dermatitis. J Invest Dermatol 2019, 139:475–478. [DOI] [PubMed] [Google Scholar]
- 30.Brauweiler AM, Goleva E, Leung DYM: Staphylococcus aureus lipoteichoic acid damages the skin barrier through an IL-1-mediated pathway. J Invest Dermatol 2019, 139:1753–1761.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brauweiler AM, Goleva E, Leung DYM: Staphylococcus aureus lipoteichoic acid initiates a TSLP-basophil-IL4 axis in the skin. J Invest Dermatol 2019. 10.1016/j.jid.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryffel B, Alves-Filho JC: ILC2s and basophils team up to orchestrate IL-33-induced atopic dermatitis. J Invest Dermatol 2019, 139:2077–2079. [DOI] [PubMed] [Google Scholar]
- 33.Liang Y, Yu B, Chen J, Wu H, Xu Y, Yang B, Lu Q: Thymic stromal lymphopoietin epigenetically upregulates Fc receptor gamma subunit-related receptors on antigen-presenting cells and induces TH2/TH17 polarization through dectin-2. J Allergy Clin Immunol 2019, 144:1025–1035.e7. [DOI] [PubMed] [Google Scholar]
- 34.Paller AS, Kong HH, Seed P, Naik S, Scharschmidt TC, Gallo RL, Luger T, Irvine AD: The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol 2019, 143:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo TE, Sibley CD: The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol 2020, 82:222–228.* This paper reports that AD has cutaneous dysbiosis and that cutaneous microbiome transplantation is a promising strategy for the treatment of AD.
- 36.Callewaert C, Nakatsuji T, Knight R, Kosciolek T, Vrbanac A, Kotol P, Ardeleanu M, Hultsch T, Guttman-Yassky E, Bissonnette R, et al. : IL-4Ralpha blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol 2020, 140:191–202.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Luo Y, Zhu Z, Zhou Y, Sun L, Gao J, Sun J, Wang G, Yao X, Li W: A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J Allergy Clin Immunol 2019, 143:2108–2119.e12. [DOI] [PubMed] [Google Scholar]
- 38.Smits JPH, Ederveen THA, Rikken G, van den Brink NJM, van Vlijmen-Willems I, Boekhorst J, Kamsteeg M, Schalkwijk J, van Hijum S, Zeeuwen P, et al. : Targeting the cutaneous microbiota in atopic dermatitis by coal tar via AHR-dependent induction of antimicrobial peptides. J Invest Dermatol 2020, 140:415–424.e10. [DOI] [PubMed] [Google Scholar]
- 39.Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, Beck LA, Guttman-Yassky E, Pariser D, Blauvelt A, et al. : Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020, 156:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faiz S, Giovannelli J, Podevin C, Jachiet M, Bouaziz JD, Reguiai Z, Nosbaum A, Lasek A, Ferrier le Bouedec MC, Du Thanh A, et al. : Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol 2019, 81:143–151. [DOI] [PubMed] [Google Scholar]
- 41.Kim BS, Howell MD, Sun K, Papp K, Nasir A, Kuligowski ME: Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol 2020, 145:572–582. [DOI] [PubMed] [Google Scholar]
- 42.Igelman S, Kurta AO, Sheikh U, McWilliams A, Armbrecht E, Jackson Cullison SR, Kress DW, Smith A, Castelo-Soccio L, Treat J, et al. : Off-label use of dupilumab for pediatric patients with atopic dermatitis: a multicenter retrospective review. J Am Acad Dermatol 2020, 82:407–411. [DOI] [PubMed] [Google Scholar]
- 43.Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, Murrell DF, Alexis A, Lindsey L, Ahmad F, et al. : Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol 2020, 145:173–182. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Oda M, Kabashima K, Nagata T: Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol 2019, 144:1575–1583. [DOI] [PubMed] [Google Scholar]
- 45.Guttman-Yassky E, Pavel AB, Zhou L, Estrada YD, Zhang N, Xu H, Peng X, Wen HC, Govas P, Gudi G, et al. : GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol 2019, 144:482–493.e7. [DOI] [PubMed] [Google Scholar]
- 46.Czarnowicki T, He H, Krueger JG, Guttman-Yassky E: Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 2019, 143:1–11.**AD pathogenesis is complex, with multiple genetic and epigenetic factors orchestrating its phenotype. This article describes precision medicine approaches which are striving for targeted, tailored, endotype-driven disease prevention and treatment.
- 47.Peppers J, Paller AS, Maeda-Chubachi T, Wu S, Robbins K, Gallagher K, Kraus JE: A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol 2019, 80:89–98.e3. [DOI] [PubMed] [Google Scholar]
- 48.Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, van der Merwe R: Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol 2019, 80:1013–1021. [DOI] [PubMed] [Google Scholar]
- 49.Simpson EL, Imafuku S, Poulin Y, Ungar B, Zhou L, Malik K, Wen HC, Xu H, Estrada YD, Peng X, et al. : A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol 2019, 139:1063–1072. [DOI] [PubMed] [Google Scholar]
- 50.Brunner PM, Pavel AB, Khattri S, Leonard A, Malik K, Rose S, Jim On S, Vekaria AS, Traidl-Hoffmann C, Singer GK, et al. : Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol 2019, 143:142–154. [DOI] [PubMed] [Google Scholar]
- 51.Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, Moate R, van der Merwe R: Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol 2019, 143:135–141. [DOI] [PubMed] [Google Scholar]
- 52.Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, de la Pena A, Nunes FP, Janes J, Gamalo M, et al. : Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol 2019, 80:913–921.e9. [DOI] [PubMed] [Google Scholar]
- 53.Guttman-Yassky E, Brunner PM, Neumann AU, Khattri S, Pavel AB, Malik K, Singer GK, Baum D, Gilleaudeau P, Sullivan-Whalen M, et al. : Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J Am Acad Dermatol 2018, 78:872–881.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]