Abstract
Nicotine is a highly addictive drug found in tobacco that drives its continued use despite the harmful consequences. The initiation of nicotine abuse involves the mesolimbic dopamine system, which contributes to the rewarding sensory stimuli and associative learning processes in the beginning stages of addiction. Nicotine binds to neuronal nicotinic acetylcholine receptors (nAChRs), which come in a diverse collection of subtypes. The nAChRs that contain the α4 and β2 subunits, often in combination with the α6 subunit, are particularly important for nicotine’s ability to increase midbrain dopamine neuron firing rates and phasic burst firing. Chronic nicotine exposure results in numerous neuroadaptations, including the upregulation of particular nAChR subtypes associated with long-term desensitization of the receptors. When nicotine is no longer present, for example during attempts to quit smoking, a withdrawal syndrome develops. The expression of physical withdrawal symptoms depends mainly on the α2, α3, α5, and β4 nicotinic subunits in the epithalamic habenular complex and its target regions. Thus, nicotine affects diverse neural systems and an array of nAChR subtypes to mediate the overall addiction process.
Introduction:
Nicotine addiction (Dani and Heinemann, 1996; De Biasi and Dani, 2011; Mansvelder and McGehee, 2002) causes more than 7 million deaths each year worldwide (WHO, 2017). This number is rising (Prochaska and Benowitz, 2016), making it the leading cause of preventable death in the world (Gowing et al., 2015). Approximately 50 million people in the United States alone are addicted to tobacco products (Creamer et al., 2019), resulting in tremendous public health, societal, and economic costs. Nicotine is the main addictive component of tobacco products (Dani et al., 2019), but there are multiple constituents that contribute to its reinforcing properties (Brennan et al., 2015). While the rate of cigarette smoking has fallen in recent years, the rise of e-cigarettes has led to a renewed use of nicotine, particularly among adolescents and young adults (CDC, 2019). This resurgence has many harmful consequences and highlights the importance of understanding the pharmacology of nicotine (Dani et al., 2019) and the properties of neuronal nicotinic acetylcholine receptors (nAChRs) (Dani and Bertrand, 2007) for devising effective addiction treatments.
Nicotine is a naturally occurring alkaloid in many plants where it serves as an insecticide (Dani et al., 2019). Nicotine is a tertiary amine that occurs in two stereoisomers and consists of a pyridine and a pyrrolidine ring. The (S)-nicotine form is found in tobacco, and is the active form that binds to diverse nAChR subtypes throughout the central and peripheral nervous systems. During smoking or heating of tobacco, some racemization of nicotine takes place and small quantities of the (R)-nicotine isoform can also be found in the consequent smoke. In addition, because nicotine is a tertiary amine, it can exist in both a charged and an uncharged form. In its uncharged state, nicotine is membrane-permeable and can enter the brain, where it then converts to the charged form and binds to receptors (Dani and Bertrand, 2007; De Biasi and Dani, 2011). Nicotine thus influences intracellular processes indirectly by acting on nAChRs, but it may also directly influence these processes when it enters into the cytoplasm (Henderson and Lester, 2015; Rezvani et al., 2007). This chapter will focus exclusively on nicotine’s actions on nAChRs on the cell surface.
Nicotinic Receptor Structure and Subtypes
Nicotinic acetylcholine receptors are ligand-gated cation channels that are widely distributed throughout the nervous system and body (Papke, 2014), but this section will focus on neuronal nAChRs (Dani and Bertrand, 2007). They are expressed in nearly every region of the brain, both pre- and post-synaptically and can be found on axon terminals, axons, dendrites, and somata (Grady et al., 2007; Henderson and Lester, 2015; McGehee et al., 1995; Nashmi and Lester, 2006). Nicotinic receptors are pentameric structures made up of five distinct subunits that together form a central aqueous pore that allows cation influx when the receptor is activated (Cooper et al., 1991; Morales-Perez et al., 2016). Each subunit is comprised of an extracellular N-terminus that contributes to ligand-binding, three hydrophobic transmembrane domains (M1-M3), an intracellular loop, a fourth hydrophobic transmembrane domain (M4), and an extracellular C-terminus. Activation of nAChRs is achieved by the binding of the endogenous neurotransmitter acetylcholine or exogenous ligands like nicotine (Karlin, 1993).
There are 12 homologous neuronal nAChR subunits found in vertebrates, resulting in an enormous amount of diversity in the subunit compositions of these receptors (Albuquerque et al., 2009; Dani and Bertrand, 2007; Zoli et al., 2015). Among them are nine α-subunits (α2-10) and three β-subunits (β2-4), and diverse combinations of these subunits form functionally distinct receptors that can vary widely in their pharmacological and biophysical properties (Figure 1). All α subunits share a highly conserved set of six amino acids, including two adjacent cysteine residues that share a disulfide bond that is important in forming the ligand-binding site (Karlin, 1993; McGehee and Role, 1995). Importantly, the β subunits lack this pair of cysteine residues (Cooper et al., 1991; McGehee and Role, 1995). As a result, at least two alpha subunits are necessary to form a functional receptor.
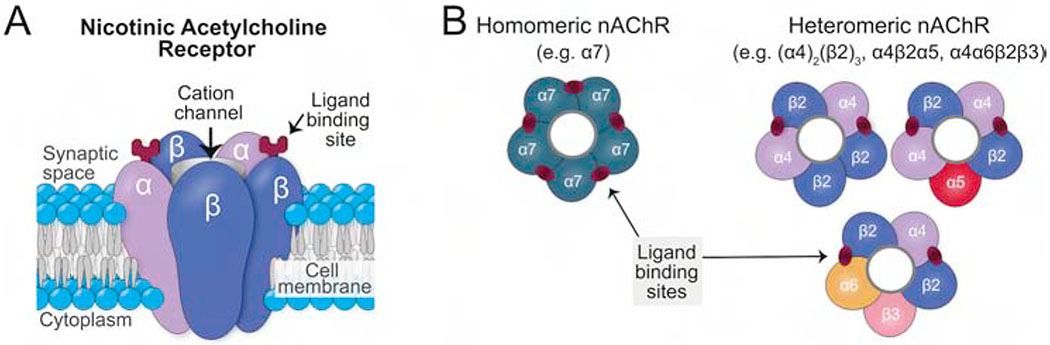
Figure 1.
Didactic structure of nAChR subtypes. (A) A side view of the subunits’ arrangement like the staves of a barrel around the central water-filled pore. In the generic heteromeric nAChR subtype shown, there are two alpha subunits and three beta subunits with the ACh binding sites located at the interfaces between α-β subunits. Note that for clarity, this schematic illustration is not drawn to scale and shows the ligand binding-sites at the apex of the subunits rather than at their actual positions deep within the structure. (B) These top down views of subunit arrangements for a homomeric α7-nAChR and for 3 of the myriad potential heteromeric nAChRs.
The resultant nAChR subtypes can be classified (Figure 1B) as either homopentameric (consisting of 5 identical subunits) or heteropentameric (consisting of at least 1 α and 1 β subunit type). Homopentameric receptors are thought to have five identical ligand binding sites, one between each pair of neighboring subunits, but it seems that only one binding site needs to be occupied to achieve some receptor activation (Figure 2) (Andersen et al., 2013). Heteropentameric receptors are believed to have only two binding sites that are located between neighboring pairs of α and β subunits (Gotti et al., 2006; Palma et al., 1996; Taly et al., 2009; Zoli et al., 2018) but unorthodox binding sites have recently been reported (Wang and Lindstrom, 2018). These receptors typically contain two αβ subunit pairs and a fifth accessory subunit. Each subunit is not entirely symmetrical and, thus, the placement of different subunits in a variety of positions within the pentameric complex can result in a wide variety of different nAChR subtypes, each with potentially different roles. Both α5 and β3 subunits function only as accessory subunits, at least in native nAChRs (Groot-Kormelink et al., 1998; Jain et al., 2016; Ramirez-Latorre et al., 1996; Wang and Lindstrom, 2018; Zoli et al., 2018). As such, they do not contribute to the agonist-binding site, but instead modify the functional properties of the nAChR complex and modify the receptor’s regulation by agonists (Kuryatov et al., 2008). For example, the α5 subunit has a regulatory role as its presence blunts the desensitization of nAChRs following nicotine exposure and is thought to be critical for controlling the expression and function of α4-containing nAChRs in the VTA (Chatterjee et al., 2013).
Figure 2.
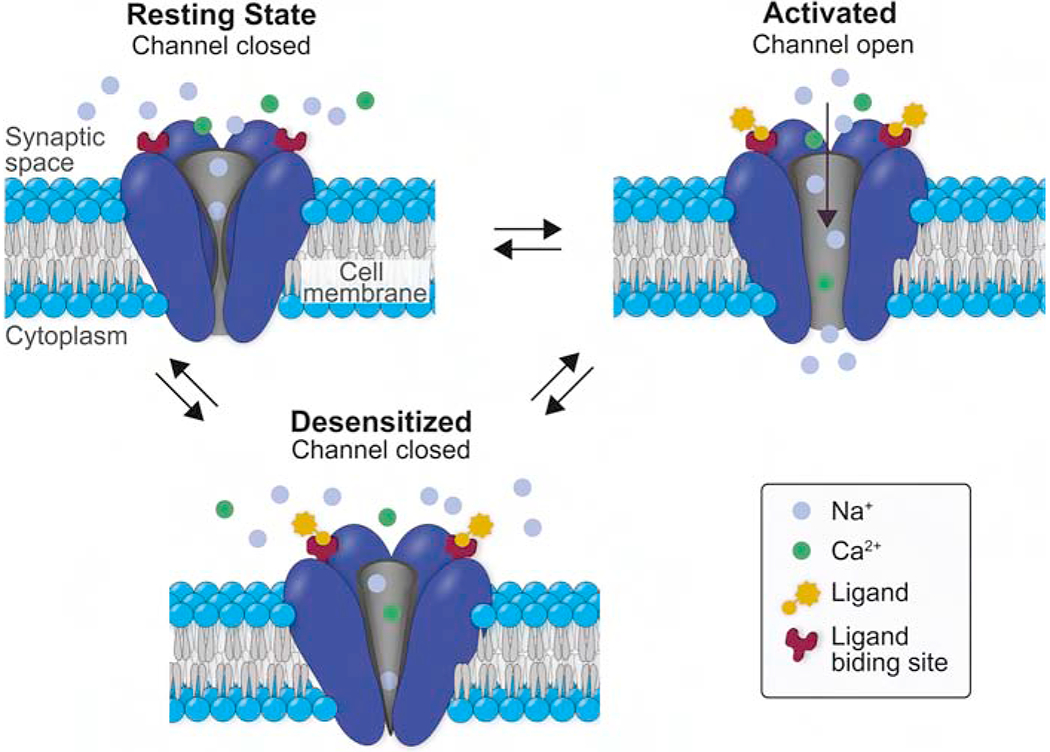
Didactic representation of the three main functional states of the nAChR. In the resting state, the ACh binding sites are not occupied, and the water-filled pore is closed and non-conducting to cations. In the open, activated state, the ion channel is open, providing a water-filled pore through the membrane that is permeable to small cations. In the desensitized state, the ACh binding sites are (usually) occupied, but the pore is closed and non-conducting. Note that for clarity, sodium to calcium ratios are lower in this schematic than in actuality and that the arrows do not indicate rate constants of conformation changes.
Despite this diversity, all mammalian neuronal nAChR subtypes share the functional property of being permeable to Na+, K+, and Ca2+ (Gotti et al., 2007; Gray et al., 1996; Shen and Yakel, 2009; Vernino et al., 1992; Vernino et al., 1994). Nicotinic receptors, like most ligand-gated ion channels, can exist in multiple conformational states (Figure 2): closed and able to be activated by ligand, open and conducting to small cations, or desensitized and closed and not able to be activated by ligand. When nicotine binds to the receptor, the ion channel is open and briefly stabilized in that conformation, allowing cation flux, which will move the membrane potential toward 0 mV, usually depolarizing the membrane. The channel then either returns to its resting state (closed and able to be activated) or enters a desensitized state in which it cannot be activated by nicotine or other agonists. The subunit composition governs the kinetics of these conformational states, the selective cationic permeability of the nAChR’s pore, and the pharmacological affinities of various agonists (Dani et al., 2019; Giniatullin et al., 2005; Picciotto et al., 2008; Quick and Lester, 2002; Wang and Lindstrom, 2018; Zoli et al., 2018). Therefore, the extraordinary diversity of nAChR subtypes results in numerous functional responses to nicotine. Among the wide array of nAChR subtype combinations in the brain, the most commonly expressed homomeric receptors are the α7 type, and the most commonly expressed heteromeric receptors are those containing the α4 and β2 subunits (Feduccia et al., 2012; Gotti et al., 2006).
Because the α7 nAChRs are the most common homomeric subtype, and because α7 subunits form heteromeric receptors relatively rarely (Gotti et al., 2006; Zoli et al., 2018), a short-hand for receptor classification is whether the receptor is an α7 nAChR or a non-α7 nAChR. Pharmacologically, this determination can be made by observing sensitivity to α-bungarotoxin (α-BTX), which in the brain is a potent and selective antagonist of the neuronal α7 homomeric receptors. Receptors sensitive to α-BTX have a relatively low affinity for nicotine and fast kinetics, while receptors insensitive to α-BTX generally have a higher affinity for nicotine, slower kinetics, are heteromeric, and desensitize to low agonist concentrations (Dani, 2015; Gotti et al., 2006). These distinct receptor properties result in different temporal, physiological, and biochemical responses to nicotine, and the significance of those different responses will be discussed in the next section.
Genetic Factors Mediating Nicotine Effects
While a complex array of environmental, pharmacological, and individual factors influence nicotine dependence and smoking behaviors, many studies have also investigated the genetics of nicotine addiction. Genetic factors are thought to play an important role in smoking initiation, progression to heavy use, and persistence of use (Fowler et al., 2007; Kendler et al., 1999; Lessov et al., 2004; Munafo and Johnstone, 2008; Sullivan and Kendler, 1999). One meta-analysis reports that genetic elements were responsible for 50% of the variation in nicotine initiation and persistence of usage (Li et al., 2003). Although gene-wide association studies (GWAS) and candidate gene studies have identified a large number of genes that can influence tobacco use, many associations between gene variants and nicotine phenotypes have not been reliably replicated. However, risk alleles in the CHRNA5-A3-B4 gene cluster of nicotinic receptor subunit genes on chromosome 15q25, which encodes the α5, α3, and β4 nAChR subunits, have consistently been associated with nicotine addiction (Bühler et al., 2015). Polymorphisms in this gene cluster have been linked to multiple smoking-related phenotypes, including nicotine dependence (Bierut et al., 2008; Chen et al., 2009a; Grucza et al., 2007; Saccone et al., 2007; Spitz et al., 2008; Thorgeirsson et al., 2008), smoking quantity (Amos et al., 2008; Berrettini et al., 2008; Keskitalo et al., 2009; Lips et al., 2010; Stevens et al., 2008; Thorgeirsson et al., 2008), smoking cessation (Freathy et al., 2009), and smoking-related diseases (Amos et al., 2008; Hung et al., 2008; Lips et al., 2010).
A number of single nucleotide polymorphisms (SNPs) in this gene cluster have been identified, but rs16969968 in CHRNA5 and rs1051730 in CHRNA3 have generated particular interest with respect to nicotine-related phenotypes, though the rs16969968 SNP has been studied in more detail. This SNP is a functional missense mutation G/A (D398N) in exon 5 and is the only SNP that has been implicated in nicotine behaviors thus far that results in a non-synonymous amino acid change in the resulting protein. The major allele produces α5 subunits with an aspartate (D) in position 398, which is swapped for an asparagine (N) in the minor allele. The minor allele (N398) has been shown to be highly associated with heavy smoking, intense craving for nicotine, and nicotine dependence (Bierut et al., 2008; Breetvelt et al., 2012; Buczkowski et al., 2015; Chen et al., 2012; Liu et al., 2010; Olfson et al., 2015; Sherva et al., 2008; Stevens et al., 2008). This polymorphism is also known to be of functional significance because in vitro studies have shown that α4β2α5 receptors with the asparagine allele show a decreased response to a nicotinic agonist, reduced calcium permeability, and faster desensitization (Bierut et al., 2008).
SNP 1051730, located within the CHRNA3 gene, has also been associated with nicotine dependence and smoking quantity (Chen et al., 2009b; Saccone et al., 2007; Thorgeirsson et al., 2008). This SNP is in perfect linkage disequilibrium with rs16969968 and the two SNPs are considered to be interchangeable, although this requires further investigation. An additional non-synonymous variant located in CHRNB4 (rs12914008) has been shown to mediate the fast transition from initial smoking to nicotine dependence, but it is a less common SNP and has been studied in much less detail. This SNP causes a missense mutation in CHRNB4 from threonine to isoleucine. While these rarer variants have been linked with nicotine dependence and susceptibility, it is currently unknown whether they represent risk factors independent of the other SNPs in the same gene cluster (Saccone et al., 2009).
Many other genes for nAChRs have also been implicated in various ways in nicotine addiction-related phenotypes (for review, see Ware et al., 2012; Yang and Li, 2016), and these findings from human genetic studies have formed the basis for a variety of animal models that have been used to study the circuit and molecular effects of these mutations. The following sections will refer to these and other animal models when discussing nicotine’s effects on various neural pathways and behaviors.
Nicotine Acts on the Mesolimbic Reward Circuit
All drugs of abuse, including nicotine, activate the ventral tegmental area (VTA) dopamine neurons that project to the nucleus accumbens (NAc). Activation of this mesolimbic pathway results in dopamine efflux in the NAc, which is important for nicotine reward-based learning and the initiation of the addiction process (De Biasi and Dani, 2011; Di Chiara and Imperato, 1988). A variety of nAChR subtypes are expressed in the neuronal populations in the VTA, as well as on the axon terminals of afferents from a number of brain regions. Therefore, nicotine’s effects in the VTA are complex. However, a variety of studies have shed some light on the mechanisms by which nicotine acts in the VTA to cause dopamine neuron activation.
Nicotine can directly activate dopamine neurons by binding to their high-affinity, β2-containing nicotinic receptors, thereby causing a net influx of cations that depolarizes the neuron (Dani and Bertrand, 2007; Mao et al., 2011; Pidoplichko et al., 1997). These effects increase dopamine neuron firing rates and phasic burst activity, elevating dopamine levels in the NAc (Placzek et al., 2009; Tsai et al., 2009; Zhang et al., 2009). As a result, the β2 subunit may be crucial in mediating the rewarding effects of nicotine since nicotine stimulates dopamine release in wild-type mice, but mice lacking the β2 subunit do not show an increase in extracellular dopamine following nicotine administration (Picciotto et al., 1998). However, VTA GABA neurons that inhibit the dopamine neurons also express β2-containing nAChRs, so GABAergic drive to the VTA dopamine neurons is transiently enhanced at the same time that the dopamine neurons themselves are directly activated by the presence of nicotine. Because the α4β2 nAChRs rapidly desensitize, GABAergic drive to the dopamine neurons is functionally reduced over a longer time frame when low levels of nicotine are continually present (Dani et al., 2019; Grieder et al., 2019; Mansvelder et al., 2002). Simultaneously, glutamatergic drive to the dopamine neurons is enhanced by activation of pre-synaptic α7 nAChRs, which are less prone to desensitization at nicotine concentrations achieved by smokers due to their low affinity for nicotine (Dani et al., 2000; Pidoplichko et al., 1997; Wooltorton et al., 2003), thus resulting in a long-lasting increase in excitatory drive to the dopamine neurons in the presence of nicotine (Mansvelder et al., 2002; Mansvelder and McGehee, 2002; Pidoplichko et al., 2004). This increased excitatory drive combined with the reduced inhibitory drive enhances activity in the dopamine neurons and facilitates Hebbian long-term potentiation of glutamatergic afferents onto the midbrain dopamine neurons (Mansvelder and McGehee, 2000; Mao et al., 2011; Ostroumov and Dani, 2018; Saal et al., 2003), which is thought to be a critical step in the initiation of addiction.
Nicotine dependence also critically involves striatal dopamine. VTA dopamine neurons express nAChRs on their presynaptic terminals in the striatum. Therefore, in addition to increasing the activity of the dopamine neurons themselves, nicotine also modulates striatal dopamine release via activation of these receptors. In the nucleus accumbens, nicotine acts via heteromeric presynaptic nAChRs that contain the α6β2 and/or α4β2 subunits (Exley et al., 2013; Gotti et al., 2010). The β2 subunit regulates dopamine release probability in the NAc core as well as in the dorsal striatum (Zhang et al., 2009; Zhou et al., 2001). Specifically in the NAc core, dopamine is predominantly regulated by α6α4β2β3 nAChRs (Exley et al., 2011). Multiple studies have shown that nicotine rapidly desensitizes these receptors, resulting in reduced tonic dopamine tone. However, this reduction in background dopamine “noise” allows for an improved signal to noise ratio when a phasic dopamine signal is transmitted (Rice and Cragg, 2004; Threlfell and Cragg, 2011; Zhang et al., 2009).
Effects of Chronic Nicotine
Chronic nicotine exposure leads to a number of neuroadaptations that can influence diverse signaling pathways and circuits, including the mesolimbic reward pathway. One such neuroadaptation is the subtype-specific upregulation of nAChRs that occurs in response to persistent desensitization of the receptors (Henderson and Lester, 2015). This desensitization occurs because unlike acetylcholine, nicotine cannot be removed from the synapse via rapid hydrolysis by acetylcholinesterase (Baker et al., 2013; De Biasi and Dani, 2011; Nashmi et al., 2007; Picciotto et al., 2008). Nicotine’s long-lasting presence in the synapse desensitizes nAChRs, rendering them unresponsive to further binding by nicotine or acetycholine and functionally inhibiting the nAChRs (Dani et al., 2000; Picciotto et al., 2008; Pidoplichko et al., 1997; Wooltorton et al., 2003). Accordingly, nAChR levels are upregulated to maintain homeostasis following chronic nicotine (Feduccia et al., 2012; Fenster et al., 1999a; Fenster et al., 1999b; Giniatullin et al., 2005).
The α4β2 nAChR is the predominant high-affinity nicotinic receptor in the brain and its upregulation has been the most thoroughly-studied (Bertrand and Terry, 2018; Feduccia et al., 2012; Henderson and Lester, 2015; Zoli et al., 2018). Comparing other nAChR subtypes to the α4β2 nAChR can therefore be useful in understanding how different nAChRs are differentially regulated by nicotine. For example, α4β2 nAChRs are upregulated by low, physiologically-relevant nicotine concentrations (Henderson et al., 2014; Peng et al., 1994), whereas α3β4 nAChRs are usually upregulated only by high nicotine concentrations that are outside of the normal range obtained by smoking (Matta et al., 2007; Mazzo et al., 2013). These changes begin within days of nicotine exposure and are thought to be significant mediators of nicotine addiction (Henderson et al., 2014; Matta et al., 2007; Nashmi et al., 2007).
The upregulation of nAChRs can be achieved by multiple processes, including changes in receptor assembly, trafficking, and degradation (Henderson and Lester, 2015; Henderson et al., 2014; Mazzo et al., 2013; Rezvani et al., 2010; Rezvani et al., 2007). It is important to note that nAChR upregulation and the mechanisms underlying this phenomenon can vary, not only for different nAChR subtypes, but also for different brain regions and nicotine administration paradigms (Baker et al., 2013; Henderson et al., 2017; Marks and Pauly, 1992; Nashmi et al., 2007; Pistillo et al., 2016; Renda and Nashmi, 2014).
In addition to altering nAChR expression levels in specific brain regions, repeated nicotine exposure can also produce a variety of additional neuroadaptations throughout the brain, including strengthening glutamatergic synapses onto dopamine neurons and onto the projection neurons of the NAc (Kenny et al., 2009; Mansvelder and McGehee, 2000; Mao et al., 2011; Ostroumov and Dani, 2018; Pidoplichko et al., 2004; Pistillo et al., 2015; Saal et al., 2003). Chronic nicotine can also dysregulate neuronal homeostatic mechanisms, modulate midbrain GABAergic circuitry, upregulate the high-affinity D2 dopamine receptors in the NAc, modulate neuronal scaffolding proteins, and alter epigenetic processes (Grilli et al., 2012; Hayase, 2017; Hwang and Li, 2006; Novak et al., 2010; Rezvani et al., 2007; Thomas et al., 2018).
Withdrawal from Chronic Nicotine
After chronic nicotine exposure, abstinence from the drug can cause unpleasant withdrawal symptoms (De Biasi and Dani, 2011; De Biasi and Salas, 2008; Mclaughlin et al., 2015). Withdrawal is defined as a combination of affective and somatic symptoms that appear soon after nicotine abstinence, reflecting a change in neurochemistry caused by the absence of the drug. In humans, the symptoms used to assess nicotine withdrawal according to the DSM-V are: irritability/frustration, anxiety, depression, increased appetite, impatience, insomnia, and restlessness (Wenzel, 2017). Because the half-life of nicotine in humans is roughly 20 hours (Matta et al., 2007), withdrawal symptoms typically peak within a week of cessation and subside over the following 3–4 weeks (Hughes, 2007). In mice, symptoms can be assessed by observing somatic signs of withdrawal, which include increased shaking, paw tremors, and scratching (Damaj et al., 2003; De Biasi and Salas, 2008; Isola et al., 1999; Salas et al., 2004), and affective withdrawal symptoms, which include anhedonia, aversion, and anxiety. These affective symptoms are probed using a variety of behavioral assays. For example, an increase in intracranial self-stimulation (ICSS) reward thresholds or reduced sucrose preference can indicate anhedonia, and reduced time spent in the open arms of an elevated-plus maze can indicate increased anxiety. Because the half-life for nicotine in mice is approximately 6 minutes, withdrawal symptoms typically peak 12-36 hours after abstinence (Matta et al., 2007; Perez et al., 2015).
While not the main focus of this section, it is interesting to note that different nAChR subtypes appear to differentially mediate the somatic vs. affective components of withdrawal (for extensive review, see Jackson et al., 2015; Mclaughlin et al., 2015). For example, β4 KO is sufficient to prevent somatic signs of nicotine withdrawal in mice, while β2 KO results in no change to somatic withdrawal signs compared to controls (Salas et al., 2004). However, β2 KO is sufficient to reduce the affective components of withdrawal (Jackson et al., 2008), and interestingly, β4 KO also prevents withdrawal-induced anhedonia (Stoker et al., 2012). Thus far, β2 (Jackson et al., 2009a; Jackson et al., 2008) and α6 (Jackson et al., 2009b) nAChRs have been implicated in the affective components of nicotine withdrawal, while α7, α3 (Jackson et al., 2013), α5 (Jackson et al., 2008; Salas et al., 2009), β4 (Jackson et al., 2013; Salas et al., 2004; Stoker et al., 2012), and α2 (Lotfipour et al., 2013; Salas et al., 2009) nAChRs have been implicated in the somatic aspects of nicotine withdrawal (De Biasi and Salas, 2008; Mclaughlin et al., 2015).
In addition to understanding the roles of various nAChR subtypes in different aspects of nicotine withdrawal, it is important to consider how neural circuits mediate withdrawal and how withdrawal serves to maintain addiction-related behaviors. Nicotine withdrawal contributes to continued nicotine use through negative reinforcement mechanisms, suggesting that both rewarding and aversive motivational signals and brain circuits are important for maintaining chronic nicotine use (Bromberg-Martin et al., 2010; Hikosaka, 2010; Matsumoto and Hikosaka, 2007). In fact, aversive nicotine withdrawal symptoms may be required to produce escalated intake of nicotine (George et al., 2007; Gilpin et al., 2014), a hallmark feature of addiction. While the positive motivational effects of nicotine are achieved by activation of the mesolimbic dopamine pathway, the negative motivational effects of nicotine withdrawal are mediated by the habenulo-interpeduncular pathway (Mclaughlin et al., 2015; McLaughlin et al., 2017; Pang et al., 2016). The habenula is an epithalamic nucleus involved in fear, anxiety, depression, and other forms of negative affect (De Biasi and Dani, 2011; Ikemoto, 2010; Lecca et al., 2014; Meye et al., 2017; Winter et al., 2011). Anatomically, the habenula is divided into medial (MHb) and lateral (LHb) nuclei, but the MHb has been most strongly implicated in nicotine withdrawal (Dao et al., 2014; Mclaughlin et al., 2015; McLaughlin et al., 2017). The MHb receives its primary inputs from the limbic system and sends almost all of its cholinergic and glutamatergic projections to the interpeduncular nucleus (IPN) (Hikosaka, 2010; Ren et al., 2011).
The MHb-IPN axis is unique in that it densely expresses almost all neuronal nAChR subtypes (Grady et al., 2009; Marks and Pauly, 1992) and many studies have established its important role in mediating the symptoms of nicotine withdrawal. For example, microinjections of the nAChR antagonist mecamylamine into the MHb or IPN of mice treated with chronic nicotine are sufficient to precipitate nicotine withdrawal symptoms (Damaj et al., 2003; De Biasi and Salas, 2008; Hildebrand et al., 1997; Malin and Goyarzu, 2009; Malin et al., 1992; Salas et al., 2004; Salas et al., 2009), and mice lacking the α2, α5 and β4 nAChR subunits, which are most densely expressed in the MHb-IPN pathway, exhibit reduced withdrawal responses after chronic nicotine. These findings indicate both the role of the MHb-IPN circuit and the role of these receptor subtypes in mediating nicotine withdrawal (Salas et al., 2004; Salas et al., 2009). More recent studies have also shown that cholinergic habenular signaling is required for nicotine withdrawal (Frahm et al., 2015), that optogenetic activation of IPN GABA neurons is sufficient to elicit withdrawal-like symptoms (Zhao-Shea et al., 2013), and that increased nAChR activity in the MHb underlies anxiety-related symptoms of nicotine withdrawal (Pang et al., 2016). Interestingly, studies have also shown that different sub-regions of the MHb and the IPN are implicated in different aspects of the nicotine withdrawal syndrome (Shih et al., 2014; Shih et al., 2015).
Although the MHb-IPN axis and its role in nicotine withdrawal are usually studied in parallel to the mesolimbic circuit that regulates nicotine reward, these pathways likely interact with each other. In addition to altering the MHb-IPN pathway, withdrawal from chronic nicotine also leads to reduced basal dopamine levels in the NAc (Zhang et al., 2012). Furthermore, many of the nAChR subtypes that are important for withdrawal-related behaviors are also expressed in the mesolimbic circuit, and a variety of studies have shown direct and indirect anatomical connections between these aversive and rewarding pathways (Quina et al., 2017; Wolfman et al., 2018; Zhao-Shea et al., 2015).
Conclusion
Nicotine addiction results from a complex and wide-ranging series of neuroadaptations in many brain regions and neurotransmitter systems. These adaptations include alterations in nicotinic receptor expression and modulation of neurotransmitter release. A great deal of preclinical research has focused on the circuits that underlie both the rewarding effects of acute nicotine exposure and the aversive effects of withdrawal from chronic nicotine exposure. These studies have confirmed the role of the mesolimbic reward pathway in the development and maintenance of nicotine addiction as well as the role of the MHb-IPN axis in mediating the nicotine withdrawal syndrome.
Highlights.
Nicotine is highly addictive and is a leading cause of premature death worldwide.
Great diversity in neuronal nicotinic acetylcholine receptors arises from their subunit composition.
Genetic factors, especially within the CHRNA5-A3-B4 gene cluster, play an important role in nicotine addiction.
Midbrain dopamine systems play an important role especially in the initiation of nicotine use.
Aspects of nicotine withdrawal are mediated by the habenulo-interpeduncular pathway.
Acknowledgments
The work from our labs is supported by the National Institutes of Health grants R01 AA026267 (National Institute on Alcohol Abuse and Alcoholism, J.A.D.), R01 NS021229 (National Institute of Neurological Disorders and Stroke, J.A.D.), and R01s DA044205 and DA049545 (National Institute on Drug Abuse, M.D.B.) and UO1 AA025931(National Institute on Alcohol Abuse and Alcoholism, M.D.B.). S.L.W. is supported in part by a grant from the Hartwell Foundation. Our work is also supported by a generous award from the Chernowitz Medical Research Foundation (J.A.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW, 2009. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiological Reviews, pp. 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K, Matakidou A, Wang Y, Mills G, Doheny K, Tsai YY, Chen WV, Shete S, Spitz MR, Houlston RS, 2008. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 40, 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen N, Corradi J, Sine SM, Bouzat C, 2013. Stoichiometry for activation of neuronal alpha7 nicotinic receptors. Proc Natl Acad Sci U S A 110, 20819–20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LK, Mao D, Chi H, Govind AP, Vallejo YF, Iacoviello M, Herrera S, Cortright JJ, Green WN, McGehee DS, Vezina P, 2013. Intermittent nicotine exposure upregulates nAChRs in VTA dopamine neurons and sensitises locomotor responding to the drug., The European Journal of Neuroscience, pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V, 2008. a5/a3 Nicotinic Receptor Subunit Alleles Increase Risk for Heavy Smoking. Molecular Psychiatry, pp. 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Terry AV, 2018. The wonderland of neuronal nicotinic acetylcholine receptors. Biochemical Pharmacology, pp. 214–225. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PAF, Mayo K, Nurnberger J, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM, 2008. Variants in the Nicotinic Receptors Alter the Risk for Nicotine Dependence. American Journal of Psychiatry, pp. 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breetvelt EJ, Numans ME, Aukes MF, Hoeben W, Strengman E, Luykx JJ, Bakker SC, Kahn RS, Ophoff RA, Boks MPM, 2012. The association of the alpha-5 subunit of the nicotinic acetylcholine receptor gene and the brain-derived neurotrophic factor gene with different aspects of smoking behavior. Psychiatric Genetics, pp. 96–98. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Crowther A, Putt F, Roper V, Waterhouse U, Truman P, 2015. Tobacco particulate matter self-administration in rats: Differential effects of tobacco type. Addiction Biology, pp. 227–235. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O, 2010. Dopamine in motivational control: rewarding, aversive, and alerting., Neuron. Elsevier Inc., pp. 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczkowski K, Sieminska A, Linkowska K, Czachowski S, Przybylski G, Jassem E, Grzybowski T, 2015. Association between Genetic Variants on Chromosome 15q25 Locus and Several Nicotine Dependence Traits in Polish Population : A Case-Control Study. BioMed Research International, pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler K-M, Giné E, Echeverry-Alzate V, Calleja-Conde J, de Fonseca FR, López-Moreno JA, 2015. Common single nucleotide variants underlying drug addiction: more than a decade of research. Addiction Biology, pp. 845–871. [DOI] [PubMed] [Google Scholar]
- CDC, C. f. D. C. a. P., 2019. Progress Erased: Youth Tobacco Use Increased During 2017-2018 | CDC Online Newsroom | CDC. Center for Desease Control and Prevention. [Google Scholar]
- Chatterjee S, Santos N, Holgate J, Haass-Koffler CL, Hopf FW, Kharazia V, Lester H, Bonci A, Bartlett SE, 2013. The α5 subunit regulates the expression and function of α4*-containing neuronal nicotinic acetylcholine receptors in the ventral-tegmental area., PloS one, p. e68300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-S, Baker TB, Grucza R, Wang JC, Johnson EO, Breslau N, Hatsukami D, Smith SS, Saccone N, Saccone S, Rice JP, Goate AM, Bierut LJ, 2012. Dissection of the phenotypic and genotypic associations with nicotinic dependence., Nicotine & Tobacco Research, pp. 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Johnson EO, Breslau N, Hatsukami D, Saccone NL, Grucza RA, Wang JC, Hinrichs AL, Fox L, Goate AM, Rice JP, Bierut LJ, 2009a. Interplay of Genetic Risk Factors and Parent Monitoring in Risk for Nicotine Dependence. Addiction 104, 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS, 2009b. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet 150B, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M, 1991. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature, pp. 235–238. [DOI] [PubMed] [Google Scholar]
- Creamer MLR, Wang TW, Babb S, Cullen KA, Day H, Willis G, Jamal A, Neff L, 2019. Tobacco Product Use and Cessation Indicators Among Adults - United States, 2018. MMWR. Morbidity and mortality weekly report, pp. 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR, 2003. Characterization of Spontaneous and Precipitated Nicotine Withdrawal in the Mouse. Journal of Pharmacology and Experimental Therapeutics, pp. 526–534. [DOI] [PubMed] [Google Scholar]
- Dani JA, 2015. Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine. Int Rev Neurobiol 124, 3–19. doi: 10.1016/bs.irn.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D, 2007. Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the Central Nervous System. Annual Review of Pharmacology and Toxicology pp. 699–729. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S, 1996. Molecular and cellular aspects of nicotine abuse. Neuron, pp. 905–908. [DOI] [PubMed] [Google Scholar]
- Dani JA, Kosten TR, Benowitz NL, 2019. Chapter 14, The Pharmacology of Nicotine and Tobacco In: The ASAM Principles of Addiction Medicine. 6th Edition, Miller SC, Fiellin DA, Rosenthal RN, Saitz R, (Eds), Wolters Kluwer/Lippincott Williams & Wilkins, pp. 190–207. [Google Scholar]
- Dani JA, Radcliffe KA, Pidoplichko VI, 2000. Variations in desensitization of nicotinic acetylcholine receptors from hippocampus and midbrain dopamine areas. European Journal of Pharmacology, pp. 31–38. [DOI] [PubMed] [Google Scholar]
- Dao DQ, Perez EE, Teng Y, Dani JA, De Biasi M, 2014. Nicotine Enhances Excitability of Medial Habenular Neurons via Facilitation of Neurokinin Signaling. Journal of Neuroscience, pp. 4273–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Dani JA, 2011. Reward, addiction, withdrawal to nicotine., Annual Review of Neuroscience, pp. 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Salas R, 2008. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Experimental Biology and Medicine, pp. 917–929. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato, a., 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats., Proceedings of the National Academy of Sciences of the United States of America, pp. 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, Mcintosh JM, Franklin M, Bermudez I, Cragg SJ, 2013. Striatal dopamine transmission is reduced after chronic nicotine with a decrease in α6-nicotinic receptor control in nucleus accumbens. European Journal of Neuroscience, pp. 3036–3043. [DOI] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux J-P, Maskos U, Cragg SJ, Faure P, 2011. Distinct contributions of nicotinic acetylcholine receptor subunit 4 and subunit 6 to the reinforcing effects of nicotine. Proceedings of the National Academy of Sciences National Academy of Sciences, pp. 7577–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia A. a., Chatterjee S, Bartlett SE, 2012. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions., Frontiers in Molecular Neuroscience, p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Hicks JH, Beckman ML, Covernton PJO, Quick MW, Lester RAJ, 1999a. Desensitization of nicotinic receptors in the central nervous system. Annals of the New York Academy of Sciences, pp. 620–623. [DOI] [PubMed]
- Fenster CR, Whitworth TL, Sheffield EB, Quick MW, Lester RAJ, 1999b. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. Journal of Neuroscience, pp. 4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, McBride A, van den Bree MB, 2007. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction 102, 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Antolin-Fontes B, Görlich A, Zander J-F, Ahnert-Hilger G, Ibañez-Tallon I, 2015. An essential role of acetylcholine-glutamate synergy at habenular synapses in nicotine dependence. eLife, pp. 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, Smith GD, Frayling TM, Hattersley AT, 2009. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet 18, 2922–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF, 2007. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences of the United States of America, pp. 17198–17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O, 2014. Nicotine vapor inhalation escalates nicotine self-administration. Addiction Biology, pp. 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL, 2005. Desensitization of nicotinic ACh receptors: Shaping cholinergic signaling. Trends in Neurosciences, pp. 371–378. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M, 2010. Nicotinic Acetylcholine Receptors in the Mesolimbic Pathway: Primary Role of Ventral Tegmental Area a6b2* Receptors in Mediating Systemic Nicotine Effects on Dopamine Release, Locomotion, and Reinforcement. Journal of Neuroscience, pp. 5311–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M, 2007. Heterogeneity and complexity of native brain nicotinic receptors., Biochemical pharmacology, pp. 1102–1111. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F, 2006. Brain nicotinic acetylcholine receptors : native subtypes and their relevance. Trends in Pharmacological Sciences, pp. 482–491. [DOI] [PubMed] [Google Scholar]
- Gowing LR, Ali RL, Allsop S, Marsden J, Turf EE, West R, Witton J, 2015. Global statistics on addictive behaviours: 2014 status report., Addiction, pp. 904–919. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C, 2009. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release., The Journal of Neuroscience, pp. 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ, 2007. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochemical Pharmacology, pp. 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA, 1996. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature, pp. 713–716. [DOI] [PubMed] [Google Scholar]
- Grieder TE, Besson M, Maal-Bared G, Pons S, Maskos U, van der Kooy D, 2019. β2* nAChRs on VTA dopamine and GABA neurons separately mediate nicotine aversion and reward. Proceedings of the National Academy of Sciences of the United States of America, pp. 25968–25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Summa M, Salamone A, Olivero G, Zappettini S, Di Prisco S, Feligioni M, Usai C, Pittaluga A, Marchi M, 2012. In vitro exposure to nicotine induces endocytosis of presynaptic AMPA receptors modulating dopamine release in rat nucleus accumbens nerve terminals. Neuropharmacology, pp. 916–926. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Luyten WH, Colquhoun D, Sivilotti LG, 1998. A reporter mutation approach shows incorporation of the “orphan” subunit beta3 into a functional nicotinic receptor. J Biol Chem, pp. 15317–15320. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Abbacchi AM, Przybeck TR, Gfroerer JC, 2007. Discrepancies in estimates of prevalence and correlates of substance use and disorders between two national surveys. Addiction 102, 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase T, 2017. Epigenetic mechanisms associated with addiction-related behavioural effects of nicotine and/or cocaine: Implication of the endocannabinoid system. Behavioural Pharmacology, pp. 493–511. [DOI] [PubMed] [Google Scholar]
- Henderson BJ, Lester HA, 2015. Inside-out neuropharmacology of nicotinic drugs. Neuropharmacology. Elsevier Ltd, pp. 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Srinivasan R, Nichols WA, Dilworth CN, Gutierrez DF, Mackey EDW, McKinney S, Drenan RM, Richards CI, Lester HA, 2014. Nicotine exploits a COPI-mediated process for chaperone-mediated up-regulation of its receptors., The Journal of General Physiology, pp. 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, Mckinney S, Lester HA, 2017. Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nachr function, nachr upregulation, and DA neuron excitability. Neuropsychopharmacology, pp. 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, 2010. The habenula: from stress evasion to value-based decision-making., Nature Reviews Neuroscience. Nature Publishing Group, pp. 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Bondjers C, Nisell M, Svensson TH, 1997. Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology, pp. 348–356. [DOI] [PubMed] [Google Scholar]
- Hughes JR, 2007. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine and Tobacco Research, pp. 315–327. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P, 2008. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452, 633–637. [DOI] [PubMed] [Google Scholar]
- Hwang YY, Li MD, 2006. Proteins differentially expressed in response to nicotine in five rat brain regions: Identification using a 2-DE/MS-based proteomics approach. Proteomics, pp. 3138–3153. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, 2010. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory., Neuroscience and Biobehavioral Reviews, pp. 129–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M, 1999. Nicotine abstinence in the mouse. Brain Research, pp. 189–196. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR, Damaj MI, 2009a. The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology 56, 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI, 2008. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI, 2009b. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther 331, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Muldoon PP, De Biasi M, Damaj MI, 2015. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology 96, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Sanjakdar SS, Muldoon PP, McIntosh JM, Damaj MI, 2013. The α3β4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the α5 subunit in the mouse., Neuropharmacology. Elsevier Ltd, pp. 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Kuryatov A, Wang J, Kamenecka TM, Lindstrom J, 2016. Unorthodox acetylcholine binding sites formed by α5 and β3 accessory subunits in α4α2*nicotinic acetylcholine receptors. Journal of Biological Chemistry, pp. 23452–23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A, 1993. Structure of nicotinic acetylcholine receptors. Current Opinion in Neurobiology, pp. 299–309. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA, 1999. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med 29, 299–308. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon W. a., Markou A, 2009. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala., Neuropsychopharmacology, pp. 266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskitalo K, Broms U, Heliovaara M, Ripatti S, Surakka I, Perola M, Pitkaniemi J, Peltonen L, Aromaa A, Kaprio J, 2009. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum Mol Genet 18, 4007–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J, 2008. Roles of accessory subunits in α4β2* nicotinic receptors. Molecular Pharmacology, pp. 132–143. [DOI] [PubMed] [Google Scholar]
- Lecca S, Meye FJ, Mameli M, 2014. The lateral habenula in addiction and depression: An anatomical, synaptic and behavioral overview. European Journal of Neuroscience, pp. 1170–1178. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Swan GE, Ring HZ, Khroyan TV, Lerman C, 2004. Genetics and drug use as a complex phenotype. Subst Use Misuse 39, 1515–1569. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE, 2003. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98, 23–31. [DOI] [PubMed] [Google Scholar]
- Lips EH, Gaborieau V, McKay JD, Chabrier A, Hung RJ, Boffetta P, Hashibe M, Zaridze D, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Field JK, Liloglou T, Xinarianos G, McLaughlin J, Liu G, Skorpen F, Elvestad MB, Hveem K, Vatten L, Study E, Benhamou S, Lagiou P, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Castellsague X, Macfarlane TV, Barzan L, Canova C, Lowry R, Conway DI, Znaor A, Healy C, Curado MP, Koifman S, Eluf-Neto J, Matos E, Menezes A, Fernandez L, Metspalu A, Heath S, Lathrop M, Brennan P, 2010. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol 39, 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, Vollenweider P, Preisig M, Wareham NJ, Zhao JH, Loos RJF, Barroso I, Khaw K-T, Grundy S, Barter P, Mahley R, Kesaniemi A, McPherson R, Vincent JB, Strauss J, Kennedy JL, Farmer A, McGuffin P, Day R, Matthews K, Bakke P, Gulsvik A, Lucae S, Ising M, Brueckl T, Horstmann S, Wichmann H-E, Rawal R, Dahmen N, Lamina C, Polasek O, Zgaga L, Huffman J, Campbell S, Kooner J, Chambers JC, Burnett MS, Devaney JM, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein S, Wilson JF, Wild SH, Campbell H, Vitart V, Reilly MP, Li M, Qu L, Wilensky R, Matthai W, Hakonarson HH, Rader DJ, Franke A, Wittig M, Schäfer A, Uda M, Terracciano A, Xiao X, Busonero F, Scheet P, Schlessinger D, St Clair D, Rujescu D, Abecasis GR, Grabe HJ, Teumer A, Völzke H, Petersmann A, John U, Rudan I, Hayward C, Wright AF, Kolcic I, Wright BJ, Thompson JR, Balmforth AJ, Hall AS, Samani NJ, Anderson C. a., Ahmad T, Mathew CG, Parkes M, Satsangi J, Caulfield M, Munroe PB, Farrall M, Dominiczak A, Worthington J, Thomson W, Eyre S, Barton A, Mooser V, Francks C, Marchini J, 2010. Meta-analysis and imputation refines the association of 15q25 with smoking quantity., Nature Genetics, pp. 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Byun JS, Leach P, Fowler CD, Murphy NP, Kenny PJ, Gould TJ, Boulter J, 2013. Targeted deletion of the mouse α2 nicotinic acetylcholine receptor subunit gene (Chrna2) potentiates nicotine-modulated behaviors., The Journal of Neuroscience, pp. 7728–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Goyarzu P, 2009. Rodent models of nicotine withdrawal syndrome., Handbook of Experimental Pharmacology, pp. 401–434. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB, 1992. Rodent model of nicotine abstinence syndrome. Pharmacology, Biochemistry and Behavior, pp. 779–784. [DOI] [PubMed] [Google Scholar]
- Mansvelder H, McGehee DS, 2000. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron, pp. 349–357. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS, 2002. Synaptic Mechanisms Underlie Nicotine-Induced Excitability of Brain Reward Areas. Neuron, pp. 905–919. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS, 2002. Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology, pp. 606–617. [DOI] [PubMed] [Google Scholar]
- Mao D, Gallagher K, McGehee DS, 2011. Nicotine Potentiation of Excitatory Inputs to Ventral Tegmental Area Dopamine Neurons. Journal of Neuroscience, pp. 6710–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M, Pauly J, 1992. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. The Journal of Neuroscience, pp. 2765–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2007. Lateral habenula as a source of negative reward signals in dopamine neurons., Nature. Nature Publishing Group, pp. 1111–1115. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins K. a., Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM, 2007. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology, pp. 269–319. [DOI] [PubMed] [Google Scholar]
- Mazzo F, Pistillo F, Grazioso G, Clementi F, Borgese N, Gotti C, Colombo SF, 2013. Nicotine-Modulated Subunit Stoichiometry Affects Stability and Trafficking of a3b4 Nicotinic Receptor. Journal of Neuroscience, pp. 12316–12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee D, Heath M, Gelber S, Devay P, Role LW, 1995. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science, pp. 1692–1696. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW, 1995. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology, pp. 521–556. [DOI] [PubMed] [Google Scholar]
- Mclaughlin I, Dani JA, Biasi MD, 2015. Nicotine Withdrawal. The Neuropharmacology of Nicotine Dependence, pp. 99–123. [Google Scholar]
- McLaughlin I, Dani JA, De Biasi M, 2017. The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. Journal of Neurochemistry, pp. 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Trusel M, Soiza-Reilly M, Mameli M, 2017. Neural circuit adaptations during drug withdrawal — Spotlight on the lateral habenula. Pharmacology Biochemistry and Behavior, pp. 87–93. [DOI] [PubMed] [Google Scholar]
- Morales-Perez CL, Noviello CM, Hibbs RE, 2016. X-ray structure of the human alpha4beta2 nicotinic receptor. Nature 538, 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC, 2008. Genes and cigarette smoking. Addiction 103, 893–904. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Lester HA, 2006. CNS Localization of Neuronal Nicotinic Receptors. Journal of Molecular Neuroscience, pp. 181–184. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA, 2007. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path., The Journal of Neuroscience, pp. 8202–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak G, Seeman P, Foll BL, 2010. Exposure to nicotine produces an increase in dopamine D2 High receptors: A possible mechanism for dopamine hypersensitivity. International Journal of Neuroscience, pp. 691–697. [DOI] [PubMed] [Google Scholar]
- Olfson E, Saccone NL, Johnson EO, Chen L-S, Culverhouse R, Doheny K, Foltz SM, Fox L, Gogarten SM, Hartz S, Hetrick K, Laurie CC, Marosy B, Amin N, Arnett D, Barr RG, Bartz TM, Bertelsen S, Borecki IB, Brown MR, Chasman DI, van Duijn CM, Feitosa MF, Fox ER, Franceschini N, Franco OH, Grove ML, Guo X, Hofman A, Kardia SLR, Morrison AC, Musani SK, Psaty BM, Rao DC, Reiner AP, Rice K, Ridker PM, Rose LM, Schick UM, Schwander K, Uitterlinden AG, Vojinovic D, Wang J-C, Ware EB, Wilson G, Yao J, Zhao W, Breslau N, Hatsukami D, Stitzel JA, Rice J, Goate A, Bierut LJ, 2015. Rare, low frequency and common coding variants in CHRNA5 and their contribution to nicotine dependence in European and African Americans. Molecular Psychiatry. Nature Publishing Group, pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A, Dani JA, 2018. Convergent Neuronal Plasticity and Metaplasticity Mechanisms of Stress, Nicotine, and Alcohol. Annu. Rev. Pharmacol. Toxicol, pp. 547–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Bertrand S, Binzoni T, Bertrand D, 1996. Neuronal nicotinic α7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. Journal of Physiology, pp. 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Liu L, Ngolab J, Zhao-Shea R, McIntosh JM, Gardner PD, Tapper AR, 2016. Habenula cholinergic neurons regulate anxiety during nicotine withdrawal via nicotinic acetylcholine receptors. Neuropharmacology, pp. 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, 2014. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochemical Pharmacology. Elsevier Inc., pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J, 1994. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Molecular Pharmacology, pp. 523–530. [PubMed] [Google Scholar]
- Perez E, Quijano-Cardé N, De Biasi M, 2015. Nicotinic Mechanisms Modulate Ethanol Withdrawal and Modify Time Course and Symptoms Severity of Simultaneous Withdrawal from Alcohol and Nicotine. Neuropsychopharmacology, pp. 2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH, 2008. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in Neurobiology, pp. 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP, 1998. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine., Nature, pp. 173–177. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, De Biasi M, Williams JT, Dani JA, 1997. Nicotine activates and desensitizes midbrain dopamine neurons. Nature, pp. 295–316. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA, 2004. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction., Learning & Memory, pp. 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistillo F, Clementi F, Zoli M, Gotti C, 2015. Nicotinic, glutamatergic and dopaminergic synaptic transmission and plasticity in the mesocorticolimbic system: Focus on nicotine effects. Progress in Neurobiology. Elsevier Ltd, pp. 1–27. [DOI] [PubMed] [Google Scholar]
- Pistillo F, Fasoli F, Moretti M, McClure-Begley T, Zoli M, Marks MJ, Gotti C, 2016. Chronic nicotine and withdrawal affect glutamatergic but not nicotinic receptor expression in the mesocorticolimbic pathway in a region-specific manner. Pharmacological Research. Academic Press, pp. 167–176. [DOI] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA, 2009. Age dependent nicotinic influences over dopamine neuron synaptic plasticity., Biochemical Pharmacology, pp. 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Benowitz NL, 2016. The Past, Present, and Future of Nicotine Addiction Therapy. Annu Rev Med 67, 467–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RAJ, 2002. Desensitization of neuronal nicotinic receptors. Journal of Neurobiology, pp. 457–478. [DOI] [PubMed] [Google Scholar]
- Quina LA, Harris J, Zeng H, Turner EE, 2017. Specific connections of the interpeduncular subnuclei reveal distinct components of the habenulopeduncular pathway. Journal of Comparative Neurology, pp. 2632–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu C, Qu X, Perlin F, Karlin A, Role L, 1996. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature, pp. 347–351. [DOI] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M, 2011. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes., Neuron. Elsevier Inc., pp. 445–452. [DOI] [PubMed] [Google Scholar]
- Renda A, Nashmi R, 2014. Chronic nicotine pretreatment is sufficient to upregulate α4* nicotinic receptors and increase oral nicotine self-administration in mice. BMC Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, De Biasi M, 2010. The ubiquitin-proteasome system regulates the stability of neuronal nicotinic acetylcholine receptors. J Mol Neurosci 40, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Shim D, De Biasi M, 2007. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. Journal of Neuroscience, pp. 10508–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, 2004. Nicotine amplifies reward-related dopamine signals in striatum., Nature Neuroscience, pp. 583–584. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC, 2003. Drugs of Abuse and Stress Trigger a Common Synaptic Adaptation in Dopamine Neurons. Neuron, pp. 577–582. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ, 2009. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet 150B, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ, 2007. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet 16, 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M, 2004. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit., The Journal of Neuroscience, pp. 10035–10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M, 2009. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice., The Journal of Neuroscience, pp. 3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J.-x., Yakel JL, 2009. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacologica Sinica, pp. 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse S. a., Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF, 2008. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction, pp. 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P-Y, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA, Drenan RM, 2014. Differential Expression and Function of Nicotinic Acetylcholine Receptors in Subdivisions of Medial Habenula. Journal of Neuroscience, pp. 9789–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P-Y, McIntosh JM, Drenan RM, 2015. Nicotine Dependence Reveals Distinct Responses from Neurons and their Resident Nicotinic Receptors in Medial Habenula. Molecular Pharmacology, pp. 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz MR, Amos CI, Dong Q, Lin J, Wu X, 2008. The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst 100, 1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE, 2008. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev 17, 3517–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A, 2012. Role of alpha7- and beta4-containing nicotinic acetylcholine receptors in the affective and somatic aspects of nicotine withdrawal: studies in knockout mice. Behav Genet 42, 423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, 1999. The genetic epidemiology of smoking. Nicotine Tob Res 1 Suppl 2, S51–57; discussion S69-70. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP, 2009. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nature Reviews Drug Discovery, pp. 733–750. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T, Dani JA, 2018. Adolescent Nicotine Exposure Alters GABAAReceptor Signaling in the Ventral Tegmental Area and Increases Adult Ethanol Self-Administration. Cell Reports. ElsevierCompany., pp. 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KKH, de Vegt F, Mulders PFA, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K, 2008. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452, 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ, 2011. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons., Frontiers in Systems Neuroscience, p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.-c., Zhang F, Adamantidis A, Stuber GD, Lecea LD, Deisseroth K, 2009. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science, pp. 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino S, Amador M, Luetje CW, Patrick J, Dani JA, 1992. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron, pp. 127–134. [DOI] [PubMed] [Google Scholar]
- Vernino S, Rogers M, Radcliffe KA, Dani JA, 1994. Quantitative measurement of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. Journal of Neuroscience, pp. 5514–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lindstrom J, 2018. Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. British Journal of Pharmacology, pp. 1805–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JJ, van den Bree M, Munafó MR, 2012. From men to mice: CHRNA5/CHRNA3, smoking behavior and disease., Nicotine & Tobacco Research, pp. 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, 2017. Tobacco Use Disorder. The SAGE Encyclopedia of Abnormal and Clinical Psychology. [Google Scholar]
- WHO, 2017. WHO Report on the Global Tobacco Epidemic. World Health Organization, https://www.who.int/tobacco/global_report/2017/en/. [Google Scholar]
- Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A, 2011. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behavioural Brain Research, pp. 463–465. [DOI] [PubMed] [Google Scholar]
- Wolfman SL, Gill DF, Bogdanic F, Long K, Al-Hasani R, McCall JG, Bruchas MR, McGehee DS, 2018. Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nature Communications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton J. R. a., Pidoplichko VI, Broide RS, Dani JA, 2003. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas., The Journal of Neuroscience, pp. 3176–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li MD, 2016. Converging findings from linkage and association analyses on susceptibility genes for smoking and other addictions. Mol Psychiatry 21, 992–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Doyon WM, Dani JA, 2012. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biological Psychiatry, pp. 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou F-M, Dani JA, 2009. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine., The Journal of Neuroscience, pp. 4035–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, Gao G, Rando OJ, Martin GE, George O, Gardner PD, Tapper AR, 2015. Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal., Nature Communications. Nature Publishing Group, p. 6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Pang X, Gardner PD, Tapper AR, 2013. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Current Biology. Elsevier Ltd, pp. 2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA, 2001. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum., Nature Neuroscience, pp. 1224–1229. [DOI] [PubMed] [Google Scholar]
- Zoli M, Pistillo F, Gotti C, 2015. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. Elsevier Ltd, pp. 302–311. [DOI] [PubMed] [Google Scholar]
- Zoli M, Pucci S, Vilella A, Gotti C, 2018. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Current Neuropharmacology, pp. 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]