Abstract
BACKGROUND:
The purpose of this research study was to evaluate the earliest markers of vocal functioning and neurological development in infants with isolated oral cleft of the lip and/or palate (iCL/P).
METHODS:
Participants were recruited through advertisements and clinic visits at a local mid-western university. A total of eight participants (four unaffected and four with iCL/P), ranging in age from 7.29 to 11.57 weeks, were enrolled and completed demographic and pre-speech measures. A subset of six males (four unaffected and two with iCL/P) successfully completed a structural magnetic resonance imaging scan.
RESULTS:
Patterns of disrupted vocal control and reduced myelinated white matter were found in participants with iCL/P.
CONCLUSIONS:
The findings of this study provide a foundation from which to build further research on the neuronal development of infants with oral clefts: the need to evaluate measures of cortical development, inclusion of information on anesthesia exposure and airway obstruction, and suggestions for avoiding identified pitfalls/blocks to obtaining data are discussed.
INTRODUCTION
Twenty years of research has used neuroimaging techniques to evaluate brain development among youth and adults with isolated oral clefts of the lip and/or palate (iCL/P).1–3 This work has documented structural differences in contrast to unaffected controls and suggests abnormal trajectories of neuronal growth and maturation. Findings demonstrate early reductions in total volume for both the cerebrum and the cerebellum, with these reductions limited to cerebral white matter in boys with iCl/P.2 Yang et al.3 found that infants (ranging from 6 to 24 months) with iCL/P had decreased volume and cortical thickness in the left auditory cortex. Neuronal abnormalities have been correlated to behavior,4 social functioning,5 cognition,6 and speech.7
There are three prevailing theories regarding the etiology of these neurological findings:
Exposure to anesthesia: Early and frequent exposure to anesthesia may be the causal factor resulting in both abnormal brain development and later functioning impairments. Research assessing the relationship of early surgery to later outcomes is limited to a retrospective chart review. Results showed increased exposure to anesthesia (total exposure prior to 6 years of age) being correlated to increased frontal lobe volume and decreased verbal intelligent quotient in later childhood and adolescence.8
Obstructed airway and low oxygenation: An emerging hypothesis is based on increased instances of obstructed airways among children with cleft, often resulting in obstructive sleep apnea.9 There is concern that reductions in oxygenation of the brain in infants may contribute to later cognitive deficits.10 Only one study to date has been conducted looking at outcomes of sleep disturbance at 3 years of age;11 issues in sleep were correlated to lower performance on cognitive tasks.
Disrupted neuronal development (cortical dysplasia): An oral cleft occurs when there is disruption in the migration of cells that make up the lips and palate.12 These cells originate from the same cells that later develop into the brain and other components of the central nervous system. It is hypothesized that there may be concurrent abnormal migration in the developing brain cells, resulting in slightly abnormal neuronal structure and connections, and consequently, disrupted brain functioning.13 Additionally, volumetric differences across the age span are reflective of disrupted neuronal development (disturbed white matter growth and inefficient cortical pruning) in childhood and adolescence.2
To adequately assess the above theories, imaging must be conducted early in life, before exposure to anesthesia and potential impact of lowered oxygenation, with a proxy measure for early language skills. Infant vocalizations (e.g., recordings of cries) have previously been used as an indicator of pre-speech development. The first developmental step of an infant on his way towards speech and language is to control and modify air flow for phonation under changing subglottal pressure condition.14 This is typically reflected by cry melody breaking, sometimes call “interrupted phonation,”15 generated through oscillatory pauses of the vocal folds or through laryngeal constriction phenomena.16,17 The infant’s ability to segment single cry utterances by complete (glottal stop) or incomplete laryngeal constriction (melody breaking) is thought to characterize early vocal development and to be an important prerequisite skill for later syllable production.18
Central integration of information from different brain regions is necessary to coordinate and fine tune respire-laryngeal interaction involved in cry production.17 This process starts during the first days of life,14 and well-coordinated interaction between phonation and supra-laryngeal activity in crying can be found starting at ~8 weeks of life.19 In contrast to the old “brainstem model” of cry production, which suggests that no structures rostral to the midbrain are required for infant crying, now scientists recognize the involvement of cortical brain structures.20 Recent findings confirmed that the production of laryngeal constriction is a regularly occurring developmental phenomenon in healthy infants’ spontaneous crying over the first 6 months,18 while an increased occurrence of melody contour breaking by one or more laryngeal constrictions within a cry utterance was found to indicate a developmental delay in vocal control.17 In a study of 21 infants with orofacial clefts and 50 typically developing infants in the second month of life,17 a significantly higher frequency of occurrence of melody breaking due to laryngeal constriction was found in the infants with iCL/P, independent of hearing performance and sex. This suggested delayed respiratory-vocal control in iCL/P infants.
The purpose of the current study was to conduct the first systematic evaluation of these theories using neural imaging and vocalization at the earliest known age. Specifically, this study sought to determine if there are differences in brain structure and vocal development between infants with iCL/P and unaffected peers at 7–11 weeks of age, before exposure to anesthesia. Given the preliminary nature of this study and small sample size, only descriptive information is presented. However, this is the first study of its kind and this information is highly significant in providing a foundation and direction for future evaluation of infant development in this population.
METHODS
Participants
Unaffected participants were recruited through an email advertisement, and participants with iCL/P were recruited through a clinic at the Local University Hospital. Interested families contacted the PI and were sent detailed information about the study procedures and screening questions. All infants with neurological issues (e.g., epilepsy, brain tumor, brain trauma), premature birth (i.e., born before 37 weeks gestation), low birth weight, or metal in their body were excluded. Additionally, infants with iCL/P could not be suspected of having a genetic disorder or syndrome.
Attempts were made to schedule appointments on the same day as clinic visits (when helpful for the family) and during the infant’s typical nap time. Testing took place during a single 3-h visit to the Local University Hospital. A total of eight participants (four iCL/P and four unaffected) were recruited and successfully scheduled. Demographic variables are present in Table 1. All infants, except one, were males. Successful completion of the magnetic resonance imaging (MRI) protocol was accomplished in all unaffected participants and two infants with iCL/P. Two infants with iCL/P did not fall asleep for the MRI scan and a subsequent attempt could not be scheduled.
Table 1.
Demographic information, cry utterances, and structural brain volumes.
| iCL/P | Unaffected | |||||||
|---|---|---|---|---|---|---|---|---|
| iCL/P1 | iCL/P2 | iCL/P3 | iCL/P4 | HC1 | HC2 | HC3 | HC4 | |
| Demographics | ||||||||
| Gestational age at birth | 36.57 | 40.71 | 40.00 | 38.57 | 40.71 | 38.00 | 40.00 | 38.00 |
| Age at participation | 11.57 | 7.43 | 7.29 | 8.71 | 7.86 | 8.14 | 7.86 | 9.71 |
| Height | 48.5 | 58.42 | 58.42 | 55.88 | 58.50 | 60.96 | 56 | |
| Weight | 5.20 | 4.74 | 4.91 | 4.68 | 5.65 | 4.38 | 5.20 | 4.74 |
| Head circumference | 36.00 | 40.00 | 38.10 | 39.50 | 38.74 | 39.00 | ||
| Parental SES | 2 | 2 | 3 | 3 | 3 | 2 | 2 | 2 |
| Age of mother at birth | 32 | 37 | 31 | 35 | 24 | 32 | 29 | 32 |
| Age of father at birth | 35 | 35 | 34 | 36 | 27 | 32 | 27 | 28 |
| Cry utterances | ||||||||
| Total utterances | 19 | 11 | 21 | 14 | 26 | 21 | 28 | 36 |
| Mean (SD) length of utterances | 1.54 (0.8) | 2.72 (1.0) | 1.54 (0.5) | 2.64 (1.6) | 1.49 (0.5) | 1.58 (0.6) | 0.96 (0.7) | 1.36 (0.5) |
| Utterances with melody breaking | 14 | 11 | 10 | 4 | 4 | 3 | 2 | 5 |
| Percentage of segmented utterances | 73.7 | 100 | 47.6 | 28.6 | 15.4 | 14.3 | 7.1 | 13.9 |
| Structural brain volumes | ||||||||
| Total brain tissue | 491,634 | 520,593 | 544,365 | 494,119 | 516,520 | 530,059 | ||
| Total cerebral tissue | 457,121 | 476,493 | 498,449 | 454,530 | 477,944 | 483,220 | ||
| Surface gray matter | 178,783 | 148,105 | 173,721 | 211,648 | 207,280 | 221,903 | ||
| Myelinated white matter | 20,295 | 23,188 | 25,994 | 32,712 | 33,219 | 27,578 | ||
| Unmyelinated white matter | 258,043 | 305,200 | 298,734 | 210,170 | 237,445 | 233,739 | ||
| Total cerebellar tissue | 34,513 | 44,100 | 45,916 | 39,589 | 38,576 | 46,839 | ||
| Gray matter | 30,794 | 40,282 | 39,336 | 35,420 | 34,007 | 43,306 | ||
| White matter | 3719 | 3818 | 6580 | 4169 | 4569 | 3533 | ||
Notes: Given that only one participant was female, sex is not reported to protect confidentiality. Gestational age and age at participation in weeks. Height and head circumference in cm. Weight in kg. Age of parents in years. Length of utterances in seconds. Brain volumes are in mm3.
SES socioeconomic status.
Measures
Vocal recording.
Spontaneously uttered crying in pain-free conditions was recorded for each infant during their research visit. The digital cry recordings (48 kHz, 16 bit) were obtained in the infant testing laboratory. All the recordings were made using a portable TASCAM (DR-100) recorder and an external Earthworks microphone (TC20) during individual mother–child interactions (mainly shortly before feeding). Cry recording began when an infant started to fuss at a time when the mother would normally feed her baby. The duration of an individual recording session ranged from ~1 to 3 min.
Magnetic resonance imaging.
All infants underwent an unsedated MRI scan of their brain. An established protocol was utilized where infants were fed and then rocked to sleep. Once asleep, they were carefully transferred to the MRI scanner and sound-reducing infant earmuffs were placed on their head. The PI remained next to the infant inside the scanner throughout each protocol to monitor movement and safety. The scanning protocol lasted 25 min. Of the eight consented participants, six (all male) successfully completed the MRI scan. All procedures were approved by the local Institutional Review Board and parents signed a consent form prior to participation. Parents were compensated monetarily for the participation of their infant.
Analyses
Vocal control.
A cry utterance was defined as the onset and the offset of identifiable acoustic energy in the waveform that occurred on the expiratory phase of a single respiratory cycle. To identify and measure cry utterances within the original audio file (*.wav), a distinction of expiratory and inspiratory phases as well as background noise is mandatory. A script for a suitable detection algorithm, based on intensity threshold, using the open-source software PRAATv. 6.1.3521 was developed and applied by one of the co-authors (M.E.). All automatic segmentations were double-checked by an audio-visual analysis. Segmentation errors by the detection algorithm, which sometimes occurred in the interrupted cry utterances (melody breaking) with several laryngeal constrictions, were manually corrected by modifying cursor positions for start and end point detection of a cry utterance (see Fig. 1). Then, the final automatic analysis routine for extracting temporal features (cry utterance duration) was applied.
Fig. 1.
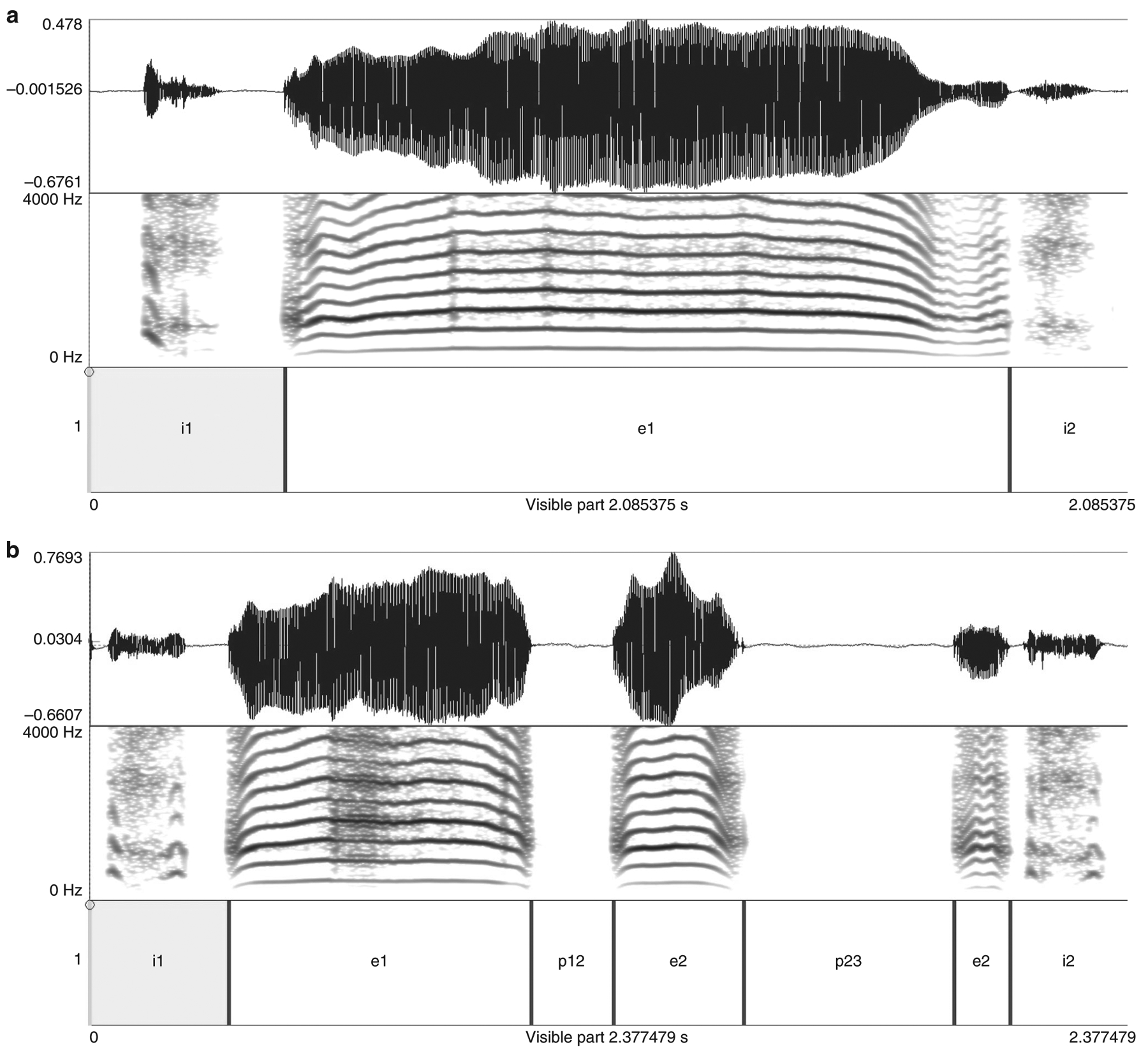
PRAAT display of a cry utterance a without and b with a two-fold melody breaking. For each figure, the top is a time representation of the original wav-file. The middle is a frequency spectrogram (lowest band = melody). The bottom is an annotation of time intervals for automatic extraction of temporal features (inspiratory intervals are marked by i#, expiratory elements are marked by e#, melody breaks are marked by p# [pauses]).
To analyze the degree of melody breaking, each cry utterance was categorized to whether it contained one or more melody-breaking (see Fig. 1) instances.17 The percentage of cry utterances containing an uninterrupted melody (no segmentation) versus those containing instances of melody breaking were calculated per subject and group as the primary outcome data.
Structural imaging and volumetric data.
All MRI data were acquired on a 3 T Siemens Trio scanner (Siemens, Erlangen, Germany) using a 12-channel head coil. The protocol acquired a three-dimensional T1-weighted magnetization-prepared rapid-acquisition gradient-echo sequence in the coronal plane with 1-mm isotropic resolution. A turbo spin-echo T2-weighted sequence was obtained in the coronal plane with 1-mm isotropic resolution.
To provide unbiased quantitative volumetric data, an automated atlas-based segmentation approach to tissue classification was implemented. The automated pipeline consisted of initial registration to bring the age-appropriate atlas into subject-specific coordinates, tissue classification using an expectation-maximization algorithm, followed by a level set segmentation developed to enhance white/gray matter (GM) differentiation in the cerebral cortex, and isolation of results to functionally specific regions for analysis. With the exception of the level set segmentation, all programs used for the pipeline are available through the open-source software suite BRAINSTools.22 The atlas comprises manually corrected tissue maps of neonate subjects taken randomly from the sample population. The pipeline was applied to the images from each subject with successful T1-and T2-weighted magnetic resonance scans.
Quantitative volumetric data were obtained using tissue measurements within the entire head, and within functionally distinct regions (e.g., basal ganglia), they were determined by Talairach atlas space.23 The original label maps generated by the automated pipeline were manually corrected by two independent observers using Slicer Version 4.8.1.24 The following structural regional volumes were generated from the manually corrected label maps for each neonate: GM, myelinated white matter (MWM), unmyelinated white matter (UWM), venous blood, cerebellar GM, and cerebellar white matter.
RESULTS
Description of vocal control (rhythmicity)
A total of 176 cry utterances (111 from unaffected infants and 65 from infants with iCL/P) were available in the originally recorded audio files. For all subjects, mean utterance duration and the proportion of interrupted (segmented) cry utterances were identified and reported (Table 1). As a group, unaffected infants had a mean percentage of 12.6% cry utterances with melody breaking (laryngeal constriction), while for infants with iCL/P, over 60% of cry utterances were interrupted. There was no significant group difference in mean cry utterance duration (Mann–Whitney, p = 0.080).
Description of structural imaging
Volumes (mm3) for gray, white, and total brain tissue are presented in Table 1. Overall, the two infants with iCL/P had a pattern of consistently lower volume for cerebral and cerebellar GM, with a higher volume of cerebral UWM (Fig. 2).
Fig. 2. Map of unmyelinated and myelinated white matter for individual participants.
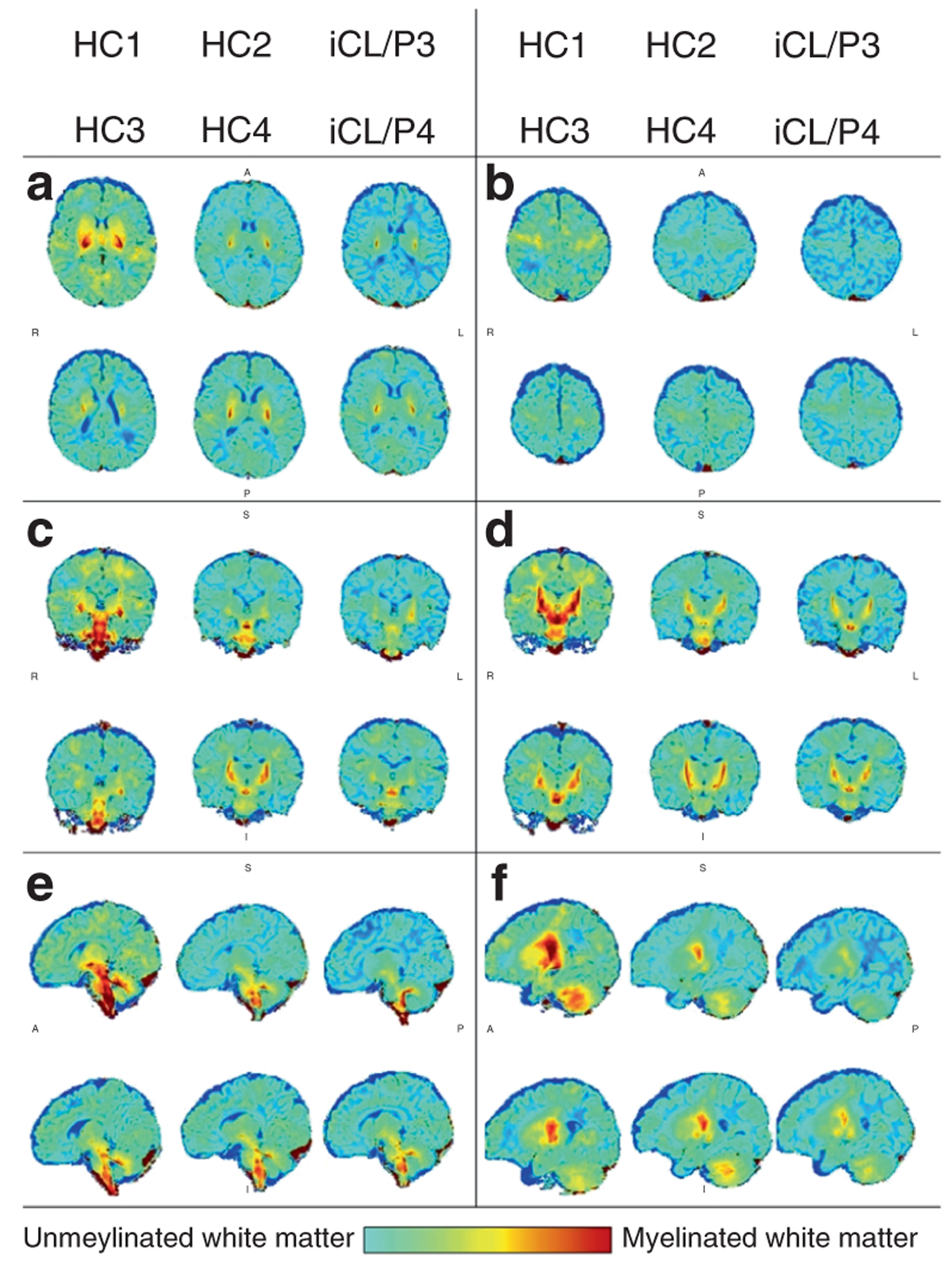
The anterior commissure (AC; 100,133,81) is the center reference point; positive values are superior, right, and anterior to the AC. The following images present individual maps with sagittal slices a (x,y,5) and b (x,y,24); coronal slices b (x,−18,z) and c (x,−22,z); and axial slices d (24,y,z) and (36,y,z).
DISCUSSION
This study introduced a suitable method to investigate the developmental state of respiratory-vocal control (rhythmicity) and structure in young infants. Preliminary data point to potential reduced vocal control and decrements in MWM in infants with iCL/P—consistent with previous research and prior to exposure to anesthesia.
The percentage of melody breaking (intra-cry utterance segmentation) in the affected infants was even higher than that of a previous study17 (mean 62% in the current study vs. 30%), while the mean percentage in the unaffected infants was very similar (mean 13% in the current study vs. 16%). This may point to an intra-individual variability caused by a variation in cleft size between both studies. An additional factor could be that some syndromes associated with oral clefts are difficult to identify at this early age and it is possible that participants in this study may be diagnosed with a syndrome later on.
However, the observed difference in vocal control reflects an existing deviation in the temporal organization (rhythmicity) of laryngeal sound production in infants with clefts. Given the limited lung capacity of infants, air flow must be maintained at a certain level to enable the production of long cry utterances (i.e., muscular coordination). This is comparable to speaking in adults, where the production of very long sentences requires a particularly controlled air flow. Respiratory efforts are considered to be maximal during crying and may push the complex control system of phonation to its limits.14 To generate cry utterances of variable durations, infants must also be able to maintain vocal control through the central nervous system. Failure in achieving this balance leads to unstable neuromuscular activity and consequently the increase of melody breaking (segmented cries). The presence and number of segmented cries, while not currently clinically significant, do act as statistically significant biomarkers of the maturity and fitness of vocal control in infants. The increased segmentation found in infants with cleft for this study could reflect either (1) the specifics of intra-uterine neurodevelopmental processes engaged in brain organization or (2) a maturational delay in neuromuscular coordination.
Similarly, the finding that the two participants with iCL/P had the highest volumes of UWM and collectively less MWM compared to controls prior to exposure to anesthesia may reflect (1) disturbed white matter growth or (2) a more global developmental delay. As discussed earlier, previous work using neuroimaging in children and adults with iCL/P has identified a pattern of disturbed cerebral white matter growth2 and inefficient cortical pruning.25 The pattern of lower cerebral and cerebellar volume is consistent with findings by Yang et al.26 in their study on infants 6 to 24 months old.
While these patterns are intriguing, there are limitations to the current study that must be addressed. Recruitment for the current study was limited by a small number of eligible participants, a tight scheduling window (i.e., 7–11 weeks of age) that fell during an often overwhelming time for families, and difficulties coordinating study procedures with clinic visits (e.g., infants were often tired after being in a clinic and were less likely to sleep deeply for the MRI, clinics frequently ran over). For those who were able to participate and undergo the MRI scan, there were further complications due to the extremely small brain sizes and trying to correctly label regions whose size was smaller than the voxels.
To further evaluate early development and the potential impact of anesthesia and airway obstruction, a longitudinal study is indispensable. For example, a larger sample, a pre-and post-surgery design, and inclusion of oxygen measures will provide more information in the potential role of biology and medical factors in developmental language deficits and brain structure differences found in patients with isolated oral clefts later in life. In designing a study, we would recommend incorporating flexibility as much as possible. This may mean using portable devices (such as functional near-infrared spectroscopy; fNIRS) that can be taken to the families’ home and assessment can take place in a comfortable environment. This would also increase the chances of obtaining successful data post surgery, after 14 months of age, when obtaining an unsedated MRI scan is much more difficult. (There is a trade-off in specificity and detail of data obtained when switching to portable devices, such as fNIRS, and this balance needs to be considered.) In planning the MRI protocol, our data would have benefited from increases in resolution. This could be accomplished through shrinking the field of view or increasing the resolution. Additionally, some protocols (such as diffusion tensor imaging) may be less useful for infants this young (e.g., not enough MWM to obtain meaningful signals). Instead, increasing the efficiency of T1 and T2 structural images and using a MWM/UWM map may provide just as useful information.
Although all postulated trends should be interpreted with caution, preliminary results proved the methodology and justify further research in this field to better understand the impact of isolated oral clefts on neurodevelopment.
IMPACT:
Research in children with isolated oral clefts has demonstrated higher rates of learning disorders connected to subtle differences in brain structure.
There is no work evaluating the potential impact of exposure to anesthesia on development.
This is the first known attempt to evaluate brain structure and function in infants with isolated oral clefts before exposure to anesthesia.
Potential trends of early vocal issues and structural brain differences (less myelinated white matter) were identified in infants with isolated oral clefts compared to unaffected controls.
Differences in brain structure and function in infants with isolated oral clefts may be present before surgery.
ACKNOWLEDGEMENTS
We thank the Cleft Palate Foundation and Dr. Deborah Kacmarynski who provided financial support to this project. We would also like to thank Joel Bruss who went above and beyond to process the youngest brain images our lab has obtained. Finally, we would like to extend our sincerest gratitude to the families who allowed their infants to participate in this study.
Footnotes
Competing interests: The authors declare no competing interests.
Patient consent: Parents/guardians of infants provided written consent for their child to participate.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Nopoulos P et al. Abnormal brain morphology in patients with isolated cleft lip, cleft palate, or both: a preliminary analysis. Cleft Palate Craniofac. J 37, 441–446 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Nopoulos P, Langbehn DR, Canady J, Magnotta V & Richman L Abnormal brain structure in children with isolated clefts of the lip or palate. Arch. Pediatr. Adolesc. Med 161, 753–758 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Yang FF, McPherson B, Shu H, Xie N & Xiang K Structural abnormalities of the central auditory pathway in infants with non-syndromic cleft lip and/or palate. Cleft Palate Craniofac. J (2011). [DOI] [PubMed] [Google Scholar]
- 4.Nopoulos P et al. Hyperactivity, impulsivity, and inattention in boys with cleft lip and palate: relationship to ventromedial prefrontal cortex morphology. J. Neurodev. Disord 2, 235–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boes AD et al. Social function in boys with cleft lip and palate: relationship to ventral frontal cortex morphology. Behav. Brain Res 181, 224–231 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shriver AS, Canady J, Richman L, Andreasen NC & Nopoulos P Structure and function of the superior temporal plane in adult males with cleft lip and palate: pathologic enlargement with no relationship to childhood hearing deficits. J. Child Psychol. Psychiatry 47, 994–1002 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Conrad AL et al. Cerebellum structure differences and relationship to speech in boys and girls with nonsyndromic cleft of the lip and/or palate. Cleft Palate Craniofac. J 47, 469–475 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad AL, Goodwin JW, Choi J, Block RI & Nopoulos P The relationship of exposure to anesthesia on outcomes in children with isolated oral clefts. J. Child Neurol 32, 308–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cielo CM et al. Evolution of obstructive sleep apnea in infants with cleft palate and micrognathia. J. Clin. Sleep Med 12, 979–987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muntz HR Management of sleep apnea in the cleft population. Curr. Opin. Otolaryngol. Head. Neck Surg 20, 518–521 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Smith CB, Walker K, Badawi N, Waters KA & MacLean JE Impact of sleep and breathing in infancy on outcomes at three years of age for children with cleft lip and/or palate. Sleep 37, 919–925 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdi AR in Cleft Lip and Palate (ed. Berkowitz S) 3–12 (Springer, New York, 2006). [Google Scholar]
- 13.Sperber GH First year of life: prenatal craniofacial development. Cleft Palate Craniofac. J 29, 109–111 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Wermke K et al. The vocalist in the crib: the flexibility of respiratory behaviour during crying in healthy neonates. J. Voice (2019). [DOI] [PubMed] [Google Scholar]
- 15.Koopmans-van Beinum F & van der Stelt J in Precursors of Early Speech (eds Lindblom B & Zetterström R) 37–50 (Palgrave Macmillan UK, London, 1986). [Google Scholar]
- 16.Wermke K in International Encyclopedia of the Social & Behavioral Sciences 2nd edn (eds. Wright J) 475–480 (Elsevier, Oxford, 2015). [Google Scholar]
- 17.Wermke K et al. Cry melody in 2-month-old infants with and without clefts. Cleft Palate Craniofac. J 48, 321–330 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Robb MP et al. Laryngeal constriction phenomena in infant vocalizations. J. Speech Lang. Hear. Res 63, 49–58 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Wermke K, Mende W, Manfredi C & Bruscaglioni P Developmental aspects of infant’s cry melody and formants. Med. Eng. Phys 24, 501–514 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Newman JD Neural circuits underlying crying and cry responding in mammals. Behav. Brain Res 182, 155–165 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boersma P & Weenink D PRAAT: doing phonetics by computer (2014).
- 22.Kim R et al. (eds). Clinical Image-Based Procedures. Translational Research in Medical Imaging. CLIP 2015. Lecture Notes in Computer Science (Springer, Cham, 2016). [Google Scholar]
- 23.Talairach J & Tournoux P Co-Planar Stereotaxic Atlas of the Human Brain (Thieme Medical Publishers, New York, 1988). [Google Scholar]
- 24.Fedorov A et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging 30, 1323–1341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nopoulos P et al. Cognitive dysfunction in adult males with non-syndromic clefts of the lip and/or palate. Neuropsychologia 40, 2178–2184 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Yang FF, McPherson B, Shu H & Xiao Y Central auditory nervous system dysfunction in infants with non-syndromic cleft lip and/or palate. Int. J. Pediatr. Otorhinolaryngol 76, 82–89 (2012). [DOI] [PubMed] [Google Scholar]


