Abstract
After decades of being neglected, broad tapeworms now attract growing attention thanks to the increasing number of reports from humans but also thanks to many advancements achieved by application of molecular methods in diagnosis and epidemiological studies. Regarding sparganosis, unfortunately general uniformity of most species, their high intraspecific variability and lack of agreement among researchers has led to confusion about the classification of Spirometra/Sparganum species. For the first time we determined adult, eggs and plerocercoid life cycle stages and the molecular phylogeny of Sparganum proliferum obtained from endangered wild felids (Panthera onca, Leopardus pardalis, Leopardus guttulus and Herpailurus yagoauroundi) in one of the largest continuous remnants of worldwide biodiversity, the Atlantic Forest from South America. Our results showed that at least 57% of total species of wild felids in this natural area could act as definitive hosts of Sparganum proliferum. We conclude that the availability of more morphological characteristics are needed in order to secure reliable characterization and diagnosis of sparganosis. The integration of these data with molecular analysis of mitochondrial DNA sequences will be useful for species discrimination.1
Keywords: Endangered fauna, Sparganum proliferum, Wild carnivores, Mitochondrial genes
Graphical abstract
Highlights
-
•
Mitochondrial sequencing and morphological data integration is useful to determine species in Sparganum.
-
•
First molecular determination of adult parasites form Sparganum proliferum.
-
•
Anatomopathological characters of adult tapeworms of Sparganum proliferum are described for the first time.
-
•
Endangered wild felids are natural hosts of Sparganum proliferum.
-
•
Sparganum proliferum is found in one of the regions with highest worldwide biodiversity.
1. Introduction
Sparganosis is an emerging parasitic zoonotic disease mainly caused by the second larva stage (plerocercoid) of diphyllobothriid cestodes such as, Spirometra ssp. and Sparganum proliferum (Noya et al., 1992; Kokaze et al., 1997; Miyadera et al., 2001; Brabec et al., 2006; Kuchta et al., 2008; Schauer et al., 2014; , Oda et al., 2016; Kikuchi, T. & Maruyama, H. 2020; Kikuchi et al., 2020; Hong et al., 2020). Sparganum proliferum is a cryptic parasite which phylogeny and life cycle are poorly understood. The adult stage of S. proliferum has not been observed and the precise taxonomic relationships of S. proliferum with other tapeworms remain unclear because few genes have been sequenced (Noya et al., 1992; Miyadera et al., 2001; Okamoto et al., 2007). Recently, the genome and transcriptomic analysis of plerocercoid of S. proliferum was reported and confirmed that S. proliferum and Spirometra erinaceieuropaei are closely related but different species (Kikuchi et al., 2020). In addition to taxonomic considerations, the pathogenicity of S. proliferum (proliferative sparganosis) and plerocercoids of Diphyllobothriidae tapeworms, including those of S. erinaceieuropaei (non-proliferative sparganosis) are different (Kikuchi and Maruyama, 2020). Sparganosis cases are reported worldwide, but it has been predominantly diagnosed in Southeast Asia, mainly in China (Dorny et al., 2009; Qiu and Qiu, 2009; Liu et al., 2015). Human sparganosis frequently occurs by consuming raw or undercooked meat of infected reptiles or amphibians, drinking water contaminated with copepods as well as direct contact with the skin of infected frogs or snakes (Li et al., 2011; Liu et al., 2015; Oda et al., 2016; Okino et al., 2017; Zhang et al., 2020). Also, a case of human infection by adult of S. erinaceieuropaei has been reported in Vietnam (Le et al., 2017). In Argentina, three cases of sparganosis have been reported in individuals from border countries. Two with cerebral location (Boero et al., 1991; Jones et al., 2012) and one with cutaneous location (De Roodt et al., 1993). Moreover, in Argentina there are few reports of Spirometra spp. in animals. Spirometra mansonoides has been found in cats (Santa Cruz and Lombardero, 1987), S. erinaceieuropaei in cats (Venturini, 1980, 1989) and dogs (Denegri, 1993). In wildlife, Martínez et al. (2010) has identified eggs of S. mansonoides in the felines F. pardalis, F. yagouaroundi, Panthera onca and Puma concolor. Spirometra has been reported in Pampas fox (Lycalopex gymnocercus) (Reigada et al., 2012; Petrigh et al., 2015). Despite numerous attempts to clarify its taxonomy, host specificity and geographic distribution (Faust et al., 1929; Wardle et al., 1974), the genus remains one of the most complicated groups of tapeworms, and several lines of evidence concluded that it is very difficult and almost impossible to distinguish some of the 50 nominal species of Spirometra based solely on morphological characteristics (Iwata, 1934, 1972; Mueller, 1974; Daly, 1981; Odening, 1985; Kuchta and Scholz, 2017). In Asia, there are several studies of S. erinaceieuropaei lineages (Zhang et al., 2016), particularly in wild frogs in China the prevalence is above 10% in some regions. (Hong et al., 2020; Zhang et al., 2017, 2020). Regarding Africa, there are molecular reports of findings of Spirometra sp. in human infections in South Sudan and Ethiopia (Eberhard, 2015). In Europe, there are also molecular records of S. erinaceieuropaei plerocercoid larvae in wild fauna from Poland (Kołodziej-Sobocińska et al., 2019). In Brazil, there have been reports of Spirometra spp. larval stages in cold-blooded animals (Rego and Schäffer, 1992) humans (Liu et al., 2015) and adult stages in wild felids (Vieira et al., 2008). The occurrence of a particular Spirometra lineage in South America has been reported (Almeida et al., 2016) and the molecular sequences obtained were phylogenetically cluster in a separate node and distant to the Asian S. erinaceieuropaei lineage.
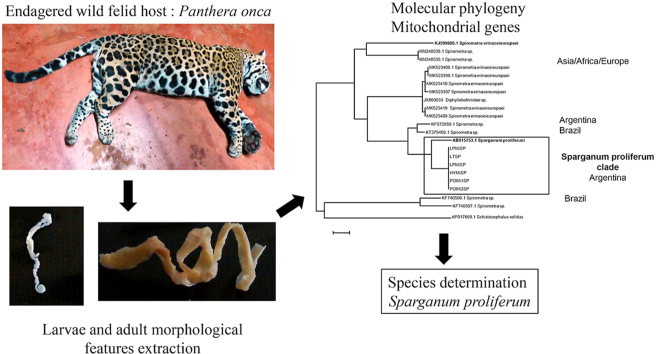
The objective of this work is to identify and characterize diphyllobothriid infections in wild animals of the Atlantic Forest of Misiones, Argentina through an integrative approach that links morphological, genetic and ecological aspects. Here we report, for the first time, the presence of adult and eggs of S. proliferum in wild animals in South America, confirmed by molecular analysis. Our results could be useful to understand some of the underlying aspects of the life cycle of S. proliferum and evaluate the zoonotic importance in the interface areas to guide prevention measures for human and animal welfare.
2. Methods
2.1. Study area
The study area contains one of the largest continuous remnants of Atlantic Forest (AF) in the World. It is located in northern Misiones province, Argentina, (54°15′30.60″W, 25°55′52.32″S). The area is 220 m in altitude and presents subtropical climate with annual rain precipitations between 1700 and 2100 mm (Ligier, 2000).
2.2. Animal samples
Road-killed animals were actively searched on national routes 101 and 12 that cross the Iguazú National Park between the years 2015–2016. Animal necropsies were carried out under approved protocols by the National Parks Administration technical office (NEA 423 Rnv ex DCM 483 Dispo 23/2015). Only 1–2 days old animal carcasses were selected for sampling. Four animals were collected and analyzed in this work and are summarized in Table 1. Each animal was individually packed and labelled with relevant information including place of origin, sampling date, age category, and sex of the animal.
Table 1.
Percentage divergence between mitochondrial sequences from samples of wildlife Sparganum proliferum and reference genes of Spirometra erinaceieuropaei (KJ599680) and Sparganum proliferum (AB015753).
| Sample ID | Host& | Parasitic stage in host | Percentage Divergence (SE)+ |
|||||
|---|---|---|---|---|---|---|---|---|
| Cox1 |
Nad1 |
Atp6 |
||||||
| Spirometra erinaceieuropaei | Sparganum proliferum | Spirometra erinaceieuropaei | Sparganum proliferum | Spirometra erinaceieuropaei | Sparganum proliferum | |||
| LPMiSP | Ocelot | Adult | 14.4 (2.4) | 4.2 (1.3) | 12.5 (2.3) | --* | 26.9 (2.6) | --* |
| LTMiSP | Tirica | Plerocercoid | 14.4 (2.4) | 4.2 (1.3) | 12.5 (2.3) | --* | 26.9 (2.6) | --* |
| HYMiSP | Yaguarundi | Adult (fragment) | 14.4 (2.4) | 4.2 (1.3) | nd | --* | nd | --* |
| POMiSP1 | Jaguar | Adult | 14.4 (2.4) | 4.2 (1.3) | nd | --* | nd | --* |
| POMiSP2 | Jaguar | Adult (fragment) | 14.4 (2.4) | 4.2 (1.3) | nd | --* | nd | --* |
*There is no sequence information from Sparganum proliferum (AB015753) nad1 and atp6 genes.
+Pairwise genetic distance was calculated with MEGA7 using Tamura and Nei (1993) model.
&POMiSP1 and POMiSP2 samples belongs to the same individual host.
2.3. Parasite samples
The intestinal tracts of the analyzed carnivores were carefully removed from each carcass and subsequently isolated by ligatures (pylorus and rectum). All samples were kept at −20 °C for at least 1 month prior to processing in order to inactivate possible parasite eggs from other species (Scioscia et al., 2013). Examination of the intestinal content was performed as previously (Arrabal et al., 2017) using the modification of the technique described originally by Eckert (2001). Briefly, the small intestine was separated from the large intestine, and then each section was placed in different trays and cut lengthwise. Coarse material and large parasites of the small intestine were removed. Then, each section was immersed in 9% saline solution at 37 °C for 30 min. Intestinal walls were scraped with a microscope slide, and all the content of each section were poured into individual glass bottles and left to stand for 20 min. The supernatant was discarded and physiological saline solution was added to dilute the sediments. This procedure was repeated several times until the supernatant was almost translucent. Obtained sediments were examined in small portions of 5–10 ml round petri dishes with magnifier lens at × 65 to identify small helminths. The helminths found were cleaned with saline solution and deposited in recipients with either 4% formalin or 70% ethanol for further taxonomic and molecular examination, respectively.
2.4. Morphology studies
Strobilas of adult tapeworms, larvae and eggs were analyzed under optical Primo Star (Carl Zeiss Gmbh, Gӧttingen, Germany) microscope using Axion Cam ERc 5s camera (Carl Zeiss Gmbh, Gӧttingen, Germany). Each sample was whole mounted and registered with 4 × , 10 × and 40 × using Carl Zeiss Vision software for image analysis. Moreover, strobilas and larvae tissue sections were prepared in paraffin and were sectioned in serial sections of 4–5 μm, mounted on glass slides, and stained with hematoxylin-eosin (HE). The slides were analyzed under optical microscope and picture was taken at 4 × , 10 × and 40 × . The main features analyzed in the larvae were pleomorphism, color, symmetry and presence or not of scolex (Noya et al., 1992). The main features analyzed in eggs were size, shape and presence or not of cap and pointed ends (Mueller, 1936).
2.5. Molecular identification and phylogenetic analysis
Total parasite genomic DNA was obtained using the DNeasy Blood &Tissue Kit (Qiagen GmbH, Hilden, Germany). Three molecular markers from mitochondrial genome were used to determine species. Cytochrome c oxidase subunit I (cox1) gene, NADH dehydrogenase subunit 1 (nad1) gene and ATP synthase subunit 6 (atp6) gene were selected since we know and used them in previously reports from cestodes (Kamenetzky et al., 2002; Arrabal et al., 2017) and were demonstrated to be useful to classify isolates of Spirometra in previously reports (Almeida et al., 2016). The genes were amplified by polymerase chain reaction according to Arrabal et al. (2017) (Supplementary Table 1). The PCR-product obtained was sequenced and firstly aligned with ClustalX (v2.0.12) with Spirometra and Sparganum sequences extracted from complete mitochondrial genomes available on GenBank and considered as reference genomes (International Helminth Genomes Consortium, 2019). To get insight into an accurate phylogenetic analysis of our parasite linage we downloaded cox1 sequences form Asia, Africa, Europe and South America totalling 275 cox1 sequences (Lavikainen et al., 2013; Zhang et al., 2017, 2020; Jeon and Eom, 2019; Kołodziej-Sobocińska et al., 2019; Hong et al., 2020). After several sequence redundancy removal 42 cox1 sequences were retained. These data set includes Spirometra cox1 sequences from wild frogs that were described to have a high pairwise genetic distance with the reference mitochondrial genome (Zhang et al., 2017). Multiple alignments were edited with BioEdit (v7.1.3). Maximum likelihood phylogeny was performed using MEGA7. A discrete gamma distribution was used to model evolutionary rate differences among sites. Branch lengths were measured as the number of substitutions per site. All positions with less than 80% site coverage were eliminated. There was a total of 296 positions in the final dataset. Additionally, Bayesian phylogeny was implemented by using BEAST. Substitution model HKY + G + X with gamma distribution was selected with PartitionFinder. Changes in the evolutionary rates among branches were performed by using random local clock model (Drummond and Suchard, 2010). For earlier tree a basic coalescent model was selected. MCMC run was performed with tree parameter values sampled every 1000 steps over a total of 100,000.000 steps (Zhang et al., 2017).
3. Results
3.1. Morphological identification of Sparganum proliferum in wild carnivores
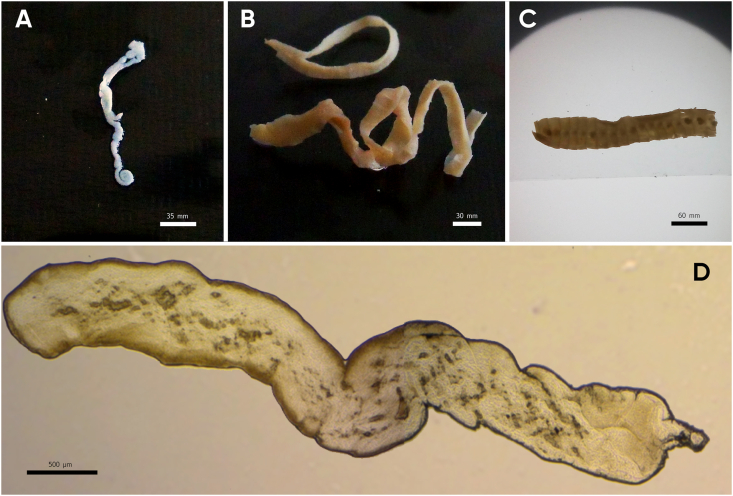
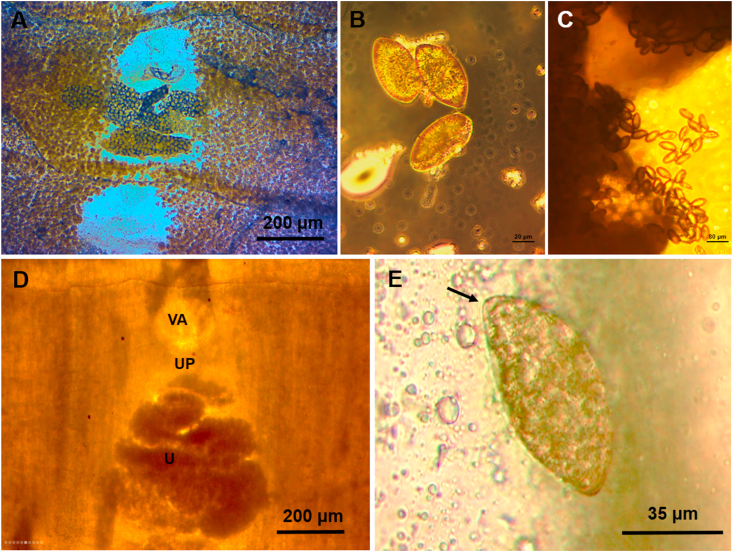
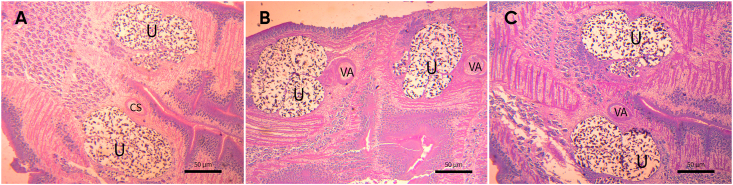
The analysis for intestinal tracts of wild carnivores allowed as isolating tapeworms morphologically compatible to Spirometra in wild carnivores, this being the first report of this parasites in the eco-region of Atlantic Forest. One Leopardus pardalis (ocelot), one Panthera onca (jaguar), one Leopardus guttulus (tirica) and one Herpailurus yagoauroundi (yaguarundi) (Fig. 1). Parasites were identified according to morphological features, the individual selected for further analysis has a resemblance with Spirometra by their general appearance and size (Fig. 2). The larva has the following major macroscopic features: pleomorphism, white color; length <5 mm, lack of bilateral symmetry and without scolex (Fig. 2D) accordingly to previously Sparganum proliferum larvae features described so far (Noya et al., 1992). Although numerous worms were found it was not feasible to identify all specimens based on morphological features because most of them were fragmented and were not suitable for morphological examination. Regarding strobilas corresponding to adult tapeworms the major differentiating features of the eggs found were land shape and the evident cap and pointed ends attributable to the genus (Mueller, 1936) (Fig. 3). The average eggs measures were 67.02 μm by 34.95 μm (n = 50). Histological sections of strobilas were performed. The main characteristics (based in mature and gravid proglottids) were i) presence of anterior and posterior uterine coils in the longitudinal median line of the proglottids ii) ventral middle uterine pore in the third of the gravid proglottid iii) uterus opened by a pore well separated from and posterior to the vagina, and presence a varying number of loops in the terminal heavy walled portion in an “S” shape (iv) uterus consisted of 5–7 loops and the dumbbell-shaped ovary connected to the uterus and situated near the posterior margin v) vagina passed traversing from its vestibule in an approximately straight path in the median line thrown into lateral undulations of different amplitude viii) cirrus surrounded by the seminal receptacle and opens out separately from vagina and near to the uterine pore (Fig. 4). In this section the ratio of width and length of gravid proglottids and uterine morphology were consistent to Spirometra spp. (Iwata., 1972; Mueller, 1974).
Fig. 1.
Animals road-killed in Iguazú National Park, Misiones, Argentina. The intestinal tracts of each carnivore felid were necropsied and parasites removed from intestine. A- Panthera onca (jaguar); B-Herpailurus yagouaroundi (yaguarundí); C-Leopardus guttulus (tirica); D-Leopardus pardalis (ocelot).
Fig. 2.
Sparganum proliferum worms from intestinal tracts of wild cats. A- Larvae from tirica; B- Adult from ocelot; C- Adult from jaguar; D- Larva or plerocercoid from tirica. (A-C. Macroscopical images, D. Stereoscopic magnifying glass image).
Fig. 3.
Whole mounted samples from Sparganum proliferum adult found in wild felids host A- Proglottids showing uterus with eggs from ocelot. B, C and E − Light brown eggs with evident cap and pointed ends attributable to the genus from jaguar. D- Gravid uterus from ocelot. *VA: vagina; UP: uterine pore; U: uterus. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Histological cut from mature proglottid showing the uterus (U) and vagina (VA) and cirrus sac (CS). Showing the cirrus (C), uterus (U), genital pore (GP), vaginal pore (VP), uterine pore (UP), and ovary (OV). Aceto carmine stain.
3.2. Molecular characterization of Sparganum proliferum in wild felids
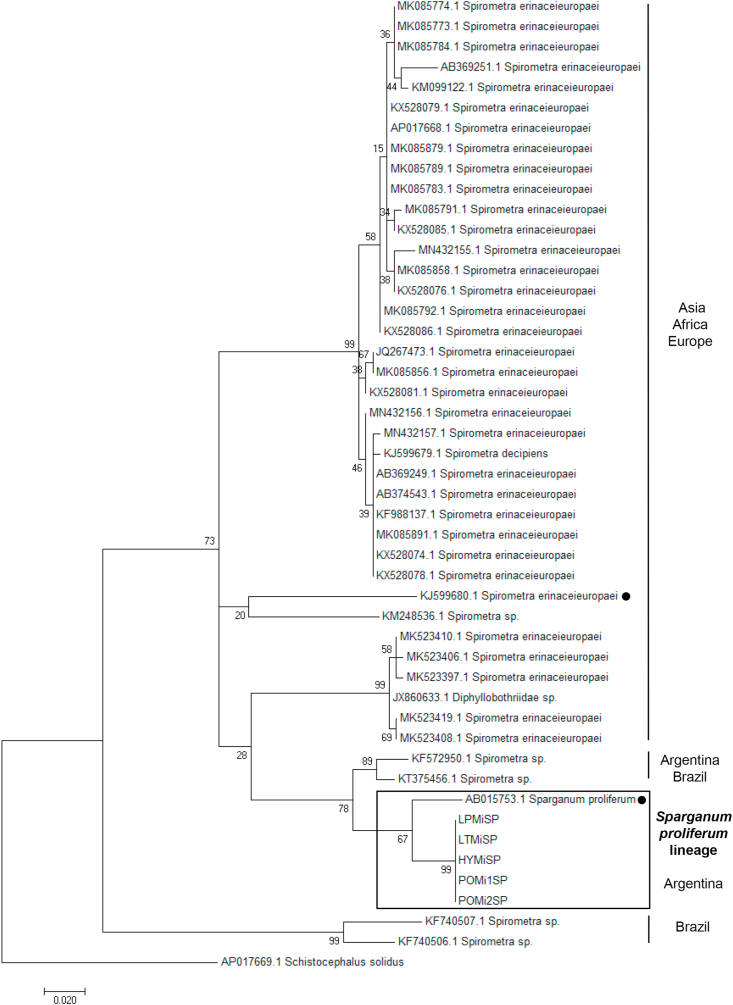
First, we analyzed by PCR and sequencing the adult obtained from the ocelot (sample LPMiSP) by three molecular markers. The sequences obtained from cox1 (295 nt), nad1 (343 nt) and atp6 (594 nt) mitochondrial genes were concatenated resulting in a dataset of 1322 nucleotides to analyze the complete information in an integrated phylogeny. Multiple sequence alignment comparisons with all mitochondrial reference genomes were performed in order to identify the ocelot mitochondrial sequence. Redundant reference sequences were removed and a total of 10 orthologous sequences from mitochondrial complete genomes were finally included (Supplementary Table 2). The phylogenetic tree constructed based on the multiple alignment showed that LPMiSP belongs to Spirometra lineage near to S. erinaceieuropaei (KJ599680) isolated from a human in Korea (Supplementary Fig. 1). The genetic divergence between LPMiSP and S. erinaceieuropaei was 14.4% for cox1, 12.5% for nad1 and 26.9% for atp6 (Table 1). Since S. erinaceieuropaei cox1 non redundant sequences available in GenBank have an average genetic divergence of 8.8% and the genetic distance obtained between LPMiSP and Spirometra spp. was relatively higher (14.4%) we couldn't classify it as belonging to the same species. To get insight the presence of Spirometra in wild felids we assessed to amplify and sequence the same three molecular makers from more samples. The cox1 sequences from jaguar (samples POMiSP1 and POMiSP2), tirica (sample LTMiSP) and yaguarundi (sample HYMiSP) and additional nad1 sequence from sample LTMiSP were obtained. The nad1 sequence obtained from tirica host was 100% identical to the previously sequenced obtained from LPMiSP-nad1. Additionally, all cox1 sequences were 100% identical to each other. Since atp6 was not possible to be amplified, we hypothesize that several SNPs are present between mitochondrial genomes from Argentinean wild felids and Spirometra spp. mitochondrial genomes reported, and may be different species. To test this hypothesis, we retrieve a broader set of cox1 sequences available for Spirometra/Sparganum in GenBank and performed multiple alignments. The number of SNPs between cox1 sequences from parasites from Argentinean wild hosts is shown in Supplementary Fig. 2. One interesting finding was that cox1 sequences from wild felids from Argentina have a genetic divergence of 4.2% with Sparganum proliferum cox1 sequence (AB015753) (Table 1). This finding was consistent with the phylogeny obtained from the multiple sequences alignment. Even the tree topology indicates that a taxonomic revision of some sample is needed (some Spirometra decipiens clustered with S. erinaceieuropaei sequences). Parasite samples obtained in this work shared common ancestor with Sparganum proliferum (Fig. 5). Besides this, the sequences that were characterized as Sparganum, including those obtained in this study, are included within the same clade as a Spirometra lineage registered in South America (KF572950 and KT375456). These sequences have 6.4% and 6.7% of genetic divergence with LPMiSP, respectively. The Spirometra sequences from the next near node (e. g. KF988137) have 12.0% genetic divergence with LPMiSP. Taking into account the tree topology and the genetic distance between Sparganum and Spirometra cox1 sequences we suggest that KF572950 and KT375456 accession numbers also belongs to S. proliferum species. We confirmed our results by Bayesian phylogenetic analysis (Supplementary Fig. 3). In this phylogeny numbers along branches indicate posterior probabilities that support the groups mentioned before. Moreover, the effective sample size (ESS) values for all parameters were above 200 giving confidence to the analysis.
Fig. 5.
Sparganum proliferum COX1 phylogeny. A total of 48 sequences from different host species and geographic origin were analyzed by Maximum Likelihood method including 296 positions in the final dataset. Genbank accession number are shown. The codes of the samples obtained in this work are the same as Table 1. References species are marked with a black dot.
4. Discussion
After decades of being neglected, broad tapeworms now attract growing attention thanks to the increasing number of human cases but also thanks to considerable advance achieved by application of molecular methods in diagnosis and epidemiological studies (Scholz et al., 2019). Regarding sparganosis, general uniformity of most species, their high intraspecific variability and lack of agreement among investigators has led to confusion about the classification of Spirometra/Sparganum (Mueller, 1974; Daly, 1981; Kuchta and Scholz, 2017). Moreover, most of the available material was obtained from host examined long time post mortem or even from decomposed carcasses, which may have caused significant morphological changes (Hernández-Orts et al., 2015). As a result, morphological and biometrical data in some species descriptions may be misleading. Similarly, most clinical samples of larval stages were not characterized molecularly and were described under different names. This work showed that S. proliferum and S. erinaceieuropaei species have dissimilar cox1, nad1 and atp6 sequences. The molecular results of this work are in concordance with previous analysis where both species were clearly distinguished by cox1, nuclear sdhB and 18 S rDNA V2 region gene sequencing (Miyadera et al., 2001; Kikuchi et al., 2020). Also, discrepancies in Spirometra phylogeny and a possible new species were also reported by Zhang et al. (2017, 2020) studying parasites from wild fauna. Moreover, the low identity and high genetic distance between atp6 sequences obtained from ocelot (LPMiSP) and the reference atp6 sequence from S. erinaceieuropaei support these findings. Mitochondrial atp6 sequences from S. proliferum are not available in public databases to make the necessary comparisons with the results obtained in the present work. The isolates analyzed in this work are not closely related to S. erinaceieuropaei or other Asian Spirometra lineage, but instead, might display close affinities to one of the lineages described as Spirometra from South America and with S. proliferum (AB015753) mitochondrial reference genome. Our source of parasites are dead animals on the road, it should be noted that the high temperature of the region under study favours the decomposition rate of carcasses. For this reason, helminth specimens not suitable for ideal morphological identification are the most common outcome. We overcome these problems by implementing an integration of morphological and molecular data analysis. Morphologically similar species presenting a spiralled uterus (S. decipiens, S. gracilis, S. longicollis, and S. mansoni) were reported in wild felids from Brazil, as well as in proglottids of Spirometra spp. (Almeida et al., 2016); however, the vagina of S. erinaceieuropei is considered to lie next to the midline and descends in waves of different amplitude (Palmer et al., 2008). Also, the shape of the uterus lacks uniformity in the number of turns (between three and seven loops) having irregular arrangement and size (Iwata, 1932; Mueller, 1974; Okino et al., 2017). Our results showed that the proglottids of S. proliferum, and also the eggs found, presented the same morphological characteristics that S. erinaceieuropei and it is because of that, these species need to be evaluated using molecular markers. Recently, Kuchta et al. (in press) suggested Sparganum proliferum belongs to a lineage of S. decipiens described in South America. However, since their results are based only in genetic data and we analyzed not only this data but also novel adult morphological features that classification may be revised. More isolates analyzed with other molecular markers such as nuclear genes or complete genome sequences are needed to confirm the presence of S. proliferum in wild hosts. meanwhile the three mitochondrial genes employed in this work could be used as molecular markers for epidemiology studies. The sequence comparison among Spirometra from Brazil and Sparganum from Argentina indicates that they are different lineages. Species of Sparganum occur in warmer latitudes similar to the region analyzed here (Mueller, 1974; Daly, 1981). Fatal proliferative sparganosis was reported from domestic cats in North America (Buergelt et al., 1984; Woldemeskel, 2014) and dogs in Europe (Stief and Enge, 2011). However, the impact of diphyllobothriid cestodes in wild animals is not clear yet. Our findings showed for the first time S. proliferum adults and larvae in the intestine track of the wild felids. In Spirometra species it was already described that once in the secondary vertebrate host, the procercoid can develop into a plerocercoid larva in different tissues, which can survive predation and reach a wide variety of vertebrates (Mueller, 1974; Opuni and Muller, 1974; Liu et al., 2015). We found S. proliferum plerocercoids larvae in tirica intestine, indicating that the preys it feeds on are harbouring plerocercoids that survive the gastric digestion. Regarding the unknown complete life cycle of S. proliferum, the human activities in the region under study like the conversion of natural landscapes to urban areas may increase predation by domestic dogs and cats on wild amphibian and reptile populations, thus potentially enhancing the incidence of proliferative sparganosis (Borteiro et al., 2015). The possible role of amphibians and reptiles may have in the occurrence of human cases in South America is an issue not being well investigated yet. In Argentina, there are few reports of Spirometra spp. mostly in domestic definitive hosts Venturini (1980, 1989); Santa Cruz and Lombardero (1987); Denegri (1993). Regarding wildlife, S. mansonoides eggs were reported in felids (Martínez et al., 2010) and Spirometra spp. in the Pampas fox (Lycalopex gymnocercus) (Reigada et al., 2012; Scioscia et al., 2014; Petrigh et al., 2015). The present work showed that at least four different species of wild felids, out of the six existing in the natural area under study are involved in the sylvatic life cycle of S. proliferum and could act as definitive hosts in the Atlantic Forest. This region is shared with other groups of carnivores (canids, mustelids and procyonids) that could also be participating in the cycle. Sparganum proliferum is a good model for ecological interaction studies, which allows us to understand and define the trophic levels of the intermediate and definitive hosts, and then to establish the distribution of parasites within a host population (Denegri, 2008). For these reasons, the knowledge of prevalence of Sparganum in wild animals from Argentina is necessary, due to ongoing changes on the environment that affect parasite ecology and its transmission dynamics. In Argentina few cases of human sparganosis have been reported but the real prevalence is unknown in the country. In the meantime, a reliable taxonomic criterion based on morphological characteristics integrated with molecular analysis of mitochondrial DNA sequences will be useful for species discrimination.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the ongoing collaboration of the Regional Technical Delegation NEA National Parks and Subtropical Ecological Research Center (CIES) for technical assistance and to “Proyecto Yaguareté” members specially to Sebastián Costa. Also, we acknowledge to the park rangers from National Park Iguazu for collaborating with monitoring and collecting road kill animals specially Emiliano Francisconi, Cecilia Moyano, Ricardo Melzew and the veterinarian Gabriel Acevedo for collaborating with animal samples. Fernanda Roca for histology assistance and Florencia Soubeste for help in english langauge and Claudia Vergara Páez for assistance with the high quality images. This work was carried out with funding from CONICET-UBA, Argentina (L.K and M.G.P.), PICT 2017 3176 ANPCyT Argentina (L. K and L.F A.), National Institute of Tropical Medicine – National Ministry of Health, Argentina (J.P.A).
Footnotes
All sequences reported in this work are available at GenBank nºAccession: MK976918-MK976920.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2020.09.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Phylogenetic analyzes of Sparganum samples. Maximum Likelihood method of three molecular markers concatenated from parasite ocelot isolate (LPMiSP) and 10 orthologous sequences from reference genomes. There were a total of 1245 positions in the final dataset.
Multiple sequence alignment of cox1 Sparganum and Spirometra species performed with ClustalX (v2.0.12). Dots indicates identical nucleotides.
Bayesian phylogeny of Spirometra and Sparganum based on the data set of cox1. The numbers along branches indicate posterior probabilities. The ESS value for all parameters were above 200. Sequences ID are the same as Fig. 5.
References
- Almeida G.G. Molecular identification of Spirometra spp. (Cestoda: Diphyllobothriidae) in some wild animals from Brazil. Parasitol. Int. 2016;65(5):428–431. doi: 10.1016/j.parint.2016.05.014. Elsevier Ireland Ltd. [DOI] [PubMed] [Google Scholar]
- Arrabal J.P. Echinococcus oligarthrus in the subtropical region of Argentina: first integration of morphological and molecular analyses determines two distinct populations. Vet. Parasitol. 2017;240:60–67. doi: 10.1016/j.vetpar.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Boero A.M.E., Garaguso P., Navarr J. A case of cerebral sparganosis in southamerica. Arq. Neuro. Psiquiatr. 1991;49(1):111–113. doi: 10.1590/s0004-282x1991000100018. [DOI] [PubMed] [Google Scholar]
- Borteiro C. Spargana in the neotropical frog Hypsiboas pulchellus (Hylidae) from Uruguay. N. West. J. Zool. 2015;11(1):171–173. [Google Scholar]
- Brabec J., Kuchta R., Scholz T. Paraphyly of the Pseudophyllidea (Platyhelminthes: cestoda): circumscription of monophyletic clades based on phylogenetic analysis of ribosomal RNA. Int. J. Parasitol. 2006;36:1535–1541. doi: 10.1016/j.ijpara.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Buergelt C.D., Greiner E.C., Senior D.F. Proliferative sparganosis in a cat. J. Parasitol. 1984;70(1):121–125. [PubMed] [Google Scholar]
- Daly J.J. Sparganosis. In: Press C., editor. CRC Handbook Series in Zoonoses. Boca Ratón. 1981. pp. 293–312. [Google Scholar]
- Denegri G.M. The complete biological cycle of Diphyllobothrium erinaceieuropei (Cestoidea, Pseudophyllidea) under experimental conditions. Helminthologia. 1993;30:177–179. [Google Scholar]
- Denegri G.M. Epistemological Foundation of Parasitology. In: EUDEM, editor. EUDEM; 2008. [Google Scholar]
- Dorny P. Emerging food-borne parasites. Vet. Parasitol. 2009;163(3):196–206. doi: 10.1016/j.vetpar.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Drummond A.J., Suchard M.A. Bayesian random local clocks, or one rate to rule them all. BMC Biol. 2010;8:114. doi: 10.1186/1741-7007-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard M.L. Thirty-seven human cases of sparganosis from Ethiopia and South Sudan caused by Spirometra spp. Am. J. Trop. Med. Hyg. 2015;93(2):350–355. doi: 10.4269/ajtmh.15-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J. Predictive values and quality control of techniques for the diagnosis of Echinococcus multilocularis in definitive hosts. Acta Trop. 2001;85(2):157–163. doi: 10.1016/s0001-706x(02)00216-4. [DOI] [PubMed] [Google Scholar]
- Faust E.C., Campbell H.E., Kellogg C.R. Morphological and biological studies on the species of diphyllobothrium in China. Am. J. Epidemiol. 1929;9(3):560–583. doi: 10.1093/oxfordjournals.aje.a121670. [DOI] [Google Scholar]
- Hernández-Orts J.S. High morphological plasticity and global geographical distribution of the Pacific broad tapeworm Adenocephalus pacificus (syn. Diphyllobothrium pacificum): molecular and morphological survey. Acta Trop. 2015;149:168–178. doi: 10.1016/j.actatropica.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Hong X. Global genetic diversity of Spirometra tapeworms. Trop. Biomed. 2020;37(1):237–250. [PubMed] [Google Scholar]
- International Helminth Genomes Consortium Comparative genomics of the major parasitic worms. Nat. Genet. 2019;51(1):163–174. doi: 10.1038/s41588-018-0262-1. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S. vol. 2805. Tokyo Med News; 1932. pp. 2784–2786. (Studies on Diphyllobothrium erinacei VI. Adult Worms of Plerocercoid Larvae from Korean Weasels, Erinaceus Koreanus Lon- Ferg). [Google Scholar]
- Iwata S. Some experimental studies on the regeneration of the Plero-cercoid of Manson's tapeworm, Diphyllobothrium erinacei (Rudolph), with special reference to its relationship with Sparganum proliferum Ijima. Jpn. J. Zool. 1934;6:139–158. [Google Scholar]
- Iwata S. Experimental and morphological studies of Manson's tapeworm Diphyllobothriumerinacei (Rudolphi) – special reference with its scientific name and relationship with Sparganumproliferum, Ijima. Progr Med Parasitol. 1972;4:536–590. [Google Scholar]
- Jeon H.K., Eom K.S. Mitochondrial DNA sequence variability of Spirometra species in Asian countries. Kor. J. Parasitol. 2019;57(5):481. doi: 10.3347/kjp.2019.57.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.C. Esparganosis cerebral. Presentación de un caso pediátrico. Archivo Argentino de Medicina. 2012;110(6):1–4. doi: 10.5546/aap.2013.e1. [DOI] [PubMed] [Google Scholar]
- Kamenetzky L., Gutierrez A.M., Canova S.G. Several strains of Echinococcus granulosus infect livestock and humans in Argentina. Infect. Genet. Evol. 2002;2(2):129–136. doi: 10.1016/s1567-1348(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Kikuchi T., Maruyama H. Human proliferative sparganosis update. Parasitol. Int. 2020;75:102036. doi: 10.1016/j.parint.2019.102036. [DOI] [PubMed] [Google Scholar]
- Kikuchi T., Dayi M., Hunt V.L., Toyoda A., Maeda Y., Kondo Y., Noya B., Noya O., Kojima S., Kuramochi T., Maruyama H. Genome analysis of the fatal tapeworm Sparganum proliferum unravels the cryptic lifecycle and mechanisms underlying the aberrant larval proliferation. bioRxiv. 2020 05.19.105387. [Google Scholar]
- Kołodziej-Sobocińska M. Factors affecting the spread of parasites in populations of wild European terrestrial mammals. Mammal Research. 2019;64:301–318. [Google Scholar]
- Kokaze A., Miyadera H., Kita K., Machinami R., Noya O., de Noya B.A., Okamoto M., Horii T., Kojima S. Phylogenetic identification of Sparganum proliferum as a pseudophyllidean cestode. Parasitol. Int. 1997;46(4):271–279. doi: 10.1016/s1383-5769(01)00071-x. [DOI] [PubMed] [Google Scholar]
- Kuchta R. Suppression of the tapeworm order Pseudophyllidea (Platyhelminthes: eucestoda) and the proposal of two new orders, Bothriocephalidea and Diphyllobothriidea. Int. J. Parasitol. 2008;38:49–55. doi: 10.1016/j.ijpara.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Kuchta R., Scholz T. Planetary Biodiversity Inventory (2008–2017): Tapeworms from Vertebrate Bowels of the Earth. University. 2017. Diphyllobothriidea; pp. 167–189. [Google Scholar]
- Kuchta R, Kołodziej-Sobocińska M, Brabec J, Młocicki D, Sałamatin R and Scholz T. (in press) ‘Sparganosis (Spirometra) in Europe in the molecular era’, Clin. Infect. Dis.. doi: 10.1093/cid/ciaa1036/5875650. [DOI] [PubMed]
- Lavikainen A. Identificación molecular de Taenia spp. En el lince euroasiático (Lynx lynx) de Finlandia. Parasitología. 2013;140(5):653–662. doi: 10.1017/S0031182012002120. [DOI] [PubMed] [Google Scholar]
- Le A.T. The first case of human infection by adult of Spirometra erinaceieuropaei in Vietnam. BMC Infect. Dis. 2017;17(1):669. doi: 10.1186/s12879-017-2786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.W. Sparganosis in mainland China. International Journal of Infectious Diseases. International Society for Infectious Diseases. 2011;15(3):e154–e156. doi: 10.1016/j.ijid.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Ligier H.D. In: Caracterización geomorfológica y edáfica de la provincia de Misiones. de T C.I.N., editor. 2000. Agropecuaria. [Google Scholar]
- Liu Q. Human sparganosis, a neglected food borne zoonosis. Lancet Infect. Dis. 2015;15(10):1226–1235. doi: 10.1016/S1473-3099(15)00133-4. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Martínez F., Laffont G., Camon Rodríguez M. Identificación de huevos de Spirometra mansonoides en felinos silvestres. Revista Veterinaria Argentina. 2010;27(263):1–6. [Google Scholar]
- Miyadera H. Phylogenetic identification of Sparganum proliferum as a pseudophyllidean cestode by the sequence analyses on mitochondrial COI and nuclear sdhB genes. Parasitol. Int. 2001;50:93–104. doi: 10.1016/s1383-5769(01)00071-x. [DOI] [PubMed] [Google Scholar]
- Mueller J. Comparative studies on certain species of Diphyllobothrium. J. Parasitol. 1936;22(5):471–478. [Google Scholar]
- Mueller J. The biology of Spirometra. J. Parasitol. 1974;60:3–14. [PubMed] [Google Scholar]
- Noya O. Sparganum proliferum: an overview of its structure and ultrastructure. Int. J. Parasitol. 1992;22(5):631–640. doi: 10.1016/0020-7519(92)90012-A. [DOI] [PubMed] [Google Scholar]
- Oda F.H. Parasitism by larval tapeworms genus Spirometra in South American amphibians and reptiles: new records from Brazil and Uruguay, and a review of current knowledge in the region. Acta Trop. 2016;164:150–164. doi: 10.1016/j.actatropica.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Odening K. Neue Erkenntnisse Zu Geographie, Ökonomie und Taxonomie von Spirometra (Cestoda: Pseudophyllidea) MILU: Wissenschaft. K. Mitteil. Tierpark. 1985;6:277–294. [Google Scholar]
- Okamoto M. Intraspecific variation of Spirometra erinaceieuropaei and phylogenetic relationship between Spirometra and Diphyllobothrium inferred from mitochondrial CO1 gene sequences. Parasitol. Int. 2007;56(3):235–238. doi: 10.1016/j.parint.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Okino Establishment of the complete life cycle of Spirometra (Cestoda: Diphyllobothriidae) in the laboratory using a newly isolated triploid clone. Parasitol. Int. 2017;66(2):116–118. doi: 10.1016/j.parint.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Opuni E.K., Muller R.L. Studies on Spirometra theileri (Baer, 1925) n. comb. 1. Identification and biology in the laboratory. J. Helminthol. 1974;4(1):15–23. doi: 10.1017/s0022149x00022550. [DOI] [PubMed] [Google Scholar]
- Palmer C.S. National study of the gastrointestinal parasites of dogs and cats in Australia. Vet. Parasitol. 2008;151(2–4):181–190. doi: 10.1016/j.vetpar.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Petrigh R.S. Research note. Cox-1 gene sequence of Spirometra in Pampas foxes from Argentina. Helminthologia. 2015;52(4):355–359. doi: 10.1515/helmin-2015-0056. [DOI] [Google Scholar]
- Qiu Ming-hua, Qiu Ming-de. Human plerocercoidosis and sparganosis: I. A historical review on aetiology. Chin. J. Parasitol. Parasit. Dis. 2009;27(1):54–60. http://europepmc.org/abstract/MED/19459502 Available at: [PubMed] [Google Scholar]
- Rego A.A., Schäffer G.V. Sparganum in some brazilian vertebrates: problems in the identification of species of Luheella (Spirometra) Mem. Inst. Oswaldo Cruz. 1992;87:213–216. doi: 10.1590/s0074-02761992000500040. [DOI] [PubMed] [Google Scholar]
- Reigada C., Bisceglia S., Miño M.H. II Congreso Latinoamericano de Mastozoología and XXV Jornadas Argentinas de Mastozoología. Buenos Aires, Argentina. 2012. Descripción preliminar de la comunidad endoparasitaria del zorro gris Lycalopex gymnocercus en el Parque Nacional Lihué Calel (La Pampa, Argentina) a partir de un análisis coproparasitológico; pp. 234–235. [Google Scholar]
- De Roodt A.R. A case of human sparganosis in Argentina. Medicina. 1993;53(3):235–238. [PubMed] [Google Scholar]
- Santa Cruz A.M., Lombardero O.J. Resultados parasitológicos de 50 necropsias de gatos de la ciudad de Corrientes. Vet. Argent. 1987;4:735–739. [Google Scholar]
- Schauer F. Travel-acquired subcutaneous Sparganum proliferum infection diagnosed by molecular methods. Br. J. Dermatol. 2014;170(3):741–743. doi: 10.1111/bjd.12679. 1951. [DOI] [PubMed] [Google Scholar]
- Scholz T., Kuchta R., Brabec J. Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: recent progress and future challenges. Int. J. Parasitol.: Parasites and Wildlife. 2019;9:359–369. doi: 10.1016/j.ijppaw.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scioscia N.P., Beldomenico P.M., Petrigh R.S. Epidemiological studies on Echinococcus in Pampas fox (Lycalopex gymnocercus) and European hare (Lepus europaeus) in Buenos Aires province, Argentina. Parasitol. Res. 2013;112:3607–3613. doi: 10.1007/s00436-013-3548-3. [DOI] [PubMed] [Google Scholar]
- Scioscia N.P. The Pampas fox (Lycalopex gymnocercus) as new definitive host for Spirometra erinacei (Cestoda: Diphyllobothriidae) Acta Trop. 2014;133:78–82. doi: 10.1016/j.actatropica.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Stief B., Enge A. Proliferative peritonitis with larval and cystic parasitic stages in a dog. Veterinary Pathology. 2011;48(4):911–914. doi: 10.1177/0300985810382092. [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Venturini L. Spirometra erinacei (Rudolphi 1819) en perro. Rev. Med. Vet. 1980;61(4):330–334. [Google Scholar]
- Venturini L. Experimental evolutive cycle of Diphyllobothrium erinaceieuropei in Paracyclops fimbriatus, tadpoles of Bufo arenarum and dogs. Rev. Inst. Med. Trop. Sao Paulo. 1989;31(5):308–312. doi: 10.1590/s0036-46651989000500003. [DOI] [PubMed] [Google Scholar]
- Vieira F.M., Luque J.L., Muniz-Pereira L.C. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa. 2008;23(1721):1–23. doi: 10.11646/zootaxa.1721.1.1. [DOI] [Google Scholar]
- Wardle R.A., McLeod J.A., Radinovsky S. University of Minnesota Press; 1974. Advances in the Zoology of Tapeworms, 1950–1970. [Google Scholar]
- Woldemeskel M. Subcutaneous sparganosis, a zoonotic cestodiasis, in two cats. J. Vet. Diagn. Invest. 2014;26(2):316–319. doi: 10.1177/1040638713517697. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang H., Cui J. The phylogenetic diversity of Spirometra erinaceieuropaei isolates from southwest China revealed by multi genes. Acta Trop. 2016;156:108–114. doi: 10.1016/j.actatropica.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Zhang X., Duan J.Y., Shi Y.L. Comparative mitochondrial genomics among Spirometra (Cestoda: Diphyllobothriidae) and the molecular phylogeny of related tapeworms. Mol. Phylogenet. Evol. 2017;117:75–82. doi: 10.1016/j.ympev.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Zhang X., Hong X., Liu S.N. Large-scale survey of a neglected agent of sparganosis Spirometra erinaceieuropaei (Cestoda: Diphyllobothriidae) in wild frogs in China. PLoS Neglected Trop. Dis. 2020;14(2) doi: 10.1371/journal.pntd.0008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic analyzes of Sparganum samples. Maximum Likelihood method of three molecular markers concatenated from parasite ocelot isolate (LPMiSP) and 10 orthologous sequences from reference genomes. There were a total of 1245 positions in the final dataset.
Multiple sequence alignment of cox1 Sparganum and Spirometra species performed with ClustalX (v2.0.12). Dots indicates identical nucleotides.
Bayesian phylogeny of Spirometra and Sparganum based on the data set of cox1. The numbers along branches indicate posterior probabilities. The ESS value for all parameters were above 200. Sequences ID are the same as Fig. 5.