Abstract
Objectives:
To evaluate the impact of an aortic root abscess on perioperative outcomes and long-term survival in patients with active infectious endocarditis treated surgically.
Methods:
From 1996-2017, 336 consecutive patients were treated with aortic valve or root replacement for active endocarditis, including patients with (n=179) or without (n=157) a root abscess. Data was obtained from the Society of Thoracic Surgery data warehouse, through chart review, patient surveys, and the National Death Index data.
Results:
Demographics were similar between groups except the root abscess group had a significantly lower prevalence of congestive heart failure (CHF) and higher rates of prosthetic valve endocarditis. The abscess group had significantly more aortic root replacements, longer cardiopulmonary bypass and cross clamp times. Operative mortality was 8.4% and 3.8% (P=.11) for the abscess and no abscess groups, respectively. Nevertheless, the root-abscess group had prolonged ventilation and longer ICU stays. Kaplan-Meier survival was similar between root abscess and no abscess groups (10-year survival 41% vs. 43%, P=.35). Significant risk factors for all-time mortality included all categories of age (hazard ratio, HR= 1.64-2.85), the presence of a root abscess (HR=1.42), IV drug use (HR=1.81), CHF (HR= 1.72), renal failure requiring dialysis (HR=3.26), and liver disease (HR=3.04). The 10-year rate of reoperation was also similar between groups (5.9% vs. 7.9%).
Conclusions:
Thorough and extensive debridement is critical for successful treatment of active endocarditis with root abscess. Bioprosthetic stented and stentless valves are valid conduits to treat endocarditis with root abscess.
INTRODUCTION
Active infectious endocarditis is a life-threatening disease that is associated with high morbidity and mortality despite the use of antibiotic therapy and often requires surgical intervention. Infection of the aortic valve, in particular, can cause necrosis of surrounding tissues and ultimately lead to root abscess formation, which may require extensive debridement of local tissues and replacement of the entire aortic root. The development of perivalvular abscesses is relatively common, occurring in approximately 7.5% and 14% of infectious endocarditis patients with native and prosthetic aortic valves, respectively[1,2].
The complexity of surgical treatment in the setting of an aortic root abscess ranges from aortic valve replacement (AVR) with patch reconstruction to a total aortic root replacement (ARR) and has been associated with a 30-day mortality of 19-20% and 5-year survival rate of around 50-60% [3,4]. This mortality is multifactorial and can be related to the patient’s comorbid factors, extent of necrotic tissue, microorganism involved, or to postoperative complications. Multiple studies have attempted to assess differences in mortality between aortic valve replacement and aortic root replacement, but have found conflicting evidence[3–5]. Other studies have focused on comparing patients with infected native valves to those with infected prosthetic valves and have found increased mortality in the latter [6]. Nevertheless, only a few studies have compared the outcomes of patients with infective endocarditis in the presence or absence of a perivalvular abscess [7]. We, therefore, report the impact of having an aortic root abscess on perioperative and long-term outcomes. We hypothesized that the presence of an aortic root abscess, while inevitably increasing risk of complications, would not confer inferior postoperative outcomes.
METHODS
This study was approved by the Institutional Review Board at Michigan Medicine (HUM00142927; March 31st, 2019) and a waiver of informed consent was obtained.
Patient Selection and data collection
Between 1996 and 2017, 336 consecutive patients with infective endocarditis underwent aortic valve or root replacement. The patients in a single institution study were divided into two groups based on the presence (n=179) or absence (n=157) of an aortic root abscess. Data was obtained through the Society of Thoracic Surgery (STS) Data Warehouse to identify the relevant cohort and to determine the preoperative, operative, and postoperative variables. This data was supplemented through medical chart review for specified variables.
Survival and reoperation data were collected by medical record review and supplemented with surveys (including letters and phone calls) and National Death Index data through December 31st, 2018 [8]. Completeness of follow up for death and reoperation was calculated based on the ratio of the observed person-time and potential person-time follow-up in the study [9]. Given the end of study period on October 1, 2019, completeness of follow up for death and reoperation was 94.0% and 94.2%, respectively.
Operative technique
In the no abscess group, the infected leaflets or prosthetic valve were/was removed, and the root was debrided. Any defect at the aortic root was patched with autologous pericardium or bovine pericardium. Routine AVR was performed with a stented or stentless bioprosthesis as modified inclusion or a mechanical valve. In the abscess group, the aortic valve or prosthetic valve was removed with aggressive debridement of the abscess. After debridement, if the cavity involved less than one-third (one sinus) of the aortic annulus, the cavity was patched with autologous pericardium or bovine pericardium followed by an AVR or modified inclusion ARR. If the cavity involved greater than one-third (2 to 3 sinuses) of the aortic annulus, then either a total root replacement with separate reimplantation of two coronary buttons (modified Bentall procedure), or patch repair of the cavity and modified inclusion ARR was performed.
In the event of fistula formation between the aortic root and right or left atrium, we removed the vegetation or thrombus from the inside of the atrium, repaired the defect from the inside of the atrium, and performed a total root replacement. If the tricuspid valve was damaged, it was either repaired or replaced. If the infection destroyed the aorto-mitral curtain and mitral valve, the mitral valve was replaced with subsequent reconstruction of the aorto-mitral curtain with bovine pericardium and total ARR. Homograft conduits were used in the late 1990s and replaced with stentless valves (Freestyle, Medtronic, MN) in 2000 for all endocarditis cases if patients were suitable or preferred a bioprosthesis for replacement of their aortic root or valve.
Statistical Analysis
Continuous data was presented as median (interquartile range, 25%, 75%) and categorical data as n (%). Univariate comparisons between groups were performed using chi-square tests for categorical data. Kolmogorov-Smirnov D test and Cramer-von Mises tests were used to test the normality of the data. Wilcoxon rank sum tests were performed for continuous data. Multivariable logistic regression was used to calculate the odds ratio (OR) of risk factors for operative mortality or new-onset renal failure requiring dialysis by adjusting for group, age, gender, preoperative liver disease, congestive heart failure (CHF), new-onset renal failure requiring dialysis (only for operative mortality), cardiogenic shock, postoperative sepsis, aortic root procedure, prosthetic valve endocarditis, and surgery time. Survival curves were estimated using the Kaplan-Meier method with the log-rank test. The Cox proportional hazards regression model was used to calculate the adjusted hazard ratios (HRs) for mortality since surgery, adjusting for group, age (modeled as a categorical variable), gender, preoperative liver disease, history of IV drug use, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), CHF, new-onset renal failure requiring dialysis, cardiogenic shock, and postoperative sepsis based on model diagnostics . Surgical time was modeled as a strata in the Cox model. Cumulative incidence function curves were adjusted for death as a competing risk using the Fine and Gray sub-distribution method to assess the incidence of reoperation over time. The Gray test was used to test the difference in the cumulative incidence function curves between groups. The variables selected into the Logistic model and Cox model were based on ones used in previous reports in the literature and in clinical practice. P<.05 was considered statistically significant. Statistical calculations were performed with SAS (SAS Institute, Cary, NC).
RESULTS
Demographics and Preoperative Data
The demographics were similar between groups, although the root abscess group had significantly more male patients (82% vs. 72%), a lower prevalence of congestive heart failure (CHF; 40% vs. 61%), and higher rates of previous aortic valve replacement (43% vs. 19%) and previous mitral valve replacement (5% vs. 0.6%). There was no difference in the rates of other cardiac valve surgeries (Table 1). The most common causative organism was Staphylococci (30%) followed by Streptococci (25%; Table 2).
Table 1:
Preoperative and demographic data
| Variable | Total (n=336) | Root abscess (n=179) | No root abscess (n=157) | P-value |
|---|---|---|---|---|
| Age | 55 (43, 66) | 55 (45,66) | 54 (41,66) | .24 |
| Sex (male) | 260(77.4) | 147 (82.1) | 113(72.0) | .04 |
| CAD | 65 (19.3) | 36 (20.1) | 29 (18.5) | .78 |
| Diabetes | 75 (22.3) | 44 (24.6) | 31 (19.7) | .30 |
| Dyslipidemia | 127 (37.8) | 73 (40.8) | 54 (34.4) | .26 |
| Hypertension | 202 (60.1) | 109 (60.9) | 93 (59.2) | .82 |
| Tobacco use | .79 | |||
| Nonsmoker | 171 (50.9) | 88 (49.2) | 83 (52.9) | |
| Current smoker | 66 (19.6) | 37 (20.7) | 29 (18.5) | |
| Former smoker | 99 (29.5) | 54 (30.2) | 45 (28.7) | |
| Lung disease | .83 | |||
| None | 283 (84.2) | 148 (82.7) | 135 (86.0) | |
| Mild | 29 (8.6) | 17 (9.5) | 12 (7.6) | |
| Moderate | 11 (3.3) | 7 (3.9) | 4 (2.6) | |
| Severe | 13 (3.9) | 7 (3.9) | 6 (3.8) | |
| Pneumonia | 36 (10.7) | 16 (8.9) | 20 (12.7) | .29 |
| IV drug use | 47 (14.0) | 25 (14.0) | 22 (14.0) | .99 |
| Depression | 39 (11.6) | 21 (11.7) | 18 (11.5) | .94 |
| Alcohol | .01 | |||
| None | 194 (57.7) | 93 (52) | 101 (64.3) | |
| <1 drink/week | 78 (23.2) | 40 (22.3) | 38 (24.2) | |
| 2-7 drinks/week | 31 (9.2) | 20 (11.2) | 11 (7.0) | |
| >8 drinks/week | 20 (6.0) | 16 (8.9) | 4 (2.6) | |
| Unknown | 13 (3.9) | 10 (5.6) | 3 (1.9) | |
| Liver disease | 31 (9.2) | 16 (8.9) | 15 (9.6) | .85 |
| Prior MI | 68 (20.2) | 36 (20.1) | 32 (20.4) | .95 |
| CHF | 167 (49.7) | 71 (39.7) | 96 (61.1) | .0001 |
| Stroke | 62 (18.5) | 33 (18.4) | 29 (18.5) | .99 |
| Sepsis* | 48 (14.3) | 16 (8.9) | 32 (20.4) | .47 |
| Cardiogenic shock | 26 (7.7) | 15 (8.4) | 11 (7.0) | .69 |
| Arrhythmia | 56 (16.7) | 35 (19.6) | 21 (13.4) | .14 |
| Previous cardiac surgery | ||||
| CABG | 41 (12.2) | 28 (15.6) | 13 (8.3) | .05 |
| Root replacement | 17 (5.1) | 11 (6.1) | 6 (3.8) | .46 |
| Ascending replacement | 14 (4.2) | 8 (4.5) | 6 (3.8) | .79 |
| Arch replacement | 3 (0.9) | 2 (1.1) | 1 (0.6) | .64 |
| Previous aortic valve surgery | <.0001 | |||
| Repair | 1 (0.3) | 1 (0.6) | 0 (0) | .35 |
| Replacement | 107 (31.8) | 77 (43.0) | 30 (19.1) | <.0001 |
| Previous mitral valve surgery | .05 | |||
| Repair | 6 (1.8) | 3 (1.7) | 3 (1.9) | .87 |
| Replacement | 10 (3.0) | 9 (5.0) | 1 (0.6) | .02 |
| Previous tricuspid valve Surgery | .73 | |||
| Repair | 2 (0.6) | 1 (0.6) | 1 (0.6) | .93 |
| Replacement | 1 (0.3) | 0 (0) | 1 (0.6) | .47 |
Data presented as median (interquartile range) for continuous variables and number (percentage) for categorical variables.
Based on retrospective chart review in patients with documented sepsis in the context of a suspected or proven infection via blood cultures and a systemic inflammatory response syndrome.
Abbreviations: CAD, coronary artery disease; IV, intravenous; MI, myocardial infarction; CHF, congestive heart failure; CABG, coronary artery bypass graft.
Table 2:
Operative data
| Variable | Total (n=336) | Root abscess (n=179) | No root abscess (n=157) | P-value |
|---|---|---|---|---|
| Causative microorganism | .03 | |||
| Staphylococci | 102 (30.4) | 59(33.0) | 43(27.4) | .29 |
| Staphylococcus aureus | 57 (17.0) | 27 (15.1) | 30 (19.1) | .38 |
| Coagulase-negative staphylococci | 45 (13.4) | 32 (17.9) | 13 (8.3) | .01 |
| Enterococci | 53 (15.8) | 25 (14.0) | 28 (17.8) | .37 |
| Streptococci | 85 (25.3) | 53 (29.6) | 32 (20.4) | .06 |
| Others* | 14 (4.2) | 6 (3.4) | 8 (5.1) | .59 |
| Culture negative | 73 (21.7) | 32 (17.9) | 41 (26.1) | .08 |
| Fungal | 9 (2.7) | 4 (2.2) | 5 (3.2) | .74 |
| Aortic insufficiency | .003 | |||
| None | 51 (15.2) | 34 (19.0) | 17 (10.8) | |
| Trivial/trace/minimal | 17 (5.1) | 15 (8.4) | 2 (1.3) | |
| Mild | 38 (11.3) | 21 (11.7) | 17 (10.8) | |
| Moderate | 55 (16.4) | 26 (14.5) | 29 (18.5) | |
| Severe | 175 (52.1) | 83 (46.4) | 92 (58.6) | |
| Aortic stenosis | 122 (36.3) | 59 (33.0) | 63 (40.1) | .21 |
| BAV | 63 (18.8) | 35 (19.6) | 28 (17.8) | .78 |
| MFS | 3 (0.9) | 1 (0.6) | 2 (1.3) | .60 |
| Calcified valve leaflets | 44 (13.1) | 22 (12.3) | 22 (14.0) | .75 |
| Root aneurysm | 8 (2.4) | 6 (3.4) | 2 (1.3) | .29 |
| Pseudoaneurysm | 7 (2.1) | 6 (3.4) | 1 (0.6) | .13 |
| Ascending aneurysm | 28 (8.3) | 20 (11.2) | 8 (5.1) | .05 |
| Arch aneurysm | 4 (1.2) | 3 (1.7) | 1 (0.6) | .63 |
| Chronic dissection | 1 (0.3) | 0 (0) | 1 (0.6) | .47 |
| Status | ||||
| Elective | 37 (11.0) | 9 (5.0) | 28 (17.8) | <.0001 |
| Urgent | 250 (74.4) | 144 (80.4) | 106 (67.5) | .008 |
| Emergent | 49 (14.6) | 26 (14.5) | 23 (14.6) | 1.0 |
| Incidence | ||||
| First cardiac surgery | 193 (57.4) | 82 (45.8) | 111 (70.7) | <.0001 |
| First reoperation | 118 (35.1) | 76 (42.5) | 42 (26.8) | .003 |
| Second reoperation | 17 (5.1) | 14 (7.8) | 3 (1.9) | .02 |
| Third reoperation | 3 (0.9) | 3 (1.7) | 0 (0) | .25 |
| Fourth reoperation | 5 (1.5) | 4 (2.2) | 1 (0.6) | .38 |
| CABG | 34 (10.1) | 20 (11.2) | 14 (8.9) | .59 |
| Cardiopulmonary bypass time | 223 (163,288) | 259 (214,323) | 173 (133, 237) | <.0001 |
| Cross clamp time | 178 (128,230) | 207 (164,261) | 140 (103,185) | <.0001 |
| Aortic valve replacement | 123 (36.6) | 39 (21.8) | 84 (53.5) | <.0001 |
| Stented bioprothesis | 107 (31.8) | 37 (20.7) | 70 (44.6) | <.0001 |
| Mechanical valve | 16 (4.8) | 2 (1.1) | 14 (8.9) | .001 |
| Root replacement | 213 (63.4) | 140 (78.2) | 73 (46.5) | <.0001 |
| Homograft | 37 (11.0) | 21 (11.7) | 16 (10.2) | |
| Stentless | 176 (52.4) | 119 (66.5) | 57 (36.3) | <.0001 |
| Total replacement | 26 (7.7) | 19 (10.6) | 7 (4.5) | |
| Modified inclusion | 142 (42.3) | 94 (52.5) | 48 (30.6) | |
| Sub-coronary | 8 (2.4) | 6 (3.4) | 2 (1.3) | |
| Mitral valve procedure | .002 | |||
| Repair | 84 (25.0) | 46 (25.7) | 38 (24.2) | |
| Replacement | 36 (10.7) | 15 (8.4) | 21 (13.4) | |
| Tricuspid valve procedure | .35 | |||
| Repair | 49 (14.6) | 28 (15.6) | 21 (13.4) | |
| Replacement | 2 (0.6) | 0 (0) | 2 (1.3) | |
| Ascending procedure | 60 (17.9) | 35 (19.6) | 25 (15.9) | .40 |
| Hemi arch procedure | 7 (2.1) | 5 (2.8) | 2 (1.3) | .46 |
| Total arch procedure | 2 (0.6) | 1 (0.6) | 1 (0.6) | .93 |
Data presented as median (interquartile range) for continuous variables and number (percentage) for categorical variables.
Organisms with positive blood cultures not otherwise categorized.
Abbreviations: MFS, Marfan syndrome; BAV, bicuspid aortic valve; CABG, coronary artery bypass graft; CVG, composite valve graft.
Intraoperative Data
The root abscess group had significantly more reoperations (54% vs 29%), aortic root replacements (78% vs. 47%), and longer cardiopulmonary bypass and aortic cross-clamp times. Stentless valves were used significantly more often in the root abscess group (66% vs 36%) while stented bioprosthesis and mechanical valves were more often used in the no abscess group. There were no differences between groups for ascending, hemi-arch, or total arch procedures (Table 2).
Perioperative Outcomes
There were no significant differences in most perioperative complications or in mortality between root abscess and no root abscess groups, including operative mortality (8.4% vs. 3.8%, P =.11). However, the root abscess group had prolonged ventilation, more blood transfusions, and longer ICU stays (all <0.001). The significant risk factors for operative mortality identified on multivariate logistic analysis included prosthetic valve endocarditis (OR =3.25), preoperative liver disease (OR =11.8), new-onset renal failure requiring dialysis (OR =3.16), and postoperative sepsis (OR =12.4). (Table 4) The presence of a root abscess was not a significant risk factor for operative mortality. Furthermore, patients with a root abscess (OR=0.36) were less likely to develop renal failure requiring dialysis. Nevertheless, prosthetic valve endocarditis (OR=3.57), cardiogenic shock (OR=4.36), and postoperative sepsis (OR=14.6) were risk factors for the development of renal failure requiring dialysis. (Table 4)
Table 4:
Logistic model for operative mortality and new-onset renal failure requiring dialysis
| Operative mortality* | New-onset renal failure requiring dialysis | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Age | 0.97 | 0.80-1.16 | .71 | 1.01 | 0.87-1.18 | .89 |
| Sex (male) | 2.80 | 0.58-13.58 | .27 | 1.07 | 0.38-3.07 | .89 |
| Root abscess | 2.32 | 0.69-7.82 | .17 | 0.36 | 0.14-0.97 | .04 |
| Aortic root replacement | 1.21 | 0.33-4.41 | .77 | 0.66 | 0.24-1.82 | .42 |
| Prosthetic valve endocarditis | 3.25 | 1.09-9.67 | .03 | 3.57 | 1.32-9.64 | .01 |
| Liver disease | 11.8 | 2.84-48.98 | .0007 | 2.28 | 0.62-8.29 | .21 |
| Congestive heart failure | 1.98 | 0.67-5.84 | .22 | 1.14 | 0.44-2.93 | .79 |
| New-onset renal failure on dialysis | 3.16 | 1.12-8.92 | .03 | N/A | N/A | N/A |
| Cardiogenic shock | 0.94 | 0.17-5.13 | .94 | 4.36 | 1.30-14.71 | .02 |
| Postoperative sepsis | 12.4 | 1.67-91.62 | .02 | 14.63 | 2.36-90.61 | .0039 |
| Surgery before 2010 | 3.89 | 1.09-13.86 | .04 | 1.55 | 0.57-4.24 | .39 |
Abbreviations: OR, odds ratio; CI, confidence interval
Operative mortality is based on the Society of Thoracic Surgeons definition and includes all deaths, regardless of cause, occurring during the hospitalization in which the operation was performed, even if after 30 days (including patients transferred to other acute care facilities); and all deaths, regardless of cause, occurring after discharge from the hospital, but before the thirtieth postoperative day.
Long-term Outcomes
The median and mean follow-up time for death was 4 (1.8, 7.5) and 5.5 (σ = 5) years. The long-term survival was similar between root abscess and no root abscess groups, (10-year survival 41% versus 43%). (Figure 1) However, the presence of a root abscess was a significant risk factor for all-time mortality (HR=1.42) as well as all categories of age (HR=1.64-2.85), history of IV drug use (HR=1.81), congestive heart failure (HR=1.72), new-onset renal failure requiring dialysis (HR=3.26), liver disease (HR=3.04), and postoperative sepsis (HR=3.00). (Table 5)
Figure 1:
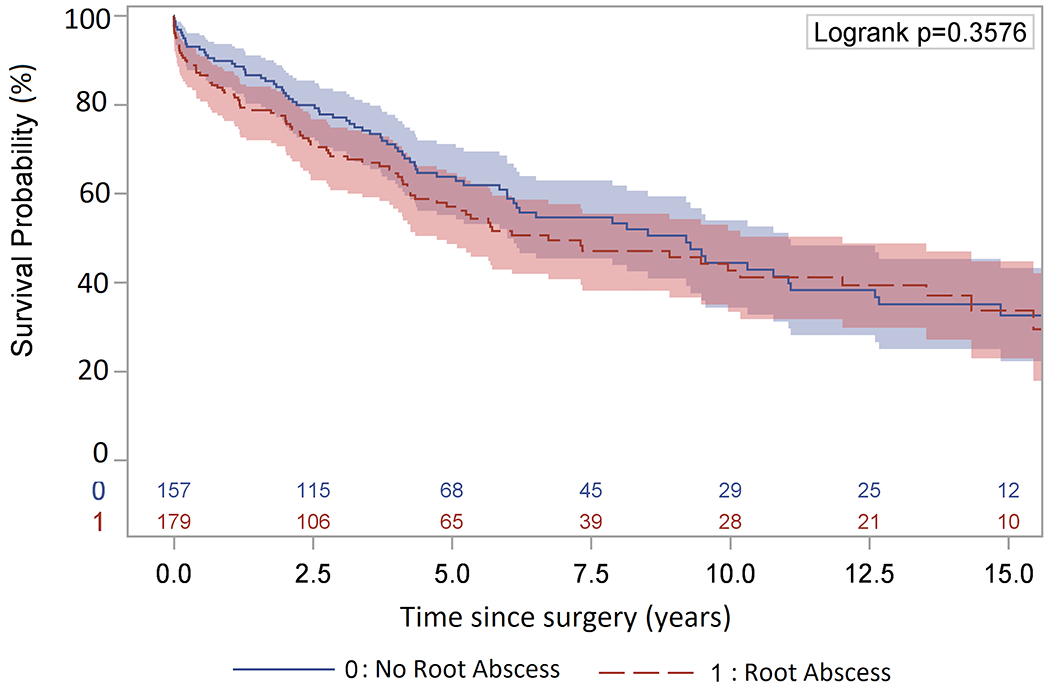
Kaplan-Meier long-term survival of endocarditis patients with a root abscess (10-year: 41%; 95% CI, 32%-50%) or no root abscess (10-year: 43%; 95% CI, 33%-53%).
Table 5:
Cox proportional hazard regression for all-time mortality
| Variable | Hazard ratio | 95% Confidence interval | P-value |
|---|---|---|---|
| Age* | |||
|
| |||
| 40-60 | 1.64 | 0.98-2.72 | .06 |
| 60-70 | 1.84 | 1.04-3.26 | .04 |
| >70 | 2.85 | 1.55-5.24 | .0008 |
| Sex (male) | 1.01 | 0.69-1.47 | .96 |
| Root abscess | 1.42 | 1.02-1.96 | .04 |
| IV drug user | 1.81 | 1.13-2.89 | .01 |
| CAD | 0.99 | 0.67-1.47 | .96 |
| COPD | 0.78 | 0.44-1.39 | .41 |
| Congestive heart failure | 1.72 | 1.22-2.42 | .0018 |
| New-onset renal failure on dialysis | 3.26 | 2.30-4.64 | < .0001 |
| Cardiogenic shock | 0.67 | 0.35-1.28 | .22 |
| Liver disease | 3.04 | 1.65-5.60 | .0004 |
| Postoperative sepsis | 3.00 | 1.30-6.93 | .01 |
Abbreviations: IV, intravenous; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease.
Modeled as a categorical variable compared to age <40.
The median and mean follow-up time for reoperation was 3.8 (1.7, 7.3) and 5.1 (σ = 4.6) years. The ten-year postoperative cumulative incidence of reoperation was not significantly different between the root abscess and no abscess groups (5.9 vs. 7.9%). (Figure 2) A total of 23 patients had reoperation. The most common indication for reoperation was structural deterioration of the bioprosthesis (10/23), followed by reinfection (5/23).
Figure 2:
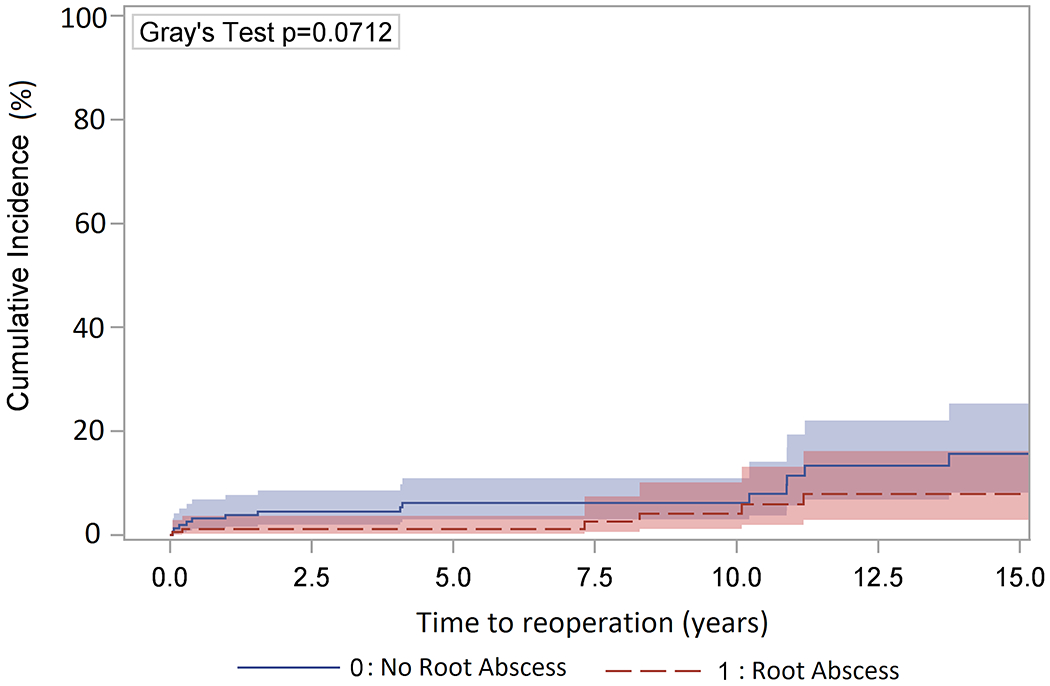
Cumulative incidence of reoperation adjusting for death as a competing factor in endocarditis patients with a root abscess (10-year: 5.9%; 95% CI, 1.9%-13.1%) or no root abscess (10-year: 7.9%; 95% CI, 3.7%-14.1 %).
DISCUSSION
In this study, we found no significant differences in operative mortality between root abscess and no root abscess groups (8.4% vs. 3.8%). Furthermore, the long-term survival was also similar between groups with a 10-year survival of 41% versus 43% that continued to decrease comparably through the follow up period. The risk factors for all-time mortality included the presence of a root abscess, age, history of IV drug use, congestive heart failure, new-onset renal failure requiring dialysis, liver disease, and postoperative sepsis.
Aortic valve endocarditis with root abscess is a dreadful disease which requires urgent or emergent surgical treatment with a 30-day mortality as high as 19-25% [3,4,6]. Traditionally, this disease has been treated with total root replacement with homograft [10–12]. At our institution, we transitioned to stentless valves (Freestyle, Medtronic) around the year 2000 due to the concern of extensive calcification of homograft, lack of immediate accessibility, and expense. For patients with a small root abscess, AVR with patch repair worked quite well. Even with large root abscesses involving more than one sinus of the aortic root, both a patch repair with modified inclusion of the root or total ARR with a stentless valve were very effective.
Our 30-day mortality of 6.7% in the root abscess group is well below what has been recently reported. This mortality rate is also better than most reported in the literature for patients with and without root abscesses treated with homograft [14–17]. Despite the increased complexity of the operation, the mortality rate in patients with a root abscess was only fractionally higher than in patients without an abscess. The key for aortic valve endocarditis is the extensive and thorough debridement. As exemplified by the variety of operative techniques in our cohort, once the aortic root is cleaned up, any reconstruction can work well. Aortic root replacement was not a risk factor for operative mortality as exemplified by our Logistic model (Table 4). Specifically for patients with a root abscess, a study by Leontyev et al. showed no significant difference in 30-day mortality between aortic root replacement and aortic valve replacement with patch reconstruction [3]. Similarly, stentless valves have been used by a few other centers with good outcomes [18,19]. Other studies have also demonstrated that the complexity of aortic reconstruction, type of valve substitute or aortic root prosthesis does not affect outcomes in patients with aortic root abscess in the setting of radical debridement [11,20–22], supporting our point that either stented or stentless valve can be used for reconstruction.
As expected, the root abscess group had more prosthetic valve endocarditis (49%) compared to the no abscess group (23%), since prosthetic valve endocarditis tends to be more aggressive, and the damage at the root is always much worse than what the echocardiogram shows. In patients with root abscess, prosthetic valve endocarditis has been reported to be associated with increased perioperative mortality [6]. Our study confirmed that the presence of a prosthetic valve was a significant risk factor for operative mortality (Table 4). Previous studies also agree with our findings that preoperative liver disease and postoperative sepsis are risk factors for perioperative mortality [6]. In addition, we found new-onset renal failure to be a risk factor for operative mortality, although not significantly different in our univariate comparison. Upon further examination of risk factors for the development of new-onset renal failure, we found prosthetic valve endocarditis, cardiogenic shock, and postoperative sepsis to be significant risk factors. (Table 4) The presence of a root abscess actually protected against the development of renal failure requiring dialysis, which was most likely due to earlier operative times compared to no abscess group, in which CHF was frequently used as an indication for operation. As such, for patients with active endocarditis and root abscess, we should operate sooner rather later before sepsis, renal failure, and heart failure develops.
In the no abscess group, we had a high rate of modified inclusion root replacement with stentless valves. (Table 2) We used this technique mainly for better hemodynamics and sometimes for treatment of root aneurysms. Operative mortality in the no abscess group was 2.7% for those who had aortic root replacement (Supplemental Table 2). In an early study, a German group reported a 10% rate of mortality in patients with aortic valve endocarditis without a root abscess [13]. In addition, the Logistic model showed that the odds ratio of aortic root replacement versus aortic valve replacement was not significant (OR =1.2, 95% CI, 0.33, 4.4; P=0.77). (Table 4) Furthermore, we only had one intraoperative death in the no abscess group (Table 3). This patient had an aortic valve replacement and a tricuspid valve repair and died from cardiogenic shock in the context of severe pulmonary hypertension. Additionally, we had a total operative mortality of six in the no abscess group, which did not appear to be related to the complexity of the aortic root replacement (Supplemental Table 1). As such, we did not think using modified inclusion root replacement in patients without root abscess increased operative mortality.
Table 3:
Postoperative data
| Variable | Total (n=336) | Root abscess (n=179) | No root abscess (n=157) | P-value |
|---|---|---|---|---|
| Red blood cell (Units) | 4 (2,6) | 4 (2,7) | 3 (1,5) | .0002 |
| Reoperation for bleeding | 14 (4.2) | 6 (3.4) | 8 (5.1) | .59 |
| Planned closure delay | 6 (1.8) | 5 (2.8) | 1 (0.6) | .22 |
| Sternal dehiscence | 1 (0.3) | 0 (0) | 1 (0.6) | .47 |
| Sepsis | 8 (2.4) | 5 (2.8) | 3 (1.9) | .73 |
| Stroke | 5 (1.5) | 3 (1.7) | 2 (1.3) | .76 |
| ICU stay (days) | 2.7 (0,7) | 3.9 (1.8, 9.4) | 1.7 (0,4.1) | <.0001 |
| Vent hours | 12 (3.1,34) | 16 (4.8, 52) | 7.1 (0,19) | <.0001 |
| Pneumonia | 25 (7.4) | 14 (7.8) | 11 (7.0) | .84 |
| Cardiac arrest | 11 (3.3) | 8 (4.5) | 3 (1.9) | .23 |
| Device | .06 | |||
| Pacemaker | 24 (7.1) | 16 (8.9) | 8 (5.1) | .21 |
| ICD | 4 (1.2) | 4 (2.2) | 0 (0) | .13 |
| New onset renal failure on dialysis | 26 (7.7) | 10 (5.6) | 16 (10.2) | .15 |
| Multi-system organ failure | 3 (0.9) | 2 (1.1) | 1 (0.6) | .64 |
| Atrial fibrillation | 84 (25.0) | 48 (26.8) | 36 (22.9) | .45 |
| Intra-operative death | 6 (1.8) | 5 (2.8) | 1 (0.6) | .22 |
| In hospital mortality | 20 (6.0) | 14 (7.8) | 6 (3.8) | .17 |
| 30 day mortality | 17 (5.1) | 12 (6.7) | 5 (3.2) | .21 |
| Operative mortality* | 21 (6.3) | 15 (8.4) | 6 (3.8) | .11 |
Data presented as median (interquartile range) for continuous variables and number (percentage) for categorical variables.
Operative mortality is based on the Society of Thoracic Surgeons definition and includes all deaths, regardless of cause, occurring during the hospitalization in which the operation was performed, even if after 30 days (including patients transferred to other acute care facilities); and all deaths, regardless of cause, occurring after discharge from the hospital, but before the thirtieth postoperative day.
Abbreviations: ICU, intensive care unit; ICD, implantable cardioverter defibrillator.
The long-term survival in our study was similar to that reported by other centers, although most had a limited number of patients at 10-years follow-up [3,6,12]. Interestingly, the Kaplan-Meier analysis did not find any difference in the long-term survival of patients with a root abscess compared to those without an abscess (Figure 1). However, multivariable Cox proportional hazard model showed that the presence of a root abscess was a significant risk factor for late mortality. As such, aggressive surgical treatment of aortic valve endocarditis is important to prevent the development of an aortic root abscess. The other significant risk factors for all-time mortality included age, history of IV drug use, the presence congestive heart failure, new-onset renal failure requiring dialysis, liver disease, and postoperative sepsis (Table 5). These are the important factors we should consider when offering patients an operation and discussing their prognosis. Some patients with severe liver disease and other comorbidities may not benefit from surgery at all, and perhaps surgery should not be offered to those patients. The hazard ratio of 1.72 for congestive heart failure suggests that we should aggressively treat aortic valve endocarditis before patients decompensate, instead of waiting for patients to develop CHF and then operate.
We should also be aware of the increasing proportion of IV drug users with infectious endocarditis and thoroughly consider their candidacy for surgery. Despite the increased incidence of IV drug use, our operative mortality did not change significantly over the last ten years of our study (data not shown). Nevertheless, recent studies showed that about 17% of patients with endocarditis are IV drug users and have reported its association with higher rates of reinfection and late complications [23], which confirm our long-term results.
Previous studies have shown a rate of reoperation of about 15% over ten years in patients with root abscesses [3]. Interestingly, in terms of reoperation, the comparable ten year cumulative incidence curves of 5.9% in the root abscess versus 7.9% in the no abscess groups substantiates the critical role of aggressive debridement in preventing graft reinfection in the presence of an abscess regardless of the type of prosthesis used for reconstruction of the aortic root (Figure 2).
Our study is a single-center retrospective experience which has all the limitations of a retrospective study. Although the study has a twenty-one-year span of data collection, the particular surgical techniques performed and the preference for stentless valves are fairly consistent. The 94.2% and 94.0% completeness of follow-up for survival and reoperation could have led us to underestimate the real cumulative incidence of these two events for both groups. The study was also limited by the possibility of Type II error. To reach powers of 80%, each group would need 432 participants for operative mortality and 537 participants for new-onset dialysis requiring dialysis. That would represent an additional death for every 22 patients treated. Nevertheless, for root abscess versus no root abscess, the odds ratios for operative mortality and for new-onset renal failure on dialysis indicate a considerable effect. Additionally, our Cox model was able to provide us with enough sensitivity to show significantly increased risk of all-time mortality in the presence of a root abscess.
CONCLUSIONS
Despite the increased complexity of aortic valve procedures and risk for postoperative complications, operative outcomes were favorable in patients with an aortic root abscess in the setting of infective endocarditis. Thorough and extensive debridement was critical for the successful treatment of this disease. Finally, both bioprosthetic stented and stentless valves were valid conduits to treat active endocarditis in the presence of a root abscess after thorough debridement of the aortic root (Figure 3).
Figure 3:
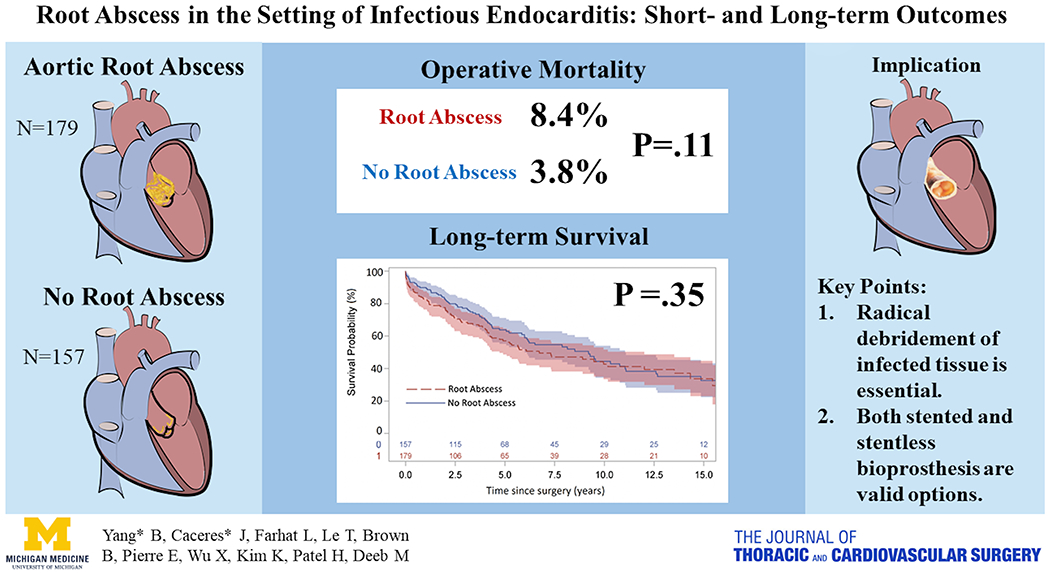
The presence of an aortic root abscess in the setting of infective endocarditis did not significantly increase perioperative mortality, but was a significant risk factor for long-term survival in the context of radical debridement of infected tissue. Both bioprosthetic stented and stentless valves were valid conduits to treat active endocarditis in the presence of a root abscess.
Supplementary Material
Video Legend: Discussion of total root replacement with a stentless valve in aortic valve endocarditis with root abscess in the setting of unroofing of an anomalous right coronary and removal of an infected thrombus in the dissected proximal descending aorta with hypothermic circulatory arrest.
PERSPECTIVE STATEMENT:
Although associated with increased procedural complexity, the presence of a root abscess did not significantly increase perioperative mortality, or cumulative incidence of reoperation in the setting of infective endocarditis when treated with a stentless or stented bioprosthesis, but was a risk factor for late mortality. Thorough and extensive debridement is critical for successful treatment of this disease.
Acknowledgement:
We thank the support from our colleagues in the department of Cardiac Surgery, including Drs Rich Prager, Steve Bolling, Frank Pagani, Jonathan Haft, Paul Tang, Matthew Romano, as well as our Aortic Research Team, including Tom Ren, Jeffrey Clemence Jr., and Alexander Makkinejad.
Funding Source: Dr. Yang is supported by the NHLBI of NIH K08HL130614 and R01HL141891, Phil Jenkins and Darlene & Stephen J. Szatmari Funds. Dr. Patel is supported by the Joe D. Morris Collegiate Professorship, the David Hamilton Fund, and the Phil Jenkins Breakthrough Fund in Cardiac Surgery. Dr. Deeb is supported by the Herbert Sloan Collegiate Professorship, Jamie Buhr Fund, and Richard Nerod Fund.
GLOSSARY OF ABBREVIATIONS
- ARR
aortic root replacement
- AVR
aortic valve replacement
- CAD
coronary artery disease
- CHF
congestive heart failure
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- HR
hazard ratio
- ICU
intensive care unit
- IV
intravenous
- MI
myocardial infarction
- OR
odds ratio
- STS
Society of Thoracic Surgery
Footnotes
Date and number of IRB approval: Mar. 31, 2018, HUM00142927
CENTRAL MESSAGE:
With thorough surgical debridement, patients with an aortic root abscess can have favorable perioperative outcomes and long-term survival.
Conflict of Interest: None related to this study
REFERENCES
- [1].Anguera I, Miro JM, Evangelista A, Cabell CH, San Roman JA, Vilacosta I, et al. Periannular complications in infective endocarditis involving native aortic valves. Am J Cardiol 2006;98:1254–60. 10.1016/j.amjcard.2006.06.016. [DOI] [PubMed] [Google Scholar]
- [2].Anguera I, Miro JM, San Roman JA, de Alarcon A, Anguita M, Almirante B, et al. Periannular complications in infective endocarditis involving prosthetic aortic valves. Am J Cardiol 2006;98:1261–8. 10.1016/j.amjcard.2006.05.066. [DOI] [PubMed] [Google Scholar]
- [3].Leontyev S, Davierwala PM, Krögh G, Feder S, Oberbach A, Bakhtiary F, et al. Early and late outcomes of complex aortic root surgery in patients with aortic root abscesses. Eur J Cardiothorac Surg 2016;49:447–54; discussion 454–455. 10.1093/ejcts/ezv138. [DOI] [PubMed] [Google Scholar]
- [4].Chen G-J, Lo W-C, Tseng H-W, Pan S-C, Chen Y-S, Chang S-C. Outcome of surgical intervention for aortic root abscess: a meta-analysis. Eur J Cardiothorac Surg 2018;53:807–14. 10.1093/ejcts/ezx421. [DOI] [PubMed] [Google Scholar]
- [5].Kirali K, Sarikaya S, Ozen Y, Sacli H, Basaran E, Yerlikhan OA, et al. Surgery for Aortic Root Abscess: A 15-Year Experience. Tex Heart Inst J 2016;43:20–8. 10.14503/THIJ-14-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leontyev S, Borger MA, Modi P, Lehmann S, Seeburger J, Doenst T, et al. Surgical management of aortic root abscess: a 13-year experience in 172 patients with 100% follow-up. J Thorac Cardiovasc Surg 2012;143:332–7. 10.1016/j.jtcvs.2010.10.064. [DOI] [PubMed] [Google Scholar]
- [7].Yoshioka D, Toda K, Yokoyama J-Y, Matsuura R, Miyagawa S, Shirakawa Y, et al. Recent Surgical Results for Active Endocarditis Complicated With Perivalvular Abscess. Circ J 2017;81:1721–9. 10.1253/circj.CJ-17-0355. [DOI] [PubMed] [Google Scholar]
- [8].Centers for Disease Control and Prevention; National Center for Health Statistics. National Death Index. n.d.
- [9].Clark TG, Altman DG, Stavola BLD. Quantification of the completeness of follow-up. The Lancet 2002;359:1309–10. 10.1016/S0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- [10].Siniawski H, Klose H, Pasic M, Hetzer R, Weng Y, Yankah AC. Homograft reconstruction of the aortic root for endocarditis with periannular abscess: a 17-year study☆. European Journal of Cardio-Thoracic Surgery 2005;28:69–75. 10.1016/j.ejcts.2005.03.017. [DOI] [PubMed] [Google Scholar]
- [11].David TE, Regesta T, Gavra G, Armstrong S, Maganti MD. Surgical treatment of paravalvular abscess: long-term results. Eur J Cardiothorac Surg 2007;31:43–8. 10.1016/j.ejcts.2006.10.036. [DOI] [PubMed] [Google Scholar]
- [12].Sultan I, Bianco V, Kilic A, Chu D, Navid F, Gleason TG. Aortic root replacement with cryopreserved homograft for infective endocarditis in the modern North American opioid epidemic. J Thorac Cardiovasc Surg 2019;157:45–50. 10.1016/j.jtcvs.2018.05.050. [DOI] [PubMed] [Google Scholar]
- [13].Bauernschmitt R, DeSimone R, Lange R, Vahl CF, Thomas G, Hagl S. [Surgery of acute aortic valve endocarditis: prognosis in paravalvular abscess]. Z Kardiol 1998;87:276–82. [DOI] [PubMed] [Google Scholar]
- [14].Yankah AC, Pasic M, Klose H, Siniawski H, Weng Y, Hetzer R. Homograft reconstruction of the aortic root for endocarditis with periannular abscess: a 17-year study. Eur J Cardiothorac Surg 2005;28:69–75. 10.1016/j.ejcts.2005.03.017. [DOI] [PubMed] [Google Scholar]
- [15].Musci M, Weng Y, Hübler M, Amiri A, Pasic M, Kosky S, et al. Homograft aortic root replacement in native or prosthetic active infective endocarditis: Twenty-year single-center experience. The Journal of Thoracic and Cardiovascular Surgery 2010;139:665–73. 10.1016/j.jtcvs.2009.07.026. [DOI] [PubMed] [Google Scholar]
- [16].Preventza O, Mohamed AS, Cooley DA, Rodriguez V, Bakaeen FG, Cornwell LD, et al. Homograft use in reoperative aortic root and proximal aortic surgery for endocarditis: A 12-year experience in high-risk patients. J Thorac Cardiovasc Surg 2014;148:989–94. 10.1016/j.jtcvs.2014.06.025. [DOI] [PubMed] [Google Scholar]
- [17].Perrotta S, Aljassim O, Jeppsson A, Bech-Hanssen O, Svensson G. Survival and quality of life after aortic root replacement with homografts in acute endocarditis. Ann Thorac Surg 2010;90:1862–7. 10.1016/j.athoracsur.2010.06.100. [DOI] [PubMed] [Google Scholar]
- [18].Schneider AW, Hazekamp MG, Versteegh MIM, Bruggemans EF, Holman ER, Klautz RJM, et al. Stentless bioprostheses: a versatile and durable solution in extensive aortic valve endocarditis. Eur J Cardiothorac Surg 2016;49:1699–704. 10.1093/ejcts/ezv463. [DOI] [PubMed] [Google Scholar]
- [19].Sponga S, Daffarra C, Pavoni D, Vendramin I, Mazzaro E, Piani D, et al. Surgical management of destructive aortic endocarditis: left ventricular outflow reconstruction with the Sorin Pericarbon Freedom stentless bioprosthesis. European Journal of Cardio-Thoracic Surgery 2016;49:242–8. 10.1093/ejcts/ezv068. [DOI] [PubMed] [Google Scholar]
- [20].Elgalad A, Arafat A, Elshazly T, Elkahwagy M, Fawzy H, Wahby E, et al. Surgery for Active Infective Endocarditis of the Aortic Valve With Infection Extending Beyond the Leaflets. Heart, Lung and Circulation 2018. 10.1016/j.hlc.2018.05.200. [DOI] [PubMed] [Google Scholar]
- [21].Jassar AS, Bavaria JE, Szeto WY, Moeller PJ, Maniaci J, Milewski RK, et al. Graft Selection for Aortic Root Replacement in Complex Active Endocarditis: Does It Matter? The Annals of Thoracic Surgery 2012;93:480–7. 10.1016/j.athoracsur.2011.09.074. [DOI] [PubMed] [Google Scholar]
- [22].Okada K, Okita Y. Surgical treatment for aortic periannular abscess/pseudoaneurysm caused by infective endocarditis. General Thoracic and Cardiovascular Surgery 2013;61:175–81. 10.1007/s11748-012-0152-x. [DOI] [PubMed] [Google Scholar]
- [23].Kim JB, Ejiofor JI, Yammine M, Ando M, Camuso JM, Youngster I, et al. Surgical outcomes of infective endocarditis among intravenous drug users. The Journal of Thoracic and Cardiovascular Surgery 2016;152:832–841.e1. 10.1016/j.jtcvs.2016.02.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Legend: Discussion of total root replacement with a stentless valve in aortic valve endocarditis with root abscess in the setting of unroofing of an anomalous right coronary and removal of an infected thrombus in the dissected proximal descending aorta with hypothermic circulatory arrest.


