Figure 4. Immunization with inactivated chimeric HA A/PR8 viruses confer cross protection against heterosubtypic influenza virus.
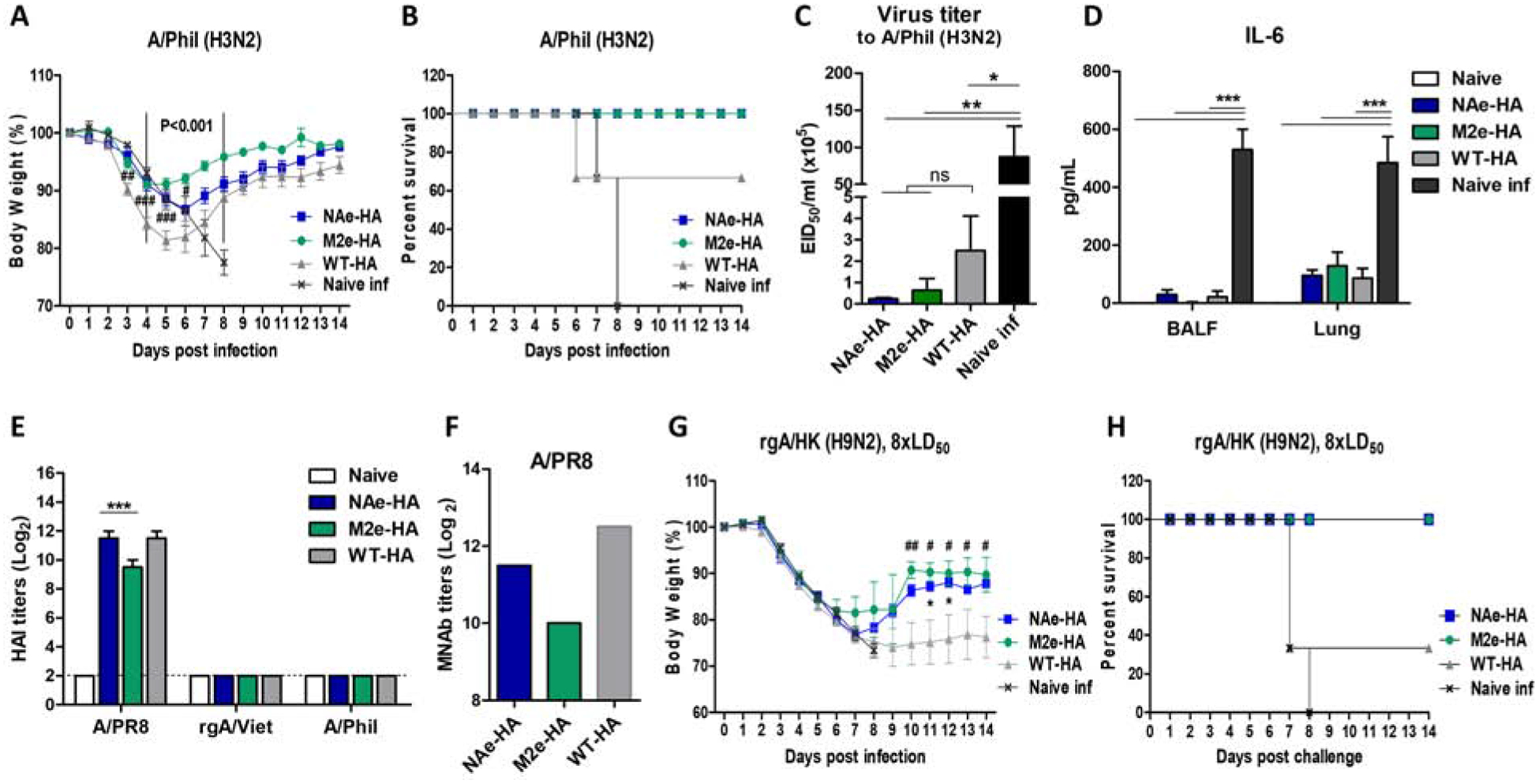
The groups of mice (n=6) immunized with chimeric HA (NAe-HA or M2e-HA) and WT-HA A/PR8 viruses were infected with A/Phil (H3N2, 8× LD50) or reassortant rgA/HK (H9N2, 8× LD50) virus and then monitored body weight changes daily for 14 days. (A) Body weight changes. P<0.001; compared between M2e-HA and WT-HA, ###; p<0.001, ##; p<0.01, #; p<0.05; compared between NAe-HA and WT-HA. (B) Survival rates. Another set of the groups of mice (n=4) was sacrificed at 6 days after infection (dpi) with the same challenge dose A/Phil (H3N2) to determine the lung viral loads and the level of IL-6 cytokine. (C) Lung viral titers at 6 dpi. Viral loads were determined by using embryonated chicken eggs and presented in EID50. (D) The level of IL-6 was determined from BALF and lung lysates by ELISA. (E) HAI titers to homologous or heterosubtypic influenza viruses. (F) Microneutralization antibody titers in pooled immune sera after second boost immunization. (G and H) Body weight changes and survival rates after challenge of mice (n=6) with rgA/HK H9N2 (8× LD50) virus. *; p<0.05 between NAe-HA and WT-HA, ##; p<0.01, #; p<0.05 between M2e-HA and WT-HA. Statistical significance was determined by using one-way ANOVA and Dunnett’s multiple comparison test. Data are representative of individual animals out of two independent experiments. Error bars indicate the means ± SEM. ***; p<0.001, **; p<0.01. *; p<0.05. ns; no significant difference between groups.
