Abstract
Perfluorobutanesulfonic acid (PFBS), a shorter chain Per- and polyfluoroalkyl substances (PFASs) cognate of perfluorooctanesulfonic acid (PFOS), has been used as replacement for the toxic surfactant PFOS. However, emerging evidences suggest safety concerns for PFBS and its effect on reproductive health is still understudied. Therefore, the current work aimed to investigate the effect of PFBS, in comparison to PFOS, on reproductive health using Caenorhabditis elegans as an in vivo animal model. PFOS (≥ 10 μM) and PFBS (≥ 1000 μM) significantly impaired the reproduction capacity of C. elegans, represented as reduced brood size (total egg number) and progeny number (hatched offspring number), without affecting the hatchability. Additionally, the preconception exposure of PFOS and PFBS significantly altered the embryonic nutrient loading and composition, which further led to abnormalities in growth rate, body size and locomotive activity in F1 offspring. Though the effective exposure concentration of PFBS was approximately 100 times higher than PFOS, the internal concentration of PFBS was lower than that of PFOS to produce the similar effects of PFOS. In conclusion, PFOS and PFBS significantly impaired the reproductive capacities in C. elegans and the preconception exposure of these two compounds can lead to offspring physiological dysfunctions.
Keywords: PFOS, PFBS, Caenorhabditis elegans, reproductive toxicity
1. Introduction
Perfluorooctanesulfonic acid (PFOS) is an anthropogenic fluorosurfactant that belongs to a larger group of chemicals called per- and polyfluoroalkyl substances (PFASs). Due to its surface-active properties, it has been used in a wide variety of applications including cookware, food packaging, and stain repellants. Due to its persistence, bioaccumulation, and toxic effects, PFOS has been recognized as a global pollutant and has been banned in many countries, but some developing countries are still producing it. Perfluorobutanesulfonic acid (PFBS), a short-chain PFASs, is considered to be a safer alternative of PFOS with a much shorter half-life (~ 1 month) than PFOS (~ 5 years) in human[1, 2]. Recently, studies have revealed a potential link of PFBS and pathological development[3–5] and thus safety of PFBS usage is called into question. However, unlike PFOS, which has been extensively researched, the effect of PFBS on health is still less understood, particularly its effect on reproductive health.
Caenorhabditis elegans is a free-living nematode, found in soil, and fed on bacteria[6]. In the lab, it can be cultured in either agar plate or liquid medium with non-pathogenic bacteria Escherichia coli as the food source. After first being adopted as a model organism for developmental biology study in 1960s, C. elegans has been widely used in numerous research including reproductive toxicity[7, 8]. It possesses many advantages over the traditional animal models including easy lab maintenance, compact size (~1 mm in length for adults), transparent body, short lifecycle (~3 days at 25°C), large progeny production (~300 by self-fertilization), completely sequenced genome and large number of mutant and transgenic strains available (from Caenorhabditis Genetics Center)[9]. Moreover, it conserves more than 65% of the human diseases-related genes[9]. All of these properties make C. elegans a convenient model for the study of environmental toxicants related pathologies. Thus, in the current study, we employed C. elegans as the in vivo animal model to examine the effect of PFOS and PFBS on the reproductive health.
2. Materials and Methods
2.1. Materials
Perfluorooctanesulfonic acid (PFOS, ~40% in H2O, catalog no. 77283, lot 101406188), and perfluorobutanesulfonic acid (PFBS, 97%, catalog no. 562629, lot MKCB6204), Analytical standard of PFOS (98%) and PFBS (97%), ampicillin and carbenicillin were purchased from Sigma-Aldrich Co. (St. Louis, MO). The internal standard (13C8 PFOS, 50 μg/mL in methanol) was purchased from Cambridge Isotope Laboratories (Andover, MA). Luria-Bertani medium, biological agar and peptone were purchased from Fisher Scientific Inc. (Pittsburgh, PA). Commercial kits (Triglyceride, Infinity™ Triglyceride Reagent) used for triglyceride measurement and Hoechst 33258 (>98%) used for DNA quantification were purchased from Thermo Fisher Scientific Inc. (Middletown, VA). For protein quantification, Bio-Rad DC protein assay kit was purchased from Bio-Rad Co. (Hercules, CA). Escherichia coli OP50 and C. elegans strains including N2, Bristol (wild type) and DH1033 (sqt-1(sc103) II; bIs1 X), were obtained from Caenorhabditis Genetics Center (CGC).
2.2. Caenorhabditis elegans Maintenance
C. elegans was cultured as previously described[10–12] with non-pathogenic bacterial Escherichia coli (E. coli) OP50 as the food source. Synchronization of the population was obtained by using the bleaching method[13]. PFOS and PFBS stock solution (1,000x) were prepared in dimethyl sulfoxide (DMSO) with a final concentration of 0.1% DMSO and 0.1% DMSO was used as the vehicle. To eliminate the potential interference of E. coli to the target compounds, heat-killed E. coli was used for all treatments, which was prepared by heat treatment at 65°C for 30 min . For F0 generation experiments, treatments were started from late L4/ young adult stage for 1 day at 20°C in S-complete liquid media (100 mM NaCl, 5.7 mM K2HPO4, 44 mM KH2PO4, 5 μg/L cholesterol, 0.1 M C6H5K3O7 pH 6.0, 3 mM MgSO4, 3 mM CaCl2, 50 μM disodium EDTA, 2.5 μM FeSO4 · 7 H2O, 1 μM MnCl2 · 4 H2O, 1 μM ZnSO4 · 7 H2O, 0.1 μM CuSO4 · 5 H2O) supplemented with test chemicals, ampicillin (100 μg/mL), carbenicillin (50 μg/mL) and heat-killed E. coli (8 mg wet weight/mL). Since C. elegans reproduction reached its peak on the 2nd day of adulthood, F0 worms were treated for 2 days in order to obtain enough eggs for the following F1 parameters analysis. Based on the previous report of PFOS and PFBS in C. elegans[14], we did a preliminary study with a range of concentrations of PFOS (0.1, 1, 10, 20 and 40 μM) and PFBS (125, 250, 500, and 1,000 μM), and used PFOS at 10, 20 and 40 μM and PFBS at 1,000 and 2,000 μM in the current study (Supplementary Figure S1).
2.3. Triglyceride and Protein Quantification
For quantification of triglyceride and protein in worms, pre-treated animals were collected and washed 3 times to get rid of treatment and E. coli. Samples were prepared by sonicating the worms in 0.05% Tween 20 for 3 min[10, 11]. Likewise, pre-treated F0 nematodes were bleached to collect eggs for triglyceride and protein quantification in embryos/eggs. Measurements of triglyceride and protein were performed with the commercial kits: Infinity™ Triglyceride Reagent and Bio-Rad DC protein assay kit, respectively. DNA was quantified as the internal control as previously reported[15] with Hoechst 33258 to normalize triglyceride and protein levels.
2.4. Reproduction, Pumping Rate, Locomotive assay, Body Size and Growth Rate
For the reproduction assay, the pre-treated worm was individually transferred to a freshly prepared E. coli seeded NGM plate every day until the reproduction was ceased[16]. Daily brood size and progeny number were recorded. Pumping rate was conducted by counting the pharyngeal contraction of randomly selected worms for 30 s under the optical microscope[10, 11]. Body size and locomotion were measured by using the WormLab Tracking system (MBF Bioscience, Williston, VT) as previously described[10, 11]. After treatment, worms were transferred to the freshly prepared E. coli seeded low peptone NGM plate and allowed to adapt for 10 min. Then a 1 min video was recorded and analyzed by the WormLab software (MBF Bioscience version 3. 1. 0, Williston, VT) for the worm length, width and average moving speed. Growth rate was determined by the number of worms at each developmental stage[12, 17]. Growth rate results were presented as a % of worms at each stage.
2.5. Measurement of Yolk Uptake into Oocytes
Yolk uptake was measured by employing the transgenic C. elegans strain DH1033 (sqt-1(sc103) II; bIs1 X). This strain labels VIT-2, one of the 6 yolk proteins that consists of yolk lipoprotein complexes with green florescence protein (GFP), therefore allows the visualization of yolk transportation. After treatment, worms were anesthetized with 10 mM NaN3, mounted on the microscopic slide with a thin layer of 3% agarose, capped with cover slip, and taken pictures using Nikon Eclipse Ti-U (Nikon Instruments Inc., Melville, NY). Since the major target of yolk lipoprotein complexes are the near mature oocytes[18], the GFP intensity of the most mature oocyte that is adjacent to spermatheca and ready to ovulate into the spermatheca is quantified by using the Image J software.
2.6. Quantification of PFOS and PFBS in worms and embryos
PFOS and PFBS internal concentrations in worms and embryos were determined by Liquid chromatography-mass spectrometry (LCMS). Synchronized L4/young adult worms were treated with control (0.1% DMSO) or PFOS (10, 20 and 40 μM) or PFBS (1,000 and 2,000 μM) for 1 day at 20°C, then harvested for the determination of internal concentrations of PFOS and PFBS in worms. For the determination of internal concentrations of PFOS and PFBS in embryos, synchronized L4/young adult worms (F0 generation) were treated with control (0.1% DMSO) or PFOS (10, 20 and 40 μM) or PFBS (1,000 and 2,000 μM) for 2 days at 20°C before the eggs were collected by the standard bleaching method[13]. The pre-treated worms and eggs were washed 5 times to remove all E. coli and treatment and collected in 2 mL polypropylene tube. 50 μL of the internal standard solution (250 ng/mL) was added. 100 μL of deionized water and 200 μL of acetonitrile were added and vortexed for 1 min, followed by sonication for 30 min. 50 mg of sodium chloride was added and the tubes were vortexed for 1 min and centrifuged for 5 min at 10.0 rpm and 4 °C. Finally, the acetonitrile (upper) layer was filtered using 0.2 μM, PTFE syringe filter and transferred to a LC vial. The obtained data were normalized to the amount of DNA present. Target compounds PFOS and PFBS were analyzed using an Acquity UPLC H-class system equipped with Acquity triple quadrupole mass spectrometer (Waters, Milford, MA). The chromatographic separation was performed on reversed-phase analytical column Atlantis T3 (2.1 mm x 100 mm and 3.0 μm particle size, Waters, Milford, MA). The column oven temperature was set at 40 °C. The mobile phase consisted of (A) 2mM ammonium acetate and (B) acetonitrile. The flow rate of mobile phase was 0.25 mL/min and the following gradient program was used: Initial conditions were 5%B, held for 0.5 min, increased to 95%B over 4.5 min then held for 2 min. The gradient returned to the initial conditions and was held for 2.5 min. The total analytical run-time was 10 min and the injection volume was 5 μL For MS/MS detection, electrospray ionization (ESI) in negative ion mode was used with the following MS parameter: Capillary voltage 4.5 kV, source temperature 150°C, desolvation line temperature 250°C and desolvation flow rate was 500 L/h (nitrogen). Tandem MS detection was achieved by multiple reaction monitoring (MRM) using the MRM transitions (Supplementary Table S1). For standard injections, 350 μL of acetonitrile and 50 μL of the internal standard solution (250 ng/mL) were mixed with 100 μL of the different concentration of the working solution (2.5 – 250 ng/mL). The ratios of signal response between PFOS/PFBS and ISTD was used to calculate the detected amount of PFOS/PFBS in samples, based on internal standard calibration.
2.7. Statistical analyses
Statistical analyses were performed by using the Statistical Analysis System (SAS Institute version 9.4, Cary, NC, USA) with one-way ANOVA (Tukey’s test for multiple comparison). Difference between groups was considered to be significant at P<0.05. Data in Figure 3G were analyzed by chi2 test. Results represent data collected from a single experiment except results in Figure 1 were from 4 independent experiments.
Figure 1.
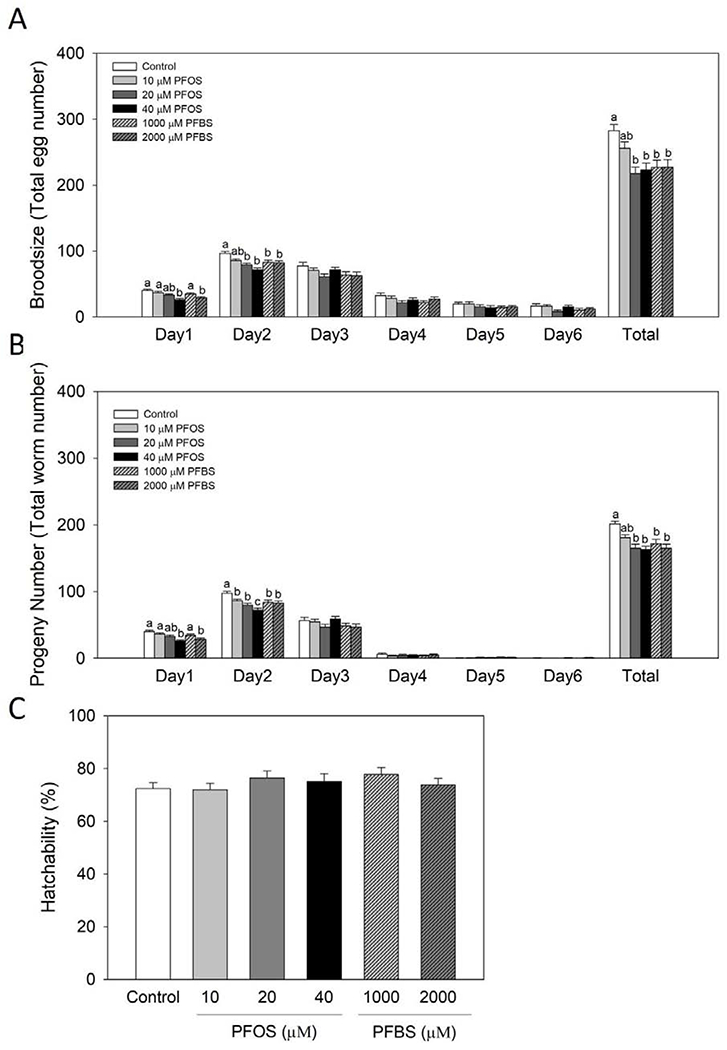
PFOS and PFBS significantly impaired the reproduction capacities of wild type C. elegans. Synchronized L4/young adult worms were treated with control (0.1% DMSO) or PFOS or PFBS for 1 day at 20°C. After treatment, worms were randomly selected for the reproduction analysis. Daily brood size and progeny number were recorded until the reproduction was ceased. Broodsize (A) was determined by counting the laid egg numbers. Progeny numbers (B) were recorded as the number of the hatched-out offspring. Hatchability (C) was calculated as progeny number/broodsize. Results are expressed as mean±S.E (n=21-24, collected from 4 independent experiments). a,b,c Means with different letters are significantly different (P<0.05).
3. Results
Progeny number and brood size were significantly reduced by PFOS and PFBS
In order to determine whether PFOS and PFBS influence the reproduction of C. elegans, we first examined their effects on the reproductive markers; brood size, progeny number and hatchability. After being treated with PFOS and PFBS for 24 hr from late L4/young adult stage, worms showed significantly impaired reproductive capacities (Figure 1). As shown in Figure 1A, the brood size, which represents the laid egg numbers, significantly declined at the first two days of reproduction period by PFOS and PFBS. Specifically, PFOS at 40 μM and PFBS at 2,000 μM significantly reduced brood size by 35% (P< 0.0001) and 28% (P= 0.0013) over the control group at day 1. At the second day of reproduction, 19% and 26% of reductions were observed at 20 μM (P= 0.0004) and 40 μM (P< 0.0001) of PFOS, while 14% and 15% of reductions were noticed in PFBS treatment groups at concentrations of 1,000 μM (P= 0.0137) and 2,000 μM (P= 0.0089), respectively, compared to the control. Similar results were observed on the progeny number during the first two days of reproduction by both PFOS and PFBS (Figure 1B). The total brood size (Figure 1A) and total progeny number (Figure 1B) were also significantly reduced by 20 μM (23%, P= 0.0002) and 40 μM PFOS (21%, P= 0.0008), as well as 1,000 μM (20%, P= 0.0022) and 2,000 μM PFBS (20%, P= 0.0025) compared to the control. No difference was observed on the hatchability for both compounds compared to the control (Figure 1C). These results suggest that PFOS and PFBS impaired the reproductive capacity of C. elegans.
PFOS and PFBS influenced embryonic nutrient loading and nutrient composition of eggs
Based on the observation that PFOS and PFBS can result in impairment on the reproduction of C. elegans, we next assessed whether this preconception exposure of PFOS and PFBS also affected the embryonic nutrient composition and uptake. The triglyceride and protein levels were significantly reduced in the PFOS and PFBS preconception exposed embryos (Figure 2A and B). As shown in Figure 2A, both PFOS (20 μM, P= 0.0247) and PFBS (1,000 μM, P= 0.0253) significantly reduced the triglyceride level by ~24% over the control. Additionally, PFOS significantly reduced protein content at 20 μM (P= 0.0024) and 40 μM (P= 0.0005), with a reduction of 30% and 34%, respectively, while PFBS at 1,000 μM significantly resulted in a 24% of reduction (P= 0.0134) compared to the control (Figure 2B). Then we monitored the effect of PFOS and PFBS on embryonic nutrient loading by measuring the oocytes yolk uptake using the transgenic strain DH1033 expressing VIT-2::GFP, a strain that has been commonly used to visualize yolk uptake. Results shown that VIT-2::GFP intensity were significantly altered by both compounds (Figure 2C). Interestingly, PFOS and PFBS exhibited different effects, as PFOS significantly increased the VIT-2::GFP intensity (45% for 20 μM and 55% for 40 μM with P< 0.0001 for both), while PFBS showed a significantly reduction (25% for 1,000 μM and 23% for 2,0 μM with P= 0.0119 and P= 0.0256, respectively) over the control (Figure 2C). These results suggest that PFOS and PFBS can significantly alter the embryonic nutrient composition and uptake in C. elegans.
Figure 2.
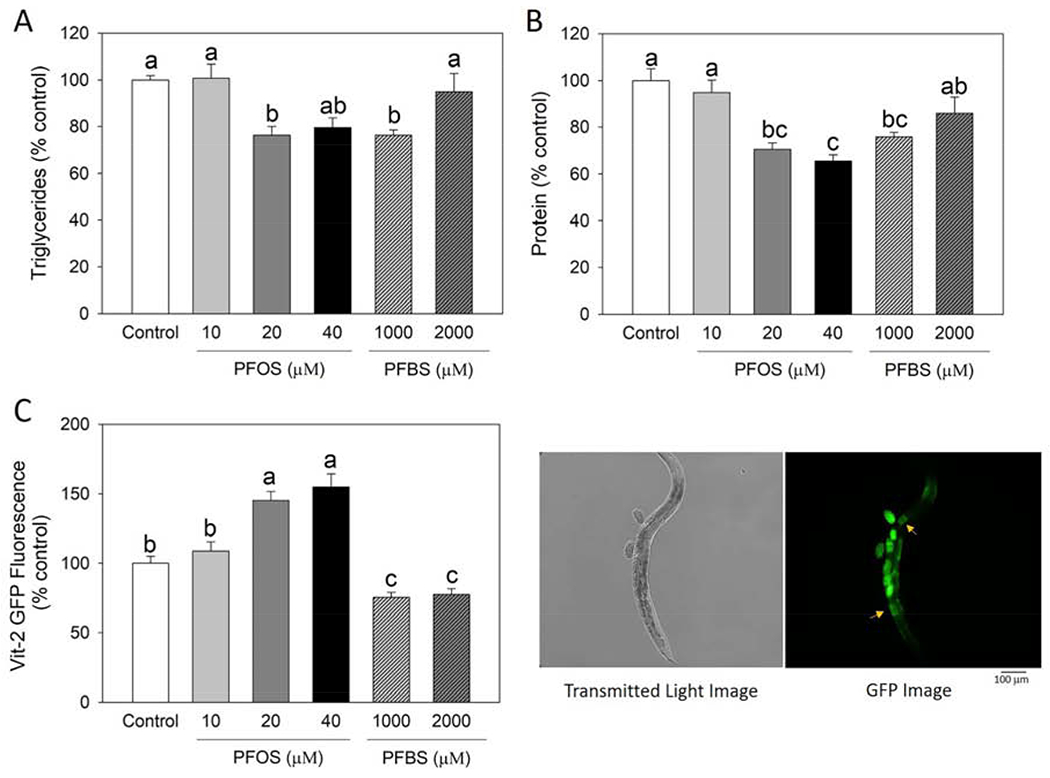
PFOS and PFBS altered the nutrient loading and nutrient composition of embryos. Synchronized L4/young adult worms were treated with control (0.1% DMSO) or PFOS or PFBS for 2 days at 20°C, then eggs were collected by bleaching method. Embryonic triglyceride (A) and protein (B) contents were measured and normalized by DNA concentration. Results are expressed as mean±S.E (n=4 plates, each plate contained >5,000 nematodes). (C) Effect of PFOS and PFBS on embryonic yolk uptake. Nutrient loading was monitored by quantifying the yolk uptake into oocytes with transgenic C. elegans strain DH1033. Synchronized L4/young adult DH1033 worms were treated for 1 day at 20°C. After treatment, worms were randomly selected for the fluorescence analysis. VIT-2::GFP expression was analyzed by Image J software by quantifying fluorescence intensity in the first pair of mature oocytes (yellow arrows) (n=115 for control, n=85 for 10 μM PFOS, n=105 for 20 μM PFOS, n=55 for 40 μM PFOS, n=107 for 1,000 μM PFBS, and n=113 for 2,000 μM PFBS). Results represent data collected from one experiment. a,b,c Means with different letters are significantly different (P<0.05).
Preconception exposure of PFOS and PFBS altered the development and locomotive activity of offspring
To investigate whether the preconception treatment of PFOS and PFBS further affect the physiological parameters of the offspring in their later life stages, we next monitored the triglyceride level, protein level, body size, growth rate, food intake and locomotive activity of the offspring F1 generation. It was observed that the growth rate of offspring from PFOS (10, 20 and 40 μM with P< 0.0001 for all groups) and PFBS (1,000 μM at P= 0.0451 and 2,000 μM at P= 0.0001) treated groups was significantly retarded compared to the control (Figure 3A), which is consistent to the reduced body size represented as worm length (Figure 3B) and width (Figure 3C). Only locomotive activity (average moving speed) was significantly decreased by PFOS treatment (24% of 40 μM at P= 0.0067) compared to the control, but not by PFBS (Figure 3D). No significance was observed in triglyceride content (Figure 3E), protein level (Figure 3F) and pumping rate (Figure 3G) by both compounds compared to the control. These results suggesting that PFOS and PFBS preconception exposure can lead to offspring physiological dysfunctions.
Figure 3.
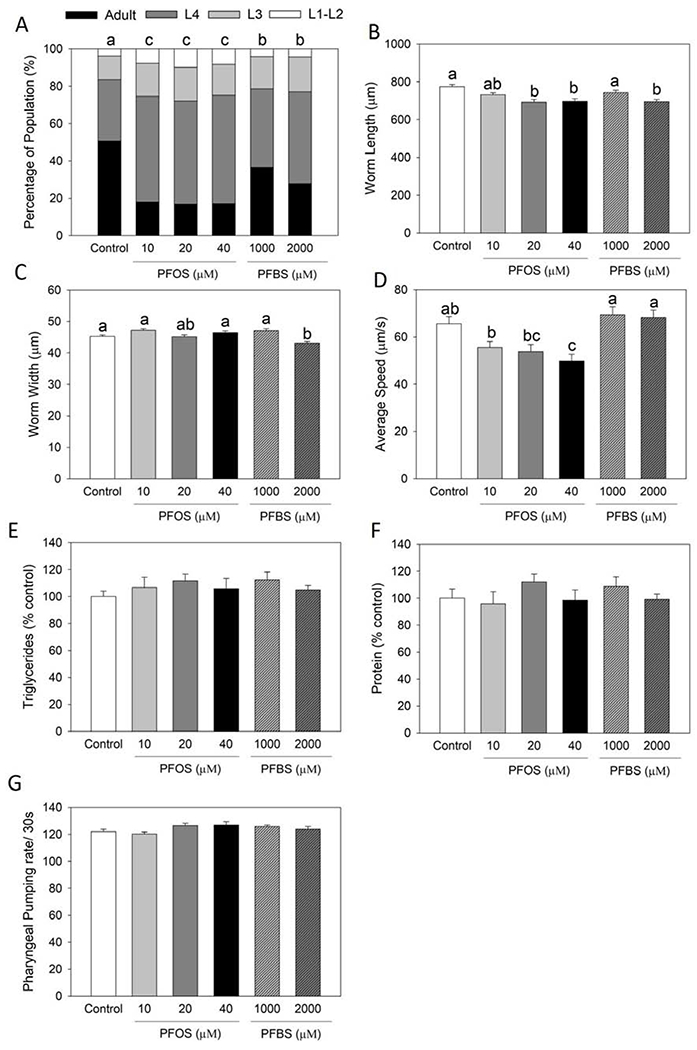
PFOS and PFBS preconception exposure led to offspring physiological dysfunctions. Synchronized L4/young adult worms (F0 generation) were treated with control (0.1% DMSO) or PFOS or PFBS for 2 days at 20°C, then harvest for eggs. Eggs were allowed to hatch out overnight and cultured without treatment till the offspring (F1 generation) reached L4 stage. Then worms were randomly selected for different parameter analyses. Growth rate (A) was scored as the percentage of worms at different developmental stages (n=211 for control, n=272 for 10 μM PFOS, n=232 for 20 μM PFOS, n=351 for 40 μM PFOS, n=173 for 1,000 μM PFBS, and n=165 for 2,000 μM PFBS). Results were analyzed by chi2 test. Worm size including length (B) and width (C) and locomotive activity as average moving speed (D) were measured and analyzed by using the WormLab Tracking System (n=143 for control, n=167 for 10 μM PFOS, n=130 for 20 μM PFOS, n=115 for 40 μM PFOS, n=131 for 1,000 μM PFBS, and n=174 for 2,000 μM PFBS). Triglyceride (E) and protein (F) contents were measured and normalized by DNA concentration. Results are expressed as mean±S.E (n=4 plates, each plate contained >1,000 nematodes). Food intake (G) was monitored by counting the pharyngeal pumping rate per 30s (n=12). All results were collected from one experiment. a,b,c Means with different letters are significantly different (P<0.05).
Internal concentrations of PFOS is greater than that of PFBS
Next, we measured the internal concentrations of PFOS and PFBS in both parent nematodes (F0 generation) and their embryos. Results showed that the internal concentrations of PFBS is much lower than that of PFOS in both parent nematodes and embryos, though the treatment concentrations of PFBS is around 100 times higher than that of PFOS (Table 1). It was also interesting to observe that with increased doses under the same treatment condition, the internal concentrations of PFOS did not increase dose-dependently, which may infer to the saturation of PFOS in C. elegans at dosages equal and higher than 20 μM. Alternatively, this may due to the reduced solubility of PFOS in aqueous buffer with potassium and sodium ions (as S-complete was used in the study) or adhesion to polypropylene and polystyrene used in experiments, while no such effects were detected in PFBS[19].
Table 1.
Internal concentrations of PFOS and PFBS after treatments.
| Dose (μM) | PFOS (ng/mg DNA) | PFBS (ng/mg DNA) | ||
|---|---|---|---|---|
| Worms | Control | 0 | N.D | N.D |
| PFOS | 10 | 174.4 ± 9.6b | N.D | |
| 20 | 238.2 ± 5.0a | N.D | ||
| 40 | 223.1 ± 7.3a | N.D | ||
| PFBS | 1,000 | N.D | 2.8 ± 0.1b | |
| 2,000 | N.D | 4.6 ± 0.2a | ||
| Embryos | Control | 0 | N.D | N.D |
| PFOS | 10 | 28.5 ± 0.4a | N.D | |
| 20 | 30.5 ± 1.1a | N.D | ||
| 40 | 23.6 ± 0.8b | N.D | ||
| PFBS | 1,000 | N.D | 1.8 ± 0.1b | |
| 2,000 | N.D | 3.3 ± 0.2a | ||
Synchronized L4/young adult worms were treated with control (0.1% DMSO) or PFOS or PFBS for 1 day at 20°C, then harvested for the determination of internal concentrations of PFOS and PFBS in worms (n=3 plates, each plate contained >1,000 nematodes). For the determination of internal concentrations of PFOS and PFBS in embryos, synchronized L4/young adult worms (F0 generation) were treated with control (0.1% DMSO) or PFOS or PFBS for 2 day at 20°C (n=3 plates, each plate contained >5,000 nematodes), then eggs were collected by the bleaching method. PFOS and PFBS concentration in worms and embryos were measured by LC/MS and normalized by DNA level. Results are expressed as mean±S.E. (n=3).
Means with different letters are significantly different (P<0.05). N.D: not detected
4. Discussion
The current study demonstrated that PFOS and PFBS can significantly impair the reproductive capacities of C. elegans by reducing the brood size and progeny number of F0 treated worms. The preconception treatment of PFOS and PFBS altered the embryonic nutrient uptake and composition, which may further contribute to the F1 offspring dysfunction including body size reduction, growth rate inhibition and reduced locomotor activity. The majority effects of PFOS and PFBS observed in the current study are similar and comparable to each other though with different dosages ranges, while the regulation of embryonic VIT-2::GFP, one of yolk uptake markers, found to be distinct between PFOS and PFBS.
As one of the most extensively studied PFASs, PFOS has been reported to exhibit many reproductive toxicities, including low birth weight[20, 21], reproductive hormone disruption[22, 23], fertility impairment[24–26] and reproductive organ defects[27]. Unlike PFOS, reduced toxicity of PFBS were reported in limited studies[14, 28–30]. In a reproduction study with adult quail, dietary concentration of 100-900 mg PFBS/kg wet weight feed did not show any significant changes on reproductive parameters[28]. Similarly, a two-generation oral gavage reproduction study conducted in rats showed that PFBS does not cause significant reproductive defects except a slight delay on the sex maturation in F1 generation with dosages up to 1,000 mg/kg/day[29]. But in a more recent mice study, it was found that exposure of pregnant mice to PFBS (200 and 500 mg/kg/day) can lead to a series of abnormalities in offspring female mice, including reduced perinatal body weight, delayed eye opening, delayed onset of puberty and impaired sexual organ development[30], which are consistent with our findings that preconception exposure of PFBS can significantly alter the normal physiological parameters of offspring generation. Likewise, our results that PFBS significantly reduced the reproductive capacity is consistent with a previous report in C. elegans published by Chen et al[14]. The current results of reduced brood size and progeny number without altering hatchability suggest that the impaired reproduction by PFASs is likely due to its impact on pre-fertilization rather than embryonic development. Moreover, as previously reported that PFOS can inhibit germline proliferation [31] and both PFOS and PFBS found to induce germ cell apoptosis in C. elegans [14], the observed effect in the current study may also in part due to effects on the reproductive tissues by PFASs.
Pharmacokinetics studies showed that, similar to PFOS, PFBS can be easily absorbed and distribute to all the tissues[32]. However, the tissue level of PFBS found to be 5–40-fold lower than that of PFOS with similar exposure levels in mice[32], reflecting its rapid elimination rate reported in many species including human[2]. PFBS has much shorter serum elimination half-life with an estimation of 4.5 hr in male rats, 95 hr in male monkeys and 26 days in humans[2, 32], while PFOS has the estimated values of 40 days in male rats, 132 days in male monkeys and 5.4 years in human[32, 33]. Though PFBS has the similar tissue distribution of PFOS, the distribution pattern differs, such as PFBS exhibits lower levels in liver (tissue to blood ratio: 1.6 for PFBS versus 4 for PFOS) and lungs (tissue to blood ratio: 0.8 for PFBS versus 2 for PFOS) relative to blood than PFOS[32]. These differences may be in part due to the difference between their solubility[19], protein binding capacity[34] and the chain length related active transport mechanisms[2, 32, 35]. Consistently, our study found that in order to produce similar effect on reproductive parameters, the dosages range (1,000 to 2,000 μM) for PFBS is around 100 times greater than that of PFOS (10 to 40 μM), whereas the internal concentrations of PFBS is still much lower than that of PFOS in both worms (174.4-238.2 ng/mg DNA for PFOS versus 2.8-4.6 ng/mg DNA for PFBS) and eggs (23.6-30.5 ng/mg DNA for PFOS versus 1.8-3.3 ng/mg DNA for PFBS). These suggest that pharmacokinetics of PFOS and PFBS is conserved between the mammals and C. elegans. It was also noted that, unlike PFOS, some results obtained from PFBS treated groups, such as embryonic nutrient composition and uptake, did not show a dose-dependent effect. The lack of dose-dependent effect by PFBS was also reported by others in different models[14, 29, 36, 37]. It is possible that the lack of dose-dependent effect of PFBS was due to the low internal concentrations of PFBS. In addition, the potential that non-monotonic dose-response relationships between PFBS and reproductive related markers exists should be considered, which needs to be elucidated by future studies.
Though it is not clear how PFOS and PFBS preconception exposure led to the dysfunction of offspring generation in current study, we infer that it is due to the altered embryonic nutrient composition by these two compounds. While C. elegans gets into adulthood and mature to reproduce, yolk, the lipoprotein complexes, by which oviparous animals provide nutrients to support the development of their progeny will be synthesized and secreted from the worm intestine, then transported to the germline[38]. While reached the germline, yolk will be loaded into the mature oocytes by receptor-mediated endocytosis which serves as the nutrient during the embryogenesis after fertilization[38]. Based on our results, we found that the embryonic nutrient composition was significantly altered in the PFOS and PFBS treated group, as they showed a significantly reduction on the embryonic triglyceride and protein levels, which may due to the reduced yolk loading into the embryos. Ezcurra et al.[39] demonstrated that yolk production is an autophagy-dependent gut-to-yolk biomass conversion, where nematodes consume their own intestines to make the yolk and promote reproduction, as such mutants deficient in making yolk will result in an increased intestinal neutral fat. Therefore, the less embryonic nutrient loading after PFOS and PFBS treatment may lead to more fat accumulate in the worm body which is consistent with our observation that the triglyceride content was increased in F0 nematodes after PFOS and PFBS treatments (Supplementary Figure S2).
Embryonic yolk abundance has been found to be positively associated with the post-embryonic survival and development[8, 40]. High yolk provisioned progeny hatched with a larger body size and increased resistance to larva starvation, while worms hatched from yolk defective embryos are smaller, more starvation sensitive, and need more time to reach adulthood[38, 40]. With reduced embryonic triglyceride and protein contents, the F1 offspring from PFOS and PFBS preconception exposure groups showed significantly reduced body size and delayed growth rate, suggesting that the observed defects in embryonic nutrient loading might be one of the contributors that leads to the F1 offspring dysfunction. In order to monitor the yolk uptake in oocytes, we employed a transgenic nematode strain that labeled one of the yolk proteins, VIT-2, with green florescence protein. By quantifying the fluorescence intensity in the oocytes, PFBS showed reduced VIT-2::GFP as expected, but interestingly PFOS showed increased uptake, which is inconsistent with the reduced embryonic triglyceride and protein levels by PFOS. One possible explanation is that since VIT-2 is only one of the 6 yolk proteins that consists of yolk lipoprotein complexes[38], the sole VIT-2::GFP may not conclusively represent the yolk accumulation in oocytes. Future studies with direct yolk quantification methods are needed to elucidate the significance of PFOS and PFBS on embryonic yolk/nutrient uptake.
PFBS has reported its occurrence in various sources including environmental medias, dietary source and consumer goods[41–44]. As such, PFBS has been detected in human serum in the general populations, but the level of PFBS is much lower than PFOS[45, 46]. The majority of human serum samples (>90%) were below the level of detection (4.2 ng/mL) from the data collected by American Red Cross in 2015[45]. Consistently, others reported 95th percentile for PFBS at below the level of detection (0.1 ng/mL) by National Health and Nutrition Examination Survey (NHANES) 2013-2014[46]. However, a study with a lower detection limit (0.013 ng/mL) reported the increasing levels of PFBS in serum of primiparous nursing Swedish women from 1996 to 2010[47], indicating the increasing concentrations of PFBS in humans over years. Although it is not currently possible to directly translate dosages from C. elegans to human and the concentration used in current study may not be achievable in human, attention still need to be paid on the potential long-term effect of PFBS cross generations as well as its potential interaction with other PFASs on reproduction.
To conclude, the current results show that PFBS, a shorter chain derivative of PFOS, significantly reduced the reproductive capacity and altered the normal physiological parameters of F1 offspring in a similar extent of PFOS with a lower internal concentration, which can provide important information for the future study elucidating the effect of PFASs on human reproductive health.
Supplementary Material
Highlights.
PFOS and PFBS impaired reproduction of C. elegans
Preconception exposure of PFOS and PFBS led to offspring physiological dysfunctions
Higher dosages are needed for PFBS to produce comparable effects of PFOS
Acknowledgments
This material is based upon work supported by NIH R01ES028201. All the strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). We would like to thank Mr. Nikolas Rodriguez for assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference
- 1.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, and Zobel LR, Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental Health Perspectives, 2007. 115(9): p. 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen GW, Chang S-C, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, and Butenhoff JL, A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology, 2009. 256(1–2): p. 65–74. [DOI] [PubMed] [Google Scholar]
- 3.Qi W, Clark JM, Timme-Laragy AR, and Park Y, Perfluorobutanesulfonic acid (PFBS) potentiates adipogenesis of 3T3-L1 adipocytes. Food and Chemical Toxicology, 2018. 120: p. 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, Zhang R, Jin F, Lou H, Mao Y, Zhu W, Zhou W, Zhang P, and Zhang J, Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environment International, 2017. 102: p. 207–212. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Tsui MM, Shi Q, Hu C, Wang Q, Zhou B, Lam PK, and Lam JC, Accumulation of perfluorobutane sulfonate (PFBS) and impairment of visual function in the eyes of marine medaka after a life-cycle exposure. Aquatic Toxicology, 2018. 201: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Shen P, Yue Y, Zheng J, and Park Y, Caenorhabditis elegans: a convenient in vivo model for assessing the impact of food bioactive compounds on obesity, aging, and Alzheimer’s disease. Annual Review of Food Science and Technology, 2018. 9: p. 1–22. [DOI] [PubMed] [Google Scholar]
- 7.Tejeda-Benitez L and Olivero-Verbel J, Caenorhabditis elegans, a biological model for research in toxicology, in Reviews of Environmental Contamination and Toxicology Volume 237 2016, Springer. p. 1–35. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PR, The C elegans model in toxicity testing. Journal of Applied Toxicology, 2017. 37(1): p. 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen P, Yue Y, and Park Y, A living model for obesity and aging research: Caenorhabditis elegans. Critical Reviews in Food Science and Nutrition, 2018. 58(5): p. 741–754. [DOI] [PubMed] [Google Scholar]
- 10.Shen P, Yue Y, Kim K-H, and Park Y, Piceatannol reduces fat accumulation in Caenorhabditis elegans. Journal of Medicinal Food, 2017. 20(9): p. 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue Y, Shen P, Chang AL, Qi W, Kim K-H, Kim D, and Park Y, trans-Trismethoxy resveratrol decreasedfat accumulation dependent on fat-6 and fat-7 in Caenorhabditis elegans. Food & Function, 2019. 10(8): p. 4966–4974. [DOI] [PubMed] [Google Scholar]
- 12.Farias-Pereira R, Oshiro J, Kim K-H, and Park Y, Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. Journal of Functional Foods, 2018. 48: p. 586–593. [Google Scholar]
- 13.Stiernagle T, Maintenance of C. elegans (February 11, 2006) WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.101.1, http://www.wormbook.org, 1999. 2: p. 51–67. [Google Scholar]
- 14.Chen F, Wei C, Chen Q, Zhang J, Wang L, Zhou Z, Chen M, and Liang Y, Internal concentrations of perfluorobutane sulfonate (PFBS) comparable to those of perfluorooctane sulfonate (PFOS) induce reproductive toxicity in Caenorhabditis elegans. Ecotoxicology and Environmental Safety, 2018. 158: p. 223–229. [DOI] [PubMed] [Google Scholar]
- 15.Labarca C and Paigen K, A simple, rapid, and sensitive DNA assay procedure. Analytical Biochemistry, 1980. 102(2): p. 344–352. [DOI] [PubMed] [Google Scholar]
- 16.Shen P, Kershaw JC, Yue Y, Wang O, Kim K-H, McClements DJ, and Park Y, Effects of conjugated linoleic acid (CLA) on fat accumulation, activity, and proteomics analysis in Caenorhabditis elegans. Food Chemistry, 2018. 249: p. 193–201. [DOI] [PubMed] [Google Scholar]
- 17.Yue Y, Shen P, Xu Y, and Park Y, p-Coumaric acid improves oxidative and osmosis stress responses in Caenorhabditis elegans. Journal of the Science of Food and Agriculture, 2019. 99(3): p. 1190–1197. [DOI] [PubMed] [Google Scholar]
- 18.Chen W-W, Yi Y-H, Chien C-H, Hsiung Κ-C, Ma T-H, Lin Y-C, Lo SJ, and Chang T-C, Specific polyunsaturated fatty acids modulate lipid delivery and oocyte development in C. elegans revealed by molecular-selective label-free imaging. Scientific Reports, 2016. 6: p. 32021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundström M, Bogdanska J, Pham HV, Athanasios V, Nobel S, McAlees A, Eriksson J, DePierre JW, and Bergman Å, Radiosynthesis of perfluorooctanesulfonate (PFOS) and perfluorobutanesulfonate (PFBS), including solubility, partition and adhesion studies. Chemosphere, 2012. 87(8): p. 865–871. [DOI] [PubMed] [Google Scholar]
- 20.Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, and Goldman LR, Cord semm concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environmental Health Perspectives, 2007. 115(11): p. 1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Zeng X-W, Qian ZM, Vaughn MG, Sauvé S, Paul G, Lin S, Lu L, Hu L-W, and Yang B-Y, Isomers of perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environment International, 2017. 102: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 22.Joensen UN, Veyrand B, Antignac J-P, Blomberg Jensen M, Petersen JH, Marchand P, Skakkebask NE, Andersson A-M, Le Bizec B, and Jørgensen N, PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Human Reproduction, 2012. 28(3): p. 599–608. [DOI] [PubMed] [Google Scholar]
- 23.Itoh S, Araki A, Mitsui T, Miyashita C, Goudarzi H, Sasaki S, Cho K, Nakazawa H, Iwasaki Y, and Shinohara N, Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health. Environment International, 2016. 94: p. 51–59. [DOI] [PubMed] [Google Scholar]
- 24.Potera C, Reproductive toxicology: study associates PFOS and PFOA with impaired fertility, 2009, National Institute of Environmental Health Sciences, p. A148–A148. [Google Scholar]
- 25.Han J and Fang Z, Estrogenic effects, reproductive impairment and developmental toxicity in ovoviparous swordtail fish (Xiphophorus helleri) exposed to perfluorooctane sulfonate (PFOS). Aquatic Toxicology, 2010. 99(2): p. 281–290. [DOI] [PubMed] [Google Scholar]
- 26.La Rocca C, Alessi E, Bergamasco B, Caserta D, Ciardo F, Fanello E, Focardi S, Guerranti C, Stecca L, and Moscarini M, Exposure and effective dose biomarkers for perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in infertile subjects: preliminaiy results of the PREVIENI project. International Journal of Hygiene and Environmental Health, 2012. 215(2): p. 206–211. [DOI] [PubMed] [Google Scholar]
- 27.Toft G, Jönsson BAG, Lindh CH, Giwercman A, Spano M, Heederik D, Lenters V, Vermeulen R, Rylander L, Pedersen HS, Ludwicki JK, Zviezdai V, and Bonde JP, Exposure to perfluorinated compounds and human semen quality in arctic and European populations. Human Reproduction, 2012. 27(8): p. 2532–2540. [DOI] [PubMed] [Google Scholar]
- 28.Newsted JL, Beach SA, Gallagher S, and Giesy J, Acute and chronic effects of perfluorobutane sulfonate (PFBS) on the mallard and northern bobwhite quail. Archives of Environmental Contamination and Toxicology, 2008. 54(3): p. 535–545. [DOI] [PubMed] [Google Scholar]
- 29.Lieder PH, York RG, Hakes DC, Chang S-C, and Butenhoff JL, A two-generation oral gavage reproduction study with potassium perfluorobutanesulfonate (K+ PFBS) in Sprague Dawley rats. Toxicology, 2009. 259(1-2): p. 33–45. [DOI] [PubMed] [Google Scholar]
- 30.Feng X, Cao X, Zhao S, Wang X, Hua X, Chen L, and Chen L, Exposure of pregnant mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in female offspring. Toxicological Sciences, 2017. 155(2): p. 409–419. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Li Q, Shi J, Shi L, Li B, Xu A, Zhao G, and Wu L, Perfluorooctane sulfonate exposure causes gonadal developmental toxicity in Caenorhabditis elegans through ROS-induced DNA damage. Chemosphere, 2016. 155: p. 115–126. [DOI] [PubMed] [Google Scholar]
- 32.Bogdanska J, Sundström M, Bergström U, Borg D, Abedi-Valugerdi M, Bergman Å, DePierre J, and Nobel S, Tissue distribution of 35S-labelled perfluorobutanesulfonic acid in adult mice following dietary exposure for 1–5 days. Chemosphere, 2014. 98: p. 28–36. [DOI] [PubMed] [Google Scholar]
- 33.Chang S-C, Noker PE, Gorman GS, Gibson SJ, Hart JA, Ehresman DJ, and Butenhoff JL, Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reproductive Toxicology, 2012. 33(4): p. 428–440. [DOI] [PubMed] [Google Scholar]
- 34.Southern Research I, Protein binding of perfluorobutane sulfonate, perfluorohexane sulfonate, perfluorooctane sulfonate and perfluorooctanoate to plasma (human, rat, and monkey), and various human-derived plasma protein fractions. Study ID 9921.7, 2003, 3M Company: St. Paul, MN. [Google Scholar]
- 35.Weaver YM, Ehresman DJ, Butenhoff JL, and Hagenbuch B, Roles of Rat Renal Organic Anion Transporters in Transporting Perfluorinated Carboxylates with Different Chain Lengths. Toxicological Sciences, 2009. 113(2): p. 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieder PH, Chang S-C, York RG, and Butenhoff JL, Toxicological evaluation of potassium perfluorobutanesulfonate in a 90-day oral gavage study with Sprague-Dawley rats. Toxicology, 2009. 255(1-2): p. 45–52. [DOI] [PubMed] [Google Scholar]
- 37.Sant KE, Venezia OL, Sinno PP, and Timme-Laragy AR, Perfluorobutanesulfonic acid disrupts pancreatic organogenesis and regulation of lipid metabolism in the zebrafish, Danio rerio. Toxicological Sciences, 2019. 167(1): p. 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez MF and Lehner B, Vitellogenins-yolk gene function and regulation in Caenorhabditis elegans. Frontiers in Physiology, 2019. 10: p. 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezcurra M, Benedetto A, Sornda T, Gilliat AF, Au C, Zhang Q, van Schelt S, Petrache AL, Wang H, and de la Guardia Y, C. elegans eats its own intestine to make yolk leading to multiple senescent pathologies. Current Biology, 2018. 28(16): p. 2544–2556. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez MF, Francesconi M, Hidalgo-Carcedo C, and Lehner B, Maternal age generates phenotypic variation in Caenorhabditis elegans. Nature, 2017. 552(7683): p. 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Lohmann R, Dassuncao C, Hu XC, Weber AK, Vecitis CD, and Sunderland EM, Source attribution of poly- and perfluoroalkyl substances (PFASs) in surface waters from Rhode Island and the New York Metropolitan Area. Environmental Science & Technology Letters, 2016. 3(9): p. 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR, and Peaslee GF, Fluorinated compounds in US fast food packaging. Environmental Science & Technology Letters, 2017. 4(3): p. 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Authority EFS, Perfluoroalkylated substances in food: occurrence and dietary exposure. EFSA Journal, 2012. 10(6): p. 2743. [Google Scholar]
- 44.Liu X, Guo Z, Krebs KA, Pope RH, and Roache NF, Concentrations and trends of perfluorinated chemicals in potential indoor sources from 2007 through 2011 in the US. Chemosphere, 2014. 98: p. 51–57. [DOI] [PubMed] [Google Scholar]
- 45.Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, Herron RM, Hanna H, Nobiletti JB, and Rios JA, Per-and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000–2015. Environmental Research, 2017. 157: p. 87–95. [DOI] [PubMed] [Google Scholar]
- 46.Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, and Needham LL, Polyfluoroalkyl chemicals in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environmental Health Perspectives, 2007. 115(11): p. 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, and Darnerud PO, Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environmental Science & Technology, 2012. 46(16): p. 9071–9079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


