Abstract
Background
Rattlesnake envenomations are a significant cause of morbidity in the USA. While pediatric rattlesnake envenomations are relatively common, data comparing adult and pediatric patients with rattlesnake envenomations remain limited.
Methods
This multi-center retrospective study used the North American Snakebite Registry (NASBR), a sub-registry of the Toxicology Investigator’s Consortium (ToxIC). All cases of rattlesnake envenomations between January 1, 2013, and December 31, 2017, which were entered into the NASBR, were reviewed. Clinical and laboratory parameters, as well as treatment and outcome measurements, were compared between adult and pediatric patients.
Results
A total of 420 unique cases were identified, including 94 pediatric patients. Adult patients were more likely to be male (76% vs. 62%; OR 1.98) and sustain upper extremity envenomations (57% vs. 25%; OR 4.4). After adjusting for bite location, adults were more likely to exhibit edema compared with pediatric patients. After controlling for envenomation location, there was no difference in rates of necrosis between adult and pediatric patients. Adults exhibited early hematologic toxicity less frequently than pediatric patients, but there was no difference in the rates of late hematologic toxicity. There were no differences in the rates of hypotension or intubation.
Conclusion
While adult and pediatric patients have some differences in envenomation characteristics and laboratory parameters, adults and pediatric patients had similar rates of systemic toxicity, severity, length of stay, and late hematologic toxicity.
Keywords: Rattlesnake, Pediatric, Snake, Envenomation, Antivenom
Introduction
In the USA, rattlesnake envenomations are the most significant source of morbidity and mortality among patients bitten by native venomous snakes. Each year, nearly 9000 patients are treated for snakebites [1]. In 2018, US Poison Control Centers received more than 4000 calls regarding rattlesnake bites [2]. Pediatric patients generally account for 15–20% of patients in published reports that include rattlesnake envenomations [2–4]. In the first 3 years of the American College of Medical Toxicology (ACMT)’s North American Snakebite Registry (NASBR), children aged 12 and younger comprised 28% of cases [5].
Rattlesnake envenomation most commonly results in tissue edema that expands from the envenomation site. Hematologic toxicity and a variety of other systemic or neurological effects can also occur. The severity of clinical effects that develop after a rattlesnake envenomation varies greatly and is not easily predicted, although the dose and composition of venom injected likely play the most important role in determining clinical severity. Some authors suggest children are at an increased risk for severe envenomation compared with adults [6, 7]. While there are some data to support increased severity in children with Vipera lebetina envenomations in Turkey, objective data supporting differences in envenomation severity between adult and pediatric patients bitten by North American rattlesnake species are lacking [8].
The primary purpose of this study is to compare pediatric to adult patients with rattlesnake envenomation, with regard to envenomation characteristics, clinical characteristics, treatment strategies, and outcomes.
Methods
This is a multi-center, retrospective study utilizing the NASBR. The NASBR is a sub-registry of the Toxicology Investigator’s Consortium (ToxIC) Registry, a toxico-surveillance registry of patients treated by physician medical toxicologists at 50 participating centers in the USA, plus three international sites. Toxicologists enter de-identified clinical data on North American snakebites into the NASBR at the time of the patient encounter. Prior to 2013, there was no prospective database detailing patients with snakebites. The NASBR sub-registry includes detailed clinical information, including patient characteristics, clinical and laboratory parameters, treatment rendered, and outcome from a select number of sites in the ToxIC registry who see the majority of snakebites. All patients in the NASBR were evaluated directly by a medical toxicologist at the bedside. The ToxIC registry has approval from the Western Institutional Review Board. Many individual sites also achieved approval from their Institutional Review Board (IRB) for participation in the registry. This study also received IRB approval.
Investigators reviewed all cases entered into the NASBR between January 1, 2013, and December 31, 2017. Envenomations from species other than North American rattlesnakes were excluded. Patients were divided into adult and pediatric groups for comparisons of demographics, envenomation and clinical characteristics, treatment strategies, and outcomes. Pediatric patients were further stratified according to age (0–6 years, 7–12 years, 13–17 years).
Definitions
Pediatric patients were defined as those less than 18 years of age. Length of stay was calculated in 24-hour blocks. Consistent with prior rattlesnake studies, coagulopathy was defined as a prothrombin time (PT) > 15 s or a fibrinogen less than 170 mg/dL, whereas thrombocytopenia was defined as platelets less than 120 × 109/L [9].
Hematologic toxicity was defined as the presence of either coagulopathy or thrombocytopenia. If hematologic toxicity occurred during the initial presentation and treatment phase (typically within 48 hours of the envenomation), it was considered to be “early.” Platelet count, fibrinogen, and PT were entered into the database at presentation to healthcare, at their nadir (for platelets and fibrinogen) or peak (for PT), and at hospital discharge. “Late” hematologic toxicity was defined as the development of thrombocytopenia or coagulopathy during the follow-up phase and after treatment was considered complete (typically after hospital discharge). Late hematologic toxicity included either delayed onset of hemotoxicity in patients who had normal laboratory values during the initial treatment phase, or recurrence of early hemotoxicity that had initially improved with treatment. Late bleeding was defined as any bleeding that developed after the initial treatment phase. We have categorized bleeding episodes as major, minor, or trivial based on previously published literature involving rattlesnake envenomation [10]. Major bleeding was defined as fatal bleeding or bleeding associated with hemodynamic instability, bleeding requiring transfusion or red blood cells or an invasive procedure, or bleeding into an enclosed space. Minor bleeding was bleeding associated with prolonged observation or admission, but not otherwise meeting criteria for major bleeding. Trivial bleeding was defined as bleeding not requiring any interventions or prolonged observation (e.g., persistent oozing from puncture wounds, or self-limited epistaxis).
A plasma fibrinogen or platelet count less than the lower limit of detection (e.g., fibrinogen less than 30 mg/dL) was considered to be zero for calculations. Similarly, a PT above the upper limit of detection (e.g., more than 120 s) was considered to be 120 s for calculation purposes. Rhabdomyolysis was defined as a CK greater than 1000 IU/L.
Data Abstraction and Analysis
Study data were collected by member toxicologists and managed using REDCap electronic data capture tools hosted at the Vanderbilt University Heath Core under contract to ACMT [11]. Data were entered by the treating medical toxicologist and/or medical toxicology fellows into REDCap. The file was exported into Excel version 14.0.7224.5000 (Microsoft Corporation, Redmond, WA). The collected data included demographic information, envenomation characteristics, key examination findings, laboratory parameters, treatment characteristics, and outcome (including presence/absence of recurrence or delayed hematologic toxicity).
Data were subsequently imported into STATA (version 15.1, StataCorp, College Station, TX). Continuous variables were reported as medians and interquartile ranges (IQR). The Kruskal-Wallis nonparametric testing was used to compare medians for the adult and pediatric patients. Categorical variables were compared with Fisher’s exact test or chi-square test, as appropriate. Logistic regression was used for dichotomous outcomes of interest, including envenomation location, hematologic toxicity, edema, late hematologic toxicity, and functional impairment. Given the paucity of patients with envenomations to locations other than extremities (e.g., face), such patients were excluded from bite location analysis. Cluster analysis was performed to ensure no single center was contributing a disproportionate number of cases of hematologic toxicity. Adjustments for multiple comparisons were not made.
Results
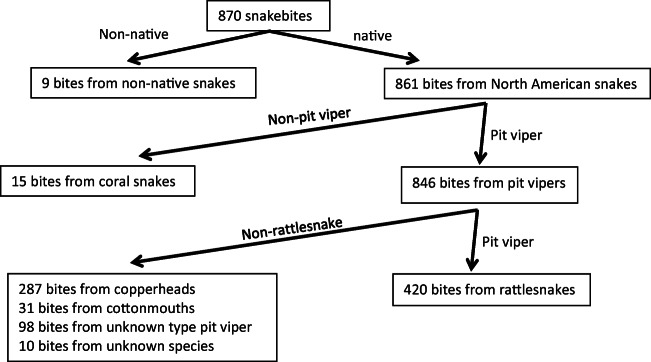
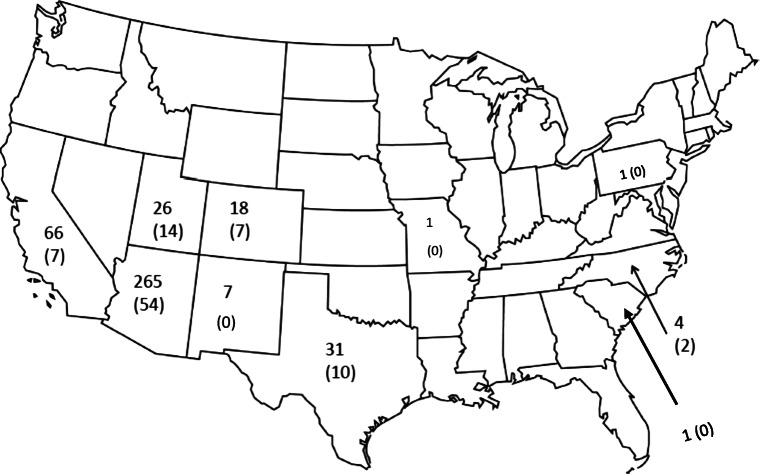
A total of 870 unique cases were identified; 450 were excluded as they involved snakes other than rattlesnakes, leaving 420 rattlesnake envenomations from 10 states (AZ, CA, CO, MO, NM, NC, PA, SC, TX, UT) (Figs. 1 and 2). The exact age was not recorded in 5 people and thus they were excluded in age-specific variables (Fig. 3).
Fig. 1.
Patient inclusion.
Fig. 2.
Map of the USA demonstrating geographical distribution of rattlesnake bites. Numbers represent total number of bites, with number of pediatric bites in parenthesis underneath the total number.
Fig. 3.
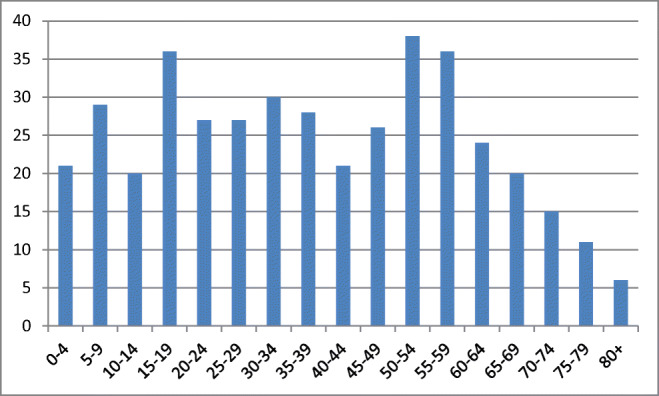
Age distribution of patients.
Overall, most patients were male (305/420; 73%) and the median age was 37 (19–56) years. Upper and lower extremity envenomations were equally distributed. A total of 208/420 (50%) envenomations involved upper extremities and 211/420 (50%) envenomations involved lower extremities. Two patients (0.5%) had head or neck bites, one of whom also had an extremity bite. The odds of having an upper extremity envenomation differed by sex. Upper extremity envenomation occurred much more frequently in males compared with females (179/208 vs. 29/208; p < 0.001).
Pediatric patients accounted for 94/415 (23%) of rattlesnake envenomation. There were several differences in bite location and presentation between children and adults. Adults were more likely to be male (76% vs. 62%; OR 1.98 [95% CI 1.2–3.2]) and sustain upper extremity bites (57% vs. 25%; OR 4.4 [95% CI 2.6–7.4]). Bite location also differed by age within each sex. Among adult women, 27 of 79 (34%) bites were on the upper extremity, compared with 2 of 36 in pediatric girls (6%; OR 8.7 [95% CI 1.9–38.8]). Among adult males, 157/244 (64%) bites occurred on the upper extremities, compared with 21/56 (38%) pediatric boys (OR 3.2 [95% CI 1.7–5.8]). Patient demographic factors and envenomation characteristics are presented in Table 1.
Table 1.
Patient demographics and clinical effects.
| Pediatric (n = 94) | Adult (n = 323) | p value | |
|---|---|---|---|
| Male (%) | 58 (61.7%) | 244 (75.5%) | 0.009 |
| Upper extremity (%) | 23 (24.5%) | 183 (56.7%) | < 0.0001 |
| Edema (%) | 82 (87.2%) | 313 (96.9%) | 0.0002 |
| Ecchymosis (%) | 69 (73.4%) | 196 (60.7%) | 0.025 |
| Acute kidney injury | 0/94 (0%) | 1/323 (0.31%) | 0.77 |
| Neurotoxicity (%) | 6 (6.5%) | 32 (10.0%) | 0.31 |
| Hypotension (%) | 3 (3.3%) | 23 (7.1%) | 0.22 |
| Intubation (%) | 0 (0%) | 7 (2.2%) | 0.36 |
| Rhabdomyolysis* | 4 (4.3%) | 19 (5.9%) | 0.80 |
*Data available for 225 subjects
Edema was common in both adult and pediatric patients, but it was more so in adults (97% vs. 87%; OR 4.7 [95% CI 1.9–10.9]). Even after adjusting for bite location (upper extremity vs. lower extremity), adults were more likely to exhibit edema compared with pediatric patients (OR 3.3; 95% CI 1.3–8.2). Children were less likely to have tissue necrosis compared with adults (OR 0.2; 95% CI 0.04–0.84). However, after controlling for bite location, the likelihood of having necrosis was no longer different between pediatric and adult patients (OR 0.4; 95% CI 0.09–1.7).
The percentage of patients with neurotoxicity, hypotension, and intubation was higher in adults than in children, but the difference was not statistically significant (Table 1). Early bleeding occurred in 36 patients, six of whom were pediatric. Bleeding was considered “trivial bleeding” in all cases (e.g., epistaxis, oozing from puncture site). There was no difference in the rate of early bleeding between adult and pediatric patients.
The rate of early hematologic toxicity was lower in the adult population than in pediatric population (45% vs. 586%; OR 0.6, 95% CI 0.4–0.97). Early hypofibrinogenemia was more common in pediatric patients than in adult patients (33% vs. 17%; 95% CI 5.9–26.4; p = 0.001). Similarly, early prolongation of the PT was also more common in pediatric patients than in adult patients (42% vs. 30%; 95% CI 0.4–22.3%; p = 0.04). There was no significant difference in the rate of early thrombocytopenia in pediatric vs. adult patients (11% vs. 19%; 95% CI − 0.45–14.9%; p = 0.06) (Table 2). On cluster analysis, the state in which the envenomation occurred did not influence development of hematologic toxicity.
Table 2.
Comparison of early hemotoxicity between pediatric and adult patients.
| Pediatric (n = 94) | Adult (n = 323) | p value | |
|---|---|---|---|
| Early thrombocytopenia | 10 (10.6%) | 61 (18.9%) | 0.06 |
| Early hypofibrinogenemia | 31 (33%) | 56 (17.3%) | 0.001 |
| Early elevation in PT | 39 (41.5%) | 98 (30.3%) | 0.042 |
| Platelets (× 109/L); median (IQR) | |||
| Initial | 255 (196–311) | 214 (163–262) | < 0.001 |
| Nadir | 217 (171–253) | 181 (137–225) | < 0.001 |
| Discharge | 237 (210–281) | 202 (172–237) | < 0.001 |
| Fibrinogen (mg/dL); median (IQR) | |||
| Initial | 238 (206–280) | 293 (241–359) | < 0.001 |
| Nadir | 202 (156–239) | 238 (191–283) | < 0.001 |
| Discharge | 255 (217–305) | 302 (252–376) | < 0.001 |
| Prothrombin time (Seconds); median (IQR) | |||
| Initial | 13 (11.7–14.5) | 12.4 (10.9–13.7) | < 0.001 |
| Maximal | 14.9 (13.7–16.6) | 14 (12.8–15.5) | 0.002 |
| Discharge | 13.6 (12–14.3) | 13.2 (12–14.1) | 0.2 |
The creatinine kinase (CK) was recorded in 225 cases. The median (IQR) CK (IU/L) among pediatric patients did not differ from that of adults (150 [112–246] vs. 158 [111–299]; p = 0.53). There was also no difference in the rate of rhabdomyolysis between adults (5.9%) and children (4.3%) (Table 1).
Time from envenomation to first evaluation at a healthcare facility was the same in adults and children: adults (1 [0.7–1.5] hour) vs. children (1 [0.5–1.5]). Median time from bite to antivenom administration was also similar (2.5 [2–4] vs. 2.5 [2–5]; p = 0.79), as was dose administered. The median (IQR) initial dose of antivenom for adults was 6 (4–6) vials, compared with 4 (4–6) vials for pediatric patients (p = 0.06). Similarly, there was no difference in total amount of antivenom administered to adult and pediatric patients (10 [6–14] vs. 12 [6–18]; p = 0.32). Adults were more likely to receive antivenom compared with children. Antivenom was administered to 307 (95%) adults, compared with 79 (87%) pediatric patients (p = 0.006). Data on maintenance therapy were not available in 2 adult cases and 1 pediatric case. For the remaining 305 adult cases and 78 pediatric cases, use of maintenance antivenom was more common in children compared with adults (31/79 [39%] vs. 63/305 [21%]; p = 0.001).
Late hematologic toxicity occurred in 89 adults (45 cases of recurrence, and 44 cases of delayed toxicity), compared with 18 pediatric patients (12 cases of recurrence, 6 cases of delayed toxicity); these were statistically similar. Late bleeding occurred in 13 cases, two of which were in pediatrics. On the first follow-up visit after initial treatment, there were four cases classified as major bleeding (epistaxis requiring packing, vaginal bleeding in two patients, and a lower gastrointestinal bleed in one patient) and two cases classified as trivial bleeding. An additional seven cases of late bleeding were identified after the first follow-up visit, all of which were classified as trivial. The median (interquartile range) length of stay was 2 (1–3) days for adult and 2 (1–2) days for pediatric patients (p = 0.14). Similarly, there was no difference in the median number of follow-up days between adults and pediatric patients (9 (5–14) and 8 (5–12) days, respectively; p = 0.17) on either univariate analysis or univariate logistic regression.
Table 3 provides clinical and envenomation characteristics among pediatric patients, stratified by age. While we did descriptively compare relevant variables among pediatric patients, stratified by predefined age groups, we did not attempt to do any statistical comparison, as we feel the numbers are relatively small in some categories, leading to too great a chance of creating either a type I or a type II error.
Table 3.
Clinical and bite characteristics among pediatric patients, stratified by age.
| 0–6 years (n = 32) | 7–12 years (n = 32) | 13–17 years (n = 30) | |
|---|---|---|---|
| Female | 11 (34%) | 16 (50%) | 9 (30%) |
| Upper extremity bites | 4 (12.5%) | 10 (31%) | 10 (33%) |
| Hematologic toxicity | 17 (53.1%) | 18 (56%) | 18 (60%) |
| Edema | 29 (90.6%) | 27 (84%) | 26 (87%) |
| Ecchymosis | 25 (78.1%) | 24 (75%) | 19 (63%) |
| Necrosis | 1 (3%) | 2 (6%) | 0 (0%) |
| Number of vials for initial control* | 5 (4–5) | 5 (4–6) | 4 (4–6) |
| Total number of vials* | 10 (6–18) | 13 (8–16) | 10 (8–16) |
| Length of stay in days* | 2 (1–3) | 2 (1–2) | 2 (1–2) |
| Late hematologic toxicity† | 8 (25%) | 3 (9%) | 7 (23%) |
| Recurrence | 7 (21.9%) | 3 (9%) | 2 (6.7%) |
| Delayed toxicity | 1 (3.12%) | 0 (0%) | 5 (16.6%) |
Values are presented as whole number and percent, unless otherwise specified
*Values represent median and interquartile range
†Number of recurrent cases plus delayed toxicity does not add to number of late hematologic toxicity as some patients had both recurrence and late toxicity (e.g., recurrent coagulopathy but late thrombocytopenia)
Discussion
Clinical findings after rattlesnake envenomation vary widely in severity. Patients may present with only mild swelling at the envenomation site and without associated venom effects or, at the other end of the clinical spectrum, they may very rapidly develop systemic toxicity with bleeding, shock, and airway compromise. Many patients with rattlesnake envenomation fall somewhere in between these extremes, with some combination of local tissue, hematologic, neurotoxic, myotoxic, or anaphylactoid effects. There are likely multiple factors that act together to determine clinical severity after a rattlesnake envenomation. Snake-specific factors, such as species, age of the snake, and venom composition, play a role, as do patient factors, such as medical comorbidities and, possibly, body weight. It makes intuitive sense that children would experience more severe consequences than adults since they generally receive a larger dose of venom per unit body weight. However, most literature to date does not support a difference in severity between adults and children, although available data are very limited [8].
Recommendations for management of patients with snakebite are the same for children and adults [12]. Patients are assessed to determine if an envenomation has occurred using a combination of clinical manifestations and serial laboratory parameters. When antivenom is indicated, the initial dose recommended varies based on severity of clinical findings rather than on body weight or age [1, 13, 14]. However, whether or not additional doses of antivenom are indicated is based on clinical progression of the envenomation and response to treatment, so total dose administered can vary tremendously, and in retrospect can be an indirect, imperfect, measure of severity [5, 15]. In a retrospective study by Tanen et al. looking at patients with rattlesnake envenomation in Arizona, 44 children under 14 years were compared with 138 adults. The mean dose of antivenom used was the same in both groups [4]. We too found no difference in timing or initial or total dose of antivenom received between children and adults. This finding suggests that envenomation severity was not worse in the pediatric age group.
We found very few differences between adults and children. Regarding local tissue effects, children appeared to fare somewhat better than adults. They had less edema reported and, at first look, a lower rate of tissue necrosis. There are several potential reasons why children might be less likely to have edema reported. One is that they might present to care and receive treatment earlier; however, this was not the case in our study. Practitioners may also have a lower threshold to treat children with antivenom, but we did not find that more children were treated than adults. Another potential reason is that children more often present with lower extremity bites. Edema and swelling after a snakebite to the leg can be subtle and more difficult to perceive than edema associated with a finger or hand envenomation. However, when controlling for bite location, the difference in this finding remained. One remaining possible explanation is that adults without edema are less likely to present for care after a snakebite than children. This may also explain why more adults were treated with antivenom. Some adults without symptoms may not present to healthcare while more children present after an envenomation regardless of symptoms.
The most common manifestation of tissue necrosis following rattlesnake envenomation is in the form of superficial hemorrhagic bullae. Bullae typically occur following upper extremity envenomations, particularly when the envenomation is to a digit [4, 16]. Not surprisingly, when we controlled for bite location, the difference in rate of necrosis between adults and children disappeared.
Children in our study were more likely to have coagulopathy than adults. The reason for this difference is not clear and it did not translate to more bleeding. Similar results were also noted in the study by Tanen et al., where more children had hypofibrinogenemia than adults, and rates of thrombocytopenia were similar between adults and children [4].
The statistically different characteristics that we found between adults and children included bite location, percent with edema and coagulopathy, use of antivenom, and use of maintenance antivenom doses. Similarities in markers of severe systemic toxicity such as hypotension or need for mechanical ventilation, total dose of antivenom used, length of stay, and outcomes such as late hematologic toxicity suggest that the statistically significant findings may not be clinically important. Our findings differ from a recently published systematic review that aimed to risk stratify patients with pit viper envenomation [6]. The authors concluded children under age 12 may have a more severe envenomation. However, this conclusion was based on two publications that may not be applicable to North American rattlesnakes The first, a Brazilian study of patients with South American rattlesnake (Crotalus durissus) envenomations, showed that children under 12 years old were more likely to have renal failure after C. durissus envenomation than older patients [17]. The South American rattlesnake produces a different clinical picture than that of a North American rattlesnake. Neurotoxicity, myotoxicity, and acute renal injury are prevalent, with C. durissus envenomations, whereas local findings are generally mild. In the Pinho study, 29% of patients experienced renal injury, compared with a single patient in our series. The second study that contributed to their conclusion was a retrospective review of 67 pit viper envenomations in Texas, which included 18 patients aged 12 and under [18]. The authors concluded that children were more likely to receive antivenom and undergo surgical procedures than were the adults. Due to the small number of patients included in this study, the retrospective nature, the failure to identify number of rattlesnake envenomation in children vs. adults, and the management approach, which is incomparable to that of modern times, the results are not reliable.
Our study provides the most comprehensive data to date regarding relative severity of North American rattlesnake envenomation between adults and children. Characteristics that best signify severity, such as systemic toxicity and bleeding, and unfavorable outcomes such as late hematologic toxicity, were all statistically similar between the groups. If anything, pediatric patients had less systemic toxicity (e.g., hypotension, need for intubation), than adults, albeit not to the level of statistical significance. It is clear that persons of any age may develop severe envenomation after rattlesnake envenomation, but age does not appear to be a predictor.
Limitations
Venom from rattlesnakes is a complex mixture of many proteins. Furthermore, there is significant inter- and intra-specific variation among various snake venom [19]. Thus, it is possible that some of the effects could be biased if there is a significant contribution from a single geographic region. However, cluster analysis failed to reveal any significant differences between sites, making limitation less likely.
In addition, while this study is a retrospective analysis, the data were collected prospectively with existing predefined definitions, thereby limiting many of the problems with retrospective studies. Because the ToxIC database contains patients that are exclusively seen by medical toxicologists, it is possible the database is limited by a referral bias, in which a toxicologist only saw select patients. There were no efforts made as part of this study to independently corroborate the type of snake. It is possible that the snake was misidentified as a rattlesnake, and still included in the study.
In addition, because multiple comparisons were made, the possibility of a type I error exists. Lastly, it is possible that the adult and pediatric patient populations were systematically different due to selection bias. It is possible that an adult may not present following a bite if there were no symptoms, whereas most parents would presumably bring their child to the hospital for evaluation, regardless of symptoms. If that is the case, the adult population may represent a slightly sicker population, thus creating a selection bias.
Conclusion
When compared with adults, children with rattlesnake envenomation were more likely to be bitten on a lower extremity and to develop coagulopathy and were less likely to have edema or be treated with antivenom. Markers of severity, such as systemic toxicity and bleeding, as well as outcomes such as length of stay and development of late hematologic toxicity, were similar between adults and children. Given the populations were overall very similar, it is important that treatment be tailored to the individual patient, regardless of age.
Acknowledgments
The ToxIC North American Snakebite Study Group:
Kim Aldy, Peter Akpunonu, Vikhyat S. Bebarta, Gillian A. Beauchamp, Michael C. Beuhler, Mary Billington, William Boroughf, Robert D. Cannon, E. Martin Caravati, Edward Cetaruk, Alex Chen, James Chenoweth, Matthew D. Cook, Lynn Farrugia, Steven Fishburn, Erik Fisher, Jonathan B. Ford, Jakub Furmaga, Spencer Greene, Stephen Alex Harding, Benjamin Hatten, Bryan Judge, Kenneth D. Katz, William P Kerns II, Kurt Kleinschmidt, Andrew L. Koons, David B. Liss, Jennifer Lowry, Kevan Meadows, Alicia Minns, Michael Mullins, Angela Padilla-Jones, Tammy Phan, Lauren Porter, Ashley Carter-Powell, Sarah Shafer, Evan S. Schwarz, Meghan Spyres, Ryan M. Surmaitis, Laura Tortora, Stephanie Weiss
Sources of Funding
ACMT receives grant support from BTG International to support the North American Snakebite Registry. None of the authors received financial compensation for their participation in this work.
Compliance with Ethical Standards
The ToxIC registry has approval from the Western Institutional Review Board.
Conflicts of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael Levine, Email: michaellevine@mednet.ucla.edu.
on behalf of the ToxIC North American Snakebite Study Group:
Kim Aldy, Peter Akpunonu, Vikhyat S. Bebarta, Gillian A. Beauchamp, Michael C. Beuhler, Mary Billington, William Boroughf, Robert D. Cannon, E. Martin Caravati, Edward Cetaruk, Alex Chen, James Chenoweth, Matthew D. Cook, Lynn Farrugia, Steven Fishburn, Erik Fisher, Jonathan B. Ford, Jakub Furmaga, Spencer Greene, Stephen Alex Harding, Benjamin Hatten, Bryan Judge, Kenneth D. Katz, William P. Kerns, II, Kurt Kleinschmidt, Andrew L. Koons, David B. Liss, Jennifer Lowry, Kevan Meadows, Alicia Minns, Michael Mullins, Angela Padilla-Jones, Tammy Phan, Lauren Porter, Ashley Carter-Powell, Sarah Shafer, Evan S. Schwarz, Meghan Spyres, Ryan M. Surmaitis, Laura Tortora, and Stephanie Weiss
References
- 1.Lavonas EJ, Ruha AM, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2. doi: 10.1186/1471-227X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gummin DD, Mowry JB, Spyker DA, et al. 2017 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th annual report. Clin Toxicol. 2018;21:1–203. doi: 10.1080/15563650.2018.1533727. [DOI] [PubMed] [Google Scholar]
- 3.Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W. 2016 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th annual report. Clin Toxicol (Phila) 2017;55(10):1072–1252. doi: 10.1080/15563650.2017.1388087. [DOI] [PubMed] [Google Scholar]
- 4.Tanen D, Ruha AM, Graeme KA. Epidemiology and hospital course of rattlesnake envenomations cared for at a tertiary referral center in Central Arizona. Acad Emerg Med. 2001;8:177–182. doi: 10.1111/j.1553-2712.2001.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 5.Ruha AM, Kleinschmidt KC, Greene S, et al. The epidemiology, clinical course, and management of snakebites in the North American Snakebite Registry. J Med Toxicol. 2017;13:309–320. doi: 10.1007/s13181-017-0633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerardo CJ, Vissoci JRN, Evans CS, et al. Does this patient have a severe snake envenomation? The rational clinical examination systematic review. JAMA Surg. 2018. 10.1001/jamasurg.2018.5069. [DOI] [PubMed]
- 7.Richardson WH, Offerman SR, Clark RF, et al. Snake envenomations. In: Erickson TB, Ahrens WR, Aks SE, et al., editors. Pediatric toxicology: diagnosis and management of the poisoned child. New York: McGraw Hill Companies, Inc; 2005. pp. 548–555. [Google Scholar]
- 8.Tekin R, Sula B, Cakirca G, et al. Comparison of snakebite cases in children and adults. Eur Rev Med Pharmacol Sci. 2015;19:2711–2716. [PubMed] [Google Scholar]
- 9.Spyres MB, Ruha AM, Kleinschmidt K, et al. Epidemiology and clinical outcomes of snakebite in the elderly: a ToxIC database study. Clin Toxicol. 2018;56:108–112. doi: 10.1080/15563650.2017.1342829. [DOI] [PubMed] [Google Scholar]
- 10.Levine M, Ruha AM, Padilla-Jones A, et al. Bleeding following rattlesnake envenomation in patients with preenvenomation use of antiplatelet or anticoagulant medications. Acad Emerg Med. 2014;21:301–307. doi: 10.1111/acem.12333. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN. REDCap Consortium. Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed]
- 12.Kannan NC, Ray J, Stewart M, et al. Wilderness Medical Society Practice Guidelines for the treatment of pitviper envenomations in the United States and Canada. Wilderness Environ Med. 2015;26:472–487. doi: 10.1016/j.wem.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Package Insert. Anavip. Rare Disease Therapeutics, Inc. Franklin, TN. https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM446175.pdf. Accessed 3 December, 2018.
- 14.Package Insert. CroFab. BTG International Inc. West Conshohocken, PA. https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM117573.pdf. Accessed 3 December, 2018.
- 15.Levine M, Offerman S, Vohra R, et al. Assessing the effect of a medical toxicologist in the care of rattlesnake-envenomated patients. Acad Emerg Med. 2018;(8):921–26. [DOI] [PubMed]
- 16.Heise CW, Ruha AM, Padilla-Jones A, et al. Clinical predictors of tissue necrosis following rattlesnake envenomation. Clin Toxicol. 2018;56:281–284. doi: 10.1080/15563650.2017.1371311. [DOI] [PubMed] [Google Scholar]
- 17.Pinho FM, Zanetta DM, Burdmann EA. Acute renal failure after Crotalus durissus snakebite: a prospective survey on 100 patients. Kidney Int. 2005;67:659–667. doi: 10.1111/j.1523-1755.2005.67122.x. [DOI] [PubMed] [Google Scholar]
- 18.White RR, Weber RA. Poisonous snakebite in central Texas: possible indicators for antivenin treatment. Ann Surg. 1991;213:466–471. doi: 10.1097/00000658-199105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex CJ, Mackessy SP. Venom composition of adult Western Diamondback Rattlesnakes (Crotalus atrox) maintained under controlled diet and environmental conditions shows only minor changes. Toxicon. 2019;164:15–60. doi: 10.1016/j.toxicon.2019.03.027. [DOI] [PubMed] [Google Scholar]