Abstract
This is a case series of 3 children from a single family who developed symptomatic elemental mercury poisoning requiring hospitalization and chelation. The mercury exposure primarily occurred in the home but the mercury was also tracked to one of their schools requiring environmental cleanup at both the home and school. The clinical assessment and management, as well as public health investigation and response, are discussed. There are many lessons learned in this difficult, often delayed, diagnosis. Early recognition of this environmental toxic exposure is essential. Communication between the clinicians and public health officials played a critical role. Public education prevented panic. Proper environmental sampling, and assessment and management of those exposed, were a few of the many challenges faced in this complicated case series.
Introduction
Mercury is a toxic heavy metal that is found in the elemental, inorganic, and organic forms. Elemental mercury is found in thermometers, dental amalgams, fluorescent light tubes, compact fluorescent lamps, and mercury added to latex paint [1]. Mercury disrupts normal cell physiology by binding to intracellular sulfhydryl-containing enzymes and proteins [2]. Elemental mercury is lipophilic and highly volatile; 70 to 85% of a dose is absorbed through the lungs [2]. Acutely, patients that inhale the mercury vapor can have chemical pneumonitis and flu-like symptoms [2]. Once elemental mercury is inhaled, it crosses the alveolar membrane in the lungs and is rapidly absorbed and distributed in the major organs. The primary target organs of elemental mercury deposition are the brain and kidney [3]. Neurotoxicity presents as a triad of erythrism, tremors, and gingivitis [3, 4]. Patients can also have acrodynia or “pink disease” [1, 5]. Nephrotoxicity includes proteinuria, acute tubular necrosis, and/or renal failure [1, 4]. Because the clinical presentation can vary, the diagnosis of mercury poisoning can be challenging and is oftentimes delayed.
This case involved three children ages 14, 11, and 9 years old following an exposure to elemental mercury. Two of the children presented to the emergency department (ED). The diagnosis was not initially made when they were sent home with a typical pediatric presentation of a viral-like illness. The third child became symptomatic the next day prompting all three children to return to the same ED. At the second ED visit, the eldest child brought a container of elemental mercury he had found outside. After further questioning, it was revealed the children had played with mercury and the concern arose for an environmental toxic exposure causing the children’s illnesses. The emergency physician consulted the university’s medical toxicologist who then recommended blood and urine mercury testing. The toxicologist also notified the ATSDR (Agency for Toxic Substances and Disease Registry) regional office and the county health department to aid the environmental and public health assessment. The university toxicology service, ATSDR regional office, EPA (Environmental Protection Agency), state and local health departments, school officials, and the regional poison control center all investigated and responded to these patients.
Consent for publication of this case was obtained with the assistance of a Spanish translator and provided to the journal in accordance with the JMT policy.
Patient 1
A 14-year-old male with a past medical history of asthma presented to the ED with fever for 1 day and a pruritic rash for a few hours. The patient was given ibuprofen and sent home from the ED with a diagnosis of viral syndrome. This patient returned the next day to the same ED with persistent fever to 40 °C, a blood pressure of 125/74 mmHg, and rash. Additional symptoms were a dry cough, malaise, sore throat, and a generalized headache. The physical exam revealed a diffuse confluent erythematous maculopapular pruritic rash (excluding the palms and soles) without desquamation (Fig. 1), petechiae localized to bilateral lower extremities, bilateral injected conjunctiva, erythematous oropharynx without exudate, coarse breath sounds diffusely, and a supple neck. The neurological examination was normal and there was no tremor.
Fig. 1.
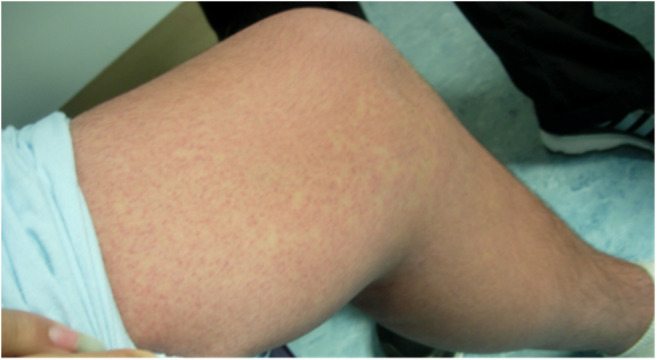
Petechiae to bilateral lower extremities of patient 1.
Patient 2
A 9-year-old female with a past medical history of asthma presented to the ED with patient 1 complaining of a fever, rash, headache, and bilateral foot pain for 1 day. Patient 2 was also sent home from the ED with a diagnosis of viral syndrome. The following day, the patient returned with patient 1 complaining of similar symptoms including fever, diffuse headache, persistent dry cough, sore throat, non-bloody diarrhea, vomiting, abdominal cramps, pruritic rash, and anorexia. On physical exam, the temperature was 37.9 °C and blood pressure was 117/62 mmHg. The patient had a similar pruritic confluent rash without petechiae (Fig. 2), bilateral injected conjunctiva, coarse breath sounds, bilateral nontender abdomen, erythematous oropharynx without exudate, supple neck, and a normal neurologic exam without tremor.
Fig. 2.
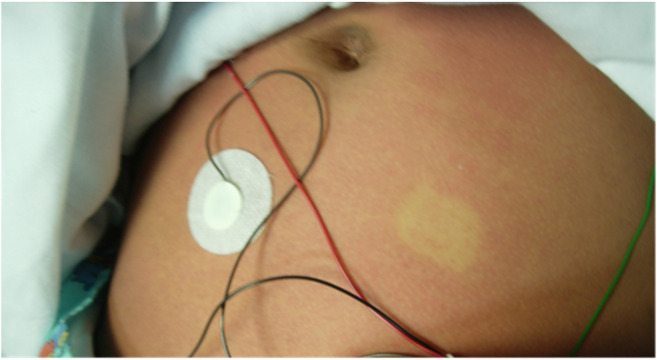
Confluent blanching erythematous maculopapular rash to abdomen of patient 2.
Patient 3
An 11-year-old female without past medical history presented to the ED 2 days after the other two siblings initial ED visit also complaining of a rash and fever. Patient 3 had a similar pruritic rash without petechiae, fever, diffuse headache, conjunctivitis, sore throat, abdominal pain, or neck pain. The patient did not have a cough. On physical exam, the temperature was 38.4 °C and blood pressure was 116/73 mmHg. The patient had clear lung sounds, a supple neck, nontender abdomen, injected bilateral conjunctiva, erythematous oropharynx without exudate, a normal neurological exam without tremor, and a diffuse pruritic confluent blanching erythematous maculopapular rash without petechiae.
What Diagnoses Should Be Considered in Children Who Present with a Rash and Fever?
Rash and fever is a common childhood presentation. Given the cluster of three similar presentations from one household, an infectious etiology is the most likely etiology. Alternatively, rash, fever, conjunctivitis, and upper respiratory symptoms can occur with adenovirus infection. Streptococcal pharyngitis may also present with rash and fever but there were no known streptococcal exposures and throat cultures were negative for streptococcus. Another less common diagnosis considered was Kawasaki Disease, an inflammatory autoimmune disease involving inflammation of medium-sized blood vessels. It is characterized by bilateral conjunctivitis, persistent fever greater than 5 days, generalized blanching rash, edema and/or desquamation of hands and feet, cervical lymphadenitis, “strawberry tongue” with injected or fissured lips, and coronary artery aneurysms.
Further History
At the time of the second ED visit, patient 1 brought a 300 g container of 99.9% elemental mercury which was found “on the street” 10 days prior to the initial ED visit (Figs. 3 and 4). After the initial ED visit, patient 1 read about mercury on the Internet and brought the mercury bottle to the hospital. He was concerned whether the mercury might have caused the acute illness. This discovery prompted the medical toxicology consultation.
Fig. 3.

Mercury bottle on a scale in grams.
Fig. 4.

Enlargement of mercury container.
What Are Some of the Key Components of the Exposure History?
Although three siblings presenting with rash and fever suggests an infectious etiology, the new history of mercury exposure suggested a possible toxic etiology. It must also be determined if others were exposed to the mercury. As such, other important factors include the determination if there was any spilled mercury, if a vacuum was used, if any mercury was disposed, and if the mercury was taken from the home, and if so, to where.
Exposure History
According to all three children, during the 10-day period when the mercury was first brought home until the children’s presentation to the hospital, all three poured some of it out of its container and played with it in their hands for approximately 15 minutes per day for at least eight of the 10 days. They also poured the mercury into other containers and stored it without lids in the kitchen and two of the siblings’ shared bedroom. Patient 1 also heated the mercury with a cigarette lighter. All denied ingesting the mercury. The family denied vacuuming the mercury. Two of the children brought the mercury to their respective schools to show their friends.
What Public Health Resources Are Available to Aid in the Investigation?
Federal, state, and local public health assets assist environmental health investigations. In particular, ATSDR and EPA lead investigations and ensure that proper environmental assessments are performed. Regional and local partners such as the state, county, or city health departments can perform the proper investigations and initiate critical risk communication outreach to involved parties.
Public Health Response
Once it became apparent that an environmental toxic exposure was the most likely etiology for the children’s illnesses, the regional ATSDR office and the county health department were contacted to assist the environmental and public health assessment. The ATSDR regional office contacted the EPA. Ultimately, the ATSDR regional office, EPA, state and local health departments, university toxicology service, school officials, and the regional poison control center were active in the investigation and response to these patients. The entire group participated in daily group calls during the event.
Where and What Environmental Sampling Should Be Done? What Are the Recommended Action Concentrations for Mercury in Residential Settings?
Portable mercury vapor analyzers (e.g., Lumex® or Jerome®) rapidly assess air for mercury and should be used if there is a suspicion of a mercury contamination. Based on the ATSDR Chronic Minimal Risk Level and EPA Reference Concentration, the recommended action concentrations to limit exposures for mercury in residential settings is 1 μg/m3 for normal occupancy and 10 μg/m3 for evacuation or limiting access of the residents [6].
Mercury vapor measurements performed by the Lumex® analyzer were elevated throughout the children’s house. Entry and living room concentrations were 42 μg/m3 and back bedrooms and bathroom were > 50 μg/m3. The home was cleaned by a private contractor with EPA funding until all measurements were below 1 μg/m3 [6].
What Are the Recommended Actions Concentrations for Mercury in the School Setting?
The exposure scenario at schools is more like a workday type of exposure (i.e., 7–10 hours) than to a residential setting (many more hours). The ATSDR recommends a mercury air concentration of ≤ 3 μg/m3 before resuming normal school operations [6].
Because the children took the mercury to their schools, the schools were assessed for mercury contamination. Mercury concentrations at the youngest siblings’ school ranged from 2 μg/m3 in hallways to > 50 μg/m3 in the janitor’s closet. The EPA cleaned the school until all measurements were below 3 μg/m3.
What Additional Procedures Needed to Be Followed When the Environmental Mercury Concentrations Testing at the School Were Elevated?
Because of the concern that children may have contaminated their shoes by walking through areas of mercury contamination prior to cleanup, the shoes that the children had worn to school prior to the cleanup were analyzed for mercury contamination.
How Is it Determined if Individual Items Are Contaminated with Mercury?
To assess for contamination, each pair of shoes was placed in its own bag and the bag was heated by direct sunlight to what might be reasonably anticipated to be maximum temperatures of normal use. Headspace readings (placing the detector probe into the air space inside the bag) for mercury vapor were then done for each bag. ATSDR states that headspace readings for belongings that may have been contaminated by vapors from a mercury spill are safe if < 6 μg/m3 [6].
The school children brought in their shoes to be tested by the EPA for mercury. Only two of the school children had shoes with mercury headspace measurements > 6 μg/m3. The EPA assessed their family cars and homes and there was no mercury contamination.
Clinical Course
All three children were hospitalized for mercury toxicity. None had the pneumonitis associated with high-dose acute elemental mercury toxicity. Chest radiographs were normal. Blood cultures and viral respiratory testing were negative in all three patients. All three patients had elevated temperatures with their maximum temperatures (40.8–41.8 °C) occurring by hospital day 2 or 3. All remained tachycardic during their hospital stay.
All had mild liver function test abnormalities during their hospitalization. The respective peaks for AST and ALT were patient 1 at 224 IU/L and 224 IU/L, patient 2 at 98 IU/L and 123 IU/L, and patient 3 at 48 IU/L and 55 IU/L. Patients 1 and 3 had thrombocytopenia with nadirs of 86 and 68 thousand/mm3, respectively.
What Other Etiologies Were Considered Based Upon their Presentations?
Two of the children developed liver transaminase elevations (AST, ALT) which in conjunction with rash and fever may result from Epstein Barr virus–induced mononucleosis. Hepatitis also presents with elevated liver transaminases along with fever, myalgias, anorexia, and abdominal pain. However, the highest peak AST and ALT were only 224 unit/L, occurring in the boy, which made hepatitis less likely.
Pheochromocytoma may present with tachycardia, mild hypertension, and high fevers. There are many reports of patients who were initially thought to have a pheochromocytoma and only later found to have mercury toxicity [7]. This is because mercury inhibits the s-adenosyl methionine (SAM) and catechol-o-methyl transferase (COMT) enzymes. COMT inactivates the catecholamines (dopamine, epinephrine, and norepinephrine) by methylation using SAM as the one-carbon donor [7]. Idiopathic thrombocytopenic purpura was also considered given the oldest sibling significant thrombocytopenia and petechiae. However, thrombocytopenia occurs as a toxic effect of mercury.
What Are some of the Typical Clinical Features of Elemental Mercury Poisoning?
Acute inhalational elemental mercury poisoning typically presents with pulmonary symptoms that may progress to respiratory distress [8]. The classic acrodynia rash features painful erythema of the palms and soles with desquamation [6, 7].
Although the children never developed pulmonary symptoms and their rashes spared the palms and soles and was not desquamating, the temporal association of these clinical presentations with the children’s handling of the mercury suggested that mercury exposure was likely the cause of their illnesses.
What Tests Should Be Ordered to Establish the Diagnosis of Mercury Poisoning and to Assess the Severity of the Mercury Exposure?
Blood and urine mercury analyses confirm the elemental mercury exposure [9]. Blood testing is useful to establish the time course of the exposure because mercury has a short half-life in blood. Thus, blood specimens are important for acute elemental mercury exposure. While urine concentrations also support acute exposure, they also reflect chronic exposures. Normal whole blood mercury concentrations are less than 10 μg/L while normal spot urine concentrations are less than 20 μg/L. However, there is poor correlation between concentrations and toxicity severity.
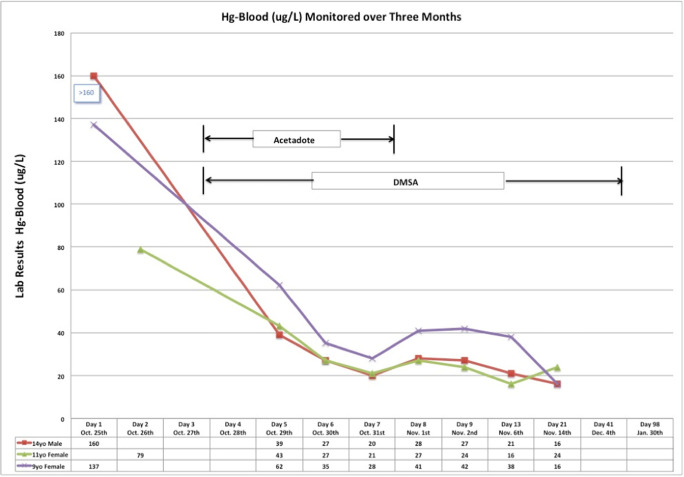
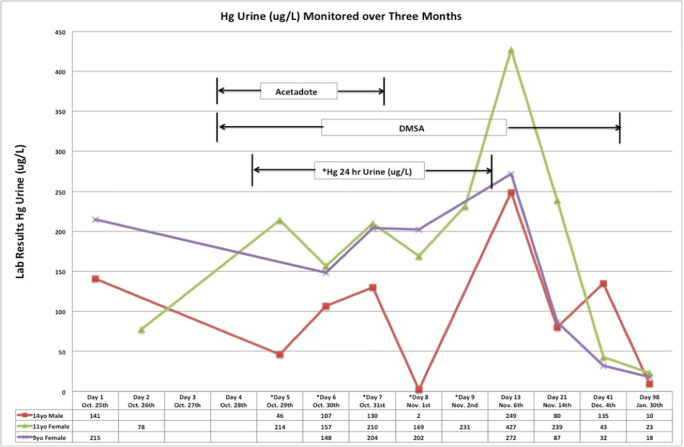
Blood and spot urine mercury tests were ordered on all three children upon hospital admission. Initial blood and spot urine mercury concentrations were as follows: patient 1, blood mercury 160 μg/L, urine mercury 141 μg/L; patient 2, blood mercury 137 μg/L, urine mercury 215 μg/L; patient 3, blood mercury 79 μg/L, urine mercury 78 μg/L.
Should There Be Concern for Mercury Exposure of Friends or Family Members? What Tests Need to Be Done on These Individuals, If Any?
Assess all family members who lived in, or frequently visited, the mercury-contaminated dwelling for mercury exposure with blood and urine mercury testing. In addition, test anyone at the school who may have been at risk for excessive mercury exposure.
Other potentially mercury-exposed individuals included the mother, two aunts, grandmother, oldest sibling of the 3 patients, students at the school, and the custodian at the school who unknowingly cleaned the mercury contaminated areas with a mop. None of these had symptoms. The mother had a blood mercury concentration of 60 μg/L and a spot urine of 34 μg/L and she had elevated liver transaminases (AST 104, ALT 88). The oldest sibling had a blood mercury concentration of 23 μg/L and urine spot mercury concentration of 14 μg/L. Liver transaminases were not tested on the oldest sibling. The blood mercury concentrations of the other family members and the school custodian were < 3 μg/L. None of them had any symptoms. None of the school children were tested because it was felt that their exposure was minimal.
Treatment
Once the blood and urine mercury concentrations confirmed the exposures, chelation therapy with DMSA (succimer) was started on the three symptomatic children on hospital day 4 to enhance the elimination of mercury. Chelation continued during the next 4 days in the hospital and after discharge for a total of 38 days. Intravenous N-acetylcysteine, which may provide some mercury-detoxifying effect [10, 11], was also administered during their hospitalization. N-Acetylcysteine serves as a cysteine donor and increases glutathione [12]. Intracellular glutathione is critical in maintaining glutathione peroxidase in the reduced state to allow for the antioxidant activity [12]. The production of additional glutathione when there is already severe oxidant stress may be partially protective [12].
Figures 5 and 6 show the blood and urine mercury concentrations in each child during treatment.
Fig. 5.
Blood mercury concentrations over 3 months in each child.
Fig. 6.
Blood mercury concentrations over 3 months in each child.
Follow-up
The children were followed in the medical toxicology clinic for 6 months. At 3 months, the spot urine mercury concentrations were as follows: patient 1, 10 μg/L; patient 2, 18 μg/L; and patient 3, 23 μg/L. During these follow-up visits, patient 1 had mild hypertension with systolic BP in 120–130s. Otherwise, all patients remained asymptomatic and their rashes and fever completely resolved. They did not have desquamation or neurotoxicity. The family moved back home after it was cleaned by the EPA.
Conclusion
We promote children’s health in the environment through this interactive discussion by addressing the challenges we faced in the treatment of three pediatric patients with elemental mercury exposure. We monitored the children for 6 months and there were no adverse events.
Mercury poisoning still occurs and remains a challenging diagnosis. It warrants multi-disciplinary and public health evaluation and action as illustrated in this case series.
Our partnership with ATSDR and other public health agencies in the cases described here is an example of ACMT’s cooperative agreement with ATSDR to address challenging pediatric environmental health exposures [13].
Sources of Funding
None
Compliance with Ethical Standards
Conflict of Interest
Consent for publication of this case was obtained with the assistance of a Spanish interpreter and provided to the journal in accordance with JMT policy.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patrick L. Mercury toxicity and antioxidants: part I: role of glutathione and alpha-lipoid acid in the treatment of mercury toxicity. Altern Med Rev. 2002;7(6):456–471. [PubMed] [Google Scholar]
- 2.Glezos JD, Albrecht MD, Gair RD. Pneumonitis after inhalation of mercury vapours. Can Respir J. 2006;13(3):150–152. doi: 10.1155/2006/898120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JD, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health. 2012;45(6):344–352. doi: 10.3961/jpmph.2012.45.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broussard L, Hammett-Stabler CA, Winecker RE, Ropero-Miller JD. The toxicology of mercury. Lab Med. 2002;33(8):614–625. doi: 10.1309/5HY1-V3NE-2LFL-P9MT. [DOI] [Google Scholar]
- 5.Mercer JJ, Bercovitch L, Muglia JJ. Acrodynia and hypertension in a young girl secondary to elemental mercury toxicity acquired in the home. Pediatr Dermatol. 2012;29(2):199–201. doi: 10.1111/j.1525-1470.2012.01737.x. [DOI] [PubMed] [Google Scholar]
- 6.ATSDR. Chemical-specific health consultation for Joint EPA/ATSDR National Mercury Cleanup Policy Workup: action levels for mercury spills. 2012.
- 7.Michaeli-Yossef Y, Berkovitch M, Goldman M. Mercury intoxication in a 2-year-old girl: a diagnostic challenge for the physician. Pediatr Nephrol. 2007;22(6):903–906. doi: 10.1007/s00467-007-0430-5. [DOI] [PubMed] [Google Scholar]
- 8.Risher JF, Nickle RA, Amler SN. Elemental mercury poisoning in occupational and residential settings. Int J Hyg Environ Health. 2003;206(4–5):371–379. doi: 10.1078/1438-4639-00233. [DOI] [PubMed] [Google Scholar]
- 9.Bose-O'Reilly S, Lettmeier B, Gothe RM, Beinhoff C, Siebert U, Drasch G. Mercury as a serious health hazard for children in gold mining areas. Environ Res. 2008;107(1):89–97. doi: 10.1016/j.envres.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Akyildiz BN, Kondolot M, Kurtoglu S, Konuskan B. Case series of mercury toxicity among children in a hot, closed environment. Pediatr Emerg Care. 2012;28(3):254–258. doi: 10.1097/PEC.0b013e3182494ed0. [DOI] [PubMed] [Google Scholar]
- 11.Trumpler S, Nowak S, Meermann B, Wiesmuller GA, Buscher W, Sperling M, et al. Detoxification of mercury species--an in vitro study with antidotes in human whole blood. Anal Bioanal Chem. 2009;395(6):1929–1935. doi: 10.1007/s00216-009-3105-1. [DOI] [PubMed] [Google Scholar]
- 12.Ballatori N, Lieberman MW, Wang W. N-acetylcysteine as an antidote in methylmercury poisoning. Envir Health Perspect. 1998;106(5):267–271. doi: 10.1289/ehp.98106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebelt E. Monograph on pediatric environmental health: a cooperative agreement award from the Agency of Toxic Substances and Disease Registry of the CDC. J Med Toxicol. 2006;2(3):120. doi: 10.1007/BF03161025. [DOI] [Google Scholar]