Abstract
Background:
Efflux pumps are involved in resistance of Acinetobacter baumannii isolates to antimicrobial agents. AdeABC efflux pump is one of the RND superfamily efflux pump and consists of adeA (membrane fusion), adeB (multidrug transporter) and adeC (outer membrane) genes. In this study, the frequency of adeA, adeB and adeC genes among A. baumannii isolates with resistance to erythromycin, trimethoprim, meropenem and imipenem was investigated.
Methods:
Overall, 79 strains of A. baumannii were isolated from patients admitted to two major hospitals in Tehran during 2016. Antibiotic susceptibility testing was determined by disc diffusion and microdilution methods according to Clinical and Laboratory Standards Institute (CLSI) guideline. The presence of adeA, adeB and adeC genes was also determined using Multiplex PCR assay.
Results:
The highest and the lowest resistance among A. baumannii isolates were to trimethoprim (93%) and erythromycin (53%), respectively. The frequency of adeA, adeB and adeC genes was 96.2%, 96.2% and 91.1 % respectively. There was a significant relationship between imipenem resistance and presence of efflux pump genes (P<0.05).
Conclusion:
According to the high prevalence of the AdeABC efflux system genes, it may involve in resistance of clinical isolates of A. baumannii to imipenem, especially.
Keywords: Acinetobacter baumannii, Antibiotic resistance, Gene
Introduction
Bacteria belong to genus Acinetobacter are important pathogenic agents of human diseases (1). Acinetobacter baumannii is known as the most frequently isolated species from human’s infection (2–5). The species is widely distributed in environments and may be found in soil, water and sewage, as well as in variety of foodstuffs (6).
Hospital environment, especially intensive care units (ICU) are the most important sites of their occurrence (7–9). As the second most commonly isolated nonfermenters bacteria in human specimens, A. baumannii is inhabitants of healthy human skin. Other common reservoirs of these organisms include moist and dry surfaces, where they can easily survive for many days or weeks (10). On the other hand, A. baumannii has been the main cause of all nosocomial infections because of its ability to acquire resistance determinants and biofilm formation on surfaces, which allow them to become resistant to a wide range of antibiotics (11).
A. baumannii causes many infections including bloodstream infections, ventilator-associated pneumonia (VAP), urinary tract infections (UTI) and wound infections (12). The risk factors of A. baumannii infections are instrumentation, mechanical ventilation, surgery, treatment with broad-spectrum antibiotics, and hospitalization at ICU (13).
Due to the vast consumption of antibiotics and the extension of A. baumannii multi-drug resistant (MDR) strains, its morbidity and mortality has increased in last decade. (14). The MDR A. baumannii strains often are susceptible only to a few antibiotic classes (carbapenems and polymyxins) and resistance to other antimicrobial classes (15). Colonization of strains in the gastrointestinal tract in hospitalized patients at ICU is an important epidemiologic reservoir for A. baumannii (MDR strains) infections in hospital outbreaks (16). In the last few decades A. baumannii has emerged as important opportunistic pathogen, especially as multiple resistant to the major antimicrobial agents which used to treat nosocomial infections.
Efflux pumps genes, β-lactamases, integrons, and insertion sequence (IS) elements are considered as the most prevalent determinants among MDR A. baumannii strains (17). Bacterial efflux pumps are complicated in clinically related resistance to antibiotics, bacterial colonization and the persistence of bacteria in the host (18). Chromosomally-encoded pump is tripartite efflux machinery belongs to the RND-type superfamily. The AdeABC efflux pump consists of adeA (membrane fusion), adeB (multidrug transporter) and adeC (outer membrane protein) genes. These genes are contiguous and adjacent by two-component regulatory systems; adeR and adeS (19).
The major efflux mechanism associated with carbapenems resistance in A. baumannii is the chromosomally-encoded tripartite efflux pump, AdeABC, found in approximately 80% of clinical strains of A. baumannii. Inappropriate antibiotic therapy can induce more expression AdeABC efflux pump. Hence, the over-expression of AdeABC efflux pump confers resistance to aminoglycosides and decreases susceptibility to fluoroquinolones, tetracycline, chloramphenicol, erythromycin, trimethoprim and ethidium bromide, netilmicin and meropenem (20). The synergy between acquired oxacillinases and the AdeABC pump has been implicated in the higher levels of resistance to β-lactams.
We aimed to investigate the frequency of adeABC genes and antibiotic resistance to erythromycin, trimethoprim, meropenem and imipenem among A. baumannii strains, from two hospitals in Tehran, Iran.
Materials and Methods
Bacterial isolation and antimicrobial susceptibility testing
During 85 days since May 4th 2016, overall 79 clinical strains of A. baumannii were subjected to the study. These isolates have been recovered previously from patients in two major hospitals (Baqiyatallah and Tehran Heart Hospitals) in Tehran, Iran. The clinical strains were cultured in triple sugar iron agar (TSI), sulfide-indole-motility (SIM), methyl red and Voges-Proskauer (MR-VP) test and identified according to the conventional biochemical tests, API20 E and Vitek2 systems (BioMerieux, Marcy-l’Etoile, France). Antimicrobial susceptibility test was also performed using disc diffusion method according to Clinical and Laboratory Standards Institute guidelines (CLSI).
Detection of AdeABC efflux pump system by PCR
A. baumannii strains were assayed for presence adeABC genes by PCR method with the specific primers showed in Table 1. DNA extraction was carried out using a procedure as described by Hassanzadeh, et al. (21). The condition for amplification of adeABC genes was as follows: initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C for 1 min, 55°C for 1 min, 72 °C for 1 min and a final amplification at 72 °C for 5 min. Amplicons were electrophoresed in gel agarose 1%, stained with ethidium bromide and visualized under UV transilluminator documentation system (Bio-Rad).
Table 1:
The primers used in study
| Primer name | Sequence (5′ →3′) | Amplicon size |
|---|---|---|
| Ade.A | F: GAAATCCGTCCGCAAGTC R: ACACGCACATACATACCC |
683 bp |
| Ade.B | F: AAAGACTTCAAAGAGCGG R: TCACGCATTGCTTCACCC |
623 bp |
| Ade.C | F: ATTTCAGGTCGTAGCATT R: CTTGATAAGTAGAGTAGGGATT |
370 bp |
Statistical Analysis
All data were analyzed using conducting Chi-square method using SPSS software, version 16 (Chicago, IL, USA). The P value less than 0.05 was used as the cutoff for significance.
Results
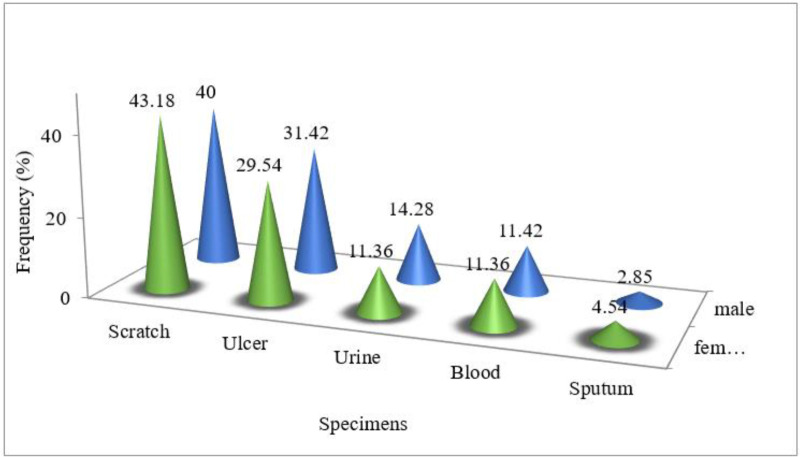
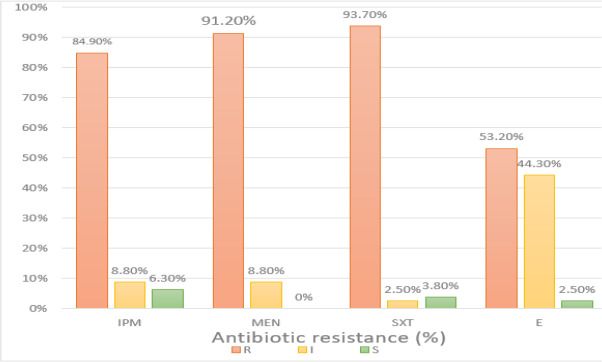
From 79 strains of A. baumannii isolated from clinical specimens, 35 isolates obtained from males (44%) and 44 strains belonged to females (56%). In fact, in terms of gender, the prevalence of A. baumannii strains was almost the same in females as males. The strains in this study were obtained from scratch, ulcer, urine, blood and sputum sources. The frequency of bacteria extracted from the specimens is shown Fig. 1. The results of antibiotics susceptibility test showed that the highest and the lowest resistance of A. baumannii were to trimethoprim (93.7%) and erythromycin (53.2%), respectively (Fig. 2).
Fig. 1:
The frequency of A. baumannii strains isolated from the clinical specimens.
Fig. 2:
Antibiotic resistance of A. baumannii isolates
Genes encoding efflux pumps in all 79 MDR isolates were determined by multiplex-PCR assay. Amplicons of adeA, adeB and adeC genes were 683bp, 623 bp and 370 bp, respectively (Fig. 3). From a total of 79 isolates, the percent of isolates with adeC gene were 91.1% (high significant differences at P < 0.01). The effect of Phe-Arg-Beta-Naphthylamide (PAβN) on MICs of A. baumannii is summarized in Fig. 4. There was no statistically significant difference between the erythromycin resistance and presence of adeA, adeB, adeC genes (chi-square test), while a significant difference was observed between the resistance to trimethoprim and adeC gene (chi-square test, P <0.05).
Fig. 3:
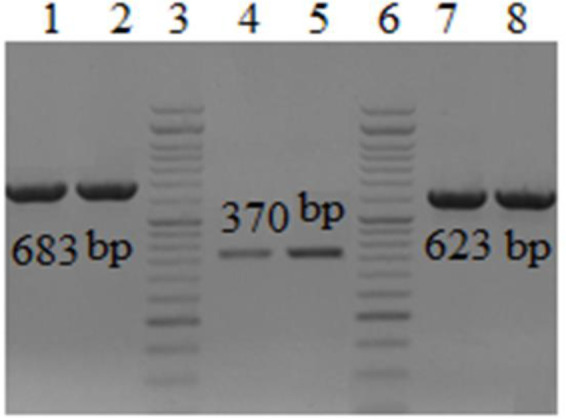
Electrophoresis of adeA, adeB and adeC amplicons of three selected isolates of A. baumannii in gel agarose 1%. Lanes 1–2, PCR product of adeA gene (683 bp); lanes 3&6, DNA ladder 100-bp; lane 4–5, PCR product of adeB gene (370 bp). Lanes 7–8, PCR product of adeC gene (623 bp)
Fig. 4:
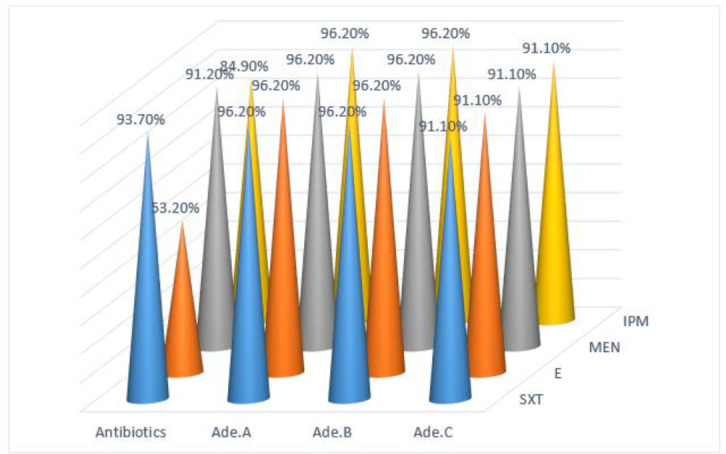
The rate of efflux pump existence in the drug resistant phenotypes in A. baumannii isolates
Discussion
The novelty of the current study was to detect and determine the prevalence of efflux pump genes in MDR strains of A. baumannii isolated from Tehran, Iran. A total of 56 multidrug resistant isolates of A. baumannii, the adeA, adeB and adeC genes detected in 100%, 100% and 96.5% of isolates, respectively. Furthermore, all isolates were resistant to cefotaxime, ceftazidime, cefepime, cefoxitin, azteronam, ciprofloxacin and imipenem.
The results revealed that all of A. baumannii strains isolated from burn units were positive for adeA and adeB genes, while adeC gene was found in 85% of isolates (22). The most of carbapenems resistant Acinetobacter spp. was positive for adeA, adeB, adeR, adeS and adeC genes (23).
It is considered the frequency of these resistance genes to be high, so that in China, 88.2% of multidrug resistant isolates of A. baumannii isolated from hospitals carried genes of AdeABC efflux system (24). The majority of imipenem resistant A. baumannii isolates (> 80%) was positive for adeB, adeR, adeS, adeJ and abeM genes (25).
The adeB efflux pump gene existed in all MDR A. baumannii strains isolated from five hospitals in Taiwan (26). Insertional inactivation of adeB in A. baumannii strain BM4454 showed that it is involved in resistance against many antimicrobial agents including aminoglycoside, tetracycline, fluoroquinolones, erythromycin, trimethoprim and ethidium bromide (27). Additionally, the overexpression of AdeABC system and mutations in the adeR/S genes (encoding a two-component regulatory system) constitutes a major mechanism of multidrug resistance in nosocomial strains of A. baumannii (28).
Our results showed that the most of multidrug resistant isolates possessed the all adeA, adeB and adeC genes but it was obvious that the adeB and adeA genes had the main role in the resistance mechanism.
The effects of efflux pump inhibitors such as carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and Phe-Arg β-naphthylamide (PAβN) on susceptibility to antimicrobial agents has been investigated, previously (29). The addition of the PAβN at different concentrations reduced the MICs of different antibiotics such as ertapenem (30).
Statistical analysis showed no significant relationship between the resistance to erythromycin and the presence of adeABC genes but in the case of three other antibiotics, there was a significant relationship between antibiotic c resistance and the presence of adeABC genes. It can be concluded that the presence of adeABC genes can stimulate the resistance to imipenem and trimethoprim in A. baumannii strains. Our results also suggested that drug efflux pumps contribute to the resistance to carbapenem in A. baumannii clinical isolates.
Our results are concordant with the findings that believed the adeC gene is not essential for A. baumannii MDR phenotypes (19). Overexpression of OXA-23 carbapenemase and AdeABC efflux pump may contribute to carbapenem resistance in clinical isolates of A. baumannii (31). Our results disagree with the data of some previous studies which indicated the AdeABC efflux pump were present in the both carbapenem-resistant and sensitive strains, therefore they might do not involve in A. baumannii carbapenems resistance (32, 33).
Conclusion
The current study demonstrated the main contribution of the adeB gene and its regulatory system in multidrug and carbapenems resistance in clinical isolates of A. baumannii.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We would like to thank the “Clinical Research Development Center of Baqiyatallah Hospital” for their kind cooperation. This study was financially supported in part by “Clinical Research Development Center of Baqiyatallah Hospital”.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Ranjbar R, Sadeghifard N, Ahmadi A, et al. (2007). Antimicrobial Susceptibility and AP-PCR Typing of Acinetobacter Spp. Strains. Iran J Public Health, 36(4):50–56. [Google Scholar]
- 2.Bouvet P, Grimont P, editors (1987). Identification and biotyping of clinical isolates of Acinetobacter. Ann Inst Pasteur Microbiol,138(5):569–78. [DOI] [PubMed] [Google Scholar]
- 3.Bombicino KA, Almuzara MN, Famiglietti AMR, Vay C. (2006). Evaluation of Pyrrolidonyl Arylamidase for the Identification of Nonfermenting Gram-negative Rods. Diagn Microbiol Infect Dis,57(1):101–3. [DOI] [PubMed] [Google Scholar]
- 4.Traub WH, Leonhard B. (1994). Serotyping of Acinetobacter-Baumannii and Genospecies-3 - an Update. Med Microbiol Lett, 3(3):120–7. [Google Scholar]
- 5.Sadeghifard N, Ranjbar R, Zaeimi J, et al. (2010). Antimicrobial susceptibility, plasmid profiles, and RAPD-PCR typing of Acinetobacter bacteria. Asian biomedicine, 4(6):901–911. [Google Scholar]
- 6.Houang ET, Chu Y, Leung C, et al. (2001). Epidemiology and Infection Control Implications ofAcinetobacter spp. in Hong Kong. J Clin Microbiol, 39(1):228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergogne-Berezin E, Towner K. (1996). Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev, 9(2): 148–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wieczorek P, Sacha P, Hauschild T, et al. (2008). Multidrug resistant Acinetobacter baumannii - the role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem Cytobiol, 46(3):257–67. [DOI] [PubMed] [Google Scholar]
- 9.Falagas M, Kasiakou S, Michalopoulos A. (2004). Antimicrobial resistance of Acinetobacter spp. in Europe. Clin Microbiol Infec, 10(8):684–704. [DOI] [PubMed] [Google Scholar]
- 10.Seifert H, Dijkshoorn L, Gerner-Smidt P, et al. (1997). Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J Clin Microbiol, 35(11):2819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gootz TD, Marra A. (2008). Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev Anti Infect Ther, 6(3):309–25. [DOI] [PubMed] [Google Scholar]
- 12.Urban C, Segal-Maurer S, Rahal JJ. (2003). Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis, 36(10):1268–74. [DOI] [PubMed] [Google Scholar]
- 13.Uwingabiye J, Lemnouer A, Baidoo S, et al. (2017). Intensive care unit-acquired Acinetobacter Intensive Care Unit-Acquired Acinetobacter baumannii Infections in a Moroccan Teaching Hospital: Epidemiology, Risk Factors and Outcome.Germs,5;7(4):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gales AC, Jones R, Forward K, et al. (2001). Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999). Clin Infect Dis, 32 Suppl 2:S104–13. [DOI] [PubMed] [Google Scholar]
- 15.Manikal VM, Landman D, Saurina G, et al. (2000). Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis, 31(1):101–6. [DOI] [PubMed] [Google Scholar]
- 16.Corbella X, Pujol M, Ayats J, et al. (1996). Relevance of digestive tract colonization in the epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clin Infect Dis, 23(2):329–34. [DOI] [PubMed] [Google Scholar]
- 17.Perez F, Hujer AM, Hujer KM, et al. (2007). Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother,51(10):3471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Bambeke F, Balzi E, Tulkens PM. (2000). Antibiotic Efflux Pumps. Biochem Pharmacol, 60(4):457–70. [DOI] [PubMed] [Google Scholar]
- 19.Marchand I, Damier-Piolle L, Courvalin P, Lambert T. (2004). Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother, 48(9):3298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbott I, Cerqueira GM, Bhuiyan S, Peleg AY. (2013). Carbapenem resistance in Acinetobacter baumannii: laboratory challenges, mechanistic insights and therapeutic strategies. Expert Rev Anti Infect Ther, 11(4):395–409. [DOI] [PubMed] [Google Scholar]
- 21.Hassanzadeh S, Pourmand MR, Afshar D, et al. (2016). TENT: a rapid DNA extraction method of Staphylococcus aureus. Iran J Public Health, 45(8):1093–1095. [PMC free article] [PubMed] [Google Scholar]
- 22.Gholami M, Hashemi A, Hakemi-Vala M, et al. (2015). Efflux pump inhibitor phenylalanine-arginine Β-naphthylamide effect on the minimum inhibitory concentration of imipenem in Acinetobacter baumannii strains isolated from hospitalized patients in Shahid Motahari Burn Hospital, Tehran, Iran. Jundishapur J Microbiol, 8(10):e19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong EW, Mohd YMY, Bt MM, et al. (2009). Disruption of adeB gene has a greater effect on resistance to meropenems than adeA gene in Acinetobacter spp. isolated from University Malaya Medical Centre. Singapore Med J, 50(8):822–6. [PubMed] [Google Scholar]
- 24.Jia W, Li C, Zhang H, et al. (2015). Prevalence of genes of OXA-23 carbapenemase and AdeABC efflux pump associated with multidrug resistance of Acinetobacter baumannii isolates in the ICU of a comprehensive hospital of Northwestern China. Int J Environ Res Public Health, 12(8):10079–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou PF, Chen XY, Yan GF, et al. (2012). Study of the correlation of imipenem resistance with efflux pumps AdeABC, AdeIJK, AdeDE and AbeM in clinical isolates of Acinetobacter baumannii. Chemotherapy, 58(2):152–8. [DOI] [PubMed] [Google Scholar]
- 26.Lin MF, Chang KC, Lan CY, et al. (2011). Molecular epidemiology and antimicrobial resistance determinants of multidrug-resistant Acinetobacter baumannii in five proximal hospitals in Taiwan. Jpn J Infect Dis, 64(3):222–7. [PubMed] [Google Scholar]
- 27.Magnet S, Courvalin P, Lambert T. (2001). Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob Agents Chemother, 45(12):3375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coyne S, Courvalin P, Périchon B. (2011). Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother, 55(3):947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardebili A, Talebi M, Azimi L, Rastegar Lari A. (2014). Effect of Efflux Pump Inhibitor Carbonyl Cyanide 3-Chlorophenylhydrazone on the Minimum Inhibitory Concentration of Ciprofloxacin in Acinetobacter baumannii Clinical Isolates. Jundishapur J Microbiol, 7(1):e8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo D, Silveira F, Hujer AM, et al. (2006). Outer membrane protein changes and efflux pump expression together may confer resistance to ertapenem in Enterobacter cloacae. Antimicrob Agents Chemother, 50(8):2833–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee Y, Yum JH, Kim CK, et al. (2010). Role of OXA-23 and AdeABC efflux pump for acquiring carbapenem resistance in an Acinetobacter baumannii strain carrying the blaOXA-66 gene. Ann Clin Lab Sci, 40(1):43–8. [PubMed] [Google Scholar]
- 32.Bratu S, Landman D, Martin DA, et al. (2008). Correlation of antimicrobial resistance with beta-lactamases, the OmpA-like porin, and efflux pumps in clinical isolates of Acinetobacter baumannii endemic to New York City. Antimicrob Agents Chemother, 52(9):2999–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Huang J, Yu F, Wang X, Li G. (2010). AdeABC efflux pump: Less important role in Acinetobacter baumannii against carbapenems. Afr J Microbiol Res, 4(20):2148–2152. [Google Scholar]