Abstract
Background:
Aortic stenosis (AS) is the most common primary valvular disease. Currently, there is no pharmacological approach for the medical management of AS. We investigated the effect of osteoporosis therapy with alendronate on hemodynamic progression in patients concurrently affected by AS and osteoporosis.
Materials and Methods:
In this observational prospective study, we enrolled 37 women more than 60 years old with diagnosis of AS and concurrent osteoporosis from August 2017 to December 2019. These patients were treated with alendronate 70 mg every week added to their routine treatment for AS, and their outcomes were compared with 33 patients only affected by AS. Echocardiographic changes and N-terminal-prohormone of brain natriuretic peptide (NT-pro-BNP) level were evaluated during about 2 years of follow-up.
Results:
The mean follow-up time for the treated and nontreated groups was 20.89 ± 2.73 and 20.84 ± 2.76 months, respectively. Mean gradient (P = 0.02) and peak gradient (P = 0.04) of aortic valve were significantly different between the groups after follow-up. Aortic valve area was decreased 0.09 cm2 in the treated group by alendronate and 0.23 cm2 in the other group (P = 0.001). Furthermore, NT-pro-BNP was significantly decreased in patients treated by alendronate (P = 0.01), but it was increased in nontreated patients (P = 0.04).
Conclusion:
Treatment with alendronate in patients with AS and concurrent osteoporosis slows down the progression of stenosis and improves their prognosis. This study could open a new pathway for the treatment of AS. Further studies, particularly randomized controlled clinical trial, should be done for providing more evidence.
Keywords: Alendronate, aortic stenosis, N-terminal-prohormone of brain natriuretic peptide, osteoporosis
INTRODUCTION
Aortic stenosis (AS) is the most common primary valvular disease leading to surgery or catheter intervention in Western countries. Its prevalence has increased significantly in recent years due to population aging. Calcific aortic valve disease (CAVD) with significant stenosis affects 2%–3% of those over 65 years of age and up to 8% of those over 85 years in Western countries.[1]
AS is a slowly progressive disorder with a prolonged period of asymptomatic latency which eventually leads to obstruction of critical left ventricular (LV) outflow tract, necessitating surgical replacement of the valve.[2] Currently, there is no pharmacological approach for the medical management of AS.[3]
Therefore, there is a clear clinical need for new pharmacological agents to treat AS before the onset of symptoms to delay the aortic valve intervention or surgery. To find a suitable pharmacological agent at first, we should notice to the pathogenesis of CAVD. Mechanical stress, endothelial dysfunction, lipid deposition, inflammation, and oxidative stress play important roles in its pathogenesis.[1] Furthermore, activation of the renin–angiotensin–aldosterone system[4] and genetic factors such as the gene coding for the synthesis of apolipoproteins responsible for the individual's lipid load[5] affects the initiation and progression of CAVD. Based on the association of CAVD and mentioned factors, some clinical studies have been established. Randomized trials have consistently shown that statins do not affect the progression of AS.[6,7] Treatment with drugs that block the renin–angiotensin–aldosterone cascade had a controversial effect in this group of patients in different studies.[8,9]
In the other hand, it has been shown that there is an association between aortic valve calcification and disorders of calcium and bone metabolism such as end-stage renal disease and osteoporosis.[10,11] Bisphosphonates are used to treat osteoporosis by blocking bone resorption. In some studies, bisphosphonates have shown a potential benefit in slowing the progression of human calcific AS.[3,12]
In this situation, the eyes notice to remodeling of the extracellular matrix and calcification of aortic valve as a target with bisphosphonates to find a possible medical treatment to slow the progression of CAVD. We, therefore, investigated the association between the use of osteoporosis treatment with bisphosphonates and hemodynamic progression of AS in an observational study.
METHODS
Study setting and participants
In this observational prospective study, we enrolled 37 women more than 60 years old with diagnosis of AS and concurrent osteoporosis from August 2017 to December 2019. Furthermore, 33 patients with AS but without osteoporosis were selected as a nontreated group. All of patients in both groups were recruited from the Echocardiography Department of Chamran Cardiology Hospital in Isfahan Province in Iran. We defined AS in our study as restricted motion in aortic valve leaflets with at least mild calcification, followed by aortic valve peak velocities more than 2 m/s and aortic valve area (AVA) <2 cm2.[13] AVA was calculated from peak velocities at the aortic valve and LV outflow tract using the continuity equation. Furthermore, we recruited patients with osteoporosis when bone mineral density T-score was <−2.5 at either the lumbar spine or total hip and more than −4.0 at both sites.[14] Patients with known bicuspid aortic valve, mitral stenosis, aortic valve surgery, hyperparathyroidism, serum creatinine level more than 1.5 mg/dl, LV ejection fraction (LVEF) <50%, serum 25-hydroxyvitamin D <20 ng/mL, history of calcium or phosphate metabolic disorder, and bisphosphonate use during the last 3 years were excluded from the study. Furthermore, if an indication for aortic valve replacement (AVR) was happen or any pathologic fracture was done, the patients were excluded from the study. All echocardiographies were done by an echocardiography specialist who is blinded to the study. After echocardiography, individuals underwent a complete examination that included physical examination and blood sampling for biochemistry measurements. Then, all patients with osteoporosis undergone with treatment of alendronate (Osteofos Alborz Darou, Iran) 70 mg every week and 1000 mg calcium and 400 IU Vitamin D every day. Appropriate medical management for AS and other comorbid diseases was done for both the groups. The nontreated group did not use alendronate or any other bisphosphonate drugs. All the patients (both the treated and nontreated groups) were visited every 2 months and sooner if needed. Echocardiography, biochemistry measurements, and follow-up were done for about 2 years for both the nontreated and treated groups.
Ethical issues
This research has been approved and funded by Baqiyatallah University of Medical Sciences (project number: 101858). Ethical approval has been obtained from the Ethical Committee of Baqiyatallah University of Medical Sciences. Before signing informed consent, the project and its aims are clearly explained to the patients and the confidentiality of the data is ascertained. Patients receive appropriate medical management during the study, and they are informed that discontinuing participation in this study will not interfere with their medical management.
Statistical analysis
Obtained data were gathered in checklist and entered IBM SPSS Statistics software (version 22, IBM Corporation, Armonk, NY, USA) for analysis. The data were expressed as mean ± standard deviation and frequency (percent) for numeric and categorical variables, respectively. The normality of the distribution of numeric variables was evaluated using the Kolmogorov–Smirnov test. Differences in numeric and categorical variables between the treated and nontreated groups were analyzed using the independent-sample t- and Chi-square tests, respectively. In the multivariate analysis, to adjust for potential confounders, analysis of covariance was carried out. P < 0.05 was considered as level of significance. All statistical analyses were performed by a blind analyzer about details of the research.
RESULTS
In this study, 173 patients with AS with no need for surgery were evaluated at the beginning of the study. From these patients, 43 patients with inclusion criteria and osteoporosis were enrolled as a treated group which was treated with alendronate. Furthermore, 37 patients who fulfilled inclusion criteria but without osteoporosis were recruited as a nontreated group. All the patients were female in both the groups. Six patients in the treated group and four patients in the nontreated group did not complete follow-up due to different causes and, therefore, were excluded from the study. The enrollment flowchart is shown in Figure 1.
Figure 1.
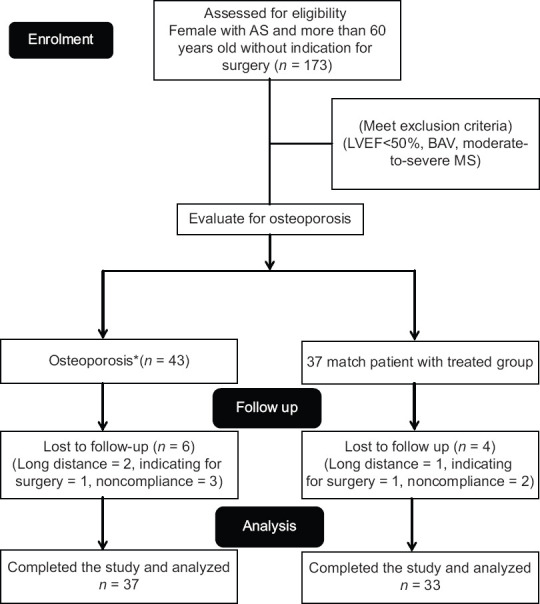
Study fellow diagram. *Osteoporosis defines as bone mineral density T-score < −2.5 at either the lumbar spine or total hip and more than −4.0 at both sites
The mean age in the treated group was 69.59 ± 5.97 and in the nontreated group was 68.72 ± 4.04 (P = 0.48). The mean of follow-up time was 20.89 ± 2.73 months in the alendronate-treated group and 20.84 ± 2.76 in the other group (P = 0.92). There was no significant difference at baseline characteristics and laboratory tests between the two groups; however, after adjusting for age, body mass index (BMI), and follow-up time, a significant difference was observed between the groups (P < 0.05) for fast blood sugar [Table 1].
Table 1.
Basic characteristics of alendronate-treated and nontreated patients
| Alendronate treated (n=37), n (%) | Nontreated (n=33), n (%) | P# | Adjusted mean difference | L | U | Adjusted P$ | |
|---|---|---|---|---|---|---|---|
| Age (years) | 69.59±5.97 | 68.72±4.04 | 0.48 | - | - | - | - |
| BMI (kg/m2) | 27.07±3.76 | 28.08±3.50 | 0.25 | - | - | - | - |
| Follow-up (months) | 20.89±2.73 | 20.84±2.76 | 0.92 | - | - | - | - |
| Hypertension (%) | 16 (43.2) | 14 (42.4) | 0.94 | - | - | - | - |
| Diabetes mellitus (%) | 10 (27) | 10 (30.3) | 0.76 | - | - | - | - |
| CAD history (%) | 24 (64.9) | 18 (54.5) | 0.38 | - | - | - | - |
| Statin use (%) | 33 (89.2) | 32 (97) | 0.21 | - | - | - | - |
| Beta-blocker use (%) | 26 (70.3) | 19 (57.6) | 0.27 | - | - | - | - |
| ACEI/ARB use (%) | 15 (40.4) | 14 (42.4) | 0.87 | - | - | - | - |
| Hemoglobin (g/dL) | 12.87±0.70 | 12.99±0.83 | 0.52 | −0.11 | −0.48 | 0.27 | 0.577 |
| Platelet (/µL) | 201.78±49.70 | 212.36±56.29 | 0.41 | −10.87 | −34.90 | 13.17 | 0.370 |
| Creatinine (mg/dL) | 1.11±0.13 | 1.39±1.6 | 0.84 | −0.30 | −0.85 | 0.25 | 0.283 |
| FBS (mg/dL) | 106.08±23.13 | 99.51±13.78 | 0.44 | 7.58 | 0.41 | 14.74 | 0.039 |
| Triglyceride (mg/dL) | 118.75±28.66 | 113.69±25.9 | 0.43 | 6.15 | −6.76 | 19.07 | 0.345 |
| LDL-C (mg/dL) | 87.48±15.16 | 85.45±15.61 | 0.58 | 1.59 | −5.85 | 9.04 | 0.671 |
| HDL-C (mg/dL) | 47.89±7.48 | 46.72±5.74 | 0.47 | 0.62 | −2.54 | 3.78 | 0.697 |
| Uric acid (mg/dL) | 5.43±0.73 | 5.19±0.58 | 0.23 | 0.27 | −0.07 | 0.60 | 0.116 |
| ALT (IU/L) | 25.24±5.58 | 24.27±6.16 | 0.31 | 0.88 | −1.98 | 3.74 | 0.540 |
| AST (IU/L) | 23.62±5.47 | 25.93±6.28 | 0.11 | −2.14 | −5.06 | 0.79 | 0.150 |
| Calcium (mg/dL) | 9.06±0.37 | 9.06±0.36 | 0.94 | 0.00 | −0.18 | 0.18 | 0.971 |
| Albumin (g/dL) | 4.06±0.24 | 4.12±0.24 | 0.29 | −0.05 | −0.17 | 0.07 | 0.393 |
| Phosphorus (mg/dL) | 3.87±0.52 | 3.77±0.29 | 0.71 | 0.09 | −0.12 | 0.30 | 0.379 |
| PTH (pg/mL) | 54.75±10.73 | 53.12±9.50 | 0.50 | 0.22 | −4.49 | 4.92 | 0.928 |
| Serum Vitamin D level (ng/mL) | 43.58±6.57 | 44.36±10.01 | 0.69 | −1.17 | −5.33 | 2.99 | 0.577 |
| NT-pro-BNP (pg/mL) | 1258±986 | 1053±741 | 0.29 | 115.39 | −305.86 | 536.63 | 0.586 |
BMI=Body mass index; FBS=Fast blood sugar; LDL-C=Low-density lipoprotein; HDL-C=High-density lipoprotein; PTH=Parathyroid hormone; CAD=Coronary artery disease; ACEI=Angiotensin-converting enzyme inhibitor; ARB=Angiotensin receptor blocker; NT-Pro-BNP=N-terminal-prohormone of brain natriuretic peptide. Data are expressed as mean±SD and frequency (%) for numeric and categorical variables, respectively. #Based on t/Chi-square test for numeric/categorical variables. $Adjusted for age, BMI, and follow-up time; L=95% CI lower bound; U: 95% CI upper bound. CI=Confidence interval; SD=Standard deviation; ALT=Alanine aminotransferase; AST=Aspartate aminotransferase
The mean of AVA is 1.46 ± 0.35 cm2 in the treated group and 1.57 ± 0.33 in the nontreated group (P = 0.22). As shown in Table 2, all echocardiographic features were not significantly different in both the groups at baseline. Furthermore, in the multivariate analysis, after adjusting for age, BMI, and follow-up time and baseline measurements, no significant differences were observed between the groups for echocardiographic features (P > 0.05).
Table 2.
Echocardiographic characteristics of patients at baseline
| Alendronate treated | Nontreated | P# | Adjusted mean difference | L | U | Adjusted P$ | |
|---|---|---|---|---|---|---|---|
| LVDD (mm) | 46.27±5.04 | 46.48±4.43 | 0.70 | −0.11 | −2.45 | 2.24 | 0.928 |
| LVEF (%) | 56.86±4.78 | 57.03±3.94 | 0.86 | −0.32 | −2.40 | 1.76 | 0.758 |
| IVS (mm) | 11.75±1.90 | 11.18±1.94 | 0.19 | 0.55 | −0.41 | 1.51 | 0.254 |
| MG (mmHg) | 22.81±9.09 | 24.87±8.04 | 0.18 | −2.90 | −6.99 | 1.19 | 0.161 |
| PG (mmHg) | 39.87±14.82 | 42.10±11.04 | 0.48 | −3.70 | −9.83 | 2.43 | 0.233 |
| AVA (cm2) | 1.46±0.35 | 1.57±0.33 | 0.22 | −0.07 | −0.23 | 0.10 | 0.404 |
LVDD=Left ventricular diastolic diameter; LVEF=Left ventricular ejection fraction; IVS=Interventricular septum; MG=Mean gradient; PG=Peak gradient; AVA=Aortic valve area. Data are expressed as mean±SD. #Based on t-test; $Adjusted for age, BMI, and follow-up time using analysis of covariance; L=95% confidence interval lower bound; U=95% confidence interval upper bound; BMI=Body mass index
Table 3 shows the echocardiographic characteristics of patients after about 2 years of follow-up and treatment. As it was shown in the multivariate analysis, after adjusting for age, BMI, and follow-up time and baseline measurements, significant differences were observed between the groups for N-terminal-prohormone of brain natriuretic peptide (NT-pro-BNP), LV diastolic dysfunction, LVEF, interventricular septum, mean gradient (MG), pressure gradient, AVA, and delta-AVA (All P < 0.05).
Table 3.
Echocardiographic characteristics and serum N-terminal-prohormone of brain natriuretic peptide level of both groups after about 2-year follow-up
| Alendronate treated | Nontreated | P# | Adjusted mean difference | L | U | Adjusted P$ | |
|---|---|---|---|---|---|---|---|
| NT-pro-BNP | 881.48±519.63 | 1185.12±591.42 | 0.009 | −384.89 | −588.36 | −181.41 | <0.001 |
| LVDD | 46.0±4.59 | 47.39±3.92 | 0.18 | −1.17 | −2.10 | −0.23 | 0.015 |
| LVEF | 56.62±4.24 | 54.48±5.94 | 0.18 | 2.29 | 0.25 | 4.32 | 0.028 |
| IVS | 11.81±1.64 | 12.30±1.42 | 0.21 | −0.86 | −1.27 | −0.44 | <0.001 |
| MG | 25.28±8.95 | 29.39±9.14 | 0.02 | −2.14 | −3.63 | −0.64 | 0.006 |
| PG | 44.70±14.51 | 51.21±11.45 | 0.04 | −4.54 | −6.16 | −2.92 | <0.001 |
| AVA | 1.37±0.33 | 1.34±0.30 | 0.69 | 0.12 | 0.05 | 0.19 | 0.001 |
| Delta-AVA | −0.09±0.17 | −0.23±0.13 | 0.001 | 0.129 | 0.054 | 0.204 | 0.001 |
BMI=Body mass index; SD=Standard deviation; NT-Pro BNP=N-terminal-prohormone of brain natriuretic peptide; LVDD=Left ventricular diastolic diameter; LVEF: Left ventricular ejection fraction; IVS=Interventricular septum; MG=Mean gradient; PG=Peak gradient; AVA=Aortic valve area. Data are expressed as mean±SD. #based on t-test; $Adjusted for age, BMI, follow-up time, and baseline measurements using analysis of covariance; L=95% confidence interval lower bound; U=95% confidence interval upper bound
Both AVA and AVA changes (delta-AVA) during 20-month follow-up were significantly different between the treated and nontreated groups (P < 0.05). Indeed, AVA was decreased 0.09 cm2 in the alendronate-treated group; however, it was decreased 0.23 cm2 in the other group (P = 0.001).
As shown in Figure 2, the mean of NT-pro-BNP, as a marker of prognosis in AS patients, was 1258 ± 986 (pg/mL) in the treated group at the beginning of the study and after treatment with alendronate was significantly decreased (P = 0.01). This marker in the nontreated group was significantly increased during follow-up (P = 0.04).
Figure 2.
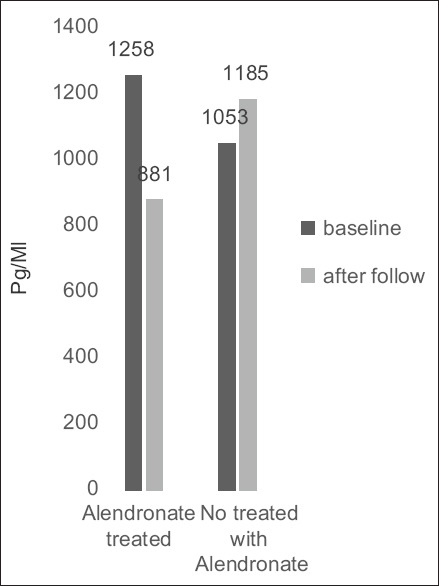
Changes in serum N-terminal-prohormone of brain natriuretic peptide level in treated and nontreated groups over time
According to the American Heart Association/American College of Cardiology classification,[13] AS with MG of <20 mmHg is defined as mild AS, MG between 20 and 39 mmHg as moderate AS, and MG more than 40 mmHg called severe AS. To this classification, 13 patients in the treated group and 8 patients in the nontreated group were classified as mild AS according to their baseline MG of aortic valve [Table 4].
Table 4.
Patient characteristics after about 20-month follow-up according to aortic stenosis classification
| AS classification | Alendronate treated | Nontreated | P# |
|---|---|---|---|
| Mild AS | |||
| n (%) | 13 (35.1) | 8 (24) | |
| NT-pro-BNP | 928.84±637.03 | 1101.37±534.82 | 0.53 |
| Delta-AVA | −0.08±0.21 | −0.21±0.11 | 0.12 |
| Nonmild AS | |||
| n (%) | 24 (64.8) | 25 (75.7) | |
| NT-pro-BNP | 855.83±457.12 | 1211.92±616.32 | 0.02 |
| Delta-AVA | −0.09±0.15 | −0.23±0.13 | 0.002 |
NT-Pro BNP=N-terminal-prohormone of brain natriuretic peptide; AVA=Aortic valve area; AS=Aortic stenosis. Data are expressed as frequency (%). #Based on Chi-square test
In our analysis, serum NT-pro-BNP level and delta-AVA after follow-up were not significantly different between the groups in mild AS subgroup of patients. However, in nonmild AS subgroup, these changes between treated and nontreated were significant, as shown in Table 4.
DISCUSSION
This study shows that alendronate treatment in women with osteoporosis and AS slows down the progression of aortic valve stenosis.
We believe that stenosis of aortic valve is a dynamic process, and chronic inflammation, renin–angiotensin system, lipid accumulation, and calcium deposition play role in its pathogenesis.[1] Studies on statins and angiotensin-converting enzyme inhibitors would not be associated with slowing of disease progression, and therefore, no appropriate therapeutic option is available to slow the rate of progression of stenosis in AS patients.[6,8]
Calcification and extracellular matrix remodeling which is promoted by the release of inflammatory cytokines is a key point in the progression of AS.[15] Calcification of aortic valve involves different mechanisms of bone mineralization and resorption that begin at the level of the fibrous layer where some fibroblasts differentiate into myofibroblasts. As a result, expression of proteins related to bone formation increases at the level of the extracellular matrix. From these proteins, osteopontin, bone-forming proteins 2 and 4, osteoprotegerin, ligand-activating factor, and nuclear factor-κB (NF-κB) ligands (receptor activator of NF-κB [RANK] and RANK ligand) are more important. Through complex interactions of these molecules with those of lipid pathways and fibroblasts, initial calcific nodules have been shown to form in regions of lipid accumulation.[1,16]
Alendronate as a bisphosphonate could affect the pathophysiology of AS in two different pathways: (1) bisphosphonates have been shown to inhibit farnesyl pyrophosphate synthase. This enzyme is involved in mevalonate/cholesterol biosynthetic pathway in which ultimately statins exert their effects.[17] Therefore, similar to statins, bisphosphonates have an effect on lipid metabolism and also exhibit anti-inflammatory actions. (2) Bisphosphonates inhibit bone resorption and slow the release of calcium phosphate particles from the bone, which could play a role in the prevention of calcium deposition in vascular and valvular tissues.[17,18]
Some previous studies have been shown that bisphosphonates slow the progression of human calcific AS.[3,12,19] Furthermore, in a multiethnic study of atherosclerosis, it has been shown that nitrogen-containing bisphosphonate was associated with decreased prevalence of aortic valve calcification in women older than 65 years old.[20]
In our study, AVA was only 0.09 cm2 decreased during 20 months treatment with alendronate. However, according to previous studies, the average rate of hemodynamic progression in adults with mild-to-moderate AS is a decrease in valve area of 0.1 cm2 per year.[21] Therefore, this amount of decrease in our patients during about 2 years might prominent the wonderful effect of alendronate in AS patients. In addition, our finding shows that alendronate effectiveness in slowing down of the progression of AS is more prominent in moderate-to-severe cases of AS. This could be due to more calcification presented on aortic leaflets in these patients.
This study illustrated that alendronate decreases serum NT-pro-BNP of the patients with AS. NT-pro-BNP is released from the myocardium with increased wall stress both from volume and pressure overload, the latter of which occurs in AS. In most studies, it has been shown that elevated levels of natriuretic peptides are associated with increased LV and left atrial dimensions, decreased LV function, and increased systolic pulmonary artery pressure.[22,23,24] Multiple studies demonstrate a strong relationship between natriuretic peptides and increased mortality in patients with AS before AVR.[25,26] Thus, this marker has the prognostic value in AS patients. In this study, alendronate decreases the NT-pro-BNP level in AS patients; therefore, it could improve outcomes of AS patients.
Controversial findings have been proved in one large-scale retrospective study.[27] In this study, the analysis of 801 female patients' findings showed that bisphosphonates do not have a significant impact on the hemodynamic or clinical progression of AS. That was a retrospective study and so its nature could affects its findings. Their authors did not have data on patient compliance with taking bisphosphonates throughout the course of follow-up. Furthermore, in that study, the patients used different types of bisphosphonates. While bisphosphonates are a large group of drugs, each with its own specific properties and affinities to distinct pathways in the calcification and inflammatory processes. In our study, the patients were evaluated in a prospective study with careful monitoring for their compliance, and all the patients in the treated group were treated with alendronate. Furthermore, our study is the first study to evaluate the effect of bisphosphonates on serum NT-pro-BNP as a prognostic marker of outcomes in AS patients.
Our study has some limitations. The first of all is that the number of patients is low and our follow-up duration is only 2 years. Furthermore, we did not evaluate the efficacy of alendronate on bone density in our follow-up. Therefore, it seems that we need other studies with more number of patients and more period of follow-up with other types of bisphosphonates to evaluate the effect of these drugs on the progression of AS.
CONCLUSION
Our study shows that treatment with alendronate in patients with AS and concurrent osteoporosis slows down the progression of stenosis and improves their prognosis. This study could open a new pathway for pharmacological treatment of AS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We appreciate Chamran Hospital staffs for their kind collaboration. This project was done under supervision of the National Elites Foundation in Iran and Baqiyatallah University, Tehran, Iran, as military service facilities for elite students. We appreciate their staffs for their warm collaboration.
REFERENCES
- 1.Izquierdo-Gómez MM, Hernández-Betancor I, García-Niebla J, Marí-López B, Laynez-Cerdeña I, Lacalzada-Almeida J. Valve calcification in aortic stenosis: Etiology and diagnostic imaging techniques. Biomed Res Int 2017. 2017:5178631. doi: 10.1155/2017/5178631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ledwoch J, Thiele H. Treatment of asymptomatic aortic valve stenosis: Watchful waiting or early intervention? Herz. 2017;42:528–35. doi: 10.1007/s00059-017-4584-z. [DOI] [PubMed] [Google Scholar]
- 3.Sterbakova G, Vyskocil V, Linhartova K. Bisphosphonates in calcific aortic stenosis: Association with slower progression in mild disease – A pilot retrospective study. Cardiology. 2010;117:184–9. doi: 10.1159/000321418. [DOI] [PubMed] [Google Scholar]
- 4.Helske S, Lindstedt KA, Laine M, Mäyränpää M, Werkkala K, Lommi J, et al. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol. 2004;44:1859–66. doi: 10.1016/j.jacc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 5.Bossé Y, Mathieu P, Pibarot P. Genomics: The next step to elucidate the etiology of calcific aortic valve stenosis. J Am Coll Cardiol. 2008;51:1327–36. doi: 10.1016/j.jacc.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–14. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 7.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–56. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien KD, Probstfield JL, Caulfield MT, Nasir K, Takasu J, Shavelle DM, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med. 2005;165:858–62. doi: 10.1001/archinte.165.8.858. [DOI] [PubMed] [Google Scholar]
- 9.Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–5. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 10.Mills WR, Einstadter D, Finkelhor RS. Relation of calcium-phosphorus product to the severity of aortic stenosis in patients with normal renal function. Am J Cardiol. 2004;94:1196–8. doi: 10.1016/j.amjcard.2004.07.095. [DOI] [PubMed] [Google Scholar]
- 11.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, et al. Human aortic valve calcification is associated with an osteo-blast phenotype. Circulation. 2003;107:2181–4. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Innasimuthu AL, Katz WE. Effect of bisphosphonates on the progression of degenerative aortic stenosis. Echocardiography. 2011;28:1–7. doi: 10.1111/j.1540-8175.2010.01256.x. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. ; ACC/AHA Task Force Members.2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–92. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 14.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kiliç R, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–7. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–26. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 17.Reszka AA, Rodan GA. Nitrogen-containing bisphosphonate mech-anism of action. Mini Rev Med Chem. 2004;4:711–9. [PubMed] [Google Scholar]
- 18.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–78. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Skolnick AH, Osranek M, Formica P, Kronzon I. Osteoporosis treatment and progression of aortic stenosis. Am J Cardiol. 2009;104:122–4. doi: 10.1016/j.amjcard.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 20.Elmariah S, Delaney JA, O'Brien KD, Budoff MJ, Vogel-Claussen J, Fuster V, et al. Bisphosphonate use and prevalence of valvular and vascular calcification in women MESA (the multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2010;56:1752–9. doi: 10.1016/j.jacc.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rashedi N, Otto CM. Aortic stenosis: Changing disease concepts. J Cardiovasc Ultrasound. 2015;23:59–69. doi: 10.4250/jcu.2015.23.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito S, Miranda WR, Jaffe AS, Oh JK. Prognostic value of N-terminal pro-form B-type natriuretic peptide in patients with moderate aortic stenosis. Am J Cardiol. 2020;125:1566–70. doi: 10.1016/j.amjcard.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Parikh V, Kim C, Siegel RJ, Arsanjani R, Rader F. Natriuretic peptides for risk stratification of patients with valvular aortic stenosis. Circ Heart Fail. 2015;8:373–80. doi: 10.1161/CIRCHEARTFAILURE.114.001649. [DOI] [PubMed] [Google Scholar]
- 24.Maréchaux S, Hattabi M, Juthier F, Neicu DV, Richardson M, Carpentier E, et al. Clinical and echocardiographic correlates of plasma B-type natriuretic peptide levels in patients with aortic valve stenosis and normal left ventricular ejection fraction. Echocardiography. 2011;28:695–702. doi: 10.1111/j.1540-8175.2011.01418.x. [DOI] [PubMed] [Google Scholar]
- 25.Pfister R, Wahlers T, Baer FM, Scherner M, Strauch J, Erdmann E. Utility of NT-pro-BNP in patients undergoing transapical aortic valve replacement. Clin Res Cardiol. 2010;99:301–7. doi: 10.1007/s00392-010-0118-x. [DOI] [PubMed] [Google Scholar]
- 26.Solberg OG, Ueland T, Wergeland R, Dahl CP, Aakhus S, Aukrust P, et al. High-sensitive troponin T and N-terminal-brain-natriuretic-peptide predict outcome in symptomatic aortic stenosis. Scand Cardiovasc J. 2012;46:278–85. doi: 10.3109/14017431.2012.687836. [DOI] [PubMed] [Google Scholar]
- 27.Aksoy O, Cam A, Goel SS, Houghtaling PL, Williams S, Ruiz-Rodriguez E, et al. Do bisphosphonates slow the progression of aortic stenosis? J Am Coll Cardiol. 2012;59:1452–9. doi: 10.1016/j.jacc.2012.01.024. [DOI] [PubMed] [Google Scholar]


