Abstract
Background:
This study aimed to evaluate the epidemiological studies on the relationship between organophosphate (OP) pesticide exposure during pregnancy and neonatal anthropometric measures.
Materials and Methods:
In this systematic review and meta analyses, a comprehensive search of the literature for the association of maternal exposure to OP pesticides and birth outcome including birth weight, birth length, and head circumference was conducted from scientific databases of MEDLINE, Scopus, Web of Science, and Cochrane library until the end of April 2019. We used the following keyword to identify the relevant studies: “birth weight,” “birth length,” “pregnancy outcome,“”birth outcome,” “organophosphate pesticides,” and “organophosphate metabolites.” Only English language studies investigating the relationship between pregnant mothers' exposure to OP metabolites and birth outcomes were examined.
Results:
Of the 10 articles reviewed, eight studies used to assess the association with birth weight, as well as five, and six studies were used in meta analysis to determine the association between OP exposure and birth length and head circumference. Pooled estimates were performed using a fixed effects model or random effects model. No significant association was observed between maternal exposure to OPs and birth weight (β = 1.520;95% confidence interval [CI] [−10.781, 13.820]), birth length (β = −0.011; [−0.132, 0.109]), and head circumference (β =0.022; 95%CI [−0.06, 0.103]).
Conclusion:
Although the effect of maternal exposure to OP on the birth outcome is not completely clear, strategies should be adopted to control the use of these substances.
Keywords: Anthropometric measures, organophosphate pesticides, pregnant women
INTRODUCTION
In the past 50 years, pesticides have been an integral part of the agricultural world. Although the demand for various pesticides that improve agricultural quality and efficiency is very explicit, the improper and inappropriate use of them is increasing. One of the most common agricultural pesticides is organophosphates (OPs) compounds.[1] OPs are a large group of biocides of which used to control insect pests on a variety of food and feed crops. These compounds can decompose in the natural environment, so they can be a suitable substitute for organochlorine pesticides. However, the high toxicity of OP pesticides is evident. Thus, there are some prohibitions of use accompanied by reduction use in Europe and the US in recent years. The use of these pesticides for agricultural and non-agricultural purposes has led to their entry into the environment, especially water, soil, and food resources.[2,3] Exposure of OPs pesticide may be due to occupational contact, pathways, routes, and agricultural applications, but the leading cause is diet exposure. Exposure to OPs causes to the bioaccumulation of these chemical compounds in adipose tissue, breast milk, and urine of humans.[4]
During pregnancy, the lipophilic type of OP can store in maternal adipose tissue and also able to pass through the placenta through the bloodstream.[5,6] Moreover, hydrolysis or oxidative desalting processes can convert the absorbed OPs into polar and water-soluble metabolites. The major ones include dimethyl phosphate (DEP), dimethyl thiophosphate (DETP), dimethyl dithiophosphate (DEDTP), dimethyl phosphate (DMP), dimethyl thiophosphate (DMTP) and dimethyl thiophosphate (DMDTP).[4,6,7,8] Recent human and animal studies show that maternal exposure to pesticides during pregnancy is associated with abnormalities in neuronal proliferation, differentiation, migration, synaptogenesis, myelin, and apoptosis.[4]
Similarly, prenatal exposure to OP pesticides is associated with adverse effects on neurodevelopment and growth in infancy and childhood.[4] As a result of research on rodents, prenatal exposure to OP pesticides associated with teratogenicity and embryotoxicity consists of decreased fetal growth in outcomes.[9,10,11] In human studies, prenatal OP pesticide exposures, as measured by metabolites of OP pesticides in the blood or urine of pregnant women, were linked to decrement in gestational duration, birth weight, birth length, and head circumference.[12,13]
This review is designed to give a systematic and meta-analysis coverage of anthropometric measures including birth weight, birth length, and head circumference resulting in OP exposure in the pregnant women group. According to our findings, no recent meta-analysis study conducted on this subject.
MATERIALS AND METHODS
Search strategy
A meta-analysis of the current literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.[14] A comprehensive search from the scientific databases of MEDLINE, Scopus, Web of Science, and Cochrane collaboration was conducted to identify the study published until the end of April 2019. Studies were selected based on the following criteria:
Participants: Women during term pregnancy (without chronic disease or long-term medication use) and their infants
Exposure: Organophosphate metabolites including dialkyl phosphates (DAPs), dimethyl phosphates (DMPs), and diethyl phosphates (DEPs)
Outcome: Studies investigating the association between organophosphate metabolites exposure of pregnant women and adverse birth outcome including birth weight, birth length, and head circumference
Study design: Studies with cohort, case–control, and cross-sectional design
Language: Studies in the English language.
The keywords used in databases, as well as medical subject heading terms in MEDLINE, included “birth weight,” “birth length,” “pregnancy outcome,” “birth outcome,” combined with the Boolean operator “OR.” Finally, the keywords of “organophosphate pesticides” or “organophosphate metabolites” were added and combined with the former search line with the Boolean operator “AND.” According to the search line, in MEDLINE, a total of 107 articles were found. Some restrictions were imposed, including human studies and English language, which ultimately, 87 studies remained. After reviewing the studies, duplicate studies and also irrelevant exposure was removed, and finally, 55 articles remained. Similarly, in other databases, this line search was used to identify eligible studies.
Study selection and data extraction
EndNote (X7) reference manager software was used in order to delete the duplicate andin vitro andin vivo articles. Two independent reviewers (SD and KE) screened all the titles and abstracts of considering publications. The full text of selected articles was checked based on inclusion criteria. Two researchers (SD and KE) did data extraction from all eligible studies. As shown in Table 1, data related to the year of publications, author's last name, location of research, type of study, sample size, sample type, OP type, outcome definition, and confounding factors extracted. Similar to the previous work, we performed a quality assessment using the STROBE guideline by two researchers (M Kh and B Sh).[15,16] For each article, study design, participant selection, variables, source, and measurement data, statistical methods, results in the primary data, and study limitations reviewed. Each of the 22 questions in the checklist rated as 0 or 1. The average quality score was 20, with scores ranging from 18 to 22. Therefore, all of the studies had high quality. In addition, there was a perfect agreement (100%) between scores of two researchers. The mean scores obtained for each study presented in Table 1.
Table 1.
Characteristics of included studies in the systematic-meta analyses review
| Number | Mean STORBE score | Year | Country | First author | Study design | Sample size | Sample type | Organophosphate type | Confounding factors |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | 2017 | Taiwan | YU- FUNG HUANG | Cohort | 162 | Maternal Urine | DMPs (DMP, DMTP, DMDTP), DEPs (DEP, DETP, DEDTP) | Maternal age, prepregnancy BMI, gestational age, weight gain, infant sex, parity, and adverse pregnancy outcomes |
| 2 | 19 | 2014 | Greece | D.Koutroulakis | Cohort | 415 | Amniotic fluid | DMP, DEP, DMTP, DETP, DEDTP | Maternal age, agricultural activities, gestational week at sampling and neonatal gender |
| 3 | 22 | 2018 | Denmark | Louise Dalsager | cohort | 858 | Maternal Urine | DAP, DMPs (DMP,DMTP, DMDTP), DEPs (DEP, DETP, DEDTP) | Education, BMI, smoking, gestational age |
| 4 | 20 | 2004 | USA | Brenda Eskenazi | cohort | 488 | Maternal Urine | DAP, DMP, DEP, MDA, chlorpyrifos, parathion, ChE, BChE | Timing of urine collection, timing of entry into prenatal care, maternal age, parity, country of birth, poverty level, infant sex, weight gain, BMI, gestational age, |
| 5 | 22 | 2019 | Netherlands | Kelly K. Ferguson | Cohort | 784 | Maternal Urine | DAP, DMP, DEP | Age, prepregnancy weight, height, education level, ethnicity, parity, smoking, alcohol use, folic acid use, gestational age at ultrasound or delivery |
| 6 | 19 | 2015 | Thailand | Warangkana Naksen | Pilot study | 59 | Maternal Urine | DMP, DMTP, DMDTP, DEP, DETP, DEDTP | Maternal age, preprenancy BMI, weight gain and gestational age |
| 7 | 22 | 2011 | USA | Kim G. Harley | Longitudinal cohort | 467 | Maternal urine | DAPs (DMP, DMDTP, DMTP), DEPs (DEP, DEDTP, DETP) | Timing of urine collection, timing of entry into prenatal care, maternal age, parity, country of birth and household income prepregnancy BMI, maternal weight gain, infant sex, gestational age |
| 8 | 20 | 2007 | USA | MARY S. WOLFF | Cohort | 404 | Maternal blood | DEDP, DMDP, DMTP | Maternal age, race/ethnicity, maternal BMI *Pregnancy weight gain, infant sex, and gestational age |
| 9 | 21 | 2012 | USA | Stephen A. Rauch | Birth cohort | 344 | Maternal urine | DMP, DMTP, DMDTP | Maternal race , gestational age |
| 10 | 19 | 2012 | china | Pei Wang | Cross sectional | 187 | Maternal urine | DMP, DMTP, DEP, DETP, DEDTP | Gestational age, maternal height, pregnancy weight gain and family income |
DEPs=Diethyl phosphates; DETP=Diethyl thiophosphate; DEDTP=Dimethyl dithiophosphate; DMTP=Dimethyl thiophosphate; DMDTP=Dimethyl thiophosphate; DMP=Dimethyl phosphate; DAP=Dialkyl phosphates; BMI=Body mass index; MDA=Dicarboxylic acid; BChE=Butyryl cholinesterase; ChE=Cholinesterase
Statistical analysis
The regression (beta) coefficient values for effects of prenatal exposure to OP pesticide metabolites on birth weight, birth length, and birth head circumference were used to effect sizes.
The meta-analysis was performed to obtain the summary measures for the effect of prenatal exposure to OP pesticide metabolites on these birth anthropometrics measures (pooled effect sizes). The potential heterogeneity across studies was evaluated using the Cochran's Q-test and expressed using the I2 index. The I2 ≥ 75% represent considerable heterogeneity.[17] Results of meta-analysis were presented using forest plots based on year of studies. The pooled results were calculated by the random-effects model because the selected studies were sampled from the different population and locations at different times.[18] Publication bias was evaluated by Egger's and Begg's tests. Subgroup analyses based on the type of metabolites were performed to seek the sources of heterogeneity. In addition, meta-regression was used for assessing the studies sample size and year of publication as the possible source of heterogeneity. The sensitivity analyses were performed by omitting one study at a time to gauge the robustness of our results. All statistical analyses conducted using software STATA 12.0 (STATA Corp, College Station, Texas, USA).
RESULTS
Study design and population
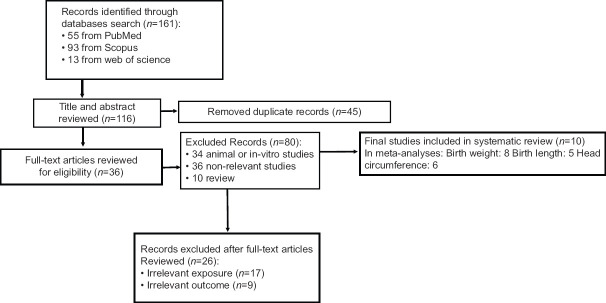
A PRISMA[14] flow diagram related to the study selection process in this meta-analyses was shown in Figure 1. As shown in Figure 1, a total of 161 articles found: 55 from MEDLINE, 93 from Scopus, and 13 from the web of science. After removing the duplicate articles, 116 studies remained. After an initial review, 34 animal or in vitro studies, 36 nonrelevant studies, and ten reviews excluded. Next, 36 studies left for full-text article review. Of these, 26 studies excluded because of exposure or irrelevant consequences. Finally, a total of 10 studies included in this systematic review and meta-analysis.
Figure 1.
A Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram
Of the 10 articles included in this systematic review, all are cohort studies except for two studies in which one of them is a pilot study, and another is cross-sectional. Studies were related to three continents of Asia, Europe, and America. Asian studies were as follows one in Taiwan,[19] one in China,[20] and one in Thailand.[21] Furthermore, European studies were as follows one in Greece,[4] one in Denmark,[22] and one in Netherland.[23] The other four studies are related to the USA.[12,24,25,26] Of these ten articles, eight selected to assess birth weight.[12,19,21,22,24,25,26] In addition, five and six studies, respectively, were selected to evaluate the birth length[12,19,20,21,25] and head circumference.[12,19,21,22,25,26] The year of publication of studies was from 2004 to 2019. Sample sizes ranged from 59[21] to 858,[22] with a total of 2503 participants for birth weight, 1166 for birth length, and 2010 for head circumference. Pregnant women in the considered studies have a mean age of 25–35 years. All considered studies except two of them, that assessed the relationship between OP exposure and neonatal anthropometric measures, have used the maternal urine.
Effect of prenatal exposure to organophosphate pesticide metabolites on birth weight
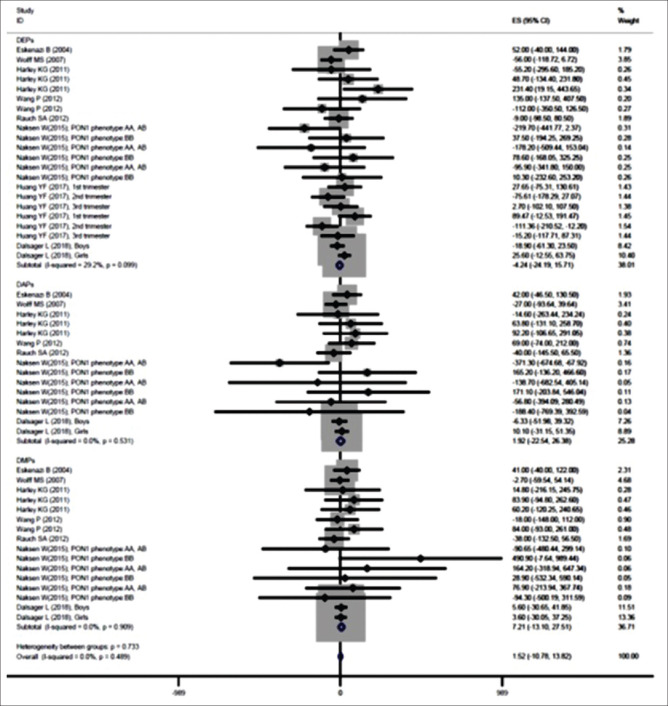
The findings of meta-analysis showed that the prenatal exposure to OP pesticide metabolites increased birth weight (β = 1.520; 95% confidence interval [CI] [−10.781, 13.820]) [Figure 2 and Table 2]. Results of subgroup analysis based on metabolites also represented that DEPs metabolites reduced birth weight (β = −6.722; 95% CI [−35.153, 21.708]), metabolites of DAPs (β = 1.922; 95% CI [−22.540, 26.384]) and DMPs (β = 7.205; 95% CI [−13.098, 27.508]) increased it [Figure 2 and Table 2]. However, none of these effects were significant. The heterogeneity was not significant for them (P > 0.05) [Figure 2 and Table 2]. The P values for Begg's test and Egger's test for birth weight were 0.872 and 0.750, respectively, that revealed no obvious publication bias among these studies. Based on the results of meta-regression analysis, the sample size (β (standard error): 0.04 (0.06); P = 0.509) and year of publication (β [standard error]: −0.24 [1.35]; P = 0.858) had no significant association with the effect of prenatal exposure to OP pesticide metabolites on birth weight (P > 0.05).
Figure 2.
Associations of prenatal exposure to organophosphate pesticide metabolites with birth weight
Table 2.
Results meta-analysis based on random effect model
| Pooled effect size | 95% CI | I2 (%) | Statistic | P | |
|---|---|---|---|---|---|
| Birth weight | |||||
| DEPs | −6.722 | −35.153-21.708 | 29.20 | 29.68 | 0.099 |
| DAPs | 1.922 | −22.54-26.384 | 0.00 | 12.94 | 0.531 |
| DMPs | 7.205 | −13.098-27.508 | 0.00 | 8.36 | 0.909 |
| Overall | 1.52 | −10.781-13.82 | 0.00 | 51.60 | 0.489 |
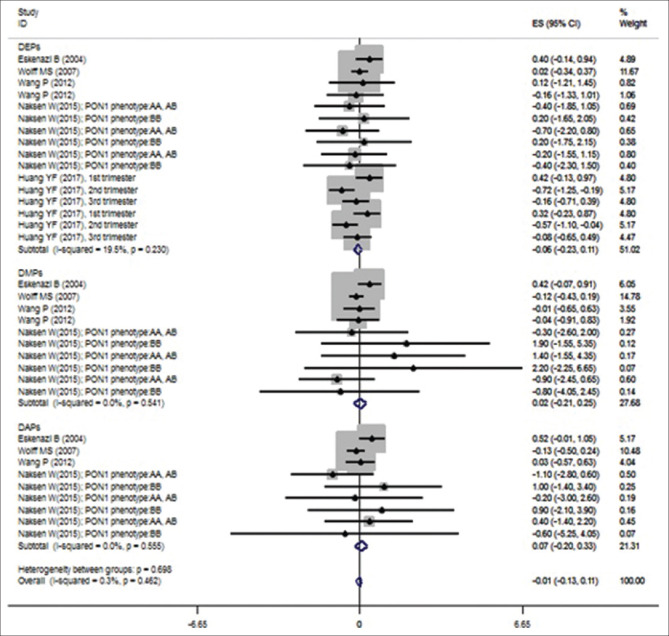
| Birth length | |||||
| DEPs | −0.067 | −0.269-0.136 | 19.50 | 18.64 | 0.23 |
| DAPs | 0.065 | −0.196-0.327 | 0.00 | 7.93 | 0.541 |
| DMPs | 0.02 | −0.209-0.249 | 0.00 | 6.83 | 0.555 |
| Overall | −0.01 | −0.132-0.11 | 0.30 | 34.12 | 0.462 |
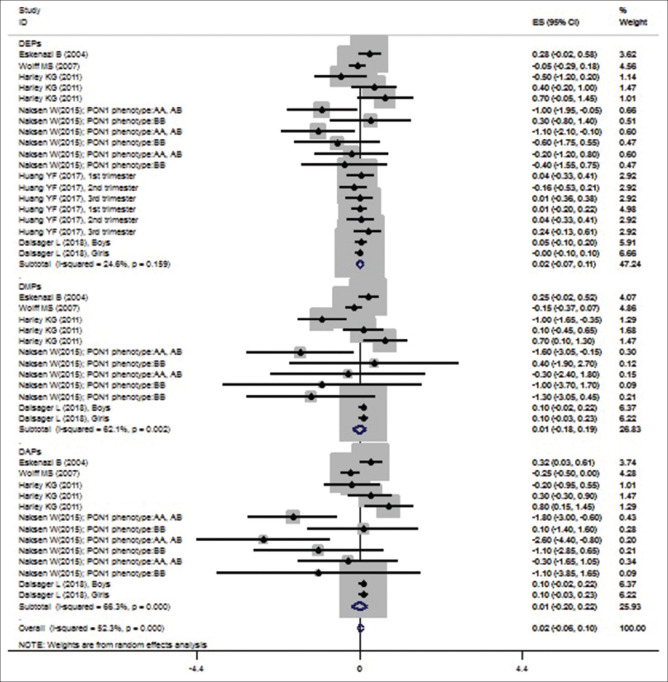
| Head circumference | |||||
| DEPs | 0.018 | −0.074-0.11 | 24.60 | 23.88 | 0.008 |
| DAPs | 0.007 | −0.203-0.217 | 66.30 | 35.64 | 0.0549 |
| DMPs | 0.006 | −0.179-0.192 | 62.10 | 29.06 | 0.0386 |
| Overall | 0.022 | −0.06-0.103 | 52.30 | 90.19 | 0.0232 |
DEPs=Diethyl phosphates; DAPs=Dialkyl phosphates; DMPs=Dimethyl phosphates; CI=Confidence interval
Effect of prenatal exposure to organophosphate pesticide metabolites on birth length
The results of meta-analysis with subgroup analysis presented that prenatal exposure to OP pesticide metabolites was not significantly associated with the birth length for DEPs metabolites (β = −0.067; 95% CI [−0.269, 0.136]), DMPs metabolites (β =0.020; 95% CI [−0.209, 0.249]), DAPs metabolites (β =0.065; 95% CI [−0.196, 0.327]) and the overall pooled effect size (β = −0.011; [−0.132, 0.109]) based on random-effect models [Table 2 and Figure 3]. Furthermore, there was no significant heterogeneity for them [Table 2 and Figure 3]. Begg's test and Egger's test revealed no obvious publication bias among these studies; The P values for these tests were > 0.05 (P = 0.551 and 0.776, respectively).
Figure 3.
Associations of prenatal exposure to organophosphate pesticide metabolites with birth length
Results of meta-regression analysis showed that sample size (β [standard error]: 0.001 [0.0005]; P = 0.020) was significantly associated with the effect of prenatal exposure to OP pesticide metabolites on birth length (P < 0.05). However, the year of publication (β [standard error]: −0.030 [0.015]; P = 0.052) was not significant.
Effect of prenatal exposure to organophosphate pesticide metabolites on birth head circumference
The results of meta-analysis based on the random-effects models showed that prenatal exposure to OP pesticide metabolites increased birth head (β = 0.022; 95% CI [−0.06, 0.103]). The heterogeneity was significant for it (P < 0.05) [Table 2 and Figure 4]. The subgroup analysis represented that metabolites of DEPs (β = 0.018; 95% CI [−0.074, 0.110]), DMPs (β = 0.006; 95% CI [−0.179, 0.192]) and DAPs (β = 0.007; 95% CI [−0.203, 0.217]) also increased birth head [Table 2 and Figure 4]. However, none of these effects were significant. The P values for Begg's and Egger's tests were 0.038 and 0.022, respectively. Therefore, there was publication bias among these studies (P < 0.05). Trim and fill analysis were conducted, but no study filled, which showed that the publication bias had a non-significant effect on the results. Based on the results of meta-regression analysis, the sample size (β [standard error]: 0.001 [0.0002]; P = 0.041) had significant association with the effect of prenatal exposure to OP pesticide metabolites on birth head (P < 0.05) but year of publication (β [standard error]: −0.003 [0.009]; P = 0.70) had no significant association.
Figure 4.
Associations of prenatal exposure to organophosphate pesticide metabolites with head circumference
Sensitivity analysis
Results of sensitivity analyses showed that with excluding the study of cross-sectional (Wang 2012) the pooled effect size for cohort studies was increased for each three outcomes. (β [95% CI] for birth weight: 5.36 [−7.75, 18.47], birth length: 0.025 [−0.14, 0.19] and birth head: 0.05 [−0.04, 0.14]). Furthermore, with excluding the study with sample of amniotic fluid (Koutroulakis et al., 2014) the pooled effect size for studies with samples of maternal urine was decreased for birth weight (β [95% CI]: 0.63 [−12.32, 13.58]) and increased for birth length (β [95% CI]: −0.01 [−0.16, 0.15]). As before, the estimates of effect size were not significant.
DISCUSSION
In this study, we examined the evidence available regarding the association of maternal OP exposure with infant anthropometric measures, including birth weight, birth length, and head circumference. Our finding revealed no significant relationship between OP exposure during pregnancy and birth weight and birth length. However, a weak but significant association observed regarding the head circumference.
Although the use of pesticides is widespread, little is known about potential adverse effects on pregnancy outcomes and anthropometric measures of neonates. Animal studies suggest that OPs may limit fetal growth, which may be due to an effect on adenylyl cyclase signaling cascade, by increasing thyroxine levels, or by its effects on placental nutrient transfer.[27,28] Various human studies have also conducted to investigate the association between maternal exposure to OP and birth outcome, although the observed results of studies are inconsistent. Rauch birth cohort study conducted on 344 pregnant women showed that exposure to DAPs metabolites had an impact on birth weight.[24] Furthermore, the results of the three cohort studies confirmed the relationship between pregnant women's exposure to OP metabolites and low birth weight.[12,25,26] Nevertheless, the cross-sectional study in china reported no association for DEPs, DAPs, and DMPs exposure by fetal growth (birth weight and birth length).[20] The results are consistent with the results of the Odense child cohort that found no association between maternal urinary concentration of OP pesticides and birth outcomes.[22] Likewise, the results of a cohort study in Taipei, Taiwan, showed that exposure to diethyl organophosphate (DEPs) metabolites in the second trimester of pregnancy not correlated with birth weight and birth length.[19] Given the disagreement between the results of various studies, the current systematic review and meta-analyses have conducted on the relationship between maternal exposure to OP pesticide and anthropometric measures.
Effects of organophosphate pesticide exposure and birth weight
The findings of this meta-analysis showed that there was no relationship between maternal exposure to OP pesticides and low birth weight. Despite differences between the types of OP metabolites, our study articles generally showed that OP pesticides did not affect birth weight. However, as mentioned above, some articles have reported different results for various OP metabolites. For instance, the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort that investigated six DAP metabolites, including DMP, DMDTP, DMTP, DEP, DEDTP, DETP, positive association was shown with birth weight. Each ten-fold increase in prenatal diethyl metabolites was associated with 258.8 g (95% CI: 23.9, 493.6) increase in birth weight.[26] Likewise, the findings of the study in Greece pregnant women showed similar consistent association. In this cohort study, the concentration of some OP metabolites, including DMP, DEP, DMTP, DETP, and DEDTP, were evaluated in amniotic fluid, results showed birth weight was positively associated with sumDMPs.[4] On the other hand, the pilot study of Naksen examined the association between urinary OP metabolites (DMP, DMDTP, DMTP, DEP, DEDTP, DETP) and birth weight, and showed significant negative relationship.[21] In the cohort study on 162 pregnant women in Taiwan, a similar association was found between levels of diethyl phosphate metabolites (DEP, DETP, DEDTP, DETP) and lower birth weight in the second trimester,[19] in contrast, some other studies did not confirm such association. The findings of a Danish child cohort study on DMP, DMDTP, DMTP, DEP, DEDTP, DETP revealed no association between OPs metabolites and birth weight.[22] Likewise, a cross-sectional study of Wang et al. conducted among 187 pregnant women with a mean (SD) age of 27.1 (4.12) years revealed that birth weight was not related to the levels of OP metabolites.[20] Moreover, the cohort study in the US pregnant women with an average of 25 years of age did not document clear patterns of association between maternal OP pesticide exposure and birth weight.[12]
Effects of organophosphate pesticide exposure and birth length
The findings of the current meta-analyses indicated that OP metabolites were not associated with birth length. However, controversial results exist in this regard. For instance, in the cohort study of Eskenzi et al. that investigated dimethyl and diethyl phosphate metabolites, higher birth length was found for two types of metabolites.[12] This result was consistent with the findings of the cohort study on 404 pregnant women conducted by Wolf et al.[25] However, in a study of 162 pregnant women, aged 18–45 years in Taiwan, a significant association was reported between diethyl phosphate (DEPs) and reduced birth length,[19] whereas some other studies did not confirm this association. A cross-sectional study of 187 pregnant women with the mean age of 27.1 years in China did not report an association between exposure to OPs and length of birth.[20] Likewise, the study on 59 pregnant women in Thailand did not reveal any association between six DAP metabolites and birth length. However, it found significant relationships of these metabolites with birth weight and head circumference.[21] Despite the controversy between the results, the relationship between OPs exposure in pregnant women and the length of birth was not significant.
Effects of organophosphate pesticide exposure and head circumference
Contrary to the results regarding the relationship between birth weight and birth length with pregnant mothers' exposure to OPs, findings of this meta-analyses showed that exposure to OPs was effective on birth head circumference. There is also conflicting evidence regarding the association between exposure of pregnant women to OP pesticides and the neonatal head circumference. Some studies have investigated the association of maternal exposure to OP metabolites with the head circumference. In a cohort study that was using data from 488 pregnant women who participated in the CHAMACOS project showed the significant association between maternal exposure to dimethyl and diethyl phosphate and increased birth head circumference.[12] This study reported that 0.32 cm (P = 0.03) increase in head circumference was associated with one long-unit increase of the DAP concentration.[12] Likewise, in a cohort study conducted among 470 pregnant women with the mean (SD) age of 25.5 (5.0) years revealed that increased head circumference was related to the DAP (dimethyl phosphate and diethyl phosphate) metabolites.[26] Harley et al. reported 0.7 cm (95% CI = 0.0, 1.5) and 0.8 cm (95% CI = 0.1, 1.4) increase in head circumference for every 1 unit increase of dimethyl phosphate and diethyl phosphate metabolites respectively.[26] In contrast, Wolf et al. reported a 0.25 cm decrease in head circumference associated with DAP metabolites,[25] although some other studies did not confirm this association. The cohort study of Huang et al. did not find any association between DEPs metabolites and head circumference.[19] Likewise, the Odense child cohort study with 858 individuals revealed no relationship between DMP, DMTP, DMDTP, DEP, DEDTP, and DETP and neonatal head circumference.[22]
Strength and limitations of the current review
Recently, the use of OP pesticides to control pests on a variety of food and feed crops is increasing. The potentially harmful effects of OPs on humans and the environment have led to much research in recent years on these compounds and their impact. However, no systematic review and meta-analysis have reported so far as to investigate the relationship between maternal OP pesticide exposure and anthropometric measures. One strength of this study is its novelty in the evaluation of this association. The large sample size is another strength of this review, which allows for a better examination of the topic. In addition, all studies that included in this review had a high quality based (≥18) on the STROBE checklist. Besides, no evidence of publication bias regarding the effect of maternal exposure to OP on the birth outcome (birth weight, birth length, head circumference) was shown in this study. There are limitations to the findings of this study. First, all of the studies except two of them used maternal urine as a biomarker of OPs exposure. Although a urine sample is a useful biomarker of OP pesticides exposure, due to the short half-lives of OPs in the body, urine sample shows short-term rather than continuous exposure.[27] The second limitation of this study was that confounding parameters were not adjusted equally in different studies.
CONCLUSION
Findings of this meta-analyses indicated that pregnant women's exposure to OP pesticides was weakly associated with the increased birth head circumference, while there is no association between pregnant women's exposure to OPs and birth weight and birth length. Given that the studies used in this meta-analysis are from all three continents of Asia, Europe, and America and also they have a large sample size, our findings can be generalized. However, since there was no significant relationship between pregnant mothers' exposure to OPs and birth weight and birth length, it is recommended that studies with a larger sample size be conducted in other countries. It is also advisable that sampling is performed at each trimester of pregnancy.
Financial support and sponsorship
This study was supported by the Isfahan University of Medical Sciences (50378).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to Research Institute for Primordial Prevention of Non-Communicable Diseases, Isfahan University of Medical Sciences for supporting this work.
REFERENCES
- 1.Yao Zw, Jiang Gb, Liu Jm, Cheng W. Application of solid-phase microextraction for the determination of organophosphorous pesticides in aqueous samples by gas chromatography with flame photometric detector. Talanta. 2001;55:807–14. doi: 10.1016/s0039-9140(01)00504-5. [DOI] [PubMed] [Google Scholar]
- 2.Zambonin CG, Quinto M, Vietro N, Palmisano F. Solid-phase microextraction gas chromatography mass spectrometry: A fast and simple screening method for the assessment of organophosphorus pesticides residues in wine and fruit juices. Food Chem. 2004;86:269–74. [Google Scholar]
- 3.Mørck TA, Andersen HR, Knudsen LE. Organophosphate Metabolites in Urine Samples from Danish Children and Women: Measured in the Danish DEMOCOPHES Population Denmark. Ministry of Environment and Food Denmark. 2016 [Google Scholar]
- 4.Koutroulakis D, Sifakis S, Tzatzarakis M, Alegakis A, Theodoropoulou E, Kavvalakis M, et al. Dialkyl phosphates in amniotic fluid as a biomarker of fetal exposure to organophosphates in Crete, Greece; association with fetal growth. Reprod Toxicol. 2014;46:98–105. doi: 10.1016/j.reprotox.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: A validation study. Environ Health Perspect. 2003;111:1779–82. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson RJ. Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: A critical review of the literature. J Toxicol Environ Health Part A Curr Issues. 1995;44:135–65. doi: 10.1080/15287399509531952. [DOI] [PubMed] [Google Scholar]
- 7.Whyatt RM, Barr DB. Measurement of organophosphate metabolites in postpartum meconium as a potential biomarker of prenatal exposure: A validation study. Environ Health Perspect. 2001;109:417–20. doi: 10.1289/ehp.01109417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsatsakis A, Tzatzarakis M, Koutroulakis D, Toutoudaki M, Sifakis S. Dialkyl phosphates in meconium as a biomarker of prenatal exposure to organophosphate pesticides: A study on pregnant women of rural areas in Crete, Greece. Xenobiotica. 2009;39:364–73. doi: 10.1080/00498250902745090. [DOI] [PubMed] [Google Scholar]
- 9.Chanda S, Pope C. Neurochemical and neurobehavioral effects of repeated gestational exposure to chlorpyrifos in maternal and developing rats. Pharmacol Biochem Behav. 1996;53:771–6. doi: 10.1016/0091-3057(95)02105-1. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava M, Raizada R. Development effect of technical dimethoate in rats: maternal and fetal toxicity evaluation. Indian J Exp Biol. 1996;34:329–33. [PubMed] [Google Scholar]
- 11.Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–56. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112:1116–24. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–32. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 16.Khoshhali M, Rafiei N, Farajzadegan Z, Shoshtari Yeganeh B, Kelishadi R. Maternal exposure to cadmium and fetal growth: A systematic review and meta analysis. Biol Trace Elem Res. 2020;195:9–19. doi: 10.1007/s12011-019-01819-y. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Chichester. England, Hoboken, NJ: Wiley-Blackwell; 2008. [Google Scholar]
- 18.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta analyses. Hoboken, NJ: John Wiley & Sons Inc; 2009. [Google Scholar]
- 19.Huang YF, Pan WC, Tsai YA, Chang CH, Chen PJ, Shao YS, et al. Concurrent exposures to nonylphenol, bisphenol A, phthalates, and organophosphate pesticides on birth outcomes: A cohort study in Taipei, Taiwan. Sci Total Environ. 2017;607:1126–35. doi: 10.1016/j.scitotenv.2017.07.092. [DOI] [PubMed] [Google Scholar]
- 20.Wang P, Tian Y, Wang XJ, Gao Y, Shi R, Wang GQ, et al. Organophosphate pesticide exposure and perinatal outcomes in Shanghai, China. Environ Int. 2012;42:100–4. doi: 10.1016/j.envint.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Naksen W, Prapamontol T, Mangklabruks A, Chantara S, Thavornyutikarn P, Srinual N, et al. Associations of maternal organophosphate pesticide exposure and PON1 activity with birth outcomes in SAWASDEE birth cohort, Thailand. Environ Res. 2015;142:288–96. doi: 10.1016/j.envres.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalsager L, Christensen LE, Kongsholm MG, Kyhl HB, Nielsen F, Schoeters G, et al. Associations of maternal exposure to organophosphate and pyrethroid insecticides and the herbicide 2, 4-D with birth outcomes and anogenital distance at 3 months in the Odense Child Cohort. Reprod Toxicol. 2018;76:53–62. doi: 10.1016/j.reprotox.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson KK, van den Dries MA, Gaillard R, Pronk A, Spaan S, Tiemeier H, et al. Organophosphate pesticide exposure in pregnancy in association with ultrasound and delivery measures of fetal growth. Environ Health Perspect. 2019;127:087005. doi: 10.1289/EHP4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano MA, et al. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environ Health Perspect. 2012;120:1055–60. doi: 10.1289/ehp.1104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff MS, Engel S, Berkowitz G, Teitelbaum S, Siskind J, Barr DB, et al. Prenatal pesticide and PCB exposures and birth outcomes. Pediatr Res. 2007;61:243. doi: 10.1203/pdr.0b013e31802d77f0. [DOI] [PubMed] [Google Scholar]
- 26.Harley KG, Huen K, Schall RA, Holland NT, Bradman A, Barr DB, et al. Association of organophosphate pesticide exposure and paraoxonase with birth outcome in Mexican-American women. PLoS One. 2011;6:23923. doi: 10.1371/journal.pone.0023923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harley KG, Engel SM, Vedar MG, Eskenazi B, Whyatt RM, Lanphear BP, et al. Prenatal exposure to organophosphorous pesticides and fetal growth: Pooled results from four longitudinal birth cohort studies. Environ Health Perspect. 2015;124:1084–92. doi: 10.1289/ehp.1409362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107:409–19. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]