Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) has become one of the major diseases plaguing worldwide. Several studies reported its association with ischemic heart disease (IHD). This study aims to determine the relationships between severity of steatosis with glycemic control and carotid intima-media thickness (CIMT) among a high-risk population of type 2 diabetes mellitus (T2DM) with proven IHD.
Materials and Methods:
This was a cross-sectional study involving patients aged between 18 and 65 years diagnosed with T2DM with IHD (n = 150). Ultrasonography of the abdomen to determine NAFLD severity category and CIMT measurements was performed by two independent radiologists. NAFLD was graded according to the severity of steatosis (NAFLD-3, NAFLD-2, NAFLD-1, and NAFLD-0). Comparison between different stages of NAFLD (NAFLD-3, NAFLD-2, NAFLD-1, and NAFLD-0) was analyzed using Chi-square and analysis of variance tests for categorical and continuous variables, respectively.
Results:
The prevalence of NAFLD was 71% (n = 107). NAFLD-1 was detected in 39% of the patients, 32% had NAFLD-2, no patients with NAFLD-3, and 29% had non-NAFLD. There were no patients with NAFLD-2 having higher systolic and diastolic blood pressure, weight, body mass index, waist circumference, total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol. Glycated hemoglobin (HbA1c) concentration was highest within the NAFLD-2. NAFLD-2 showed higher mean CIMT. Every 1% rise in HbA1c for patients with NAFLD significantly increases the CIMT by 0.03 mm (95% CI: 0.009, 0.052, P = 0.006).
Conclusion:
These findings suggest additional atherosclerotic risks within the NAFLD-2 group with significantly higher HbA1c and CIMT compared to the NAFLD-1 and NAFLD-0 groups. It is, therefore, vital to incorporate stricter glycemic control among patients with T2DM and IHD with moderate NAFLD as part of atherosclerotic risk management strategy.
Keywords: Carotid intima-media thickness, glycated hemoglobin A, myocardial ischemia, nonalcoholic fatty liver diseases
INTRODUCTION
Obesity has become a worldwide epidemic to the point, and it is now considered a public health crisis as it carries with its risks for coronary artery disease (CAD) such as hypertension, glucose intolerance, and dyslipidemia. In addition to these, nonalcoholic fatty liver disease (NAFLD) has become one of the major diseases plaguing the nation and world. NAFLD is a spectrum of liver conditions ranging from simple steatosis to the more severe form of inflammation called steatohepatitis.[1] The defining characteristic of the disease is the presence of greater than normal lipid deposition within the liver with the absence of excessive alcohol consumption. Steatosis, on the other hand, is the presence of lipid within the cytoplasm of hepatocytes, the criteria for which are defined in the literature as being either hepatic lipid levels above the 95th percentile for healthy individuals (~55 mg/g liver), >5% of the liver's weight, or found in >5% of hepatocytes histologically. Approximately 10%–29% of patients with nonalcoholic steatohepatitis (NASH) will develop cirrhosis within a 10-year period. The prevalence of NAFLD within the general population ranges between 10% and 24%,[2,3,4,5] and the relationships between NAFLD, ischemic heart disease (IHD) risk factors, and markers of subclinical atherosclerosis have been demonstrated.[6,7,8,9,10] Regarding the growing prevalence of NAFLD, several supplementations and dietary have been recently studied to treat this disease.[11,12,13]
Carotid intima-media thickness (CIMT) is a well-established tool to identify subclinical atherosclerosis and has been proven to be a reliable predictor of major cardiovascular events.[14] A recent comprehensive meta-analysis reported a strong association between NAFLD identification either by imaging or biopsy and markers of subclinical atherosclerosis.[15] The latest epidemiological data indicate that CIMT >1 mm at any age is associated with a significantly increased risk of myocardial infarction and cerebrovascular disease.[16] However, among type 2 diabetes mellitus (T2DM) patients, a lower cutoff level of 0.8 mm has been demonstrated to be associated with a higher prevalence of IHD,[17] and thus, in spite of studies showing the reliability of CIMT in determining subclinical atherosclerosis, the association between NAFLD and CIMT is not well established, especially in patients with high coronary risk. Furthermore, because it is closely linked to insulin resistance,[3,4,18] metabolic syndrome, obesity, dyslipidemia, and hypertension,[19,20,21] current efforts to elucidate the mechanism of this association and the extent of NAFLD on coronary risk are warranted. However, despite these studies, there are still several areas of uncertainties remaining about NAFLD. Unresolved issues relating to NAFLD include the absence of effective diagnostic tools and risk stratification strategies which could potentially improve diagnostic outcomes of NAFLD.
Therefore, this study aimed to address these knowledge gaps which are to determine the correlation between severity of NAFLD with glycemic control and CIMT. Positive findings could potentially improve risk stratification and initiation of earlier and more effective treatment to reduce the risk of progression of NAFLD and IHD.
MATERIALS AND METHODS
This was a cross-sectional single-center study involving 150 participants with T2DM with proven IHD who attended the Cardiology or Endocrine Clinics at the Universiti Teknologi MARA (UiTM) Medical Center from November 1, 2015, to April 30, 2016. The sample size was determined using a single proportion based on the prevalence of NAFLD among T2DM of 49.6%, a precision of 5%, confidence interval of 95%, power of 80% and with the consideration of normal attrition, the calculated minimum sample size was 170. The study was approved by our regional ethics committee (RMI Code number: [REF: 600-RMI (5/1/6)]), and written informed consent was obtained before the commencement of the study protocol.
T2DM patients between the ages of 18 and 65 years with a prior diagnosis of IHD were recruited. IHD was defined by the presence of previous hospital admission for acute coronary syndrome or coronary revascularization or a positive diagnostic test including coronary angiogram, exercise stress test, or positive dobutamine stress echocardiogram. All patients received stable doses of relevant medications and were able to comply with the study protocol. They were also adjusted for age, gender, and smoking. We excluded patients with significant alcohol consumption, liver cirrhosis, presence of hepatitis B surface antigen (HepBsAg), and presence of hepatitis C antibody and pregnant or lactating women.
Alcohol intake was estimated using the quantity-frequency method, based on patients' self-report of three main types of alcohol intake, namely beer, wine, and spirit, that was translated into a certain amount of unit of alcohol. A significant amount of alcohol is considered as >21 units/week which translates into >140 g/week for males and >14 units/week (>70 g/week) for females.[2]
Patients' weight and height were recorded. Waist circumference (WC) was measured at the midpoint between the lowest margin of the least palpable rib and the top of the lilac crest in standing position. Central obesity was defined as WC >90 cm for men and >80 cm for women. Systolic and diastolic blood pressures (SBP and DBP, respectively) were obtained while the patients were sitting using Omron ambulatory blood pressure (BP) monitoring device and taking an average of three BP readings.
Venous blood was drawn following 8–10 h overnight fast for analysis of glycated hemoglobin, lipid profile, and liver enzymes (aspartate aminotransferase, alanine aminotransferase [ALT,] and gamma-glutamyl transferase [GGT]) on an automated platform (Cobas 400 PLUS, Roche Diagnostics, USA). The status of HepBsAg and hepatitis C antibody was obtained from the patient records. All biochemical tests analyzed in this study have been accredited with MS ISO 15189:2012 (SAMM No: 688).
Abdominal ultrasound examinations were performed by two independent radiologists. Patients were scanned in the supine position. All measurements were made at the time of the scan on frozen images of longitudinal scans using the machine's electronic caliper. Evidence of NAFLD was confirmed with radiological technique of liver-kidney contrast and further divided into four grades where severe (NAFLD-3) is where echogenic liver obscures the diaphragmatic outline indicating fatty infiltration, moderate (NAFLD-2) when the echogenic liver obscures the echogenic walls of portal vein branches, mild (NAFLD-1) when echogenicity is just increased higher than the kidneys, and normal (non-NAFLD).[2]
Participants were scanned in the supine position by two independent radiologists using a high frequency 7.5 MHz linear array transducer using Philips iU22 imaging system. The distance between the two lines gives a reliable index of the thickness of the intima-medial complex. All measurements were made at the time of the scan on frozen images of longitudinal scans using the machine's electronic caliper. Carotid segments for far (posterior) walls of each common carotid artery at a distance of 1 cm from the bulb will be examined. The average of the right and left CIMT was calculated and recorded into millimeters (mm).
The study protocol followed the principles governed by the World Medical Association Declaration of Helsinki. The study was conducted after ethical approval from UiTM Research Ethics Committee (REF: 600-RMI [5/1/6]).
Statistical analysis
The Statistical Package for the Social Sciences (SPSS for Windows version 22.0, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Data on patients' sociodemographics and characteristics are presented as mean and standard deviations (SDs) for parametric data. For nonparametric data, the results are presented as a median and interquartile range (IQR). Categorical data were presented as numbers of patients and percentages. A comparison between different stages in NAFLD was analyzed using the Chi-square test for categorical variables and analysis of variance test for continuous variables.
RESULTS
Table 1 highlights the demographic data of the study population. The prevalence of NAFLD was 71% with greater obesity observed in this group compared to non-NAFLD by body mass index (BMI) (mean + SD: 31.4 kg/m2 ± 7.6 vs. 25.9 kg/m2 ± 3.8, P < 0.001, respectively) and WC (mean + SD: 104.1 ± 12.3 cm vs. 89.3 ± 14.4 cm, P < 0.001, respectively). They also had higher mean SBP and DBP (mean + SD: 136 ± 17 mmHg vs. 128 ± 16 mmHg, P < 0.001; 82 ± 5 mmHg vs. 77 ± 13 mmHg, P < 0.001, respectively).
Table 1.
Baseline characteristics between nonalcoholic fatty liver disease and nonalcoholic fatty liver disease groups
| Variables | NAFLD | NO NAFLD | P |
|---|---|---|---|
| Number (%) | n=107 (71%) | n=43 (28%) | |
| Age, median (IQR), years | 59 (IQR13) | 57 (IQR12) | 0.117 |
| Gender, n (%) | 0.690 | ||
| Male | 82 (77%) | 32 (74%) | |
| Female | 25 (23%) | 11 (26%) | |
| Smoking status, n (%) | 0.596 | ||
| Current | 27 (25%) | 10 (23%) | |
| Former | 33 (31%) | 9 (21%) | |
| Never | 47 (44%) | 24 (56%) | |
| Family history premature | 0.674 | ||
| CAD, n (%) | 25 | 9 | |
| Yes No |
(23%) 8 (77%) |
(21%) 34 (79%) |
|
| Diabetic complications, n (%) | |||
| Stroke | 2 (2%) | 0 | 0.209 |
| Chronic Kidney Disease | 20 (19%) | 8 (19%) | 0.889 |
| Diabetic retinopathy | 9 (8%) | 4 (9%) | 0.980 |
| Peripheral neuropathy | 16 (15%) | 15 (35%) | 0.005 |
| SBP, mean (SD) (mmHg) | 136±17 | 128±16 | <0.001 |
| DBP, mean (SD) (mmHg) | 82±11 | 77±13 | 0.001 |
| Weight, mean (SD) (kg) | 87±18 | 77 kg±13.3 | 0.001 |
| Waist circumference, mean (SD) (cm) | 104.1 (12.3) | 89.3 (14.4) | <0.001 |
| Body Mass Index, mean (SD) kg/m2 | 31.4±7.6 | 25.9±3.8 | <0.001 |
| Obese | 78 (73%) | 11 (26 %) | |
| Overweight | 26 (24%) | 25 (58 %) | |
| Normal | 7 (16%) | 7 (16%) | |
| HbA1c median (IQR) (%) | 9.2[4.8] | 8.0 [2.2] | <0.001 |
| ALT median (IQR) U/L | 22.0 [21.3] | 23.6 [17.1] | 0.33 |
| GGT median (IQR) U/L | 41.7 [37.0] | 36.0[25.8] | 0.42 |
| ALP median (IQR) U/L | 82.0 [31.0] | 81.5 [32.3] | 0.60 |
| Total Cholesterol mean(SD) mmol/L | 4.8 (1.4) | 4.6 (1.1) | <0.001 |
| TG median (IQR) mmol/L | 2.0 [1.7] | 1.5 [1.1] | 0.001 |
| HDL-C mean(SD) mmol/L | 1.0 (0.3) | 1.1 (0.3) | 0.01 |
| LDL-C mean(SD) mmol/L | 2.9 (1.3) | 2.4 (0.9) | 0.034 |
| Carotid- IMT mean(SD) mm | 0.71±0.17 | 0.69±0.31 | 0.013 |
p value < 0.05 is considered statistically significant
HbA1c was higher in NAFLD patients compared to non-NAFLD (median [IQR]: 9.2 [4.8]% vs. 8.0 [2.2]%, P < 0.001), whereas there were no differences in serum GGT, ALT, and ALP concentrations between both the groups. The NAFLD group had higher total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) concentrations compared to non-NAFLD patients (mean + SD: 4.8 ± 1.4 mmol/L vs. 4.6 ± 1.1 mmol/L, P < 0.001; median [IQR]: 2.0 [1.7] mmol/L vs. 1.5 [1.1] mmol/L, P = 0.001; mean + SD: 1.0 ± 0.3 mmol/L vs. 1.1 ± 0.3 mmol/L, P = 0.01; and mean + SD: 2.9 ± 1.3 mmol/L vs. 2.4 ± 0.9 mmol/L, P = 0.034, respectively). The mean CIMT was higher in the NAFLD group compared to the non-NAFLD group (mean + SD: 0.74 ± 0.17 mm vs. 0.69 ± 0.31 mm, P = 0.013).
Logistic regression analysis was performed to ascertain the effects of BP, BMI, glycemia, dyslipidemia, and CIMT on the likelihood of T2DM and proven IHD patients having NAFLD. The results have been summarized in Table 2. The logistic regression model was statistically significant χ2 (8) =47.2, P < 0.001. The model explained 39% (Nagelkerke R2) of the variance in NAFLD and correctly classified 80% of the cases. Of the eight positive predictors, only BMI, HbA1c, and HDL-C were statistically significant. Obese T2DM and established IHD patients were 4.6 times likely to have NAFLD compared to patients who are not obese (P = 0.006). Patients with IHD and an HbA1c reading of >8% were 2.8 times more likely to have NAFLD compared to those with HbA1c <8% (P = 0.032). T2DM and established IHD patients with HDL-C < 1.1 mmol/L were 3.1 times prone to have NAFLD as compared to HDL-C > 1.1 mmol/L (P = 0.012).
Table 2.
Logistic regression analysis of factors associated with nonalcoholic fatty liver disease in type 2 diabetes mellitus and established ischemic heart disease patients
| Variables | OR (CI) | P | ||||
|---|---|---|---|---|---|---|
| SBP | 2.5 (0.9–6.8) | 0.07 | ||||
| DBP | 1.5 (0.5–3.9) | 0.43 | ||||
| BMI | 4.6 (1.6–13.6) | 0.006 | ||||
| HbA1c | 2.8 (1.1–6.9) | 0.032 | ||||
| TG | 0.2 (0.2–1.3) | 0.51 | ||||
| LDL | 0.6 (0.2–1.8) | 0.55 | ||||
| HDL-C | 3.1 (1.2–7.5) | 0.012 | ||||
| CIMT | 2.5 (0.8–7.9) | 0.126 | ||||
| Clinical parameters according to severity of ultrasonography-diagnosed NAFLD | ||||||
| Variables (n) | NAFLD-2 (n=48; 32%) | NAFLD-1 (n=59; 39%) | Non-NAFLD (n=43; 29%) | P | ||
| SBP (mmHg), mean±SD | 140±19 | 133±15.0 | 128±15.7 | <0.001 | ||
| DBP (mmHg), mean±SD | 81±13.0 | 83±13 | 77±13 | 0.001 | ||
| BMI (kg/m2), mean±SD | 33.8±6.7 | 30.0±7.8 | 26.0±3.8 | <0.001 | ||
| WC (cm), mean±SD | 106±13 | 102±10 | 89±14 | <0.001 | ||
| HbA1c (%), median (IQR) | 9.3 (2.4) | 8.2 (5.8) | 8.0 (2.2) | <0.001 | ||
| ALT (U/L), median (IQR) | 33 (25) | 20 (20.8) | 22 (17) | 0.601 | ||
| GGT (U/L), median (IQR) | 59 (39) | 38 (31) | 36 (26) | 0.079 | ||
| ALP (U/L), median (IQR) | 80 (40) | 83 (27) | 82 (32) | 0.707 | ||
| TG (mmol/L), median, (IQR) | 1.8 (0.9) | 1.6 (0.8) | 1.5 (1.1) | 0.002 | ||
| LDL (mmol/L), mean±SD | 3.2±1.5 | 2.6±1.1 | 2.4±0.9 | 0.04 | ||
| CIMT (mm), mean±SD | 0.77±0.19 | 0.69±0.14 | 0.68±0.32 | 0.018 | ||
Data are presented as mean±SD for parametric data and median (IQR) for nonparametric data. Categorical data are presented as numbers of patients and percentages. Comparison for different stages in NAFLD was analyzed using Chi-square test for categorical variables and ANOVA test for continuous variables. P<0.05 is considered statistically significant. SD=Standard deviation; ANOVA=Analysis of variance; IQR=Interquartile range; NAFLD=Nonalcoholic fatty liver disease; OR=Odds ratio; CI=Confidence interval; SBP=Systolic blood pressures; DBP=Diastolic blood pressures; BMI=Body mass index; HbA1c=Glycated hemoglobin; TG=Triglyceride; LDL=Low-density lipoprotein; HDL-C=High-density lipoprotein cholesterol; CIMT=Carotid intima-media thickness; WC=Waist circumference; ALT=Alanine aminotransferase; GGT=Gamma-glutamyl transferase; ALP=Alkaline phosphatase
We further analyzed the cohort based on the severity of steatosis [Table 2]. NAFLD-1 was detected in 39% of the patients, 32% had NAFLD-2, whereas 29% had non-NAFLD. There were no patients with NAFLD-3. SBP, DBP, BMI, and WC were highest in NAFLD-2, followed by NAFLD-1 and NAFLD-0 (SBP: 140 mmHg ± 19 vs. 133 mmHg ± 15 vs. 128 mmHg ± 16, P < 0.001; DBP: 81 mmHg ± 13 vs. 83 mmHg ± 13 vs. 77 mmHg ± 13, P = 0.001; BMI: 33.8 kg/m2 ± 6.7 vs. 30.0 kg/m2 ± 7.8 vs. 26.0 8 kg/m2 ± 3.8, P < 0.001; and WC: 106 cm ± 13 vs. 102 cm ± 10 vs. 89 cm ± 14, P < 0.001).
The HbA1c was highest among NAFLD-2, followed by NAFLD-1 and non-NAFLD (median [IQR]: 9.3 (2.4)% vs. 8.2 (5.8)% vs. 8.0 (2.2)%, P < 0.001, respectively). There were no differences in ALT and ALP between the NAFLD-2, NAFLD-1, and non-NAFLD groups (median [IQR]: 33 (25) U/L vs. 19 (20) U/L vs. 22 (17) U/L, P = 0.601, and 80 (25) U/L vs. 83 (27) U/L vs. 82 (32) U/L, P = 0.707, respectively).
Serum TC, TG, HDL-C, and LDL-C concentrations were higher in the NAFLD-2 group compared to the NAFLD-1 and non-NAFLD groups (mean + SD: 4.9 ± 1.4 mmol/L vs. 4.7 ± 1.4 mmol/L vs. 4.6 ± 1.1 mmol/L, P < 0.001; median [IQR]: 1.8 (0.9) mmol/L vs. 1.6 (0.8) mmol/L vs. 1.5 (1.1) mmol/L P = 0.002; mean + SD: 0.9 ± 0.3 mmol/L vs. 1.0 ± 0.3 mmol/L vs. 1.1 ± 0.3 mmol/L, P = 0.02; and 3.2 ± 1.5 mmol/L vs. 2.6 ± 1.1 mmol/L vs. 2.4 ± 0.9 mmol/L, P = 0.04, respectively). The patients within the NAFLD-2 group showed significantly higher mean CIMT compared to patients within the other two groups (0.81 mm ± 0.29 vs. 0.74 mm ± 0.25 vs. 0.66 mm ± 0.23, P = 0.018).
Positive correlation was observed between CIMT and HbA1c (r = 0.335, P = 0.02) in the NAFLD-2 group, which was not seen within the NAFLD-1 group (r = 0.224, P = 0.09) [Figure 1].
Figure 1.
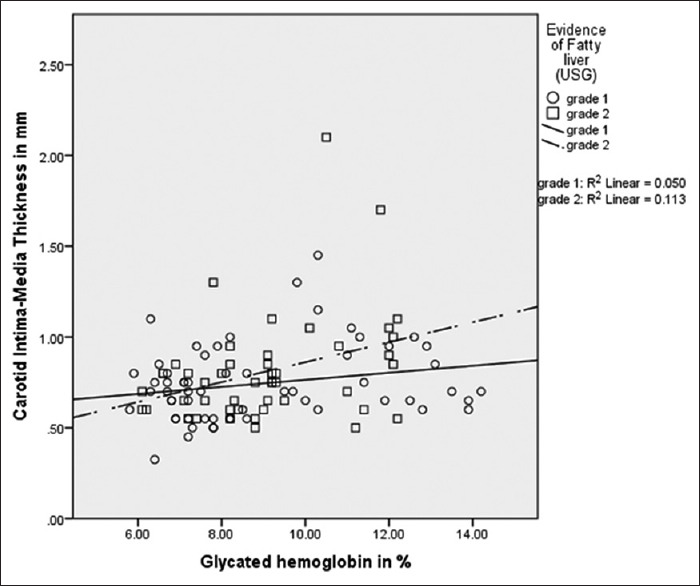
Scatter plot showing positive correlation between carotid intima-media thickness and glycated hemoglobin in nonalcoholic fatty liver disease-2 (n = 48, r = 0.335, P = 0.02) which was not demonstrated in nonalcoholic fatty liver disease-1 (n = 59, r = 0.224, P = 0.08)
A univariate analysis was carried out to determine the independent predictors among NAFLD patients whose CIMT is >0.8 mm. The presence of NAFLD (odds ratio [OR], 5.1; 95% confidence interval [CI], 1.8–14.0; P < 0.001), SBP >135 mmHg (OR, 2.4; 95% CI, 1.2–4.9; P = 0.01), obesity (OR, 6.5; 95% CI, 1.4–29.1; P = 0.005), HbA1c > 8% (OR, 3.2; 95% CI, 1.5–6.7; P = 0.002), and HDL-C <1.0 mmol/L (OR, 2.4; 95% CI, 1.1–5.5; P = 0.03) were found to be significant predictors.
Simple linear regression analysis showed that for every 1% rise in HbA1c among patients with NAFLD, there was an increase in CIMT by 0.03 mm (95% CI: 0.009, 0.052, P = 0.006). This was not demonstrated within the non-NAFLD group. The association between CIMT with SBP and DBP was not statistically significant in regression model.
DISCUSSION
The study demonstrated a 71% prevalence of NAFLD in T2DM with proven IHD which is higher compared to a previous study by Chan et al. who reported a prevalence of 49.6% among diabetic patients in Malaysia.[20] The most probable reason for this difference is the specific inclusion of patients with high cardiovascular risk in our cohort compared to Chan et al., which included mainly T2DM only. The prevalence of ultrasound-diagnosed NAFLD in patients with T2DM has, however, been similarly high as reported to be between 69% and 75%, in the Western population.[4,5,8,19,22] Although liver biopsy is the gold standard for the diagnosis of NAFLD and MRI is a superior diagnostic tool, the ultrasonographic diagnosis of NAFLD has been shown to have equal specificity and sensitivity compared to MRI with no additional values to detect inflammation.[23] Our data demonstrated similar predictors for NAFLD in T2DM with proven IHD which include obesity, poor glycemic control, and low HDL-C. We also concurred with previous studies which suggested that liver enzymes are poor indicators of NAFLD.[6]
Logistic regression analysis showed that patients who are obese and had higher SBP and DBP are more likely to have NAFLD. These findings suggest that, collectively, they could be utilized as a screening tool to identify those at risk of NAFLD as well as using them as targets in the prevention of NAFLD and subsequently CAD.
There are currently limited data on the differences in metabolic components between various stages of steatosis which this study addressed. In comparison between the three groups of NAFLD-0, NAFLD-1, and NAFLD-2, we demonstrated incremental relationships in weight, BMI, WC, SBP, HbA1c, TC, TG, and LDL-C between them. However, the most relevant finding was that patients with a higher degree of steatosis had higher mean CIMT compared to those with NAFLD-1 and NAFLD-0. This concurs with a previous study by Fracanzani et al. who found steatosis as an independent risk predictor for increased CIMT.[24] Histologically confirmed NASH in patients with T2DM in a study by Ekstedt et al. demonstrated that NASH is associated with a more severe inflammatory and insulin-resistant state which resulted in accelerated atherosclerosis.[25] These findings imply that the determination of severity of steatosis in NAFLD is warranted. Furthermore, metabolic targets such as BP, HbA1c, TC, LDL-C, and TG could possibly be used to determine the severity levels of steatosis in NAFLD. In addition, it further infers that the management for NAFLD should include metabolic profile targets (TC, TG, LDL-C, and BP) established according to the severity of steatosis in order to improve coronary outcomes.
We also note the positive correlations observed between CIMT and SBP, DBP, and HbA1c within the NAFLD cohort, which was not seen among the non-NAFLD. This is consistent with earlier studies which reported relationships between NAFLD and IMT and/or plaques of the carotid artery.[26] Fracanzani et al. found that in patients with hepatic steatosis, independent risk predictors for increased CIMT were presence of steatosis (OR = 6.9; 95% CI: 4.3–12; P < 0.001), age (OR = 6.0, 95% CI: 3.2–8.4, P = 0.001), and increased SBP (OR = 2.3, 95% CI: 1.3–3.4, P ≤ 0.001).[24] Lankarani et al. subsequently reported NAFLD as a possible independent risk factor for CIMT in a population-based, case–control study (OR: 1.90; 95% CI: 1.17–3.09; P = 0.009).[27] In a systematic review of NAFLD with carotid atherosclerosis by Sookoian and Pirola, there was a higher CIMT in the NAFLD group compared to healthy controls.[28] Hanafi et al. reported in their case–control study that with adjustment of age and gender, patients with T2DM and NAFLD had a higher CIMT compared to patients with T2DM and normal controls (0.6219 ± 0.13 mm vs. 0.6076 ± 0.12 mm vs. 0.5647 ± 0.12 mm, P = 0.02).[29] Therefore, this current study further reaffirms that the cardiovascular risks of T2DM patients with underlying IHD are further increased by the detection of NAFLD.
Data on the relationship between CIMT and glycemic control in NAFLD patients are still limited which this study addressed. We noted a direct relationship between glycemia and CIMT. Contrary to that, there was no relationship between CIMT and glycemic control in the non-NAFLD group. Furthermore, linear regression analyses suggested that with every 1% rise of HbA1c in the NAFLD group, CIMT increases by 0.03 mm. This relevant observation further underscores the need to diagnose NAFLD and optimize metabolic parameters in this population of T2DM with IHD.
To date, less emphasis has been placed on the impact of degree of steatosis in NAFLD patients on atherosclerotic disease. The result of this study, which demonstrated higher CIMT levels with increasing SBP and HbA1c, seen only within the NAFLD-2 group but not the NAFLD-1 and non-NAFLD groups, poses serious questions on whether patients with moderate steatosis should have more stringent targets, of BP and HbA1c, at the very least.
We acknowledge certain limitations of this study which include time constraint causing inability to achieve the calculated sample size and lack of patients within the Grade 3 steatosis group which deterred further analysis on the impact of severe steatosis on CIMT. We were also unable to conduct serological testing to exclude viral or autoimmune hepatitis due to financial limitation but instead obtained this information through medical records and patients' disclosure. Furthermore, as this was a cross-sectional study, we were unable to also follow-through with the patients to determine the association between improvement of NAFLD category and CIMT where such association could further strengthen the need for risk stratification and target achievements in NAFLD patients. Future studies involving larger sample size, prospective, case-controlled study will be able to confirm our findings on the significance of mild-to-moderate steatosis on atherosclerotic disease (2746).
NO contributed in the conception of the work, conducting the clinical study, preparing and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work; MK contributed in the conducting the clinical study, data acquisition and analysis, statistical analysis, preparing and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work; MHK contributed in the conducting the clinical study, data acquisition, approval of the final version of the manuscript, and agreed for all aspects of the work; SFWH contributed in the conducting the clinical study, data acquisition, approval of the final version of the manuscript, and agreed for all aspects of the work; FZMS contributed in the conception of the work, conducting the clinical study, data acquisition, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work; BJ contributed in the conception of the work, data and statistical analysis, preparing and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work; IZ contributed in data and statistical analysis, approval of the final version of the manuscript, and agreed for all aspects of the work; SSK contributed in the conception of the work, data and statistical analysis, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work; TAR contributed in preparing and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work; and RAG contributed in the conception and design of the work, preparing and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Financial support and sponsorship
The authors acknowledge the assistance of all the technical staff of the Faculty of Medicine, UiTM, for their laboratory services and co-operation. This project was funded by an internal research grant from the Endocrine Unit, Faculty of Medicine, UiTM.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–5. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal AK, Jain V, Singla S, Baruah BP, Arya V, Yadav R, et al. Prevalence of non-alcoholic fatty liver disease and its correlation with coronary risk factors in patients with type 2 diabetes. J Assoc Physicians India. 2011;59:351–4. [PubMed] [Google Scholar]
- 5.Öztürk H, Gümrükçüoǧlu HA, Yaman M, Akyol A, Öztürk Ş, Akdaǧ S, et al. Hepatosteatosis and carotid intima-media thickness in patients with myocardial infarction. J Med Ultrason (2001) 2016;43:77–82. doi: 10.1007/s10396-015-0649-x. [DOI] [PubMed] [Google Scholar]
- 6.Leite NC, Villela-Nogueira CA, Pannain VL, Bottino AC, Rezende GF, Cardoso CR, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: Prevalences and correlated factors. Liver Int. 2011;31:700–6. doi: 10.1111/j.1478-3231.2011.02482.x. [DOI] [PubMed] [Google Scholar]
- 7.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–9. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 8.Mohan V, Farooq S, Deepa M, Ravikumar R, Pitchumoni CS. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84:84–91. doi: 10.1016/j.diabres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 10.Byrne CD, Targher G. NAFLD: A multisystem disease. J Hepatol. 2015;62:S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Haghighatdoost F, Salehi-Abargouei A, Surkan PJ, Azadbakht L. The effects of low carbohydrate diets on liver function tests in nonalcoholic fatty liver disease: A systematic review and meta-analysis of clinical trials. J Res Med Sci. 2016;21:53. doi: 10.4103/1735-1995.187269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asgharian A, Askari G, Esmailzade A, Feizi A, Mohammadi V. The effect of symbiotic supplementation on liver enzymes, C-reactive protein and ultrasound findings in patients with non-alcoholic fatty liver disease: A Clinical Trial. Int J Prev Med. 2016;7:59. doi: 10.4103/2008-7802.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karandish M, Tamimi M, Shayesteh AA, Haghighizadeh MH, Jalali MT. The effect of magnesium supplementation and weight loss on liver enzymes in patients with nonalcoholic fatty liver disease. J Res Med Sci. 2013;18:573–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Musso G, Gambino R, Bo S, Uberti B, Biroli G, Pagano G, et al. Should nonalcoholic fatty liver disease be included in the definition of metabolic syndrome. A cross-sectional comparison with Adult Treatment Panel III criteria in nonobese nondiabetic subjects? Diabetes Care. 2008;31:562–8. doi: 10.2337/dc07-1526. [DOI] [PubMed] [Google Scholar]
- 15.van den Oord SC, Sijbrands EJ, ten Kate GL, van Klaveren D, van Domburg RT, van der Steen AF, et al. Carotid intima-media thickness for cardiovascular risk assessment: Systematic review and meta-analysis. Atherosclerosis. 2013;228:1–11. doi: 10.1016/j.atherosclerosis.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Kasliwal RR, Bansal M, Desai D, Sharma M. Carotid intima-media thickness: Current evidence, practices, and Indian experience. Indian J Endocrinol Metab. 2014;18:13–22. doi: 10.4103/2230-8210.126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal AK, Gupta PK, Singla S, Garg U, Prasad A, Yadav R. Carotid intimomedial thickness in type 2 diabetic patients and its correlation with coronary risk factors. J Assoc Physicians India. 2008;56:581–6. [PubMed] [Google Scholar]
- 18.Ahmed MH, Barakat S, Almobarak AO. Nonalcoholic fatty liver disease and cardiovascular disease: Has the time come for cardiologists to be hepatologists? J Obes. 2012;2012:483135. doi: 10.1155/2012/483135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Non-alcoholic fatty liver disease in diabetics-prevalence and predictive factors in a multiracial hospital clinic population in Malaysia. J Gastroenterol Hepatol. 2013;28:1375–83. doi: 10.1111/jgh.12204. [DOI] [PubMed] [Google Scholar]
- 21.Goh SC, Ho EL, Goh KL. Prevalence and risk factors of non-alcoholic fatty liver disease in a multiracial suburban Asian population in Malaysia. Hepatol Int. 2013;7:548–54. doi: 10.1007/s12072-012-9359-2. [DOI] [PubMed] [Google Scholar]
- 22.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: The plot thickens. Diabet Med. 2007;24:1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7392–402. doi: 10.3748/wjg.v20.i23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, et al. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121:72–8. doi: 10.1016/j.amjmed.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 26.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–6. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 27.Lankarani KB, Mahmoodi M, Lotfi M, Zamiri N, Heydari ST, Ghaffarpasand F, et al. Common carotid intima-media thickness in patients with non-alcoholic fatty liver disease: A population-based case-control study. Korean J Gastroenterol. 2013;62:344–51. doi: 10.4166/kjg.2013.62.6.344. [DOI] [PubMed] [Google Scholar]
- 28.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: A systematic review. J Hepatol. 2008;49:600–7. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Hanafi M, Cina M, Zakerkish M, Rahim F, Saki-Malehi A, Nissi Q. Correlation between non-alcoholic fatty liver disease and carotid intima media thickness in patient with type 2 diabetes. Int J Osteoporos Metab Disord. 2015;2:35–41. [Google Scholar]


