Abstract
Background:
It has been proven that probiotic Lactobacillus bacteria have inhibitory effects on human cancer cell lines. The aim of this study is to isolate and characterize the antioxidant probiotic Lactobacillus and determine the possible anticancer activities of the selected strain.
Methods:
One of the Lactobacillus strain isolated from camel doogh sample showed the high antioxidant activity by using of different methods such as resistance to hydrogen peroxide, hydroxyl radical and superoxide anions. The antioxidant strain was characterized by sequencing of 16S rRNA V2-V3 regions and the 16S-23S intergenic spacer (ITS). The methanol extract of this strain supernatant was fractionated using thin layer chromatography (TLC) and antioxidant activity of fractions was detected by 0.1% of DPPH through TLC-DPPH bioautography. In vitro anticancer activity of each fraction was investigated by using MTT and flow cytometry methods.
Results:
According to the phylogenetic results, the antioxidant Lactobacillus strain was closely related to Lactobacillus hilgardii strain E91 (Accession No. EF536365). After fractionation and anti-proliferation assessments of Lactobacillus hilgardii strain AG12a extracellular materials, one of the antioxidant fraction (F4) showed maximum DPPH radical scavenging activity (IC50 of 535.27 μg/mL). MTT assay of the F4 fraction demonstrated cytotoxic activity against Caco-2 with the IC50 value of 299.05 μg/mL. The cell death activity of the fraction was confirmed by flow cytometry with 30.925.
Conclusions:
In this study, the anticancer and apoptotic properties of Lactobacillus hilgardii against Caco-2 cell line was reported for the first time. The isolated bioactive fraction from the extracellular methanol extract needs to be further investigated in human studies of cancer therapy.
Keywords: Apoptosis, cancer, Lactobacillus, thin layer chromatograghy
Introduction
There is increasing evidence to suggest that some lactobacilli are able to decrease the risk of accumulation of reactive oxygen species (ROS) during ingestion of food due to antioxidant activities[1] and therefore, shrink the incidence of various degenerative diseases which are caused by free radicals.[2] Some studies have focused on health benefits of probiotics such as cancer prevention and treatment.[3,4] It has been shown that lactic acid bacteria (LAB) have inhibitory effects on human cancer cell lines[5,6] and play an important role in the delay of carcinogenesis by their effect on metabolic, immunologic, and protective functions.[7,8]
Colorectal cancer incidence is significantly rising in developing countries.[9] The preventive action of probiotics against colorectal cancer is thought to be done through several mechanisms including inactivation of cancerogenic compounds, alteration of the intestinal microflora, competition with putrefactive and pathogenic microbiota, antiproliferative effects via regulation of apoptosis and cell differentiation, fermentation of undigested food, improvement of the host's immune response, and inhibition of tyrosine kinase signaling pathways.[10] Altonsy et al. (2010) described induction of apoptosis in human colonic carcinoma cell line (Caco-2) incubated with probiotics such as Lactobacillus rhamnosus or Bifidobacterium lactis.[11]
In the current study, we focus on the isolation of the probiotic Lactobacillus with antioxidant and anticancer activities from camel doogh. Camel doogh is the homemade fermented camel milk made by Turkmens in Iran.[12] Our previous study showed that most of the Lactobacillus species isolated from camel dough have the potential of antimicrobial and antioxidant activities.[13] The aim of the present study is to investigate the anticancer activity of these Lactobacillus strains and separate a bioactive fraction from their extracellular methanol extracts by thin layer chromatography (TLC) and evaluate the apoptotic effect of the fraction against cancer cell lines.
Methods
Strains and culture media
Different 20 traditional doogh samples were collected from various parts of Iran (Isfahan, Khorasan, Ghorghan, and Tehran provinces) in fall, winter, and spring seasons. The samples immediately transferred to the laboratory on ice-packs and diluted in Ringer solution at 1:9 dilution and cultured in plates containing de Man, Rogosa, and Sharpe (MRS) medium (Merck, Germany). The samples were incubated anaerobically at 37°C for 72 h. Finally, the purified colonies were frozen in a MRS broth containing 30% glycerol.
It has been found that exhaustion of an essential nutrient (like minimum growth medium) limits the growth of a culture which can increase the antioxidant production. Therefore, for possible increase production of antioxidant and anticancer properties, the isolate was cultured anaerobically in a minimum growth medium like plantarum minimal medium (PMM5) at 37°C for 48 h.[14]
Antioxidant screening of the isolates
The antioxidant activity of the isolates was investigated by using of different methods according to Kim et al. 2006.[1]
Bacterial identification
Biochemical and physiological identification were assessed according to Vos et al. (2009).[15] The acid and bile tolerance of the bioactive isolate was determined according to Ryan et al. (2015).[16]
The DNA of antioxidant Lactobacillus strain was extracted by using of Gene Transfer Pioneers (GTP, Iran) kit according to the manufacturer's instruction. The extracted genome was quantified using a Picodrop Spectrophometer (Picodrop, UK).
Amplification of the V2-V3 regions of 16S rRNA and the 16S-23S intergenic spacer (ITS) region was performed on total DNA from the antioxidant Lactobacillus. Primers were designed as described by Jose et al. (2015)[17]: 16-1A (5 ,-GAATCGCTAGTAATCG-3 ,) and 23-1B (5 ,-GGGTTCCCCCATTCGGA-3 ,) (Pioneer, Korea). PCR was carried out in a 50 μl reaction volume containing 5 U of Taq DNA polymerase, 5 μl of 10 × PCR reaction buffer, 0.2 mM each primer, 0.5 mM MgCl2, 0.2 mM dNTPs, and 200 ng of purified genomic DNA. The amplified products were separated on 2.0% agarose gels containing 1 × TAE electrophoresis buffer. All the PCR reagents were purchased from CinnaGen, Iran. PCR product were gel purified individually using the GeneJET PCR purification kit (Thermo Scientific, USA) and confirmed amplicon was sequenced using Bioneer sequencing methods, Bioneer Inc. (Daejeon, South Korea). The phylogenetic tree of Lactobacillus strain was constructed using the MEGA 7.0.14 software program by Neighbor-joining (NJ).[18]
Preparation of extracellular extract of lactobacillus
Supernatants of fermented PMM5 broth by the antioxidant isolates was acquired by centrifuging at 4,000 ×g for 15 min at 20°C and then were passed through sterile 0.22 μm pore-size filters (Sartorius, Germany) and freeze dried (Pishtaz Engineering, Iran). Methanol extracts of the extracellular materials were prepared by mixing1 mg freeze-dried of samples in 1 mL methanol and kept at the tight container at 4°C, from which different concentration of samples (20, 40, 80, and 120 μg/mL) were prepared.[19]
DPPH assay
In DPPH (2, 2-diphenyl-1-picryl-hydrazyl-hydrate) assay, the odd electron of nitrogen atom in DPPH is reduced by getting a hydrogen atom from antioxidants that developed the reduced form of DPPH with the loss of violet color which was determined by absorbance at 517 nm.[20]
For performing the DPPH assay, DPPH solution was freshly prepared by dissolving 40 μg DPPH powder (Sigma, USA) in 1 mL methanol and kept in dark place. 5 dilution series of samples (250 μL) added in the wells of a 96-well plate. DPPH solution (63 μL) was added to all of wells. Methanol extract of PMM5 broth was used as blank. The mixture was shaken and kept at room temperature in the dark for 30 min. All the experiments were repeated for three times. Then the absorbance was read at 517 nm in a Power wave XS2 Microplate spectrophotometer (Bio-Tek Instruments Inc., USA). Butylated hydroxytoluene (BHT) (Merck, Germany) (1 mg/mL) was used as the standard antioxidant reference. The radical scavenging activity of the samples was calculated by the following equation:
Inhibition of DPPH radical (%) = [(control – sample)/control] × 100
Where control is the absorbance of all reagents except the test compound, and sample is the absorbance of the sample. IC50 was calculated from the graph plotted of inhibition percentage against samples concentration and equals to sample concentration providing 50% inhibition.[21]
Fraction isolation by TLC
A fixed amount and concentration of methanol extract (10 μL of 10 mg/mL) was applied each time on a pre-coated silica gel plate 2 × 10 cm, F254, 0.25 mm (E. Merck, Darmstadt, Germany). Each supernatant extract was developed using different solvent systems [such as chloroform:methanol (2:1), methanol:chloroform (1:1), chloroform:methanol:ethyl acetate (3:1:1), acetone:chloroform (4:1), methanol:aceton:chloroform (2:1:1) and methanol:chloroform:dichloromethane (3:1:1)]. All the solvent was purchase from Merck, Germany. The developed plates were air dried and observed under visible and UV light (240 and 300 nm). A bioautographic evaluation was conducted to check the antioxidant activity of separated compounds on TLC plate. For this purpose, the developed air dried plate was sprayed with the methanol solution of 0.1% DPPH antioxidant reagent.
The solvent system which can separate the antioxidant fractions from each other approximately in the middle of the TLC plate was selected. The active antioxidant constituents were noted according to their Rf(Maintenance Coefficient) values.[14] RF is defined as the ratio of stain motion to the solvent motion.[22] By using of TLC-DPPH autography, nine total bands were found.
After precise setting up the solvent system, the procedure repeated for 10 times until the appropriate amount of each fraction was obtained for further investigations.
The scratched samples with the same Rf was dissolved in HPLC grade methanol (Merck, Germany) and centrifuged at 12,000 ×g for 15 min to remove silica; then the combined extracts were evaporated to near dryness in the rotary (Heidolph, Germany) below 40°C.
Cell lines and cultures
AGS (human gastric carcinoma), Caco-2, and MCF-7 (breast cancer cell lines) were obtained from Iranian Biological Resource Centre. The AGS cells were cultured in Ham's F12 (Inoclon, Iran), MCF-7, and Caco-2 cells were cultured in Dulbecco's Minimum Essential Medium (DMEM): Ham's F12 (Inoclon, Iran). All cell lines were maintained in 90% medium supplemented with 100 μg/mL streptomycin (ATOCEL, Australia) and 10% heat-inactivated fetal bovine serum (ATOCEL, Australia). The cells were incubated at 37°C in CO2 incubator (Innova CO-170, USA) in an atmosphere of humidified 5% CO2 and 95% air.
Determination of cytotoxicity by MTT assay
Exponentially growing cell lines were seeded into 96-well plates at the concentration of ~ 1 × 104 cells per well and allowed to attach for 24 h. Test fractions isolated from antioxidant Lactobacillus strain were prepared in dimethyl sulfoxide (DMSO) (Sigma, USA) and serially diluted with basic media to obtain appropriate concentrations (400, 200, 100, 50, 25 μg/mL) in such way that DMSO concentration was lower than 0.2%.[23]
Cells were treated with different concentrations of fractions and incubated for 24 h. Cells in the control group received only media containing 0.2% DMSO. The test compound containing media was removed and washed with 200 μl of PBS followed by addition of 20 μl of 3-(4, 5-dimethyl-2-thiazolyl)-2 and 5-diphenyl-2H-tetrazolium bromide (MTT) reagent (5 mg/mL MTT in PBS) (Sigma, UK) and incubated for 3h at 37°C. The medium was removed and 100 μl DMSO was added into the wells to solubilize the purple crystal formazan and the absorbance was measured using a microplate reader (Bio-Tek Instruments Inc., USA) at the wavelength of 570 nm.[24] The effect of the samples on the proliferation of cell lines was expressed as the% cell viability, using the following equation:
% cell viability = [(At–A0)/(Ac-A0)] ×100.
Where Ac = Absorbance of cells treated with 0.2% DMSO medium, At = Absorbance of cells treated with extract/fractions, and A0= Absorbance of background. 0.1% (v/v) DMSO in the medium was used as negative control. Each treatment was performed in triplicate. The 50% growth inhibition concentrations (IC50) of the partial-purified fractions were estimated from the graphical interpolation.[25]
Apoptosis assessment by flow cytometry
Apoptotic cancer cells were detected by flow cytometry after double staining with annexin V-FITC and PI using the phosphatidyl serine detection kit (IQP-116F, UK). The samples were subjected to PARTEC Flow Cytometric analysis (Partec GmbH, Germany). Untreated cells were used as a negative control.
Component characterization
The bioactive fractions of the antioxidant and anticancer Lactobacillus strain supernatant were tested for the presence or absence of different compounds by using the methods of Jayashree (2013).[26]
Results
Among 14 Lactobacillus isolates that were isolated from 20 traditional doogh samples, Lactobacillus strain AG12a was isolated from camel doogh that was collected from Agh-Ghala, Golestan province, Iran.
Antioxidant screening of the isolates
Lactobacillus strain AG12a was shown as an antioxidative activity, and it survived for 6 h in the presence of hydrogen peroxide and hydroxyl radical. Superoxide-dependent growth inhibition was not established in the case of Lactobacillus strain AG12a.[13] It means that it is resistant to superoxide anions.
Bacterial identification
The anticancer Lactobacillus strain AG12a was primarily identified by biochemical and physiological tests and was assigned as L. hilgardii. The strain was confirmed to be a probiotic by survival at bile salt concentration of 0.3% and different initial culture pH values. The result of biochemical tests is confirmed by the result of genotypic identification by 99% similarity to L. hilgardii strain E91 (Accession No. EF536365). The V2-V3 regions of 16S rRNA and the 16S-23S intergenic spacer (ITS) region nucleotide sequences of the isolate was deposited in GenBank under accession numbers KU922755. The phylogenetic tree of this strain is shown in Figure 1.
Figure 1.
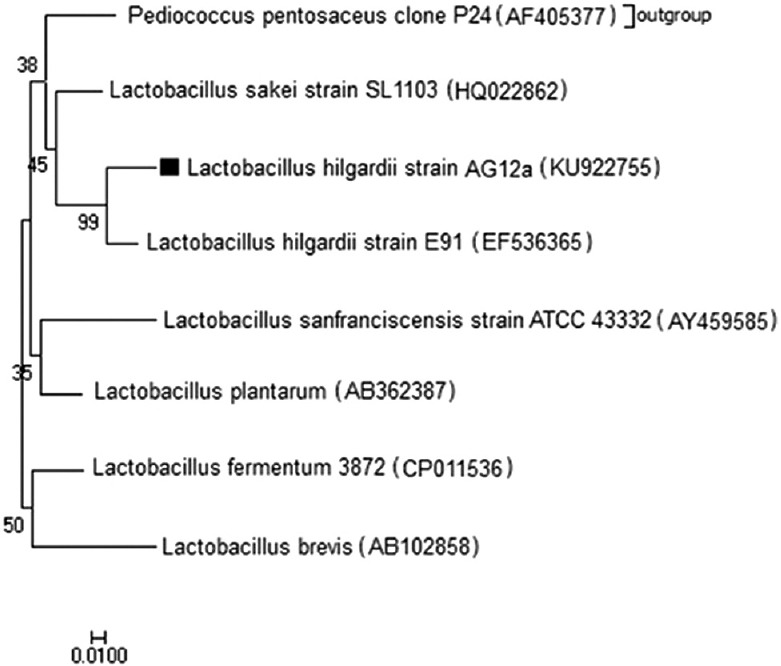
Phylogenetic tree based on the V2-V3 regions of 16S rRNA and the 16S-23S intergenic spacer (ITS) region nucleotide sequences of the Lactobacillus strain AG12a using the MEGA v7.0.14 program by neighbor-joining (NJ) method
DPPH assay
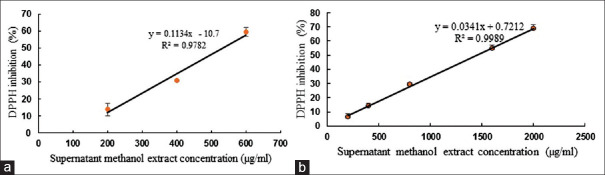
The DPPH scavenging activity of methanol extract of Lactobacillus strain AG12a whole cell-free supernatants after 48 h anaerobic cultivation in PMM5 broth (IC50 of 535.27μg/mL) was higher than MRS broth (1445.13 μg/mL) [Figure 2]. So, the PMM5 broth was used for further investigation.
Figure 2.
Antioxidant activity of extracellular L. hilgardii strain AG12a cultivated in (a) PMM and (b) MRS media by DPPH assay. Results are expressed as mean ± SD, P = 0.05
Fraction isolation by TLC
Among all of used solvent systems, methanol: chloroform: dichloromethane (3:1:1) was selected for Lactobacillus strain AG12a that shows the best fractionation of the extracellular materials. Table 1 demonstrate the bioactive groups and Rf values of fractions from Lactobacillus strain AG12a by TLC-bioautography.
Table 1.
Rf values and partial characterization of fractions isolated from extracellular materials of Lactobacillus hilgardii strain AG12a. IC50 values against Caco-2 cell line was evaluated by MTT assay
| Fractions | Rf | IC50 (µg/mL) | Bioactive groups | ||||
|---|---|---|---|---|---|---|---|
| Carbohydrate | Protein | Alkaloids | Terpenoids | Phenols | |||
| F1 | 0.11 | - | - | + | - | - | - |
| F2 | 0.3 | - | - | + | - | - | - |
| F3 | 0.35 | - | - | + | - | - | - |
| F4 | 0.41 | 299.05 | - | + | - | w* | - |
| F5 | 0.46 | - | - | - | - | W | - |
| F6 | 0.51 | 402.17 | w | w | + | W | - |
| F7 | 0.53 | - | - | w | - | + | - |
| F8 | 0.64 | - | - | - | - | + | - |
| F9 | 0.65 | 366.17 | - | - | - | + | - |
*w weak reaction
Determination of cytotoxicity by MTT assay
By using of MTT assay, the anticancer activity of antioxidant fractions against Caco-2 cell lines are displayed in Table 1. The criteria used to classify the anticancer activity of Lactobacillus extracellular methanol fraction against human cancer cell lines, based on IC50 values, were revised from those of National Cancer Institute (NCI).[27]
All the fractions are extracted from extracellular materials of Lactobacillus strain AG12a were inactive with undetectable IC50 levels for MCF-7 and AGS cell lines. Three fractions were weakly active (IC50201-500 μg/mL) against Caco-2 cell lines [Table 1 and Figure 3]. The lowest IC50 belonged to fraction F4 that was investigated for further analysis of apoptosis assessment.
Figure 3.
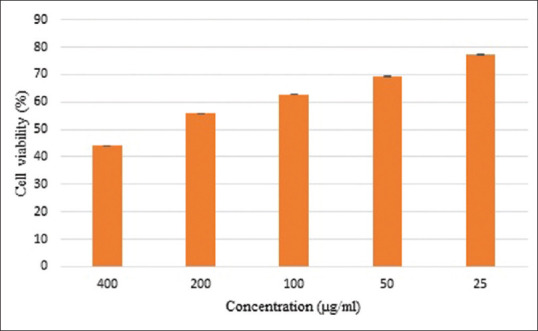
Effect of F4 fraction of L. hilgardii strain AG12a extracellular on Caco-2 cell lines by MTT assay. Results are expressed as mean ± SD, P = 0.05
Apoptosis assessment by flow cytometry
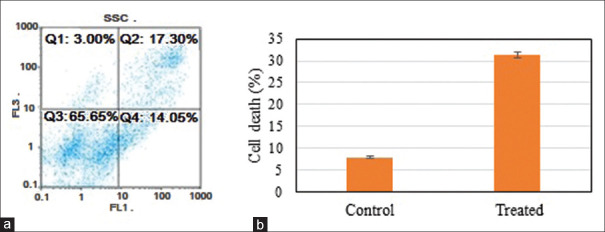
According to the MTT assay, fraction (F4) of Lactobacillus strain AG12a extracellular is only effective against Caco-2 cell line, so the further analysis continued by this cell line. The results of flow cytometric analysis of the most effective fraction (F4) against Caco-2 cell line (with 17.30% apoptosis and 14.05% necrosis in the “FL3 –”graph) and untreated Caco-2 cell line (as blank) are shown in Figure 4 (with 3.33% apoptosis and 4.57% necrosis in the “FL3 – “ graph).
Figure 4.
(a) Flow cytometry of Caco-2 cell line incubated with fraction 4 of L. hilgardi strain AG12a for 24 h stained with 10 μL Annexin V and 10 μL propidium iodine that shows 17.30% apoptosis and 14.05% necrosis in the “FL3 –”graph. (b) Effect of Lactobacillus hilgardii strain AG12a extracellular extracts on Caco-2 cell lines. Results are expressed as mean ± SD, P = 0.05
Component characterization
The partial characterization of the active component was shown in Table 1. The F4 fraction with the highest anticancer activity has the primary and secondary amines and reacts with ninhydrin reagent [Figure 5].
Figure 5.

TLC-bioautography of Ag12a methanol extract supernatant. From right to left: Folin, DPPH, ninhydrin, flavonoids, chlorides, terpenoids and Benedict reagents
Discussion
Recently, the beneficial effect of healthy diet was confirmed on different kind of cancers.[28,29] There is great interest in the potential function of probiotics as a complement therapy in cancer treatment. Several studies have revealed the relation between the dairy products consumption and the colon cancer risk and concerned the activity of probiotic bacteria in cancer prevention. There is increasing evidence that probiotics may have an effect on the cancer prevention by proliferation regulation and apoptosis.[30]
In this work, we investigate the antioxidant, anticancer, and possible apoptotic properties of Lactobacillus strains isolated from traditional doogh samples. Lactobacillus strain AG12a showed high antioxidant properties which was isolated from camel doogh. The bioactive compounds secreted from Lactobacillus strain AG12a in the PMM5 medium after 48 h fermentation are mainly polar which can be extracted by polar solvents such as methanol and chloroform. Among the compounds isolated from extracellular materials of Lactobacillus strain AG12a by using of TLC-DPPH bioautography, nine bands showed high antioxidant property and three bands exhibit weak cytotoxic activity against the Caco-2 cell lines [Table 1]. According to partial characterization, the F4 fraction of Lactobacillus strainAG12a which has the highest anticancer activity against Caco-2 cell line (IC50299.05 μg/mL) has the peptide bonds (primary and secondary amines) without any carbohydrate, phenol, and alkaloid compounds in its structure. The flow cytometry results showed that the probable mechanisms of cytotoxic activity of this fraction were apoptosis (17.30%) and necrosis (14.05%). In this study, the anticancer and apoptotic properties of Lactobacillus hilgardii against Caco-2 cell line was reported for the first time.
Several studies reported the inhibitory effects of probiotics on the proliferation of colon cancer cell lines. Ewaschuk et al. (2006) noted that Lactobacillus acidophilus, L. bulgaricus, L. casei, L. plantarum, Bifidobacterium breve, B. infantis, B. longum, and Streptococcus thermophiles reduced the viability and induced apoptosis of HT-29 and Caco-2 cells.[31] Sevda et al. (2015) demonstrated the antiproliferative effects of the cell-free filtrate and the cell-free lyophilized filtrate of 3 LAB (L. plantarum, Pediococcus pentosaceus, and Weissella confusa) on the Caco-2 cell line in a dose-dependent manner as detected by the MTT assay.[32] Awaisheh et al. (2016) stated that L. acidophilus LA102 and L. casei LC232 exhibited a strong cytotoxic activity against Caco-2 cell line.[33] Kim et al. (2008) states that colon cancer cell lines treatment with Bifidobacterium adolescentis SPM0212 cell-free supernatant was the most potent inhibitor than whole cells and heat-killed cells, against the growth of SW 480, HT-29, and Caco-2 cells by 32%, 36%, and 47%, respectively.[34]
Vamanu et al. (2006) reported that probiotic ingestion might lessen colon carcinogenesis by carcinogenic compounds inactivation, immune response stimulation, and enzymatic activity reduction in the gastrointestinal tract which are known to convert procarcinogens into carcinogens.[35] In vitro studies have shown that, in general, live cells of probiotic bacteria possessed higher anti-mutagenic activity.[36] Most animal and human studies indicate that feeding certain LAB decreases fecal enzyme levels that may be involved in the formation of carcinogens.[37]
Because of weak anticancer activity of isolated fractions from L. hilgardii, we highly recommended the live cell intake of lactobacilli for cancer prevention and treatment.
Considering that the samples used were semi-purified fractions, it is important to note that the pure active compound(s) would possibly show stronger cytotoxic effects. Further isolation and purification is essential and going to identify these bioactive compounds.
In conclusion, a fraction of Lactobacillus hilgardii extracellular extract contains molecules that demonstrates in vitro antioxidant and anticancer activities and seems to be a promising approach in the probiotic use of L. hilgardii strains as a supportive therapy or disease prevention. Additional in vivo studies are strongly required in order to validate these findings and provide protocols for the application of Lactobacillus hilgardii strain AG12a or its purified compounds into the pharma foods, and the real usefulness of probiotics for preventing cancer disease.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kim HS, Chae HS, Jeong SG, Ham JS, Im SK, Ahn CN, Lee JM. In vitro antioxidative properties of Lactobacilli. Asian Australas J Anim Sci. 2006;19:262–5. [Google Scholar]
- 2.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–72. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo JY, Gu W, Kim KA, Jang SE, Han MJ, Kim DH. Lactobacillus pentosus var. plantarum C29 ameliorates memory impairment and inflammaging in a D-galactose-induced accelerated aging mouse model. Anaerobe. 2014;27:22–6. doi: 10.1016/j.anaerobe.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Patel A, Prajapati JB. Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv Dairy Res. 2013;1:1–7. [Google Scholar]
- 6.Abedin-Do A, Taherian-Esfahani Z, Ghafouri-Fard S, Ghafouri-Fard S, Motevaseli E. Immunomodulatory effects of Lactobacillus strains: Emphasis on their effects on cancer cells. Immunotherapy. 2015;7:1307–29. doi: 10.2217/imt.15.92. [DOI] [PubMed] [Google Scholar]
- 7.Amer MN, Mansour NM, El-Diwany AI, Dawoud IE, Rashad FM. Isolation of probiotic lactobacilli strains harboring l-asparaginase and arginine deiminase genes from human infant feces for their potential application in cancer prevention.Ann. Microbiol. 2013;63:1121–9. [Google Scholar]
- 8.Wang Y, Waters J, Leung ML, Unruh A, Roh W, Shi X, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature. 2014;512:155–60. doi: 10.1038/nature13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uccello M, Malaguarnera G, Basile F, D'agata V, Malaguarnera M, Bertino G, et al. Potential role of probiotics on colorectal cancer prevention. BMC Surgery. 2012;12:S35. doi: 10.1186/1471-2482-12-S1-S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altonsy MO, Andrews SC, Tuohy KM. Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: Mediation by the mitochondrial pathway. Int J Food Microbiol. 2010;137:190–203. doi: 10.1016/j.ijfoodmicro.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Fallah Z, Feizi A, Hashemipour M, Kelishadi R. Effect of fermented camel milk on glucose metabolism, insulin resistance, and inflammatory biomarkers of adolescents with metabolic syndrome: A double-blind, randomized, crossover trial. J Res Med Sci. 2018;23:32. doi: 10.4103/jrms.JRMS_1191_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pourramezan Z, Kasra Kermanshahi R, Oloomi M, Aliahmadi A, Rezadoost H. In vitro study of antioxidant and antibacterial activities of Lactobacillus probiotic spp. Folia Microbiol. 2017:6331–42. doi: 10.1007/s12223-017-0531-x. [DOI] [PubMed] [Google Scholar]
- 14.Wegkamp A, Teusink B, De Vos WM, Smid EJ. Development of a minimal growth medium for Lactobacillus Plantarum. Lett Appl Microbiol. 2010;50:57–64. doi: 10.1111/j.1472-765X.2009.02752.x. [DOI] [PubMed] [Google Scholar]
- 15.Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, et al. Bergey's Manual of Systematic Bacteriology. New York: Springer; 2009. [Google Scholar]
- 16.Ryan KA, Jayaraman T, Daly P, Canchaya C, Curran S, Fang F, et al. Cytotoxic effects of various lactic acid bacteria on Caco-2 cells. Turk J Biol. 2015;39:23–30. [Google Scholar]
- 17.Jose NM, Bunt CR, Hussain MA. Comparison of microbiological and probiotic characteristics of lactobacilli isolates from dairy food products and animal rumen contents. Microorganisms. 2015;3:198–212. doi: 10.3390/microorganisms3020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saadatzadeh A, Fazeli MR, Jamalifar H, Dinarvand R. Probiotic properties of lyophilized cell free extract of Lactobacillus casei. Jundishapur J Nat Pharm Prod. 2013;8:131–7. doi: 10.17795/jjnpp-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–22. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li WJ, Cheng XL, Liu J, Lin RC, Wang GL, Du SS, Liu ZL. Phenolic compounds and antioxidant activities of Liriope muscari. Molecules. 2012;17:1797–808. doi: 10.3390/molecules17021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randerath K, Libman D. Thin-Layer Chromatography. Jstor. 1966 [Google Scholar]
- 23.Arullappan S, Rajamanickam P, Thevar N, Narayanasamy D, Yee HY, Kaur P. Cytotoxic effect and antioxidant activity of bioassay- Guided fractions from solanum nigrum extracts Trop. J harm Res. 2015;14:1199–205. [Google Scholar]
- 24.Longo-Sorbello Giuseppe SA, Saydam G, Banerjee D, Bertino JR. Cytotoxicity and cell growth assays. Elsevier Sci. 2006:315–24. [Google Scholar]
- 25.Chaudhary S, Chandrashekar KS, Sreedhara K, Pai R, Setty MM, Devkar RA, et al. Evaluation of antioxidant and anticancer activity of extract and fractions of Nardostachys jatamansi DC in breast carcinoma. BMC Complement Altern Med. 2015;10:15–50. doi: 10.1186/s12906-015-0563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayashree D. Phytochemical analysis and TLC fingerprinting of methanolic extracts of three medicinal plants. Int Res J Pharm. 2013;4:123–6. [Google Scholar]
- 27.Srisawat T, Chumkaew P, Heed-Chim W, Sukpondma Y, Kanokwiroon K. Phytochemical screening and cytotoxicity of crude extracts of vatica diospyroides symington type LS. Trop J Pharm Res. 2013;12:71–6. [Google Scholar]
- 28.Miraghajani M, Rafie N, Hajianfar H, Larijani B, Azadbakht L. Aged garlic and cancer: A systematic review. Int J Prev Med. 2018;9:84. doi: 10.4103/ijpvm.IJPVM_437_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamilian M, Khademi L, Vahedpoor Z, Bahmani F, Mahmoodi S, Taghizadeh M, et al. Effects of flaxseed oil omega-3 fatty acids supplementation on regression and metabolic status in endometrial hyperplasia: A randomized, double-blind, placebo-controlled trial. Int J Prev Med. 2019;10:61. doi: 10.4103/ijpvm.IJPVM_73_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Górska A, Przystupski D, Niemczura MJ, Kulbacka J. Probiotic bacteria: A promising tool in cancer prevention and therapy. Curr Microbiol. 2019;76:939–49. doi: 10.1007/s00284-019-01679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewaschuk JB, Walker JW, Diaz H, Madsen KL. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J Nutr. 2006;136:1483–7. doi: 10.1093/jn/136.6.1483. [DOI] [PubMed] [Google Scholar]
- 32.Sevda ER, Koparal AT, KivanḈ M. Cytotoxic effects of various lactic acid bacteria on Caco-2 cells. Turk J Biol. 2015;39:23–30. [Google Scholar]
- 33.Awaisheh SS, Obeidat MM, Al-Tamimi HJ, Assaf AM, EL-Qudah JM, Al-khaza'leh JM, et al. In vitro cytotoxic activity of probiotic bacterial cell extracts against Caco-2 and HRT-18 colorectal cancer cells. Milk Dairy Prod Hum Nutr. 2016;69:27–31. [Google Scholar]
- 34.Kim Y, Lee D, Kim D, Cho J, Yang J, Chung M, et al. Inhibition of proliferation in colon cancer cell lines and harmful enzyme activity of colon bacteria by Bifidobacterium adolescentis SPM0212. Arch Pharm Res. 2008;31:468–73. doi: 10.1007/s12272-001-1180-y. [DOI] [PubMed] [Google Scholar]
- 35.Vamanu A, Vamanu E, Drugulescu M, Popa O, Campeanu G. Identification of a lactic bacterium strain used for obtaining a pollen-based probiotic product. Turk J Biol. 2006;30:75–80. [Google Scholar]
- 36.Lee NK, Park JS, Park E, Paik HD. Adherence and anticarcinogenic effects of Bacillus polyfermenticus SCD in the large intestine. Lett Appl Microbiol. 2007;44:274–8. doi: 10.1111/j.1472-765X.2006.02078.x. [DOI] [PubMed] [Google Scholar]
- 37.Kumar M, Kumar A, Nagpal R, Mohania D, Behare P, Verma V, et al. Cancer-preventing attributes of probiotics: An update. Int J Food Sci Nutr. 2010;61:473–96. doi: 10.3109/09637480903455971. [DOI] [PubMed] [Google Scholar]