Abstract
Objective
To compare survival of individuals with coronavirus disease 2019 (COVID-19) treated in hospitals that either did or did not routinely treat patients with hydroxychloroquine or chloroquine.
Methods
We analysed data of COVID-19 patients treated in nine hospitals in the Netherlands. Inclusion dates ranged from 27 February to 15 May 2020, when the Dutch national guidelines no longer supported the use of (hydroxy)chloroquine. Seven hospitals routinely treated patients with (hydroxy)chloroquine, two hospitals did not. Primary outcome was 21-day all-cause mortality. We performed a survival analysis using log-rank test and Cox regression with adjustment for age, sex and covariates based on premorbid health, disease severity and the use of steroids for adult respiratory distress syndrome, including dexamethasone.
Results
Among 1949 individuals, 21-day mortality was 21.5% in 1596 patients treated in hospitals that routinely prescribed (hydroxy)chloroquine, and 15.0% in 353 patients treated in hospitals that did not. In the adjusted Cox regression models this difference disappeared, with an adjusted hazard ratio of 1.09 (95% CI 0.81–1.47). When stratified by treatment actually received in individual patients, the use of (hydroxy)chloroquine was associated with an increased 21-day mortality (HR 1.58; 95% CI 1.24–2.02) in the full model.
Conclusions
After adjustment for confounders, mortality was not significantly different in hospitals that routinely treated patients with (hydroxy)chloroquine compared with hospitals that did not. We compared outcomes of hospital strategies rather than outcomes of individual patients to reduce the chance of indication bias. This study adds evidence against the use of (hydroxy)chloroquine in hospitalised patients with COVID-19.
Keywords: Chloroquine, Coronavirus disease 2019, Hydroxychloroquine, Mortality, Severe acute respiratory syndrome coronavirus 2
Introduction
The spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leading to the current pandemic of coronavirus disease 2019 (COVID-19), has had a profound global impact on daily life, morbidity and mortality. Several preliminary studies have reported that the antimalarial agents hydroxychloroquine and chloroquine, or (H)CQ, alone or in combination with the antibiotic azithromycin, can have a suppressive effect on viral replication, and might decrease mortality from COVID-19 [[1], [2], [3], [4], [5]]. So far, clinical studies have been hampered by confounding by indications [1,2,4,5], monocentre set-up [2,3], and small numbers of included patients [3]. A recently published systematic review [6], a published randomized controlled trial (RCT) [7] and an RCT only available in pre-print [8], suggested that (H)CQ is not effective in patients admitted to hospital. The side effects of (H)CQ are well-known, and include fever and cardiac arrhythmias. While we are awaiting definitive results from more RCTs, cohort studies can provide quick closure of existing knowledge gaps. When treatment assignment in cohort studies is based on prescriber discretion, the risk of indication bias (even after covariate adjustment) remains high. However, our database of Dutch hospitals contains data of patients from hospitals that either routinely prescribed (H)CQ or did not prescribe it at all, offering a unique opportunity to compare both strategies. The comparison of different treatment strategies among hospitals leads to a significant reduction of (indication) bias. The objective of this study was to compare the effect of hospital-wide COVID-19 treatment strategies with or without routine (H)CQ use on all-cause 21-day mortality.
Methods
We used data from the ongoing CovidPredict Clinical Course Cohort containing over 2000 persons with COVID-19 [9], from nine hospitals in the Netherlands, including two university hospitals. Included in the database were all individuals admitted to hospital with positive SARS-CoV-2 PCR of nasopharynx, throat, sputum or bronchoalveolar lavage samples, or CT-scan abnormalities that were typical for COVID-19 (CO-RADS 4 and 5) [10], without an explanation for the abnormalities other than COVID-19. Inclusion dates ranged from the first admitted case in the Netherlands on 27 February to 15 May 2020, when the Dutch national guidelines no longer advised the use of (H)CQ. We excluded patients <18 years and those who were transferred to or from another hospital. Dosage of chloroquine base was: loading dose of 600 mg, followed by 300 mg twice a day for a total of 5 days. Dosage of hydroxychloroquine sulphate was 400 mg twice daily on the first day, followed by 200 mg twice daily on days 2 to 5. Among the seven (H)CQ hospitals, the timing of start of (H)CQ treatment differed; three hospitals started at the moment of COVID-19 diagnosis, four started after diagnosis but only when the patients clinically deteriorated, for example, when there was an increase in respiratory rate or increase in use of supplemental oxygen. The two hospitals that did not routinely treat patients with (H)CQ (i.e. the non-(H)CQ hospitals), offered best supportive care, including oxygen therapy and potentially antibiotic therapy, according to local guidelines and prescriber discretion. Participating hospitals did not routinely prescribe other experimental medication (e.g. lopinavir/ritonavir, remdesivir or steroids, see Table 1 ). Individuals who were incidentally treated with these drugs were included in the study. Primary outcome was 21-day all-cause mortality, defined as hospital mortality, or discharge to a hospice care facility. A waiver for the use of hospital record data was obtained through the Institutional Review Board of Amsterdam UMC; however, patients were given the opportunity to opt out. We collected data according to the collection protocol of the WHO. Missing covariates were imputed using multiple imputation with the MICE package (version 3.8.0) and the outcomes were determined by pooling the results of 25 imputed data sets [11]. We performed regression analyses and determined the pooled effect. Missing data for all covariates was less than 2.8%, except for obesity (missing data 6.2%) and use of corticosteroids (22.3%). In the primary analysis, we compared effectiveness of (H)CQ versus non-(H)CQ hospital strategies, irrespective of actual individual (H)CQ treatment. We performed a survival analysis using log-rank test and Cox regression with adjustment for age, sex, time in the pandemic (i.e. the number of elapsed days after 1 March 2020 at hospital admission), and covariates based on premorbid health (i.e. history of lung, kidney and cardiovascular disease, diabetes mellitus, obesity and neoplasms or haematological disease), disease severity during presentation (respiratory rate, oxygen saturation) and the use of steroids, including dexamethasone, for acute respiratory distress syndrome [12,13]. We repeated the analyses comparing actually received treatment, with (H)CQ. In a secondary analysis, we used a composite end point (either mechanical ventilation or all-cause mortality) at 21 days. As a sensitivity analysis, we performed a complete case analysis using inverse probability weighting of propensity scores (determined using the same covariates). We performed a subgroup analysis in (H)CQ hospitals that started (H)CQ directly from the moment of diagnosis versus outcomes in non-(H)CQ hospitals. All statistical analyses were performed using R versions 3.6.3 (R Foundation, Vienna, Austria).
Table 1.
Baseline characteristics
| Overall | Non-(H)CQ hospital | (H)CQ hospitals | |
|---|---|---|---|
| N | 1949 | 353 | 1596 |
| Age (years), mean (SD) | 66.71 (14.60) | 62.02 (15.14) | 67.75 (14.28) |
| Women, n (%) | 771 (39.6) | 155 (43.9) | 616 (38.6) |
| Chronic cardiac disease, n (%) | 587 (30.7) | 75 (21.3) | 512 (32.8) |
| Hypertension, n (%) | 915 (47.6) | 162 (46.2) | 753 (47.9) |
| Asthma or chronic pulmonary disease, n (%) | 510 (26.7) | 78 (22.1) | 432 (27.7) |
| Chronic kidney disease, n (%) | 221 (11.6) | 38 (10.8) | 183 (11.8) |
| Diabetes, n (%) | 501 (26.4) | 96 (27.2) | 405 (26.2) |
| Malignancy or chronic haematological disorder, n (%) | 194 (10.2) | 44 (12.5) | 150 (9.6) |
| Smoking, n (%) | 92 (6.2) | 18 (6.3) | 74 (6.2) |
| Obesity, n (%) |
556 (30.4) |
107 (35.3) |
449 (29.4) |
| Use of (H)CQ, n (%) | 648 (42.6) | 7 (2.0) | 641 (54.7) |
| Use of steroids for ARDS, n (%) | 120 (7.9) | 8 (2.3) | 112 (9.6) |
| Participation in drug trial, n (%) |
85 (5.7) |
39 (11.3) |
46 (4.0) |
| Respiratory rate, mean (SD) | 23.20 (6.94) | 24.29 (7.32) | 22.95 (6.83) |
| Temperature (°C), median (IQR) | 37.80 (37.00–38.60) | 37.30 (36.50–38.20) | 38.00 (37.10–38.70) |
| Peripheral oxygen saturation (%), median (IQR) | 94.00 (91.00–96.00) | 95.00 (91.00–97.00) | 94.00 (91.00–96.00) |
| CRP (mg/L) median (IQR) | 79.00 (40.38–135.00) | 82.60 (40.72–134.62) | 78.00 (40.25–135.00) |
| WBC (109/L), median (IQR) |
79.00 (40.38–135.00) |
82.60 (40.72–134.62) |
78.00 (40.25–135.00) |
| PCR positive, n (%) | 1844 (95.7) | 314 (89.2) | 1530 (97.1) |
| Time between onset of symptoms and hospital admission (days), median (IQR) | 7.00 (5.00–12.00) | 8.00 (5.00–13.00) | 7.00 (5.00–12.00) |
| ICU-admission, n (%) | 348 (17.9) | 70 (19.8) | 278 (17.4) |
| In patients admitted to the ICU; days between admission and start of mechanical ventilationa> | 1.00 (0.00–3.00) | 1.00 (0.00–3.00) | 1.00 (0.00–3.00) |
CRP, C-reactive protein; (H)CQ, (hydroxy)chloroquine; ICU, intensive care unit; PCR-positive, a positive test for COVID-19 based on PCR; WBC, white blood cell count.
Data of one centre were missing.
Results
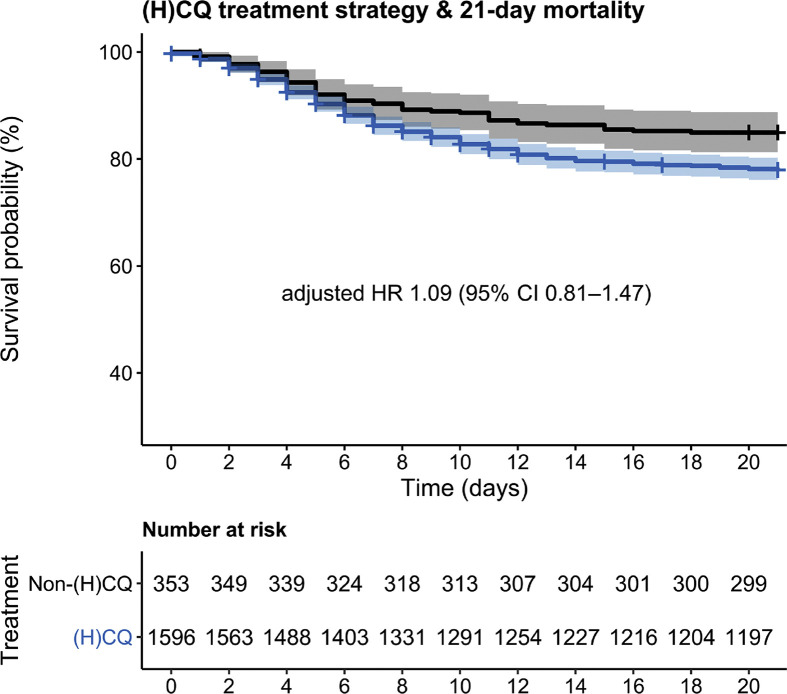
We analysed results from 1949 of the 2152 individuals admitted before 15 May 2020; 203 were excluded because they were transferred from another hospital. No patient opted out. Demographic data are shown in Table 1. Follow-up data were missing for 20 (1.0%) individuals. The individuals with missing outcome data are included in Table 1 and in the survival analysis, and were censored at the last day at which clinical information was available in the database. In total, 1596 individuals were treated in hospitals where (H)CQ was a standard part of treatment strategy (the (H)CQ hospitals) and 353 in non-(H)CQ hospitals. The two non-(H)CQ hospitals were both university hospitals. In (H)CQ hospitals, 54.7% of the patients received (H)CQ, compared with 2.0% of the individuals in the non-(H)CQ hospitals. In (H)CQ hospitals that routinely starting (H)CQ at the moment of COVID-19 diagnosis, 48.3% of patients received (H)CQ, in hospitals that started (H)CQ at clinical deterioration, 61.9% received (H)CQ. Among the seven (H)CQ hospitals, two used hydroxychloroquine during the first half and chloroquine during the second half of the epidemic, whereas five hospitals used chloroquine only. Patients in (H)CQ hospitals were older (mean ± SD: 68 ± 14 versus 62 ± 15 years) and had a higher prevalence of chronic pulmonary disease (27.7 versus 22.1) than individuals in the non-(H)CQ hospitals. Respiratory rate and peripheral oxygen saturation during admission were similar in both hospital groups (see Table 1). In the (H)CQ hospitals, 9.6% of patients received corticosteroids for adult respiratory distress syndrome and 4.0% were in a study protocol of an experimental SARS-CoV-2-directed antiviral (e.g. lopinavir/ritonavir) or immunomodulatory drug trial (e.g. imatinib, anti-complement C5), compared with 2.3% and 11.3% in non-(H)CQ hospitals, respectively. Fig. 1 shows the survival of patients in (H)CQ hospitals versus non-(H)CQ hospitals. Unadjusted mortality at day 21 was significantly higher in the (H)CQ hospitals (343/1596, 21.5%) compared with the non-(H)CQ hospitals (53/353, 15.0%, p 0.008). However, in the Cox regression models, this difference disappeared, with an adjusted hazard ratio (HR) of 1.09 (95% CI 0.81–1.47, Fig. 1, Table 2 ). When stratified by actually received treatment, the use of (H)CQ was associated with an increased 21-day mortality (HR 1.58; 95% CI 1.24–2.02, Table 3 ) in the full model. In the secondary analysis with either mechanical ventilation or all-cause mortality at 21 days, there were no statistically significant differences between the (H)CQ and non-(H)CQ hospitals (crude p 0.055, adjusted HR 1.00, 95% CI 0.75–1.35, see Supplementary material, Fig. S1). The complete analysis using propensity scores for treatment strategy and actual treatment showed similar results (see Table 4 ). An overview of the distribution of the propensity scores is given in the Supplementary material (Fig. S2) [14]. The sensitivity analysis of hospitals routinely starting (H)CQ treatment from the moment of COVID-19 diagnosis (i.e. (H)CQ hospitals without the hospitals that initiated (H)CQ treatment upon clinical deterioration) compared with non-(H)CQ hospitals, showed similar results with a significantly higher unadjusted 21-day mortality in the (H)CQ hospitals (154/670, 23.0%), compared with non-(H)CQ hospitals (53/353, 15.0%, p 0.002). This was attenuated towards an HR of 0.98 (95% CI 0.70–1.37) after adjustment for age, sex, co-morbidities and disease severity at presentation (see Supplementary material, Fig. S3).
Fig. 1.
Kaplan–Meier analysis of 21-day mortality of patients in the (hydroxyl)chloroquine ((H)CQ) hospitals (blue) versus non-(H)CQ hospitals (black), showing a significantly higher 21-day mortality in (H)CQ hospitals, p 0.004. This was attenuated towards a hazard ratio of 1.09 (95% CI 0.81–1.47) in the full regression model (see Table 2). Shaded areas indicate 95% CI.
Table 2.
Results of Cox regression models for treatment strategy
| HR | 95% CI | p value | |
|---|---|---|---|
| (H)CQ treatment strategy | 1.09 | 0.81–1.47 | 0.568 |
| Women | 1.04 | 0.84–1.29 | 0.715 |
| Age | 1.07 | 1.06–1.08 | <0.001 |
| Chronic cardiac disease | 1.23 | 0.98–1.53 | 0.068 |
| Asthma or chronic pulmonary disease | 1.14 | 0.91–1.42 | 0.250 |
| Chronic kidney disease (%) | 0.99 | 0.74–1.31 | 0.919 |
| Malignant neoplasm or chronic haematological disorder (%) | 1.34 | 1.00–1.79 | 0.051 |
| Diabetes | 1.34 | 1.07–1.68 | 0.010 |
| Hypertension | 1.06 | 0.85–1.33 | 0.577 |
| Obesity | 1.23 | 0.97–1.57 | 0.087 |
| Peripheral oxygen saturation | 0.95 | 0.94–0.97 | <0.001 |
| Respiratory rate | 1.04 | 1.03–1.06 | <0.001 |
| Use of steroids for ARDS | 1.78 | 1.26–2.52 | 0.001 |
| Time in pandemic | 0.98 | 0.97–0.99 | <0.001 |
ARDS, acute respiratory distress syndrome; (H)CQ, (hydroxy)chloroquine; HR, multivariable hazard ratios.
Table 3.
Results of Cox regression models for actual treatment
| HR | 95% CI | p-value | |
|---|---|---|---|
| (H)CQ treatment | 1.58 | 1.24–2.02 | <0.001 |
| Women | 1.06 | 0.86–1.31 | 0.587 |
| Age | 1.07 | 1.06–1.08 | 0.000 |
| Chronic cardiac disease | 1.26 | 1.01–1.57 | 0.041 |
| Asthma or chronic pulmonary disease | 1.10 | 0.89–1.37 | 0.377 |
| Chronic kidney disease (%) | 1.00 | 0.75–1.32 | 0.977 |
| Malignancy or chronic haematological disorder (%) | 1.36 | 1.02–1.82 | 0.037 |
| Diabetes | 1.33 | 1.06–1.66 | 0.014 |
| Hypertension | 1.06 | 0.85–1.32 | 0.610 |
| Obesity | 1.25 | 0.98–1.59 | 0.074 |
| Peripheral oxygen saturation | 0.95 | 0.94–0.97 | 0.000 |
| Respiratory rate | 1.04 | 1.02–1.06 | 0.000 |
| Use of steroids for ARDS | 1.62 | 1.14–2.28 | 0.007 |
| Time in pandemic | 0.99 | 0.98–0.99 | 0.001 |
ARDS, acute respiratory distress syndrome; (H)CQ, (hydroxy)chloroquine; HR, multivariable hazard ratios.
Table 4.
Complete cases analysis using inverse probability weighting
| HR | 95% CI | p-value | |
|---|---|---|---|
| For treatment strategy | |||
| (H)CQ treatment strategy | 1.17 | 0.99–1.40 | 0.072 |
| For actually received treatment | |||
| (H)CQ treatment received | 1.41 | 1.19–1.66 | <0.001 |
(H)CQ, (hydroxy)chloroquine; HR, hazard ratio.
Discussion
Mortality in individuals treated in hospitals that routinely prescribed (H)CQ was not significantly different from those treated in hospitals that routinely did not prescribe (H)CQ after adjustment for age, sex, medical history, disease severity at presentation and steroid use during treatment. Similarly, we found an increased risk of death among individuals who had actually received treatment with (H)CQ, which has probably been driven by indication bias, as in four of the seven (H)CQ hospitals, (H)CQ was only prescribed upon clinical deterioration. The unique characteristics of our study cohort enabled a study design that minimized indication bias. Our results add further weight to existing evidence against the use of (H)CQ for the treatment COVID-19.
The strength of this study is that data were collected in nine hospitals, including two university hospitals, in the Netherlands during the COVID-19 pandemic. Data collection was set up prospectively and the database included data on all consecutive patients admitted to general medicine and pulmonology wards, and to intensive care units. The database was set up according to WHO standards, which enabled data comparison and uniformity of data among the different participating centres. The comparison of hospital-defined treatment strategies rather than the treatment actually received led to a lower risk of indication bias compared with previous studies [1,2,4,5]. We roughly estimate the extent of the effect of indication bias to be the difference in outcome between the uncorrected and the corrected models. Further strengths include the multicentre set-up [2,3], as mentioned above, and the relatively large numbers of individuals included [3].
There are some limitations to address. Although health care in the Netherlands has a homogeneous set-up, there was some variability in standard protocols among the hospitals that could have led to residual confounding. The two non-(H)CQ hospitals were tertiary (university) centres, whereas the (H)CQ hospitals comprised both secondary and tertiary care hospitals. Before the COVID-19 pandemic, the tertiary care hospitals and their intensive care units functioned as referral centres for local secondary care hospitals. As we excluded patients transferred to and from other hospitals, the referral role of the tertiary care hospitals, including the university hospitals, was minimized. Furthermore, patients in the (H)CQ hospitals were more likely to receive steroid treatment, whereas those in the non-(H)CQ hospitals were more likely to receive other experimental immunomodulatory drugs. The numbers of individual types of medication were small, making it impossible to draw conclusions from these differences. The results of the RECOVERY trial suggested lower mortality in patients treated with dexamethasone [15]. Treatment with dexamethasone could therefore have resulted in a lower mortality in the group of (H)CQ hospitals. We did not find such an effect, even after correction in the full model. We also used extensive covariate adjustments, using various methods to minimize influence of differences in patient populations among hospitals, and the similarity in outcomes between these methods is reassuring in this regard. Finally, because not every patient in the (H)CQ hospitals actually received (H)CQ, the current efficacy estimate in our study is probably an underestimation of the true (H)CQ effect. Performing an instrumental variable analysis would have provided an approximation of this true effect, but because the current efficacy point estimates point toward harm rather than benefit of (H)CQ, this probably would not have changed our conclusions [16].
Despite the positive results of some studies resulting in widespread use of (H)CQ, our study did not show a benefit of (H)CQ treatment. This may be explained by the timing of the administration of the drug and its specific working mechanism. Chloroquine binds in silico and in vitro with high affinity to sialic acids and gangliosides of SARS-CoV-2. These bindings inhibit the interaction at non-toxic plasma levels with angiotensin converting enzyme 2 receptors and could hypothetically stop the cascade from formation of pulmonary infiltrations to full-blown adult respiratory distress syndrome and death [[17], [18], [19]]. The antiviral activity might be more effective in the pre-clinical setting as the deterioration in the hospital is more an effect of the cytokine storm provoked by SARS-CoV-2 than an effect of the viral infection itself. This hypothesis might explain why the clinical benefit for admitted patients was absent in our study, although we did not observe a difference in outcome among individuals treated early (at diagnosis) and among those treated later upon clinical deterioration.
Our results are in line with recently published studies. One RCT suggests a similar lack of effect of H(CQ) with higher rate of adverse effect than in supportive care [7]. Another RCT, published in preprint only, suggested a higher mortality in patients treated with H(CQ) compared with those treated with supportive care [8]. Given the current evidence, we would argue against the use of (H)CQ in hospitals outside the setting of clinical RCTs.
Transparency declaration
The authors declare that they have no conflicts of interest.
Funding
D. Collard is supported by a ZonMw grant (project no.: 10430022010002).
Contribution of authors
All authors have made substantial contributions to the following: the conception and design of the study, or acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be submitted.
Acknowledgements
We would like to acknowledge the contribution of the CovidPredict Study Group for the data collection, and Dr B.J.H. van den Born and Prof. Dr A.H. Zwinderman for their help with the analysis and interpretation of the data.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.10.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covid Risk, Treatments Collaboration Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: findings from the observational multicentre Italian CORIST study. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catteau L., Dauby N., Montourcy M., Bottieau E., Hautekiet J., Goetghebeur E. Low-dose hydroxychloroquine therapy and mortality in hospitalized patients with COVID-19: a nationwide observational study of 8075 participants. Int J Antimicrob Agents. 2020;56:106144. doi: 10.1016/j.ijantimicag.2020.106144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:19–27. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 2020.07.15.20151852. [Google Scholar]
- 9.2020. https://covidpredict.nl/en
- 10.Prokop M., van Everdingen W., van Rees Vellinga T., Quarles van Ufford H., Stoger L., Beenen L. CO-RADS—a categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020;296:E97–E104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wulff J., Ejlskov L. Multiple imputation by chained equations in Praxis: guidelines and review. Electron J Business Res Meth. 2017;15:41–56. [Google Scholar]
- 12.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J., Little T.D. A practical guide to propensity score analysis for applied clinical research. Behav Res Ther. 2017;98:76–90. doi: 10.1016/j.brat.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 [Google Scholar]
- 16.Instrumental variable analysis. 2020. https://journals.lww.com/epidem/Fulltext/2006/05000/Evaluating_Short_Term_Drug_Effects_Using_a.11.aspx Available at:
- 17.Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan J., Zhang X., Liu J., Yang Y., Zheng N., Liu Q. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In Vitro Antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.