Abstract
In this perspective, we propose to leverage reactive oxygen species (ROS) induction as a potential therapeutic measure against viral infections. Our rationale for targeting RNA viral infections by pro-oxidants is routed on the mechanistic hypothesis that ROS based treatment paradigm could impair RNA integrity faster than the other macromolecules. Though antiviral drugs with antioxidant properties confer potential abilities for preventing viral entry, those with pro-oxidant properties could induce the degradation of nascent viral RNA within the host cells, as RNAs are highly prone to ROS mediated degradation than DNA/proteins. We have previously established that Plumbagin is a highly potent ROS inducer, which acts through shifting of the host redox potential. Besides, it has been reported that Plumbagin treatment has the potential for interrupting viral RNA replication within the host cells. Since the on-going Corona Virus Disease - 2019 (COVID-19) global pandemic mediated by Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) exhibits high infectivity, the development of appropriate antiviral therapeutic strategies remains to be an urgent unmet race against time. Therefore, additional experimental validation is warranted to determine the appropriateness of repurposable drug candidates, possibly ROS inducers, for fighting the pandemic which could lead to saving many lives from being lost to COVID-19.
Keywords: ROS inducers, RNA degradation, Plumbagin, SARS-CoV-2, COVID-19
Graphical abstract
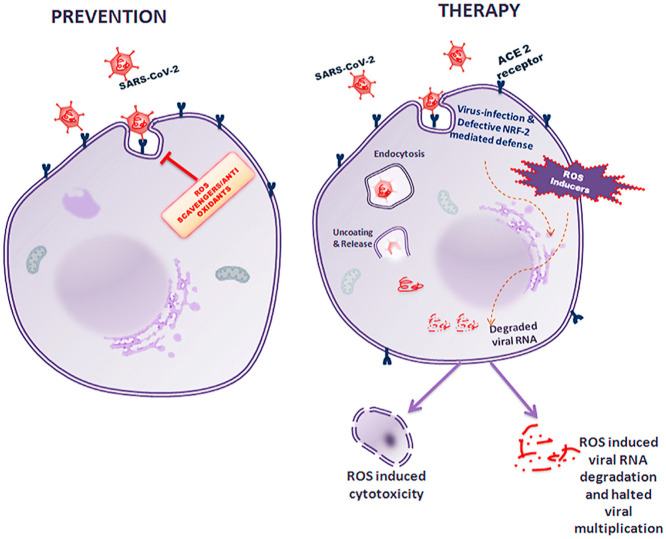
The Prevention and Therapeutic Strategies against SARS-CoV-2 employing ROS: The figure depicts the prevention as well as therapeutic strategies by using ROS scavengers and ROS inducers respectively. While ROS scavengers/antioxidants prevent the entry of the virus into the host cell, ROS inducers by appropriately adjusting the concentrations would alter the redox potential within the host cells to induce oxidative stress, thereby resulting in either nascent RNA degradation that would largely affect viral RNA or ROS induced cytotoxicity in viral infected cells as their DNA repair mechanism would be compromised.
Highlights
-
•
Viral entry causes low levels of oxidative stress in the host cells.
-
•
Strong ROS inducers would be damaging RNA, at lower concentrations, than DNA.
-
•
Plumbagin is a potent ROS inducer.
-
•
Plumbagin could be used for SARS-CoV-2 therapy.
1. Introduction
It is noteworthy that viruses lack metabolic or signaling capabilities; thus, viral replication is entirely reliant on host metabolic and signaling functions. The host mechanisms aiding and abetting viral infections, including Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2), provides broad opportunities for blocking viral infections with targeted drugs. There exists an extensive compendium of drugs currently undergoing clinical trials, which can be envisaged for the treatment of viral infections. Interestingly, these include targeted drugs that overlap with viral infection pathways like viral entry into the host cells, commandeering of the host metabolic pathways (nucleotide, amino acid and energy metabolism) critical for viral RNA replication and synthesis of viral protein envelope, as well as the progression of viral infections (Xiaowei Li, 2020).
Reactive oxygen species (ROS) inducers are extensively leveraged as therapeutics against several human diseases. Quinones are a class of chemical compounds that exhibit a variety of biological properties including antiviral, antimicrobial, antifertility, anti-atherosclerotic, anticoagulant, cardiotonic, insecticidal, antimalarial, leishmanicidal, trypanocidal, neuroprotective, hepatoprotective, anti-inflammatory and even antitumor activities, in part mediated through ROS generating capabilities. Plumbagin, 5-hydroxy-2-methyl-1,4-naphthoquinone, is a natural compound, isolated from the roots of the Plumbago species of plants, which has been comprehensively investigated as antimicrobial, antiviral and anticancer agents, through in vitro and in vivo studies. Our laboratory has extensively examined the role of Plumbagin mediated ROS as a potential mechanism for exerting its antitumor properties (Nair et al., 2016; Sinha et al., 2013; Somasundaram et al., 2016; Thasni et al., 2008). Since the RNA viruses focused in this perspective have single-stranded RNA as its genetic material, we propose that the nascent viral RNAs, which are synthesized in the cytoplasm, are highly susceptible to the oxidative stress induced by potent ROS inducers like quinones, 3′-azido-2′,3′-deoxythymidine (AZT) etc., resulting in viral RNA degradation, thus, contributing to their antiviral properties (Chang et al., 2008; Manda et al., 2011; Nunomura et al., 1999, 2002 bib_Nunomura_et_al_2002 bib_Nunomura_et_al_1999). In this perspective, we aim to summarize the possibilities of exploiting the pro-oxidant functions of ROS inducer, Plumbagin and its potential implications for targeting RNA viral infections.
2. Insights into targeting viral RNA by ROS inducers
ROS inducers like quinones have been previously shown to induce RNA degradation of the RNA viruses, Hepatitis C Virus (HCV) and Human Immunodeficiency Virus (HIV) (Hassan et al., 2016; Min et al., 2002). Single-stranded RNAs are vulnerable to damages in comparison with other macromolecules like DNA/protein; moreover, as endogenous RNAs are synthesized in multiple copies, cells could afford to lose the damaged RNAs, which are transient nucleic acids, through degradation mediated by RNA surveillance mechanisms (Chang et al., 2008; Nunomura et al., 1999, 2002 bib_Nunomura_et_al_2002 bib_Nunomura_et_al_1999). However, for the DNA, efficient repair mechanisms exist, which can protect them from degradation (Li et al., 2006). Although alkylation damages to RNAs are repairable, oxidative damages to RNA is generally unrepairable (Aas et al., 2003). Since viral RNA replication occurs within the cytosol of the host cell, we hypothesize that replicating viral RNAs, which are more susceptible than the nuclear or mitochondrial DNA, could be damaged by ROS mediated cellular stress, which is a possible strategy for the anti-viral therapy (Chang et al., 2008; Nunomura et al., 1999, 2002 bib_Nunomura_et_al_2002 bib_Nunomura_et_al_1999).
Why do we hypothesize that ROS inducers act as therapeutic agents, though the free radical scavengers like Vitamin C prevent viral infections like pneumonia? Viruses entering the host cell induce low levels of oxidative stress to promote mitogenic activity and simultaneously induce NRF-2 mediated antioxidant responses to protect the host cell from ROS induced cytotoxicity and suppression of the immune activity (Lee, 2018b). Maintenance of a delicate oxidant-antioxidant balance is imperative for the promotion of viral replication, protection of viral genetic material and host cell from excessive oxidative damage and restraining the antiviral immune responses (Lee, 2018b; Reshi et al., 2014). Thus, tipping oxidant-antioxidant balance using pharmacological agents towards antioxidant state should promote viral replication and if shifted toward pro-oxidant state should result in enhanced oxidative stress and inhibition of the viral replication (Chen et al., 2020a; Lee, 2018b; Nakamura et al., 2010). Hence, the administration of antioxidants like Vitamin C, which has been proved to be preventive against viral pneumonia, can serve as an ideal prevention strategy against viral infections; however, ROS induction can be superlative in disease therapy (Hemilä and Louhiala, 2013; Kim et al., 2013). Further, there are several host redox and other associated genes which are modulated during a viral infection enabling the viral propagation and pathogenesis as depicted in Table 1 ((Ahmed and Rahman, 2006; Bender and Hildt, 2019; Bottino-Rojas et al., 2018; Checconi et al., 2020; Chen et al., 2020a; Cuadrado et al., 2020; Jacoby and Choi, 1994; Lee, 2018a; Simenauer et al., 2019; Zhang et al., 2020).
Table 1.
The table depicts various redox and other associated genes which are being modulated in the host cells during a viral infection.
| S. No. | Redox and other associated genes activated during RNA viral infections | Reference |
|---|---|---|
| 1 | Superoxide dismutase 3 (SOD3) | (Ahmed and Rahman, 2006) |
| 2 | Activating transcription factor 4 (ATF4) | |
| 3 | Metallothionein 2A (M2TA) | |
| 4 | Nuclear factor erythroid 2-related factor 2 (NRF-2) | (Bottino-Rojas et al., 2018) |
| 5 | Heme oxygenase (HMOX1) | (Cuadrado et al., 2020) |
| 6 | Sulfiredoxin-1 (SRXN-1) | (Simenauer et al., 2019) |
| 7 | NADPH quinone dehydrogenase 1 (NQO1) | |
| 8 | Glutamate cysteine ligase catalytic and regulatory subunits (GCLC and GCLM) | (Lee, 2018a) |
| 9 | Glutathione S-transferase (GST) | |
| 10 | Uridine diphosphate glucuronosyltransferase (UDPGT) | |
| 11 | Catalase (CAT) | |
| 12 | Glucose 6 phosphate dehydrogenase (G6PD) | |
| 13 | Glutathione peroxidase-1 (GPx) | |
| 14 | Glutathione disulfide reductase (GSR) | Checconi et al. (2020) |
| 15 | Copper/zinc superoxide dismutase (Cu/ZnSOD) | (Jacoby and Choi, 1994) |
| 16 | Manganese superoxide dismutase (MnSOD) | |
| 17 | Indole- amine dioxygenase (IDO) | |
| 18 | Melatonin | Zhang et al. (2020) |
| 19 | Cytochrome P450 E1 (CYP2E1) | (Bender and Hildt, 2019) |
| 20 | ER oxidoreductin 1α (Ero1α) | |
| 21 | Mitogen-activated protein kinase (MAPK) | |
| 22 | Insulin receptor substrate (IRS1/2) | |
| 23 | Insulin receptor (IR) | |
| 24 | Insulin-like growth factor 1 (IGF-1) | |
| 25 | Insulin-like growth factor 1 receptor (IGF-1R) | |
| 26 | c-Jun-N-terminal kinase (JNK) | |
| 27 | γ-glutamylcysteine synthetase (γ-GCS) | |
| 28 | Xanthine oxidase (XO) | (Chen et al., 2020a) |
| 29 | NADPH-cytochrome P450 reductase (CPR) | |
| 30 | Inducible nitric oxide synthase (iNOS) | |
| 31 | NADPH oxidase 1 (NOX1) | |
| 32 | NADPH oxidase 2 (NOX2) | |
| 33 | NADPH oxidase 3 (NOX4) | |
| 34 | Cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) | |
| 35 | Cytochrome P450, family 1, subfamily A, polypeptide 2 (CYP1A2) | |
| 36 | Cytochrome P450, family 1, subfamily B (CYP1B) | |
| 37 | Aryl hydrocarbon receptor (AhR) | |
| 38 | Dual Oxidase 1 (DUOX1) | |
| 39 | Dual Oxidase 2 (DUOX2) | |
| 40 | Myeloperoxidase (MPO) |
During a viral infection, as viral entry into the host cells is a continuous process, there arises a question of selecting the suitable redox modulated antiviral strategy, i.e. whether to use the ROS scavengers to prevent the viral entry into each of the host cells or to employ the pro-oxidants to target the viral RNA degradation. Though this decision stands imperative, it is indeed dependent on the extent of viral infection in the patients. However, there appears a concern about whether the ROS induction would affect the normal cells. Since the normal cells have a stable redox homeostatic system as well as efficient machinery to repair the nucleic acid damages resultant of ROS induction, they would strategically strive the detrimental mechanistic effects of ROS. Conversely, the viral infected cells, which would already have a viral-induced oxidative stress as well as compromised repair system (owing to the viral evasion of the host repair systems) to overcome these detrimental nucleic acid damages, would succumb to ROS induced cytotoxicity and resultant cell death owing to the additive effects of ROS produced by pro-oxidants. Besides, when viral infection occurs, it results in minimal oxidative stress inside the host cells, triggering numerous antioxidant mechanisms, the prominent of which is the NRF-2 mediated ones. However, this is a failed defense system in cases of high viral titres in the infected cells, while this defense system remains active in the normal cells. Thus, NRF-2 mediated antioxidant mechanism additively acts in preventing the damaging cellular effects owing to ROS induction in the normal/infection-resistant cells, while the failed NRF-2 system in viral infected cells wouldn't interfere with the ROS induced damages, which adds up with viral-induced oxidative stress to selectively target these viral infected cells. The aforesaid properties substantiate the leverage of ROS inducers than the ROS scavengers as a suitable anti-viral therapeutic strategy. Therefore, the pro-oxidants possess the ability to selectively induce enhanced lethal oxidative damages in RNA than that could be induced in the DNA, thus stalling the translation process, which sufficiently and satisfactorily calls for proposals in employing ROS inducers as lead molecules for the viral RNA infection treatment, including SARS-CoV-2 (Fig. 1 ).
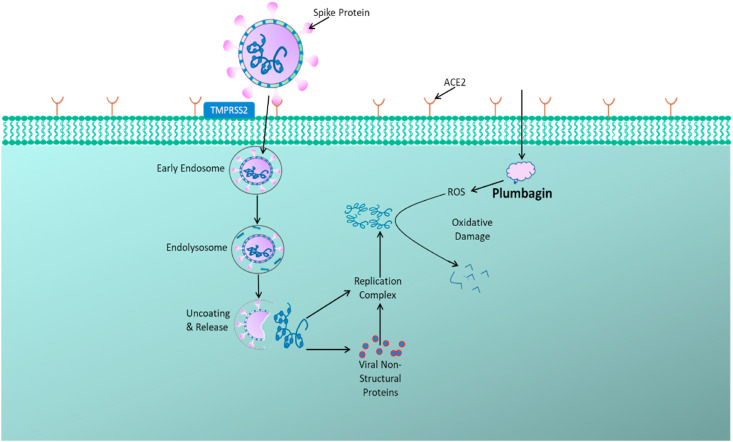
Fig. 1.
Targeting SARS-CoV-2 Infection by Plumbagin: SARS-CoV-2 viral particle anchors onto the host cell via its spike proteins through its interaction with host cell receptors like ACE2 and enters into the cell by endocytosis. The uncoating and release of viral RNA into the host cell cytoplasm triggers the viral RNA replication, transcription and translation for its propagation. Plumbagin, which is a potent oxidative stress inducer, generates ROS within the host cell, that is capable of promoting viral RNA degradation.
3. ROS mediated and allied cytotoxicity by Plumbagin
Quinones, in general, are strong ROS inducers; however, among structurally similar quinones, Plumbagin is not only an excellent ROS inducer but also has a higher ability to alter the redox potential which is mechanistically responsible for antiviral, antimicrobial, anti-inflammatory, antimalarial and antitumor properties. Since ages, Plumbago roots have been used in traditional medicine for treating topical infections in the Indian subcontinent ( Fig. 2 ) (Database., Available from: http://bodd.cf.ac.uk/.; de Lima et al., 1968; Fournet et al., 1992; Grieve, 1931; Sharma and Kaushik, 2014). Plumbagin has been extensively explored for altering the redox potential in numerous microbial systems. Plumbagin was reported to increase the rate of superoxide generation through a diaphorase-mediated reduction in certain lactic acid bacteria (Archibald and Fridovich, 1981; DiGuiseppi and Fridovich, 1982). Although Plumbagin induced lethality is an oxygen-dependent process, cellular damage and repair responses to Plumbagin has been demonstrated to be unique compared to responses observed with superoxide or hydrogen peroxide (H2O2) exposure (DiGuiseppi and Fridovich, 1982; Farr et al., 1985; Hassan and Fridovich, 1979). Moreover, Plumbagin exposure produced more strand scissions in endonuclease deficient cells, while the presence of superoxide scavenger offered only a limited protection (Denq and Fridovich, 1989). In vitro, Plumbagin, via intercepting the electrons attenuate NADH dehydrogenase, resulting in a respiratory arrest causing metabolic dysfunction in E.Coli cultured in glucose-containing media. This observed respiratory failure and growth inhibition of E.coli, with Plumbagin, occurred in the presence of excess superoxide dismutase (SOD) and catalase (Imlay and Fridovich, 1992). Additionally, Plumbagin treatment in E. coli K-12 mutant strains with diminished levels of SOD, resulted in hypersensitivity and increased DNA damage suggesting a direct role for superoxide in all these processes (Prieto-Alamo et al., 1993).
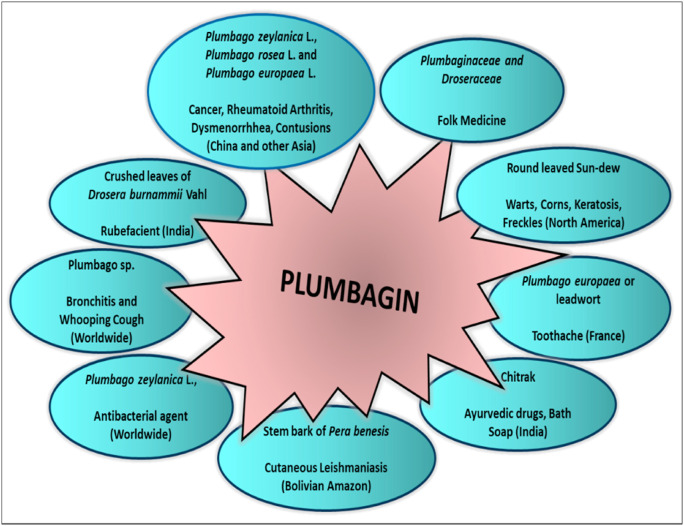
Fig. 2.
Traditional Applications of Plant Extracts with Plumbagin: The figure represents various medicinal applications of crude extracts from natural plant sources containing Plumbagin used across different regions worldwide as traditional medical practices (Database., Available from: http://bodd.cf.ac.uk/.; de Lima et al., 1968; Fournet et al., 1992; Grieve, 1931; Sharma and Kaushik, 2014).
Not just with the microbial systems, Plumbagin modulates the metabolism and signaling in the mammalian cells too, mediated through ROS induced cytotoxicity. We had proved for the first time that Plumbagin induces ROS mediated apoptosis in cervical cancer cells, which could be reversed by the pre-treatment of the cells with a free radical scavenger, N-acetyl-L-cysteine (NAC) (Srinivas et al., 2004). We had also reported through in vitro and in vivo studies that amongst the several naphthoquinones analyzed, Plumbagin selectively targets the BRCA1 defective cancers, through its ROS inductive activity. As these cancers are DNA damage repair defective, they will be more sensitive to oxidative damage induced by Plumbagin, than the normal cells, since the latter possesses an efficient DNA repair system (Nair et al., 2016; Sinha et al., 2013; Somasundaram et al., 2016; Thasni et al., 2008). Thus, the aforesaid evidence is suggestive of the fact that ROS generation has been one of the prominent mechanisms through which Plumbagin mediates its cytotoxicity.
Though Plumbagin exerts its effects mainly through ROS induction, such as the generation of superoxides, which are well proven, there are several other modes of mechanistic activities too (Tripathi et al., 2019). Plumbagin acts as a spindle poison as well as human Topoisomerase II inhibitor, thus affecting the cellular replication (Fujii et al., 1992). In affecting the mitochondrial electron transport chain (ETC), Plumbagin binds to the ubiquinone binding sites of Complexes I-III, thereby interfering with the ETC downstream of Complex II, resulting in a reduction in oxygen consumption rate, NADPH/FAD redox ratio as well as ATP synthesis (Kapur et al., 2018). ATP synthesis remains to be one of the cardinal factors of cellular energetics which is essential for viral RNA replication, capsid assembly and virus survival in the infected host cells and hence, the property of Plumbagin in blocking the ETC would be advantageous in exerting its antiviral property (Chang et al., 2009). Further, it also serves as a lysine acetyltransferase inhibitor as well as affects various signaling pathways including the EGFR, NF-κB, STAT, AKT and so on (Ravindra et al., 2009; Tripathi et al., 2019; Vasudevarao et al., 2014). Currently, Plumbagin is in its Phase I clinical trial for the treatment of metastatic castrate-resistant prostate cancers, which is under evaluation of a higher dosage (Kyriakopoulos et al., 2019). In addition to ROS induction, based on the evidence showing the impact of Plumbagin on multiple biological pathways in mammalian cells and since such mechanisms are also relevant for viral propagation within the host cells, we hypothesize that Plumbagin could be utilized for targeting RNA viruses as well as the viral infected host cells in a concentration-dependent manner, such that it remains non-toxic to the normal/uninfected cells.
4. Our perspective on prospective inhibition of SARS-CoV-2 by Plumbagin
SARS-CoV-2 has torpified the entire world; lingering of this pandemic for a longer duration would lead to widespread human catastrophe affecting for decades (L et al., 2020). SARS-CoV-2 is a single-stranded positive-sense RNA virus of the β corona virus genus. The viral spike ‘S’ protein binds to the Angiotensin-converting enzyme 2 (ACE2) receptors on the host human cells initiating the infection (Walls et al., 2020). The treatment protocol involves the use of antipyretics, antitussives, antibiotics and respiratory support for the symptomatic relief while antiviral drugs like oseltamivir, lopinavir/ritonavir and remdesivir are also administered with limited success. Several other antiviral drugs like darunavir, ribavirin, favipiravir, arbidol etc., are under experimental trials (Cai et al., 2020; Tang et al., 2020). Recently, remdesivir, which has been reported to inhibit the RNA dependent RNA polymerase activity of SARS-CoV-2 virus and thus the viral RNA replication, has been reported to show better outcomes by shortening the time for recovery; however, guidelines defining therapeutic regimens specific to SARS-CoV-2 for worldwide adoption remains to be established and thus the scope for integration of novel therapeutic drugs remains wide open both for targeting SARS-CoV-2 RNA virus and reduction of co-morbidities in these patients (Beigel et al., 2020; Chen and Li, 2020; Gordon et al., 2020a; Grein et al., 2020; Wang et al., 2020; Yang and Wang, 2020).
Hydroxychloroquine, which is a quinine derivative (a natural cinchona alkaloid, unlike quinones), is an anti-malarial drug, which was actively participating in solidarity trials being studied for repurposing it for the treatment of SARS-CoV-2. It was reported to exert its antiviral activity by increasing the pH in lysosomes/endosomes in host cells to alter the enzyme activity and resultant post-translational modifications of ACE2 receptor, as well as glycosylation of sialic acid linked gangliosides, which are the receptors for SARS-CoV-2 viral spike S protein, aiding the viral attachment to host human cells (Devaux et al., 2020). This prevented the binding of the viral spike S protein, thereby inhibiting the viral entry into the host human cells. Though several clinical trials of Hydroxychloroquine for Corona Virus Disease – 2019 (COVID-19) were carried out, the unfavorable side-effects reported from various studies have led to decisions on withdrawing the drug from solidarity trials across various parts of the world; however, the evaluation studies involving Hydroxychloroquine in non-hospitalized patients as well as prophylactic analysis on pre- and post-exposure for COVID-19 are still progressing (Chen et al., 2020b; Mehra et al., 2020; Zou et al., 2020).
As we have discussed above, cellular RNA, in comparison with DNA, is exceedingly damaged upon ROS induced oxidative stress, since RNA is more sensitive, especially the cytoplasmic RNA adjacent to mitochondria (major site of ROS synthesis), as the levels of oxidative RNA damage is greater than the oxidative DNA damages in the cell. Considering that cellular RNA is highly vulnerable to oxidative damage when compared to DNA, this property is a likely strategy for attenuating RNA viruses (Li et al., 2006); hence, we envision that Plumbagin via ROS could induce oxidative RNA damages and stall viral replication directly along with interrupting cellular metabolism to create an unfavorable environment for SARS-CoV-2 viral replication in the host cells.
Antiviral effects of Plumbagin against RNA viruses have been previously noted against HCV, which is an enveloped positive-sense single-stranded RNA virus. On the analysis of intracellular HCV RNA by RT-PCR, it was demonstrated that Plumbagin inhibited the HCV replication at an IC50 (half-maximal inhibitory concentration) of 0.57 μM/L and a CC50 (50% cytotoxic concentration) of 30.65 μM/L, thus resulting in a selectivity index (SI) of 53.7, in comparison with the standard antiviral drug, telaprevir, which exhibited an SI of 2127. Plumbagin administration also caused an enhanced expression of anti-HCV cellular host factor hA3G protein, a cytidine deaminase and a reduction in NS3 HCV non-structural protein levels to compromise the viral replication machinery, in a dose-dependent manner, more effectively when compared with telaprevir (Hassan et al., 2016). Further, studies were carried out on extracts from Plumbago indica (a natural source of Plumbagin) and Allium sativum (commonly known as garlic) to analyze their antiviral properties against Influenza A (H1N1) pdm09 employing two principles, which are either simultaneous exposure assays (to analyze if the compound inhibits viral adsorption onto the cell surface receptors) or the post-exposure treatment assays (to analyze if the compound inhibits viral replication inside the host cells or prevent budding from the infected cells). They have reported that both these extracts independently exhibit the inhibition of polymerase activity, impairing the viral nucleoprotein synthesis, blockage of viral attachment and the activity of viral hemagglutinin envelope protein of Influenza A (H1N1) pdm09, to interrupt the infection by this single-stranded RNA virus; however, the Plumbago extracts displays greater antiviral activity in comparison with the Garlic extracts (Chavan et al., 2016). Although the impact of ROS caused by Plumbagin is very apparent in RNAs, ROS is also known to attack and incapacitate protein functions, as well, in exerting its antiviral potential. This has been evident in the publication by Min et al., where the authors report the inhibition of RNase H activity in the presence of Plumbagin, resulting in an impaired reverse transcriptase function in HIV-1 (a single-stranded RNA virus) as well as weakly inhibiting the RNA dependent- and DNA dependent- DNA polymerase activities (Min et al., 2002). The reports on the antiviral activity of Plumbagin sprout its possibility as a lead drug against RNA viruses, including SARS-CoV-2. Further, Plumbagin is also thought to inhibit Chromosome Region Maintenance 1 (CRM1), a nuclear export transport receptor, via direct interaction and disruption of its activity, thereby interfering with the CRM-1 mediated export (Liu et al., 2014). This is particularly interesting because ORF9b protein, a component of corona viruses are known to interact with CRM1 to facilitate cytoplasmic translocation, viral assembly and induction of pro-inflammatory factors (Sharma et al., 2011). Moreover, sequence annotation in SARS-CoV-2 has also revealed the presence of ORF9b, wherein the latter bears 73.2% similarity with those in Human Severe Acute Respiratory Syndrome (SARS) and 74.23% similarity with those in Bat Corona Virus (Bat CoV) (Giri et al., 2020; Mathew and Ghildyal, 2017). Therefore, Plumbagin's inhibitory activity on CRM1 could be advantageous for interrupting SARS-CoV-2 viral entry into host cells and resultant viral propagation. Overall, Plumbagin could act as a poly-functional inhibitor of viral RNA replication, which along with the direct and indirect effects mediated by ROS induction could be exploited for exerting its antiviral effects against SARS-CoV-2.
Since SARS-CoV-2 infections are alarmingly increasing across the world, mapping of cardinal human interaction protein partners of the viral proteins would figure out key small molecules for targeted therapies. A recent report has predicted the gene ontology analysis of various protein components of SARS-CoV-2 virus with several interacting protein partners, significant of which are its interaction with those involved in nuclear transport, regulation of ROS, cell cycle regulation, cytoplasmic ubiquitin ligase complex and cell death in response to oxidative stress (Gordon et al., 2020b). Plumbagin, being a potent ROS inducer, would affect these process and involved proteins as well as RNA degradation; hence, should have the potential of being an antiviral agent forefront, against SARS-CoV-2 (Fig. 1).
5. Safety concerns of treating COVID-19 patients with pro-oxidizing agents
Although Plumbagin has not been evaluated in humans, results from in vivo experiments reported non-toxic effects along with slight weight loss at 2 mg/kg body weight (intraperitoneal administration for 5 days/week for 3 weeks) (Cao et al., 2018; Sakpakdeejaroen et al., 2019; Sandur et al., 2006). Further experiments are required to determine the minimal effective dose of Plumbagin required for achieving efficient degradation of viral RNA activity in humans. Results from animal studies show that Plumbagin at less than 2 mg/kg body weight is sufficient to elicit anticancer activity, while toxic side effects are observable only at >10 mg/kg body weight in mouse models (Nair et al., 2016). Thus, establishing an effective dosage and determining the safety profiles for Plumbagin is absolutely necessary, prior to evaluation for COVID-19 targeted therapy.
6. Conclusion
In conclusion, though RNA viruses keep on evolving mechanisms to escape viral degradation by host cells, plant-based natural bioactive compounds, such as Plumbagin, with lesser toxicity, enhanced bioavailability and a broad spectrum of activities, might prove to be potent molecules with antiviral properties against SARS-CoV-2 RNA viruses. Additionally, cross-checking and identification of mechanistically applicable drugs (and combinations) from the compendium of clinically approved ROS inducing drugs and repurposing them for SARS-CoV-2 viral infection treatment could be a faster route for implementing therapies for preventing tragic loss of human lives to this COVID-19 pandemic.
CRediT authorship contribution statement
Revathy Nadhan: Data curation, Writing - original draft. Dipyaman Patra: Visualization. Neethu Krishnan: Methodology. Arathi Rajan: Visualization. Srinivas Gopala: Conceptualization, Validation, Writing - review & editing. Dashnamoorthy Ravi: Conceptualization, Validation, Writing - review & editing. Priya Srinivas: Conceptualization, Validation, Writing - review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no conflicts of interest and have nothing to disclose.
Acknowledgements
P.S. acknowledges the financial support from the Department of Science and Technology, Government of India (EMR/2017/002222) and the Council of Scientific and Industrial Research, Government of India (No. 27(0372)/20/EMRII). The financial support by the intra-mural funding from Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala, India, for R.N, the University Grants Commission, Government of India for N.K. and D.P. and the Department of Science and Technology, Government of India (DST-INSPIRE), for A.R. are acknowledged. P.S., D.P., N.K. and A.R. also acknowledge the University of Kerala, Thiruvananthapuram, for aiding the research.
References
- Aas P.A., Otterlei M., Falnes P.O., Vagbo C.B., Skorpen F., Akbari M., Sundheim O., Bjoras M., Slupphaug G., Seeberg E., Krokan H.E. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature. 2003;421:859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- Ahmed M., Rahman N. ATM and breast cancer susceptibility. Oncogene. 2006;25:5906–5911. doi: 10.1038/sj.onc.1209873. [DOI] [PubMed] [Google Scholar]
- Archibald F.S., Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J. Bacteriol. 1981;146:928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L C., JM L., L S., I W., E K., S R., S B., S H., G B., O G., A B., M T., CL W., J D., K M. - Parenting in a time of COVID-19. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30736-4. 30736-30734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.-d., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of Covid-19 — Preliminary report. N. Engl. J. Med. 2020;383(10):992–994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- Bender D., Hildt E. Effect of hepatitis viruses on the Nrf2/Keap1-signaling pathway and its impact on viral replication and pathogenesis. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino-Rojas V., Talyuli O.A.C., Carrara L., Martins A.J., James A.A., Oliveira P.L., Paiva-Silva G.O. The redox-sensing gene Nrf2 affects intestinal homeostasis, insecticide resistance, and Zika virus susceptibility in the mosquito Aedes aegypti. J. Biol. Chem. 2018;293:9053–9063. doi: 10.1074/jbc.RA117.001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. 2020. Experimental Treatment with Favipiravir for COVID-19: an Open-Label Control Study. Engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.-Y., Yu J., Liu T.-T., Yang K.-X., Yang L.-Y., Chen Q., Shi F., Hao J.-J., Cai Y., Wang M.-R., Lu W.-H., Zhang Y. Plumbagin inhibits the proliferation and survival of esophageal cancer cells by blocking STAT3-PLK1-AKT signaling. Cell Death Dis. 2018;9:17. doi: 10.1038/s41419-017-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Kong Q., Shan X., Tian G., Ilieva H., Cleveland D.W., Rothstein J.D., Borchelt D.R., Wong P.C., Lin C.L. Messenger RNA oxidation occurs early in disease pathogenesis and promotes motor neuron degeneration in ALS. PloS One. 2008;3:e2849. doi: 10.1371/journal.pone.0002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-W., Li H.-C., Hsu C.-F., Chang C.-Y., Lo S.-Y. Increased ATP generation in the host cell is required for efficient vaccinia virus production. J. Biomed. Sci. 2009;16:80. doi: 10.1186/1423-0127-16-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan R.D., Shinde P., Girkar K., Madage R., Chowdhary A. Assessment of anti-influenza activity and hemagglutination inhibition of Plumbago indica and Allium sativum extracts. Pharmacogn. Res. 2016;8:105–111. doi: 10.4103/0974-8490.172562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checconi P., De Angelis M., Marcocci M.E., Fraternale A., Magnani M., Palamara A.T., Nencioni L. Redox-modulating agents in the treatment of viral infections. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21114084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li L. The Lancet. Infectious Diseases. 2020. SARS-CoV-2: virus dynamics and host response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.K., Minakuchi M., Wuputra K., Ku C.C., Pan J.B., Kuo K.K., Lin Y.C., Saito S., Lin C.S., Yokoyama K.K. Redox control in the pathophysiology of influenza virus infection. BMC Microbiol. 2020;20:214. doi: 10.1186/s12866-020-01890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Shen T., Zhong L., Liu Z., Dong X., Huang T., Wang Q., Xiao H. Research progress of chloroquine and hydroxychloroquine on the COVID-19 and their potential risks in clinic use. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A., Pajares M., Benito C., Jiménez-Villegas J., Escoll M., Fernández-Ginés R., Garcia Yagüe A.J., Lastra D., Manda G., Rojo A.I., Dinkova-Kostova A.T. Trends in Pharmacological Sciences. 2020. Can activation of NRF2 Be a strategy against COVID-19? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Database., B.D. http://bodd.cf.ac.uk/. . BotDermFolder/Bott DermP/DROS.html [cited in 1999b], Droseraceae (Sundew family) ((date of last modiþ cation, 1984).). Available from:
- de Lima O.G., d' Albuquerque I.L., Maciel G.M., Maciel M.C. Vol. 8. Revista do Instituto de Antibioticos, Universidade Federal de Pernambuco; 1968. pp. 95–97. ([Antimicrobial substances of superior plants. XXVII. Isolation of plumbagin from Plumbago scandens L]). [PubMed] [Google Scholar]
- Denq R.Y., Fridovich I. Formation of endonuclease III-sensitive sites as a consequence of oxygen radical attack on DNA. Free Radic. Biol. Med. 1989;6:123–129. doi: 10.1016/0891-5849(89)90109-3. [DOI] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGuiseppi J., Fridovich I. Oxygen toxicity in Streptococcus sanguis. The relative importance of superoxide and hydroxyl radicals. J. Biol. Chem. 1982;257:4046–4051. [PubMed] [Google Scholar]
- Farr S.B., Natvig D.O., Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J. Bacteriol. 1985;164:1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet A., Barrios A.A., Muñoz V., Hocquemiller R., Cavé A. Effect of natural naphthoquinones in BALB/c mice infected with Leishmania amazonensis and L. venezuelensis. Trop. Med. Parasitol. 1992;43:219–222. [PubMed] [Google Scholar]
- Fujii N., Yamashita Y., Arima Y., Nagashima M., Nakano H. Induction of topoisomerase II-mediated DNA cleavage by the plant naphthoquinones plumbagin and shikonin. Antimicrob. Agents Chemother. 1992;36:2589–2594. doi: 10.1128/aac.36.12.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri R., Bhardwaj T., Shegane M., Gehi B.R., Kumar P., Gadhave K., Oldfield C.J., Uversky V.N. Understanding COVID-19 via comparative analysis of dark proteomes of SARS-CoV-2, human SARS and bat SARS-like coronaviruses. Cell. Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., O'Meara M.J., Guo J.Z., Swaney D.L., Tummino T.A., Hüttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Naing Z.Z.C., Zhou Y., Peng S., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Shen W., Shi Y., Zhang Z., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Ramachandran R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Lin Y., Wankowicz S.A., Bohn M., Trenker R., Young J.M., Cavero D., Hiatt J., Roth T., Rathore U., Subramanian A., Noack J., Hubert M., Roesch F., Vallet T., Meyer B., White K.M., Miorin L., Agard D., Emerman M., Ruggero D., García-Sastre A., Jura N., Zastrow M.v., Taunton J., Schwartz O., Vignuzzi M., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S., Fraser J.S., Gross J., Sali A., Kortemme T., Beltrao P., Shokat K., Shoichet B.K., Krogan N.J. 2020. (A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing). bioRxiv, 2020.vol. 2003.2022.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve M. 1931. A modern herbal; the medicinal, culinary, cosmetic and economic properties, cultivation and folk-lore of herbs, grasses, fungi, shrubs, & trees with all their modern scientific uses. New York : Harcourt, Brace & company, [1931] [Google Scholar]
- Hassan H.M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 1979;196:385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan S.T., Berchova-Bimova K., Petras J. Plumbagin, a plant-derived compound, exhibits antifungal combinatory effect with amphotericin B against Candida albicans clinical isolates and anti-hepatitis C virus activity. Phytother Res. : PTR. 2016;30:1487–1492. doi: 10.1002/ptr.5650. [DOI] [PubMed] [Google Scholar]
- Hemilä H., Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD005532.pub2. [DOI] [PubMed] [Google Scholar]
- Imlay J., Fridovich I. Exogenous quinones directly inhibit the respiratory NADH dehydrogenase in Escherichia coli. Arch. Biochem. Biophys. 1992;296:337–346. doi: 10.1016/0003-9861(92)90581-g. [DOI] [PubMed] [Google Scholar]
- Jacoby D.B., Choi A.M. Influenza virus induces expression of antioxidant genes in human epithelial cells. Free Radic. Biol. Med. 1994;16:821–824. doi: 10.1016/0891-5849(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Kapur A., Beres T., Rathi K., Nayak A.P., Czarnecki A., Felder M., Gillette A., Ericksen S.S., Sampene E., Skala M.C., Barroilhet L., Patankar M.S. Oxidative stress via inhibition of the mitochondrial electron transport and Nrf-2-mediated anti-oxidative response regulate the cytotoxic activity of plumbagin. Sci. Rep. 2018;8:1073. doi: 10.1038/s41598-018-19261-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Kim H., Bae S., Choi J., Lim S.Y., Lee N., Kong J.M., Hwang Y.-I., Kang J.S., Lee W.J. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 2013;13:70–74. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos C., Paller C.J., Verma A., Kader K., Kittrelle J., Borgström P.G., Vaishampayan U.N. A phase I dose escalation study of PCUR-101 in men with metastatic castration-resistant prostate cancer (mCRPC) J. Clin. Oncol. 2019;37:e16517. [Google Scholar]
- Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxid. Med. Cell. Longev. 2018:6208067. doi: 10.1155/2018/6208067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxid. Med. Cell. Longev. 2018;31 doi: 10.1155/2018/6208067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wu J., Deleo C.J. RNA damage and surveillance under oxidative stress. IUBMB Life. 2006;58:581–588. doi: 10.1080/15216540600946456. [DOI] [PubMed] [Google Scholar]
- Liu X., Niu M., Xu X., Cai W., Zeng L., Zhou X., Yu R., Xu K. CRM1 is a direct cellular target of the natural anti-cancer agent plumbagin. J. Pharmacol. Sci. 2014;124:486–493. doi: 10.1254/jphs.13240fp. [DOI] [PubMed] [Google Scholar]
- Manda K.R., Banerjee A., Banks W.A., Ercal N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radic. Biol. Med. 2011;50:801–810. doi: 10.1016/j.freeradbiomed.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew C., Ghildyal R. CRM1 inhibitors for antiviral therapy. Front. Microbiol. 2017;8:1171. doi: 10.3389/fmicb.2017.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/s0140-6736(20)31180-6. ISSN: 0140-6736 (Print) 0140-6736 PMID: 32450107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Min B.S., Miyashiro H., Hattori M. Inhibitory effects of quinones on RNase H activity associated with HIV-1 reverse transcriptase. Phytother Res. : PTR. 2002;16(Suppl. 1):S57–S62. doi: 10.1002/ptr.808. [DOI] [PubMed] [Google Scholar]
- Nair R.S., Kumar J.M., Jose J., Somasundaram V., Hemalatha S.K., Sengodan S.K., Nadhan R., Anilkumar T.V., Srinivas P. Increased sensitivity of BRCA defective triple negative breast tumors to plumbagin through induction of DNA Double Strand Breaks (DSB) Sci. Rep. 2016;6:26631. doi: 10.1038/srep26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Saito H., Ikeda M., Hokari R., Kato N., Hibi T., Miura S. An antioxidant resveratrol significantly enhanced replication of hepatitis C virus. World J. Gastroenterol. 2010;16:184–192. doi: 10.3748/wjg.v16.i2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A., Perry G., Pappolla M.A., Wade R., Hirai K., Chiba S., Smith M.A. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A., Chiba S., Kosaka K., Takeda A., Castellani R.J., Smith M.A., Perry G. Neuronal RNA oxidation is a prominent feature of dementia with Lewy bodies. Neuroreport. 2002;13:2035–2039. doi: 10.1097/00001756-200211150-00009. [DOI] [PubMed] [Google Scholar]
- Prieto-Alamo M.J., Abril N., Pueyo C. Mutagenesis in Escherichia coli K-12 mutants defective in superoxide dismutase or catalase. Carcinogenesis. 1993;14:237–244. doi: 10.1093/carcin/14.2.237. [DOI] [PubMed] [Google Scholar]
- Ravindra K.C., Selvi B.R., Arif M., Reddy B.A., Thanuja G.R., Agrawal S., Pradhan S.K., Nagashayana N., Dasgupta D., Kundu T.K. Inhibition of lysine acetyltransferase KAT3B/p300 activity by a naturally occurring hydroxynaphthoquinone, plumbagin. J. Biol. Chem. 2009;284:24453–24464. doi: 10.1074/jbc.M109.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshi M.L., Su Y.C., Hong J.R. RNA viruses: ROS-mediated cell death. Int. J. Cell Biol. 2014:467452. doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakpakdeejaroen I., Somani S., Laskar P., Mullin M., Dufès C. Transferrin-bearing liposomes entrapping plumbagin for targeted cancer therapy. J. Interdisp. Nanomed. 2019;4:54–71. doi: 10.1002/jin2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur S.K., Ichikawa H., Sethi G., Ahn K.S., Aggarwal B.B. Plumbagin (5-hydroxy-2-methyl-1, 4-naphthoquinone) suppresses NF-κB activation and NF-κB-regulated gene products through modulation of p65 and IκBα kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J. Biol. Chem. 2006;281:17023–17033. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- Sharma N., Kaushik P. Medicinal, biological and pharmacological aspects of Plumbago zeylanica (Linn.) J. Pharmacogn. Phytochem. 2014;3:117–120. [Google Scholar]
- Sharma K., Åkerström S., Sharma A.K., Chow V.T., Teow S., Abrenica B., Booth S.A., Booth T.F., Mirazimi A., Lal S.K. SARS-CoV 9b protein diffuses into nucleus, undergoes active Crm1 mediated nucleocytoplasmic export and triggers apoptosis when retained in the nucleus. PloS One. 2011;6 doi: 10.1371/journal.pone.0019436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simenauer A., Assefa B., Rios-Ochoa J., Geraci K., Hybertson B., Gao B., McCord J., Elajaili H., Nozik-Grayck E., Cota-Gomez A. Repression of Nrf2/ARE regulated antioxidant genes and dysregulation of the cellular redox environment by the HIV Transactivator of Transcription. Free Radic. Biol. Med. 2019;141:244–252. doi: 10.1016/j.freeradbiomed.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Pal K., Elkhanany A., Dutta S., Cao Y., Mondal G., Iyer S., Somasundaram V., Couch F.J., Shridhar V., Bhattacharya R., Mukhopadhyay D., Srinivas P. Plumbagin inhibits tumorigenesis and angiogenesis of ovarian cancer cells in vivo. Int. J. Canc. 2013;132:1201–1212. doi: 10.1002/ijc.27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram V., Hemalatha S.K., Pal K., Sinha S., Nair A.S., Mukhopadhyay D., Srinivas P. Selective mode of action of plumbagin through BRCA1 deficient breast cancer stem cells. BMC Canc. 2016;16:336. doi: 10.1186/s12885-016-2372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas P., Gopinath G., Banerji A., Dinakar A., Srinivas G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol. Carcinog. 2004;40:201–211. doi: 10.1002/mc.20031. [DOI] [PubMed] [Google Scholar]
- Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thasni K.A., Rakesh S., Rojini G., Ratheeshkumar T., Srinivas G., Priya S. Estrogen-dependent cell signaling and apoptosis in BRCA1-blocked BG1 ovarian cancer cells in response to plumbagin and other chemotherapeutic agents. Ann. Oncol. 2008;19:696–705. doi: 10.1093/annonc/mdm557. [DOI] [PubMed] [Google Scholar]
- Tripathi S.K., Panda M., Biswal B.K. Emerging role of plumbagin: cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019;125:566–582. doi: 10.1016/j.fct.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Vasudevarao M.D., Mizar P., Kumari S., Mandal S., Siddhanta S., Swamy M.M.M., Kaypee S., Kodihalli R.C., Banerjee A., Naryana C., Dasgupta D., Kundu T.K. Naphthoquinone-mediated inhibition of lysine acetyltransferase KAT3B/p300, basis for non-toxic inhibitor synthesis. J. Biol. Chem. 2014;289:7702–7717. doi: 10.1074/jbc.M113.486522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaowei Li M.G., Yizhao Penga Liesu Meng. Lu Shemin. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharmaceut. Anal. 2020;5 doi: 10.1016/j.jpha.2020.03.001. March 2020 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Wang X. COVID-19: a new challenge for human beings. Cell. Mol. Immunol. 2020;17(5):555–557. doi: 10.1038/s41423-020-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Dai L., Zhang X., Zhang Z., Zhang Z. Hydroxychloroquine and chloroquine: a potential and controversial treatment for COVID-19. Arch Pharm. Res. (Seoul) 2020;43:765–772. doi: 10.1007/s12272-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]