Highlights
-
•
We retrospectively included 297 hospitalized COVID-19 patients and explore factors associated with clinical outcomes.
-
•
23.0% were detectable for SARS-CoV-2 RNA in annal swabs and/or blood samples, higher in severe/critical cases than in mild/moderate cases.
-
•
The CD4/CD8 ratio was higher in severe/critical cases than in mild/moderate cases (1.84 vs. 1.50, P=0.022).
-
•
Presence of extrapulmonary virus and higher CD4/CD8 ratio were independent risk factors of respiratory failure and ICU admission.
Keywords: COVID-19, SARS-CoV-2, Extrapulmonary, Virus, CD4/CD8 ratio, Respiratory failure, ICU admission
Abstract
Background
Coronavirus Disease 2019 (COVID-19) is threatening billions of people. We described the clinical characteristics and explore virological and immunological factors associated with clinical outcomes.
Methods
297 COVID-19 patients hospitalized in Guangzhou Eighth People's Hospital between January 20 and February 20, 2020 were included. Epidemiological, clinical and laboratory data were collected and analyzed. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA in respiratory tract, blood samples and digestive tract was detected and lymphocyte subsets were tested periodically.
Result
Among the 297 patients (median age of 48 years), 154 (51.9 %) were female, 245 (82.5 %) mild/moderate cases, and 52 (17.5 %) severe/critical cases. 270 patients were detected for SARS-CoV-2 RNA in anal swabs and/or blood samples, and the overall positive rate was 23.0 % (62/270), higher in severe/critical cases than in mild/moderate cases (52.0 % vs. 16.4 %, P < 0.001). The CD4/CD8 ratio on admission was significantly higher in severe/critical cases than in mild/moderate cases (1.84 vs. 1.50, P = 0.022). During a median follow-up period of 17 days, 36 (12.1 %) patients were admitted to intensive care unit (ICU), 16 (5.4 %) patients developed respiratory failure and underwent mechanical ventilation, four (1.3 %) patients needed extracorporeal membrane oxygenation (ECMO), only one (0.34 %) patients died of multiple organ failure. Detectable SARS-CoV-2 RNA in anal swabs and/or blood samples, as well as higher CD4/CD8 ratio were independent risk factors of respiratory failure and ICU admission.
Conclusions
Most of COVID-19 patients in Guangzhou are mild/moderate, and presence of extrapulmonary virus and higher CD4/CD8 ratio are associated with higher risk of worse outcomes.
1. Background
Since December, 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused an outbreak of respiratory illness termed Coronavirus Disease 2019 (COVID-19) globally [1,2]. The disease has become a global pandemic, threatening billions of people and leading to over 730,000 death worldwide [3]. At the late March, the epidemic had been under control in China with the strong and sustained efforts of the whole country. Despite many previous articles report mainly concentrating on the epidemiological findings and clinical characteristics of patients with COVID-19 in Hubei province and outside of Hubei [[4], [5], [6]], the clinical experience in Guangzhou, a non-epidemic area but with high risk of imported cases from abroad, is still very valuable for controlling this emerging disease.
Previous study shows that most cases of COVID-19 are mild with good prognosis, however, the proportion of organ failure, especially respiratory failure and mortality rate of severe patients is considerable [7]. One of the main challenges for the clinicians is how to quickly identify COVID-19 patients at high risk for worse outcomes. However, effective early warning indicators are still limited so far.
In this study, we comprehensively described the clinical characteristics, and explore virological and immunological factors associated with outcomes of hospitalized patients confirmed with COVID-19, exploring valuable experiences for controlling COVID-19 in non-endemic regions.
2. Methods
2.1. Study design and participants
This was a retrospective, observational study conducted at Guangzhou Eighth People’s Hospital. We analyzed hospitalized COVID-19 patients between January 20 and February 20, 2020. The end of follow up was June 1 st, 2020, or the day when patients recovered and discharged from hospital, or transferred to the designated hospital for critically ill patients, or died.
This study was approved by the ethics committee of Guangzhou Eighth People’s Hospital (Approval No. 202001134). Written informed consent was obtained from all patients.
2.2. Data collection
The medical records, nursing records and laboratory reports of all patients with COVID-19 were retrospectively collected and reviewed by two physicians, and all radiological images were reviewed by two radiologists. Peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood of studied patients. PBMCs were stained with the following antibodies: CD3-Pacific-Blue, CD4-APC/CY7 and CD8-Percp/CY5.5 to obtain percentage of CD3 + T cells, CD4 + T cells and CD8 + T cells, and CD4/CD8 ratio by flow cytometry.
The specimens of throat swabs, anal swabs and blood samples were obtained every 3–6 days. SARS-CoV-2 nucleic acid was detected by real-time fluorescence reverse transcriptional polymerase chain reaction (RT-PCR) on the platform of Da’an Gene Corporation, Sun Yat-sen University, Guangzhou, China, which has been described [8,9]. Viral RNA was extracted with Nucleic Acid Isolation Kit on an automatic workstation Smart 32, both were provided by Da’an Gene Corporation. Two sets of primers were used for two target genes (open reading frame 1ab [ORF1ab] and nucleocapsid protein [N]) according to the protocol issued by the National Institute for Viral Disease Control and Prevention in China [10].
2.3. Definition of disease status
Based on "Diagnosis and treatment of pneumonitis caused by new coronavirus (trial version 7)" issued by the National Health Commission of China on March 3, 2020, clinical classifications of patients with COVID-19 were defined as follows [11]. Mild status was defined as having mild clinical symptoms but no signs of pneumonia on imaging. Moderate status was defined as having fever and respiratory symptoms, and pneumonia on imaging. Severe status must meet any of the following conditions: 1) experiencing respiratory distress, RR ≥ 30 times/minute; 2) in the resting state, the oxygen saturation ≤93 %; 3) arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa). Critical status must meet any of the following conditions: 1) developing respiratory failure requiring mechanical ventilation; 2) occurrence of shock; 3) in need of intensive care unit (ICU) monitoring and treatment because of complicating with other organ failures.
2.4. Statistical analysis
Data were expressed as counts and percentages for categorical variables and as mean and standard deviation or median and interquartile range (IQR) for continuous variables. Qualitative and quantitative differences between subgroups were analyzed using χ 2 test or Fisher’s exact-tests for categorical parameters and the Student’s t-test or Mann-Whitney U test for continuous parameters, as appropriate. Cox regression model was performed to analyze the association of baseline parameters with clinical outcomes. All statistical tests were 2-sided. Statistical significance was taken as P < 0.05. All analyses were performed with SPSS software, version 26.0 (IBM, Armonk, NY).
3. Results
3.1. Basic characteristics on admission
From January 20, 2020 to February 20, 2020, 297 consecutive patients who had been confirmed with COVID-19 and admitted to Guangzhou Eighth People’s Hospital were included in this study. The median age was 48 years, and 154 (51.9 %) were female. 245 (82.5 %) patients were diagnosed with mild/moderate cases, 52 (17.5 %) severe/critical cases. The baseline characteristics are shown in Table 1 .
Table 1.
Demographic and Clinical Characteristics of the Studied Patients, According to Disease Severity*.
| Characteristic | All patients (n = 297) | Mild/Moderate cases (n = 245) | Severe/Critical cases (n = 52) | P value † |
|---|---|---|---|---|
| Age (IQR) — yr | 48 (35–62) | 44 (30–58) | 60 (51–68) | <0.001 |
| Male sex — no. (%) | 143 (48.1) | 111 (45.3) | 32 (61.5) | 0.033 |
| Cases imported from Hubei— no. (%) | 185 (62.3) | 148 (60.4) | 37 (71.2) | 0.146 |
| Any comorbidity — no. (%) | 111 (37.4) | 80 (32.7) | 31 (59.6) | <0.001 |
| Days from illness onset to admission (IQR) | 4 (2–7) | 4 (3–7) | 6 (4–10) | 0.142 |
| Symptoms on admission | ||||
| Fever — no. (%) | 211 (71.0) | 169 (69.7) | 42 (80.8) | 0.098 |
| Highest temperature (IQR) — °C | 38.0 (37.6–38.6) | 38.1 (37.7–38.5) | 38.5 (38.0–40.0) | 0.006 |
| Cough — no. (%) | 178 (59.9) | 142 (58.0) | 36 (69.2) | 0.132 |
| Dyspnea — no. (%) | 34 (11.4) | 16 (6.5) | 18 (34.6) | <0.001 |
| Diarrhea — no. (%) | 19 (6.4) | 11 (4.5) | 8 (15.4) | 0.004 |
| Other symptoms — no. (%) ‡ | 140 (47.1) | 107 (43.7) | 33 (65.3) | 0.009 |
| Vital signs on admission | ||||
| SaO2 (IQR) — % | 98 (97–99) | 98 (97–99) | 93 (88–96) | <0.001 |
| SaO2 ≤ 93 % — no. (%) | 10 (3.4) | 0 (0.0) | 10 (19.2) | <0.001 |
| Respiratory rate (IQR) — bpm | 20 (18–20) | 20 (18–20) | 20 (19–22) | 0.003 |
| Heart rate (IQR) — bpm | 84 (78–93) | 86 (80–92) | 89 (77–100) | 0.498 |
| Systolic pressure (IQR) — mm Hg | 125 (117–137) | 125 (117–134) | 133 (120–143) | 0.120 |
| Diastolic pressure (IQR) — mm Hg | 82 (75–90) | 80 (74–86) | 86 (68–93) | 0.916 |
| Laboratory findings | ||||
| C-reactive protein (IQR) — mg/L | 10 (10–26) | 10 (10–24) | 34 (20–57) | <0.001 |
| Procalcitonin — ng/mL | 0.04 (0.03-0.07) | 0.04 (0.03-0.06) | 0.09 (0.05-0.20) | 0.001 |
| Leukopenia — no. (%) | 70/272 (25.5) | 61/223 (27.4) | 9/49 (18.4) | 0.193 |
| Neutropenia — no. (%) | 48/272 (17.6) | 44/223 (19.7) | 4/49 (8.3) | 0.054 |
| Eosinophils — ×10⁹ per L | 0.02 (0.00-0.06) | 0.01(0.00-0.05) | 0.00 (0.00-0.01) | <0.001 |
| Lymphocytes — ×10⁹ per L | 1.4 (1.0–1.9) | 1.4 (1.1–1.8) | 1.0 (0.7–1.4) | <0.001 |
| Thrombocytopenia — no. (%) | 33/272 (12.0) | 25/223 (11.2) | 8/49 (16.3) | 0.321 |
| Total bilirubin (IQR) — μmol/L | 9 (7–14) | 9 (7–13) | 17 (7–27) | 0.020 |
| Albumin (SD) — g/L | 40 (37–43) | 40 (37–43) | 35 (33–39) | <0.001 |
| ALT elevation — no. (%) | 39/261 (14.9) | 25/214 (11.7) | 14/47 (29.8) | 0.002 |
| AST elevation — no. (%) | 47/269 (17.5) | 428/221 (12.7) | 19/48 (39.6) | <0.001 |
| Increased creatinine — no. (%) | 46/255 (18.0) | 34/211 (16.1) | 12/44 (27.3) | 0.080 |
| Increased creatine kinase — no. (%) | 28/258 (10.9) | 14/211 (6.6) | 14/47 (29.8) | <0.001 |
| Lactate dehydrogenase (IQR) — U/L | 189 (152–244) | 188 (153–229) | 297 (266–354) | <0.001 |
| Imaging findings | ||||
| Pneumonia — no. (%) | 241 (81.1) | 193 (78.8) | 52 (100.0) | 0.023 |
| Hydrothorax — no. (%) | 20/284 (7.0) | 14/236 (5.9) | 6/48 (12.5) | 0.105 |
| Pulmonary consolidation — no. (%) | 22/284 (7.7) | 16/236 (6.8) | 6/48 (12.5) | 0.177 |
The denominators of patients who were included in the analysis are provided if they differed from the overall numbers in the group. Percentages may not total 100 because of rounding. The increase and decrease of laboratory indicators are compared with the normal range of local laboratory testing. IQR denotes interquartile range, SD standard deviation, bpm beats per minute, sec second, ALT alanine aminotransferase, AST aspartate aminotransferase.
Qualitative and quantitative differences between mild / moderate cases and severe cases were analyzed using χ 2 test or Fisher’s exact-tests for categorical parameters and the or Student’s t-test or Mann-Whitney test for continuous parameters, as appropriate. All statistical tests were 2-sided.
Other symptoms included myalgia, fatigue, sore throat, headache, nausea and vomiting.
3.2. Extrapulmonary distribution of the SARS-CoV-2 RNA
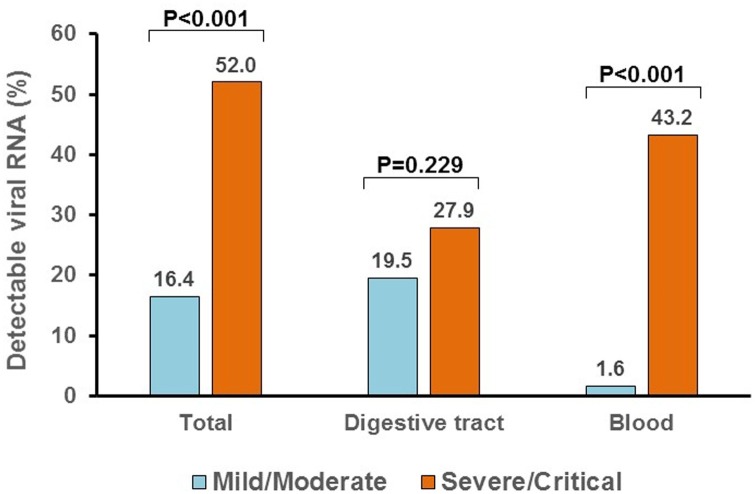
A total of 270 patients were detected for SARS-CoV-2 RNA in anal swabs and/or blood samples, and the overall positive rate was 23.0 % (62/270), higher in severe/critical cases than in mild/moderate cases (52.0 % vs. 16.4 %, P < 0.001) (Fig. 1 ). 217 patients were detected for viral RNA in anal swabs at a median of 8 days (QIR, 4-19) after admission, of which 21.2 % (46/217) were positive. The proportions of anal swabs positive were 27.9 % in severe/critical cases and 19.5 % in mild/moderate cases, but the difference is not statistically significant (P = 0.229). 234 patients were detected for viral RNA in blood samples at a median of 5 days (QIR, 2-8) after admission, and 9.4 % (22/234) were positive. The proportions of blood samples positive were significantly higher in severe/critical cases than in mild/moderate cases (43.2 % vs. 1.6 %, P < 0.001). 181 patients were detected for viral RNA both in anal swabs and blood samples, and 6 (3.3 %) patients were double positive (all were male, age ranged from 30 to 82 years). Notably, three of the six double positive patients were critical cases who developed respiratory failure and required mechanical ventilation in intensive care unit (ICU), two patients were severe cases who need high-flow nasal cannula and close monitoring, only one patient was moderate case.
Fig. 1.
Positive rate of SARS-CoV-2 RNA in anal swabs and blood samples between mild/moderate cases and severe/critical cases.
3.3. Percentage of T cells in PBMC
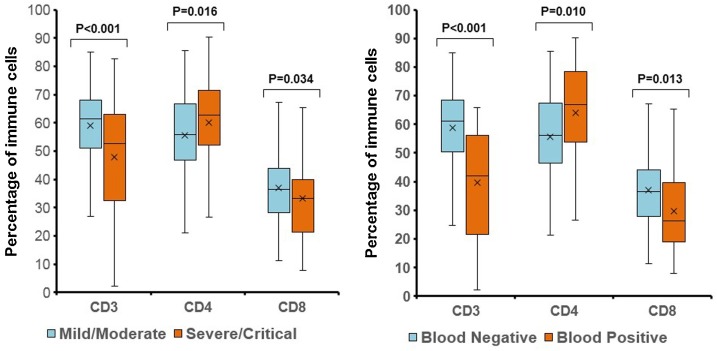
286 of the 297 patients (96.3 %) were analyzed for the percentage of CD3+ T cells, CD4+ T cells and CD8+ T cells, and CD4/CD8 ratio by flow cytometry in peripheral blood mononuclear cell (PBMC). Compared to the mild/moderate cases, the severe/critical cases had lower percentage of CD3+ T cells (52.6 % vs. 61.4 %, P < 0.001) and CD8+ T cells (33.2 % vs. 36.5 %, P = 0.034), but higher percentage of CD4+ T cells (62.8 % vs. 55.8 %, P = 0.016) (Fig. 2A). The CD4/CD8 ratio on admission was significantly higher in severe/critical cases than in mild/moderate cases (1.84 vs. 1.50, P = 0.022). In addition, patients who were detectable for viral RNA in blood samples had lower percentage of CD3+ T cells (42.1 % vs. 61.2 %, P < 0.001) and CD8+ T cells (26.4 % vs. 33.6 %, P = 0.013), but higher percentage of CD4+ T cells (66.9 % vs. 56.2 %, P = 0.010) (Fig. 2B), and higher CD4/CD8 ratio (2.57 vs. 1.49, P = 0.008). However, there was no significant difference in the percentage of CD3+ T cells, CD4+ T cells and CD8+ T cells between admission and the end of follow up (60.0 % vs. 60.1 %; 57.3 % vs. 57.2 %; 35.4 % vs. 34.3 %; all P values>0.05).
Fig. 2.
Percentage of CD3+ T cells, CD4+ T cells and CD8+ T cells. A: Between mild/moderate cases and severe/critical cases. B: Between cases with detectable and undetectable viral RNA in blood samples.
3.4. Treatment and clinical outcomes
Among the 297 patients, nearly two thirds received oxygen inhalation, antibiotic therapy, traditional Chinese medicine and antiviral therapy (including lopinavir/ritonavir, arbidol and chloroquine phosphate). In addition, 66 (22.2 %) patients were treated with oseltamivir, 65 (21.9) with corticosteroids, and 35 (11.9 %) with immunoglobulin, respectively (Supplemental Table 1).
During a median follow-up period of 17 days (IQR, 13-24), 36 (12.1 %) patients were admitted to ICU for high-flow nasal cannula or higher-level oxygen support measures to correct hypoxemia (Table 2 ). 16 (5.4 %) patients developed respiratory failure and underwent mechanical ventilation. Extracorporeal membrane oxygenation (ECMO) was performed in 4 (1.3 %) patients. One female patient with age of 30 years received successful operation because of ruptured ovarian cyst.
Table 2.
Clinical Process and Outcomes of the Studied Patients.
| Variable * | All patients (n = 297) | Mild/Moderate cases (n = 245) | Severe/Critical cases (n = 52) | P value |
|---|---|---|---|---|
| Follow-up days (IQR) — d | 17 (13–24) | 17 (12–23) | 22 (15–29) | 0.005 |
| Admission to intensive care unit — no. (%) | 36 (12.1) | 0 (0.0) | 36 (69.2) | <0.001 |
| Mechanical ventilation — no. (%) | 16 (5.4) | 0 (0.0) | 16 (30.8) | <0.001 |
| Use of ECMO — no. (%) | 4 (1.3) | 0 (0.0) | 4 (7.7) | 0.001 |
| Outcomes | <0.001 | |||
| Recovered and discharge from hospital — no. (%) | 284 (95.6) | 245 (100.0) | 39 (75.0) | — |
| Transferred for advanced treatment — no. (%) | 12 (4.0) | 0 (0.0) | 12 (23.1) | — |
| Death — no. (%) | 1 (0.3) | 0 (0.0) | 1 (1.9) | — |
IQR denotes interquartile range, ECMO extracorporeal membrane oxygenation.
As of June 1 st, 2020, 284 (95.6 %) patients had recovered and discharged from Guangzhou Eighth People’s hospital, one (0.3 %) 82-year-old patient died of multiple organ failure even though receiving ECMO treatment, twelve (4.0 %) patients were transferred to the designated hospital for critically ill patients in Guangzhou due to the deterioration of their illness within 8 days (IQR, 5-18) after admission (Table 2). All of the twelve transferred patients had recovered and discharged from hospital. No medical staff in our hospital had nosocomial infection of COVID-19 since January.
3.5. Factors associated with clinical outcomes
Cox regression model was performed to analyze the association of baseline parameters including age, gender, comorbidities, clinical symptoms, laboratory index, imaging findings and extrapulmonary virological detection with the probability of respiratory failure (Table 3 ). In the multivariate analysis, detectable viral RNA in anal swabs and/or blood samples (HR: 17.91, 95 % CI: 3.90–82.20, P < 0.001), CD4/CD8 ratio (hazard ratio [HR]: 1.24, 95 % CI: 1.07–1.44, P = 0.005) and dyspnea (HR: 3.97, 95 % CI: 1.34–11.79, P = 0.013) were independently associated with respiratory failure. We further analyzed factors associated with ICU admission, and found that detectable viral RNA in anal swabs and/or blood samples (HR: 18.95, 95 % CI: 5.59–64.27, P < 0.001), and CD4/CD8 ratio (HR: 1.25, 95 % CI: 1.00–1.56, P = 0.046) were also independent predictors (Supplemental Table 2).
Table 3.
Baseline Variables Associated with Respiratory Failure among Patients with COVID-19.
| Variable* | Respiratory failure |
|||
|---|---|---|---|---|
| Univariate |
Multivariate† |
|||
| HR (95 % CI) | P | HR (95 % CI) | P | |
| Age (>60 vs. ≤ 60 years) |
4.05 (1.47–11.15) | 0.007 | ||
| Sex (Male vs. Female) |
4.71 (1.34–16.53) | 0.016 | ||
| Comorbidity (Yes vs. No) |
3.82 (1.33–10.99) | 0.013 | ||
| Fever (Yes vs. No) |
1.17 (0.38–3.61) | 0.791 | ||
| Dyspnea (Yes vs. No) |
4.95 (1.80–13.61) | 0.002 | 3.97 (1.34–11.79) | 0.013 |
| Diarrhea (Yes vs. No) |
3.36 (0.96–11.80) | 0.058 | ||
| C-reactive protein (>10 vs. ≤ 10 mg/L) |
3.46 (1.10–10.87) | 0.034 | ||
| ALT elevation (Yes vs. No) |
0.39 (0.51–2.96) | 0.362 | ||
| Increased LDH (Yes vs. No) |
2.68 (0.97–7.40) | 0.057 | ||
| CD4/CD8 ratio | 1.19 (1.07–1.32) | 0.001 | 1.24 (1.07–1.44) | 0.005 |
| Pneumonia (Yes vs. No) |
0.71 (0.16–3.13) | 0.651 | ||
| Detectable viral RNA in anal swabs and/or blood samples (Yes vs. No) |
22.08 (4.98–97.94) | <0.001 | 17.91 (3.90–82.20) | <0.001 |
HR denotes hazard ratio, Cl confidence interval, ALT alanine transaminase, LDH lactate dehydrogenase.
Factors associated with severe/critical status were analyzed by Cox regression model (forward LR).
4. Discussion
The first two cases with COVID-19 in Guangzhou were admitted to Guangzhou Eighth People’s Hospital, the provincial and municipal designated hospital located in Guangzhou, in January 20, 2020. We found most of COVID-19 patients in this study were mild/moderate ones. Compared with those COVID-19 patients in the epidemic area Wuhan city, the proportions of patients with severe/critical clinical status and patients who needed ICU care or mechanical ventilation in this study were relatively lower [6]. This is mainly because Guangzhou can screen all suspected patients and has enough medical resources to ensure that all confirmed COVID-19 patients can be admitted to the designated hospital as soon as possible.
SARS-CoV-2 RNA can be detected not only in respiratory tract, but also in blood, digestive tract and feces [[12], [13], [14]]. Patients with more severe disease tended to have a higher detection rate of extrapulmonary SARS-CoV-2 RNA [15]. Recently published research showed that SARS-COV-2 RNA in serum was associated with multiple organ damages and higher mortality rate [16]. Our previous cross-sectional study indicated that detectable SARS-CoV-2 RNA in blood was an indicator for the further clinical severity, another study showed that detectable SARS-CoV-2 RNA in the digestive tract was a potential warning indicator of severe disease [8,9]. In this larger sample size longitudinal study, we found patients with detectable extrapulmonary SARS-CoV-2 RNA had an approximately 18-fold increase in the risk of respiratory failure and a 19-fold increase in the risk of ICU admission. In addition, patients with extrapulmonary virus had higher chance of progression from mild/moderate to severe/critical status (22.6 % vs. 8.7 %, P = 0.003). More importantly, we found the double positive patients (anal swabs positive plus blood samples positive) had much more severe disease (5/6 patients were severe/critical cases), highlighting the need to perform routine examination of digestive tract and blood virus in the clinic.
Lymphocytopenia is often found in patients with COVID-19, especially in severe ones [7,17]. Chen et al. found lower CD4+ T cells count was associated with ICU admission [18]. Liu et al. found the more serious the disease and the worse the prognosis, the lower were the T cell, CD4+ T cell, and CD8+ T cell counts on admission [19]. A recent published study found CD4/CD8 ratio was significantly higher in critically ill than in non-critically ill patients [20]. We found severe/critical patients had lower levels of lymphocytes, lower percentage of CD3+ T cells and CD8+ T cells but higher percentage of CD4+ T cells than mild/moderate ones. Notably, higher CD4/CD8 ratio was independently associate with higher risk of respiratory failure and ICU admission. This result indicated that lymphocyte subsets could be a useful parameter for early prediction of prognosis of COVID-19. We also found that there was no significant change in the percentage of CD3+ T cells, CD4+ T cells and CD8+ T cells in the convalescent phase of COVID-19 compared to the baseline, so further research is needed to explore the repair process of the immune system.
The underlying mechanism between extrapulmonary distribution of the virus and CD4/CD8 ratio and disease severity is not completely clear. It could be explained by the following reasons. First, the extrapulmonary distribution of the virus may reflect the rampant coronavirus replication in pulmonary alveolus, which break through the alveolar vessel, leakage into the blood flow and spread throughout the body [9]. Further analysis showed that the cycle threshold (Ct) values (Ct = ORF1ab + N) of the throat swabs were lower in patients with extrapulmonary virus than those without (median: Ct = 36 + 34.5 vs. Ct = 40 + 39), suggesting a higher viral load in extrapulmonary virus positive patients. Second, counts of lymphocyte and lymphocyte subset are of great value to ensure immune system functionality, so the decreased lymphocyte, especially CD8+ T cells, might reflect immune injuries caused by virus attachment and/or inflammatory mediators. Third, the strong viral damage and the weakened immune system would make it difficult to eliminate the virus. We found that the duration from admission to positive-to-negative conversion of throat swabs viral RNA was longer in patients with extrapulmonary virus (11 days vs. 8 days, P = 0.032), despite they have similar antiviral treatment strategy. The persistence of the virus and the prolonged course of the disease may also be one of the reasons for the aggravation of the disease.
In this study, around 75 % of mild/moderate patients and nearly 100 % of the severe/critical patients received antiviral treatment including Lopinavir/ritonavir (LPV/r), abidol and chloroquine. However, these antiviral regimens perform little benefit for improving the clinical outcomes including virus clearance of hospitalized mild/moderate COVID-19 beyond supportive treatment [[12], [13], [14], [15],[21], [22], [23]]. The median days from admission to positive-to-negative conversion of viral RNA exceeded 10 days (11 days [IQR, 8-17]) in severe/critical cases disregard LPV/r or abidol or chloroquine, which indicates the antiviral failure of these medicines. Even so, our study in another way implies that comprehensive therapy scheme could successfully prevent and treat COVID-19 even without effective antiviral regimens currently.
Our study has some limitations. First, the comparison of different antiviral regimens was not randomized nor blinded, the baseline health status between antiviral and non-antiviral patients were not comparable. Second, we did not collect information on absolute values of T cells, B cells, NK cells, monocytes and dendritic cells. In addition, we were unable to provide information on the changes in the absolute number of immune cells in these patients. Further studies are needed to explore the relationship between these immunological indexes and outcomes of COVID-19.
5. Conclusions
In summary, most of COVID-19 patients in Guangzhou are in mild/moderate clinical status, and comprehensive therapy scheme could successfully treat COVID-19 even without effective antiviral regimens currently. Presence of extrapulmonary SARS-CoV-2 RNA and higher CD4/CD8 ratio are associated with higher risk of worse outcomes such as respiratory failure and ICU admission in patients with COVID-19.
Authors' contributions
Contributors: L Li, C Lei and W Lin conceived the study and designed the protocol. X Tang and X Deng gave instruction. F Hu contributed to statistical analysis and interpretation of data. C Lei, W Lin and L Li drafted the manuscript. F Hu and F Li were in charge of nuclear acid RT-PCR. X Tang, F Chen and C Wai reviewed the data independently. Y Li, C Wen, Y Guan, J Wang, X Chen and Yi Cao contributed to conducting the study and collecting data. All authors had full access to the final version of the manuscript and agreed to its submission.
Funding
This study was supported by Chinese 13th Five-Year National Science and technology major project (2018ZX10302103-002, 2017ZX10202102-003-004), and Infectious Disease Specialty of Guangzhou High-level Clinical Key Specialty (2019-2021).
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We thank all patients who participated in this study and all staff of Guangzhou Eighth People’s Hospital for their clinical care given to patients and facilitating access to the relevant medical records. We also thank Ruihua Xu and ZiXian Wang for giving us suggestions on the revision of the article who come from Sun Yat sen University Cancer center, Guangzhou, China.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104661.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . 2020. World Health Organization Coronavirus Disease 2019 (COVID-19) Situation Report - 204. [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin W., Xie Z., Li Y. Association between detectable SARS‐COV‐2 RNA in anal swabs and disease severity in patients with coronavirus disease 2019. J. Med. Virol. 2020 doi: 10.1002/jmv.26307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W., Lan Y., Yuan X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg. Microbes Infect. 2020;9:469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu H., Wu J., Hong L. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Diseases. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.China NHC . 2020. Diagnosis and Treatment of Pneumonitis Caused by New Coronavirus (Trial Version 7) (in Chinese) 2020*2020. [Google Scholar]
- 12.Wu J., Liu J., Li S. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med. Infect. Disease. 2020 doi: 10.1016/j.tmaid.2020.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin L., Jiang X., Zhang Z. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 15.Wong M.C., Huang J., Lai C. Detection of SARS-CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J. Infect. 2020;81:e31–e38. doi: 10.1016/j.jinf.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D., Zhou F., Sun W. Relationship between serum SARS-CoV-2 nucleic acid(RNAemia) and organ damage in COVID-19 patients: a cohort study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J., Qi T., Liu L. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z., Long W., Tu M. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallotto C., Suardi L.R., Esperti S. Increased CD4/CD8 ratio as a risk factor for critical illness in coronavirus disease 2019 (COVID-19): a retrospective multicentre study. Infect. Diseases (London, England) 2020:1–3. doi: 10.1080/23744235.2020.1778178. [DOI] [PubMed] [Google Scholar]
- 21.Cao B., Wang Y., Wen D. A trial of Lopinavir–Ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Xie Z., Lin W. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med. 2020 doi: 10.1016/j.medj.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.